Synthesis and Study of Sorption Properties of Zinc-Imprinted Polymer
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Zinc-Imprinted Polymer
2.3. Characterization Studies
2.4. Adsorption of Zn2+ Ions
2.5. Selectivity Studies
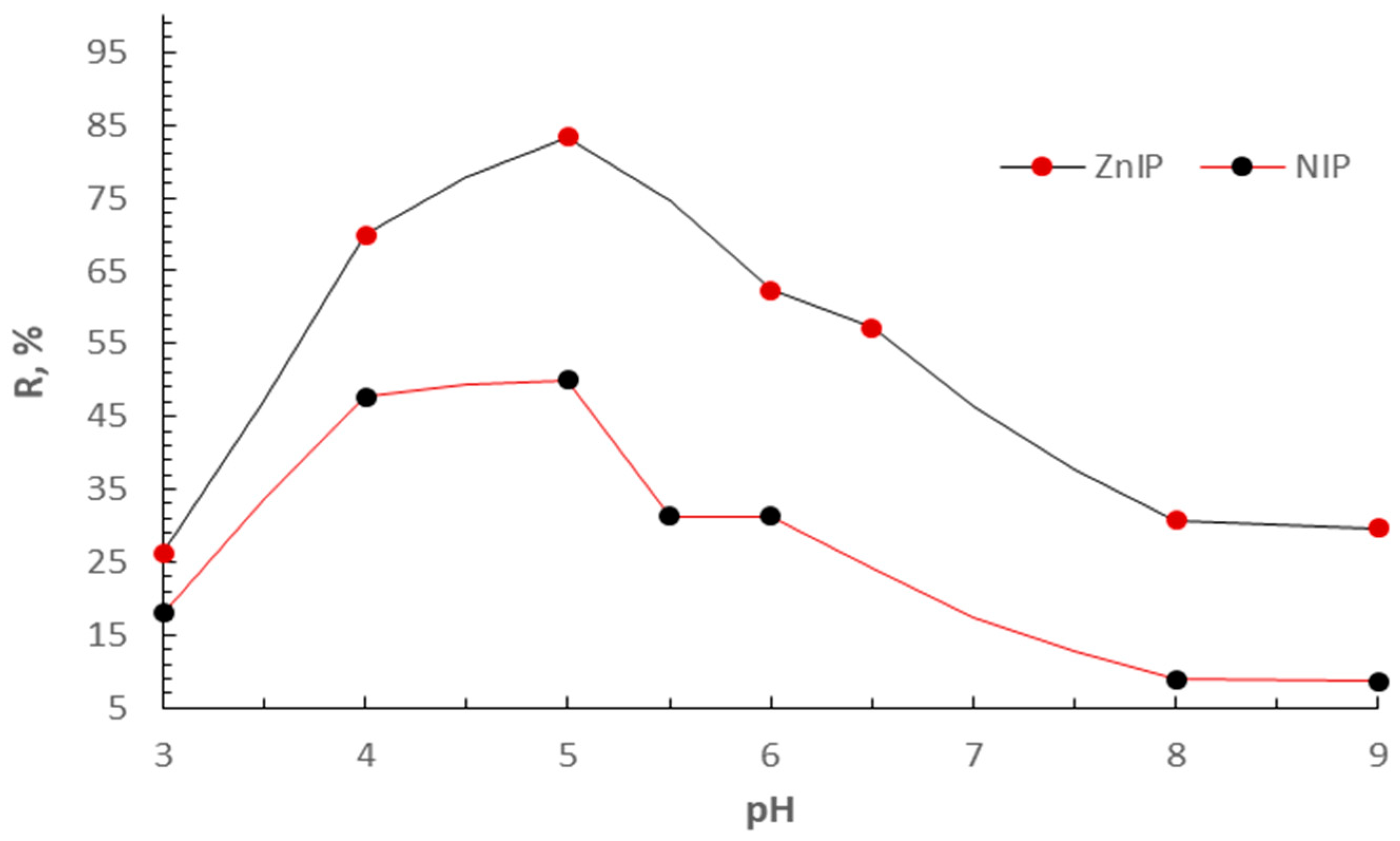
3. Results and Discussion
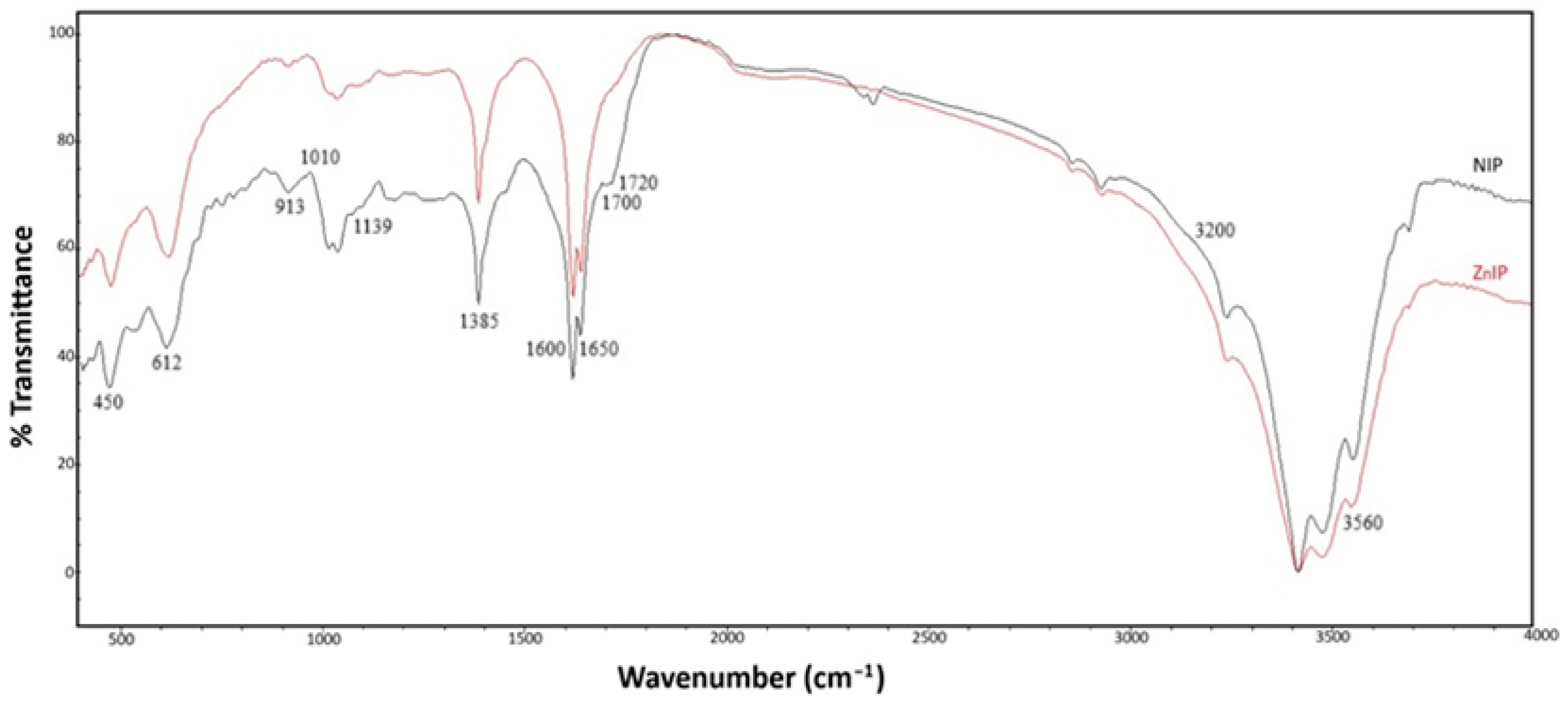
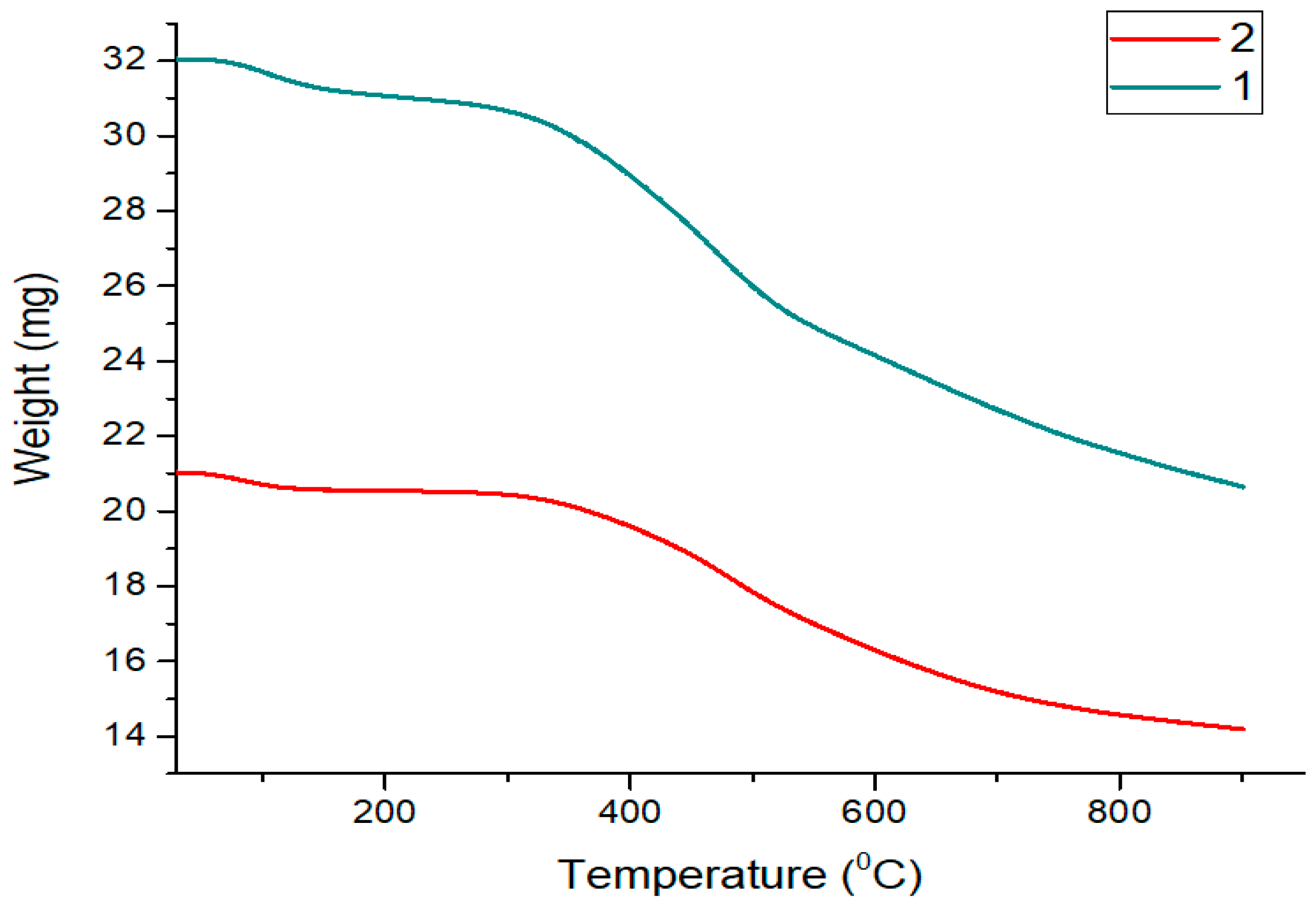
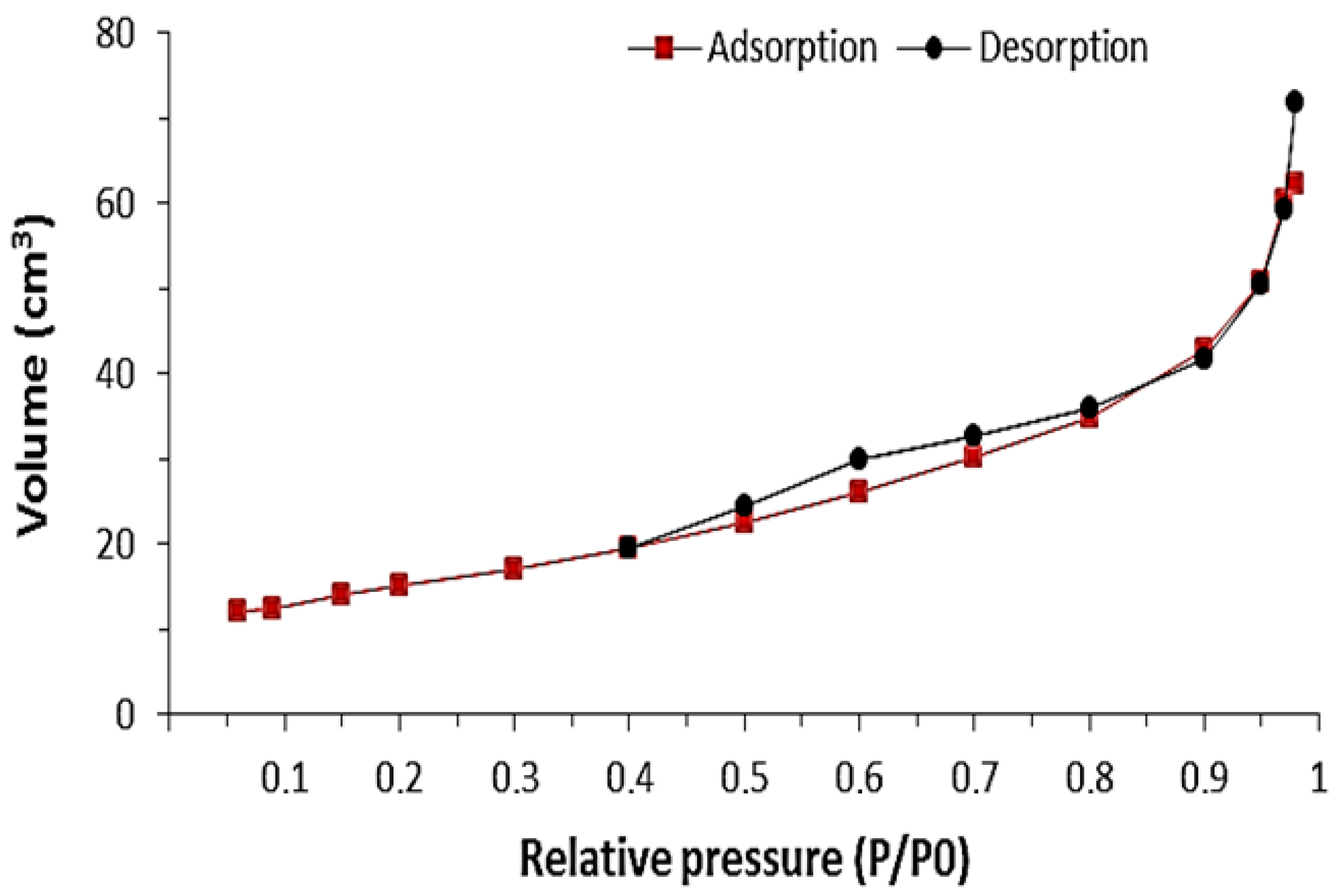
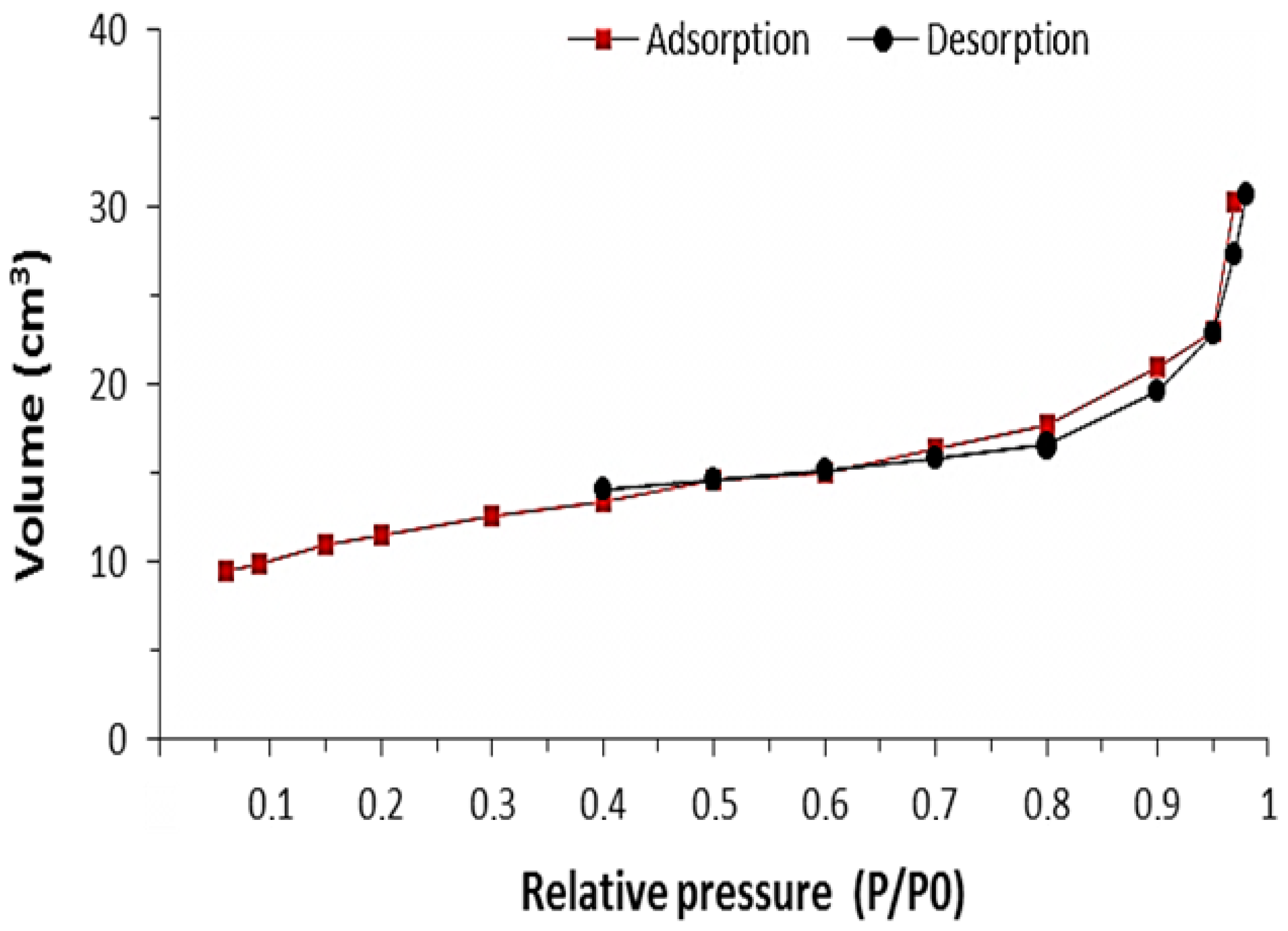
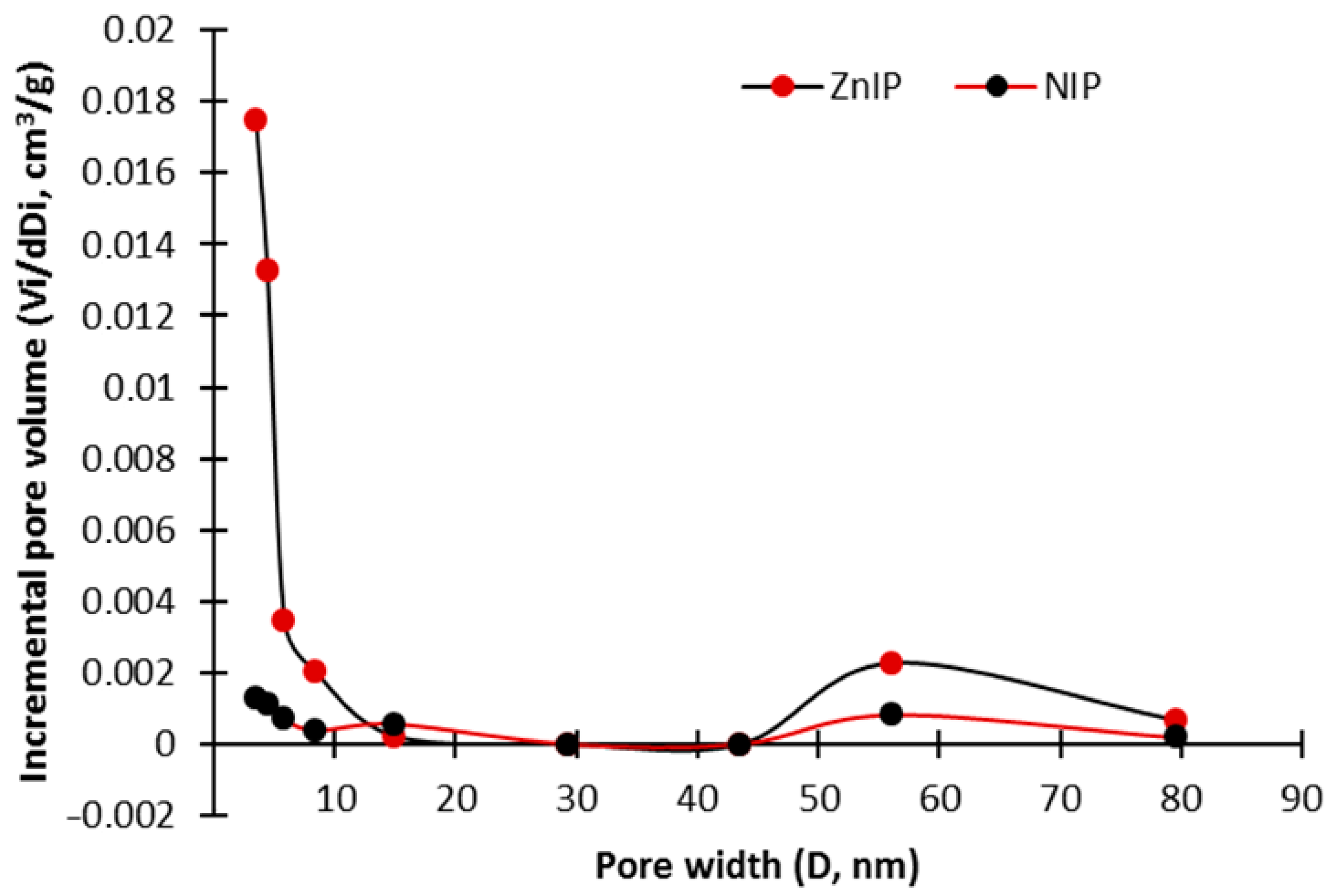
3.1. Characterization Analysis
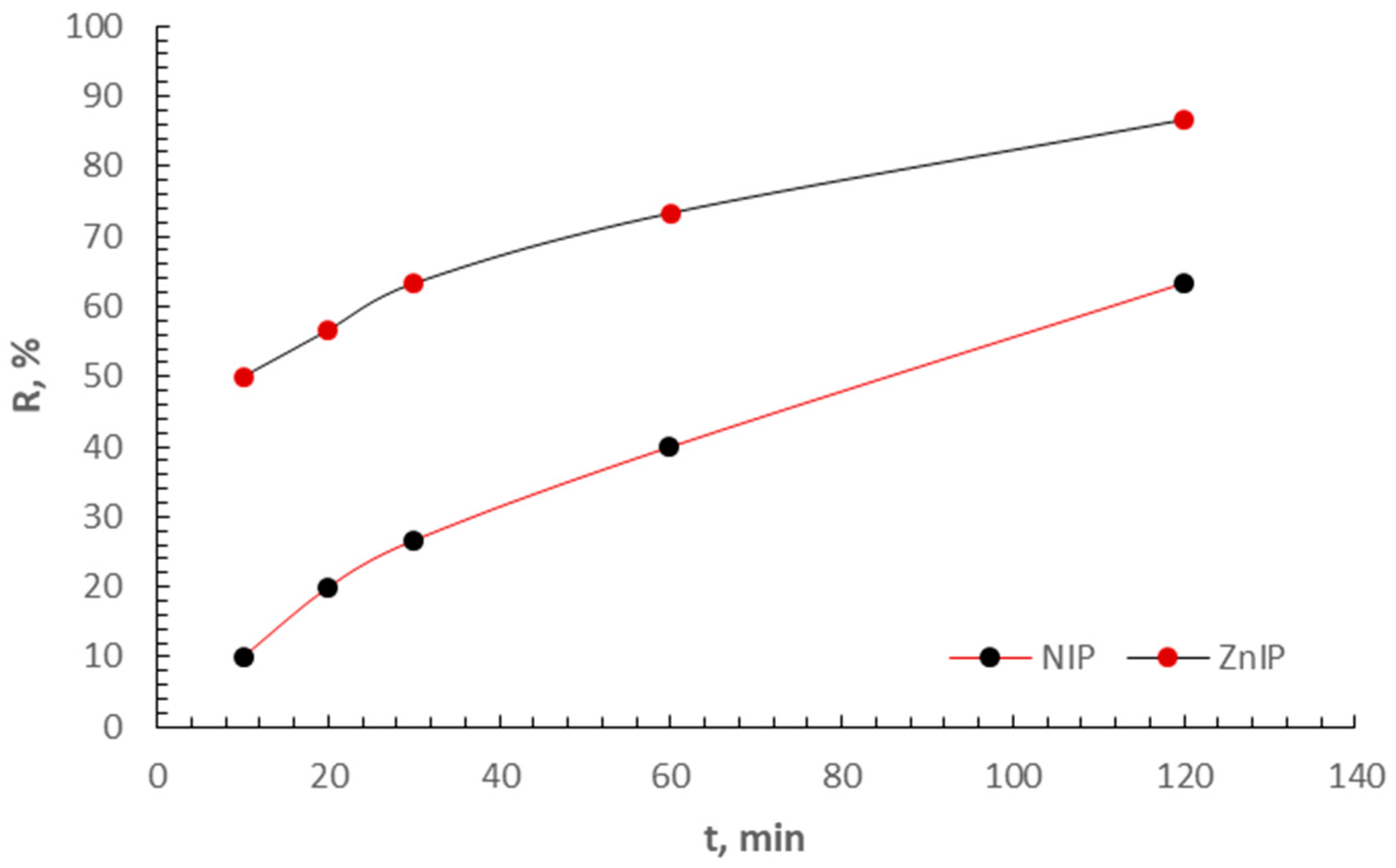
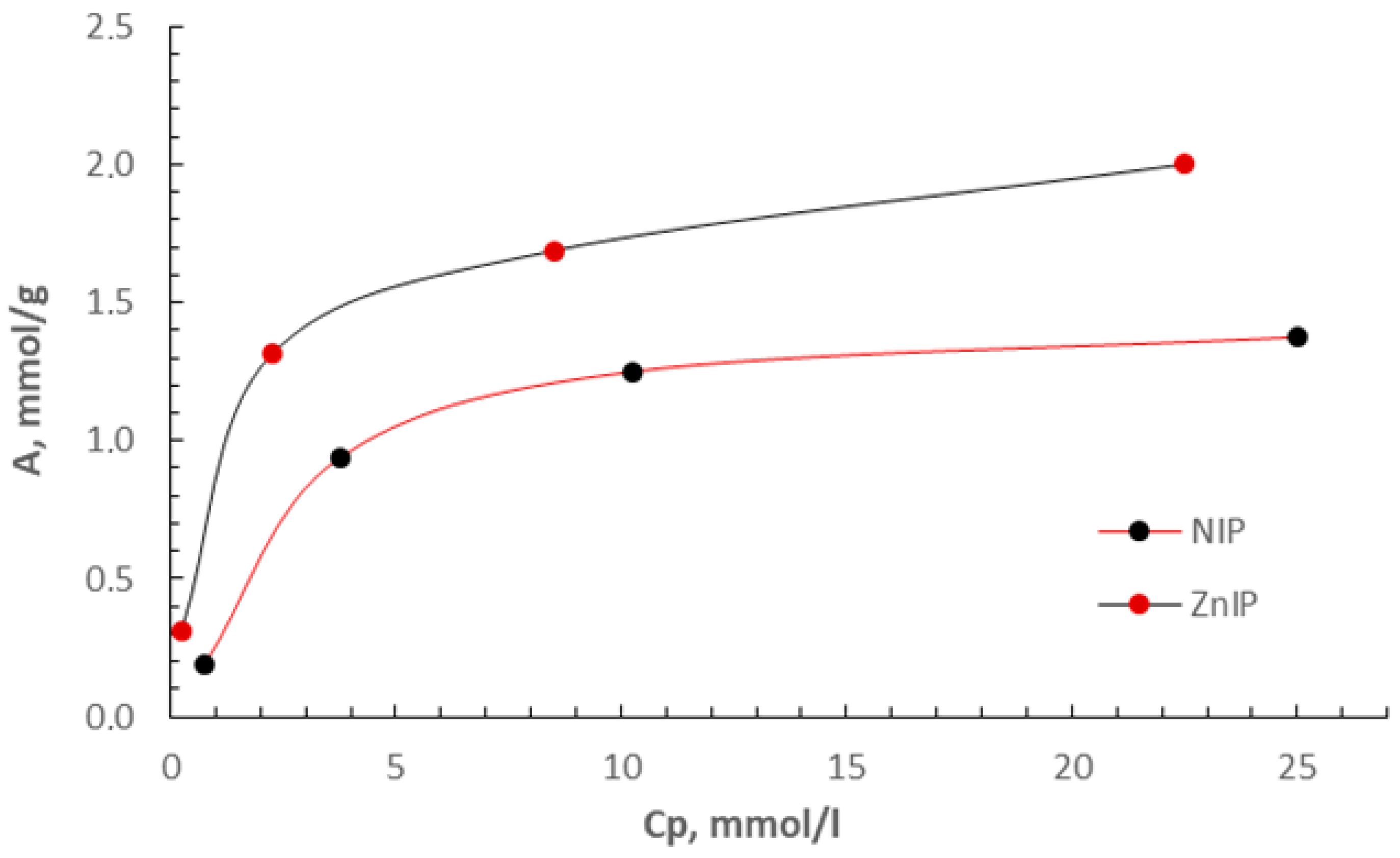
3.2. Adsorption Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Okutucu, B. Waste in Textile and Leather Sectors; Körlü, A., Ed.; IntechOpen: Izmir, Turkey, 2020; pp. 1–12. [Google Scholar] [CrossRef]
- Stevens, M.G.F.; Batlokwa, B.S. Multi-templated Pb-Zn-Hg Ion Imprinted Polymer for the Selective and Simultaneous Removal of Toxic Metallic Ions from Wastewater. Int. J. Chem. 2017, 9, 58114. [Google Scholar] [CrossRef]
- Kuras, M.J.; Perz, K.; Kołodziejski, W.L. Synthesis, characterization and application of a novel zinc(II) ion-imprinted polymer. Polym. Bull. 2017, 74, 5029–5048. [Google Scholar] [CrossRef]
- Wirawan, T.; Supriyanto, G.; Soegianto, A. Adsorption of Zinc(II) onto Zn(II)-Ionic Imprinted Polymer. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; Volume 546, p. 022036. [Google Scholar] [CrossRef]
- Lazar, M.M.; Ghiorghita, C.-A.; Dragan, E.S.; Humelnicu, D.; Dinu, M.V. Ion-Imprinted Polymeric Materials for Selective Adsorption of Heavy Metal Ions from Aqueous Solution. Molecules 2023, 28, 2798. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Huang, Y.; Han, G.; Guo, Z.; Liu, F. Study of the Adsorption of Humic Acid with Zn2+ by Molecular Dynamic Simulation and Adsorption Experiments. In Characterization of Minerals, Metals, and Materials 2019; The Minerals, Metals & Materials Series; Li, B., Li, J., Ikhmayies, S., Zhang, M., Kalay, Y.E., Carpenter, J.S., Hwang, J.-Y., Monteiro, S.N., Bai, C., Escobedo-Diaz, J.P., et al., Eds.; Springer: Cham, Switzerland, 2019; pp. 31–41. [Google Scholar] [CrossRef]
- Putri, T.; Zulfikar, M.A.; Wahyuningrum, D.; Saputra, I.S. Synthesis and Characterization of Molecularly Imprinted Polymers (MIPs) Using Humic Acid. J. Pure Appl. Chem. Res. 2022, 11, 175–181. [Google Scholar] [CrossRef]
- Bakhshi, A.; Daryasari, A.P.; Soleimani, M. A Molecularly Imprinted Polymer as the Adsorbent for the Selective Determination of Oxazepam in Urine and Plasma Samples by High-Performance Liquid Chromatography with Diode Array Detection. J. Anal. Chem. 2021, 76, 1414–1421. [Google Scholar] [CrossRef]
- Bivián-Castro, E.Y.; Zepeda-Navarro, A.; Guzmán-Mar, J.L.; Flores-Alamo, M.; Mata-Ortega, B. Ion-Imprinted Polymer Structurally Preorganized Using a Phenanthroline-Divinylbenzoate Complex with the Cu(II) Ion as Template and Some Adsorption Results. Polymers 2023, 15, 1186. [Google Scholar] [CrossRef] [PubMed]
- Özgecan, E.; Yeşeren, S.; Müge, A.; Adil, D. Molecularly Imprinted Polymers for Removal of Metal Ions: An Alternative Treatment Method. Biomimetics 2018, 3, 38. [Google Scholar] [CrossRef] [PubMed]
- Hasanah, A.N.; Safitri, N.; Zulfa, A.; Neli, N.; Rahayu, D. Factors Affecting Preparation of Molecularly Imprinted Polymer and Methods on Finding Template-Monomer Interaction as the Key of Selective Properties of the Materials. Molecules 2021, 26, 5612. [Google Scholar] [CrossRef]
- Abdou, M.M.; Soliman, A.-G.A.; Kobisy, A.S.; Abu-Rayyan, A.; Al-Omari, M.; Alshwyeh, H.A.; Ragab, A.H.; Al Shareef, H.F.; Ammar, N.S. Preparation and Evaluation of Phenol Formaldehyde-Montmorillonite and Its Utilization in the Adsorption of Lead Ions from Aqueous Solution. ACS Omega 2024, 9, 12015–12026. [Google Scholar] [CrossRef] [PubMed]
- Meza López, F.d.L.; Hernández, C.J.; Vega-Chacón, J.; Tuesta, J.C.; Picasso, G.; Khan, S.; Sotomayor, M.D.P.T.; López, R. Smartphone-Based Rapid Quantitative Detection Platform with Imprinted Polymer for Pb (II) Detection in Real Samples. Polymers 2024, 16, 1523. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, N.H.; Monier, M.; Alatawi, R.A.S.; Albalawi, M.A.; Alhawiti, A.S. Preparation of Chromium (III) Ion-Imprinted Polymer Based on Azo Dye Functionalized Chitosan. Carbohydr. Polym. 2022, 284, 119139. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Berrones, K.; Ocampo-Perez, R.; Rodríguez-Torres, I.; Medellín-Castillo, N.A.; Flores-Ramírez, R. Molecularly Imprinted Polymers (MIPs) as Efficient Catalytic Tools for the Oxidative Degradation of 4-nonylphenol and its by-products. Environ. Sci. Pollut. Res. 2023, 30, 90741–90756. [Google Scholar] [CrossRef]
- Singhal, A.; Singh, A.; Shrivastava, A.; Khan, R. Epitope Imprinted Polymeric Materials: Application in Electrochemical Detection of Disease Biomarkers. J. Mater. Chem. B 2023, 11, 936–954. [Google Scholar] [CrossRef] [PubMed]
- Dietl, S.; Sobek, H.; Mizaikoff, B. Epitope-imprinted polymers for biomacromolecules: Recent Strategies, Future Challenges and Selected Applications. TrAC Trends Anal. Chem. 2021, 143, 116414. [Google Scholar] [CrossRef]
- Cruz, A.G.; Haq, I.; Cowen, T.; Di Masi, S.; Trivedi, S.; Alanazi, K.; Piletska, E.; Mujahid, A.; Piletsky, S.A. Design and Fabrication of a Smart Sensor Using in Silico Epitope Mapping and Electro-Responsive Imprinted Polymer Nanoparticles for Determination Of Insulin Levels in Human Plasma. Biosens. Bioelectron. 2020, 169, 112536. [Google Scholar] [CrossRef]
- Piletska, E.; Magumba, K.; Joseph, L.; Cruz, A.G.; Norman, R.; Singh, R.; Tabasso, A.F.S.; Jones, D.J.L.; Macip, S.; Piletsky, S. Molecular Imprinting as a Tool for Determining Molecular Markers: A Lung Cancer Case. RSC Adv. 2022, 12, 17747–17754. [Google Scholar] [CrossRef] [PubMed]
- Piletsky, S.S.; Piletska, E.; Poblocka, M.; Macip, S.; Jonese, D.J.L.; Braga, M.; Cao, T.H.; Singh, R.; Spivey, A.C.; Aboagye, E.O.; et al. Snapshot Imprinting: Rapid Identification of Cancer Cell Surface Proteins and Epitopes Using Molecularly Imprinted Polymers. Nano Today 2021, 41, 101304. [Google Scholar] [CrossRef]
- Piletska, E.; Veron, P.; Bertin, B.; Mingozzi, F.; Jones, D.; Norman, R.L.; Earley, J.; Karim, K.; Garcia-Cruz, A.; Piletsky, S. Analysis of Adeno-Associated Virus Serotype 8 (AAV8)-Antibody Complexes Using Epitope Mapping by Molecular Imprinting Leads to the Identification of Fab Peptides That Potentially Evade AAV8 Neutralisation. Nanomed. Nanotechnol. Biol. Med. 2023, 52, 102691. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.-Z.; Cheng, S.-W.; Xu, L.-B.; Liu, H.-Y.; Huang, K.; Li, L.; Zhai, Y.-Y.; Zeng, Y.-B.; Liu, H.-Q.; Shao, Y.; et al. Highly Sensitive And Selective Sensor for Sunset Yellow Based on Molecularly Imprinted Polydopamine-Coated Multi-Walled Carbon Nanotubes. Biosens. Bioelectron. 2018, 100, 565–570. [Google Scholar] [CrossRef]
- Choi, D.Y.; Yang, J.C.; Hong, S.W.; Park, J. Molecularly Imprinted Polymer-Based Electrochemical Impedimetric Sensors on Screen-Printed Carbon Electrodes for the Detection of Trace Cytokine IL-1β. Biosens. Bioelectron. 2022, 204, 114073. [Google Scholar] [CrossRef]
- Erdem, Ö.; Eş, I.; Saylan, Y.; Atabay, M.; Gungen, M.A.; Ölmez, K.; Denizli, A.; Inci, F. In situ Synthesis and Dynamic Simulation of Molecularly Imprinted Polymeric Nanoparticles on a Micro-Reactor System. Nat. Commun. 2023, 14, 4840. [Google Scholar] [CrossRef] [PubMed]
- Brueckl, H.; Shoshi, A.; Schrittwieser, S.; Schmid, B.; Schneeweiss, P.; Mitteramskogler, T.; Haslinger, M.J.; Muehlberger, M.; Schotter, J. Nanoimprinted Multifunctional Nanoprobes for a Homogeneous Immunoassay in a Top-Down Fabrication Approach. Sci. Rep. 2021, 11, 6039. [Google Scholar] [CrossRef] [PubMed]
- Gaurav, S.; Balasubramanian, K. Molecularly Imprinted Polymers for Selective Recognition and Extraction of Heavy Metal Ions and Toxic Dyes. J. Chem. Eng. 2020, 65, 396–418. [Google Scholar] [CrossRef]
- Huang, D.L.; Wang, R.Z.; Liu, Y.G.; Zeng, G.-M.; Lai, C.; Xu, P.; Lu, B.-A.; Xu, J.-J.; Wang, C.; Huang, C. Application of molecularly imprinted polymers in wastewater treatment: A review. Environ. Sci. Pollut. Res. 2015, 22, 963–977. [Google Scholar] [CrossRef] [PubMed]
- Mabaso, N.B.; Nomngongo, P.N.; Nyaba, L. Recent Advances in Synthesising and Applying Magnetic Ion-Imprinted Polymers to Detect, Pre-Concentrate, and Remove Heavy Metals in Various Matrices. Processes 2024, 12, 1601. [Google Scholar] [CrossRef]
- Wirawan, T.; Supriyanto, G.; Soegianto, A. Synthesis, characterization, and application of novel Zn (II)-ionic imprinted polymer for preconcentration of Zn (II) ions from aqueous solution. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018; Volume 349, p. 012064. [Google Scholar]
- Çıtlakoğlu, M.; Yolcu, Z. Synthesis and characterization of zinc(II) methacrylate monomer complex and adsorption properties of zinc(II) ion-imprinted polymer. Inorg. Chim. Acta 2023, 555, 121605. [Google Scholar] [CrossRef]
- Zia, T.H.; Qureshi, M.H.; Ara, B.; Gul, K.; Ghazali, D.K. Evaluating the electrokinetic behavior of the acrylonitrile based Zn(II) ion-imprinted cross-linked polymer adsorbent for the Zn(II) ions selective removal from aqueous medium. Desalination Water Treat. 2022, 268, 67–88. [Google Scholar] [CrossRef]
- Klučáková, M.; Pavlíková, M. Lignitic Humic Acids as Environmentally-Friendly Adsorbent for Heavy Metals. J. Chem. 2017, 2017, 7169019. [Google Scholar] [CrossRef]
- Linnik, R.P.; Zaporozhets, O.; Mariychuk, A.R.; Lisnyak, V.V. Zinc Removal from Waters by Hybrid Sorbent with Humic Acid. In Proceedings of the 2019 International Council on Technologies of Environmental Protection (ICTEP), Starý Smokovec, Slovakia, 23–25 October 2019; pp. 164–168. [Google Scholar] [CrossRef]
- Masoud, M.S.; Zidan, A.A.; El Zokm, G.M.; Elsamra, R.M.I.; Okbah, M.A. Humic acid and Nano-Zeolite NaX as low cost and Eco-Friendly Adsorbents for removal of Pb (II) and Cd (II) From Water: Characterization, Kinetics, Isotherms and Thermodynamic Studies. Biomass Conv. Bioref. 2024, 14, 3615–3632. [Google Scholar] [CrossRef]
- Menad, K.; Feddag, A.; Juhna, T. Copper(II)–Humic Acid Adsorption Process Using Microporous-Zeolite Na-X. J. Inorg. Organomet. Polym. 2019, 29, 1–16. [Google Scholar] [CrossRef]
- Faisal, A.A.H.; Abdul-Kareem, M.B.; Mohammed, A.K.; Naushad, M.; Ghfar, A.A.; Ahamad, T. Humic Acid Coated Sand as a Novel Sorbent in Permeable Reactive Barrier for Environmental Remediation of Groundwater Polluted with Copper and Cadmium Ions. J. Water Process Eng. 2020, 36, 101373. [Google Scholar] [CrossRef]
- Zhakina, A.K.; Rakhimova, B.B.; Vassilets, Y.P.; Arnt, O.V.; Muldakhmetov, Z. Synthesis and Modification of a Natural Polymer with the Participation of Metal Nanoparticles, Study of Their Composition and Properties. Polymers 2024, 16, 264. [Google Scholar] [CrossRef] [PubMed]
- Muldakhmetov, Z.M.; Gazaliev, A.M.; Zhakina, A.K.; Vassilets, Y.P.; Arnt, O.V. Synthesis of a Composite Based on Humic Acid Tuned to Sorbed Copper Ion. Bull. Univ. Karaganda Chem. Ser. 2022, 108, 182–189. [Google Scholar] [CrossRef]
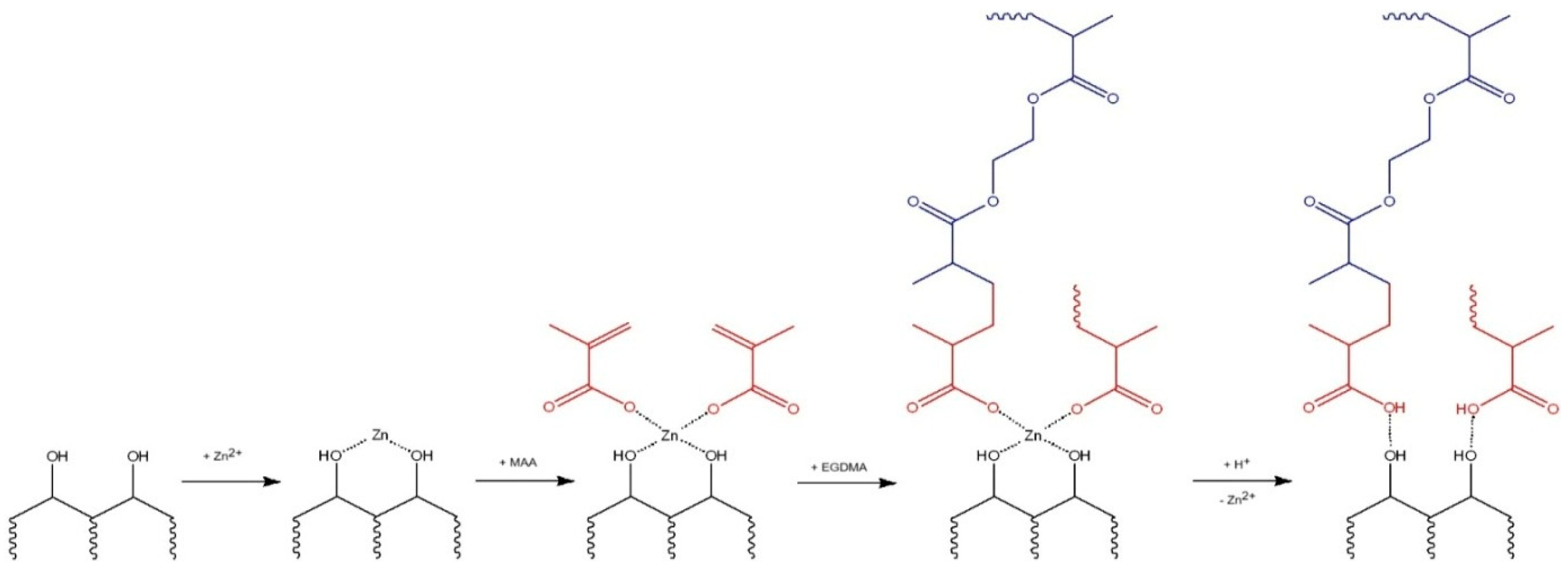
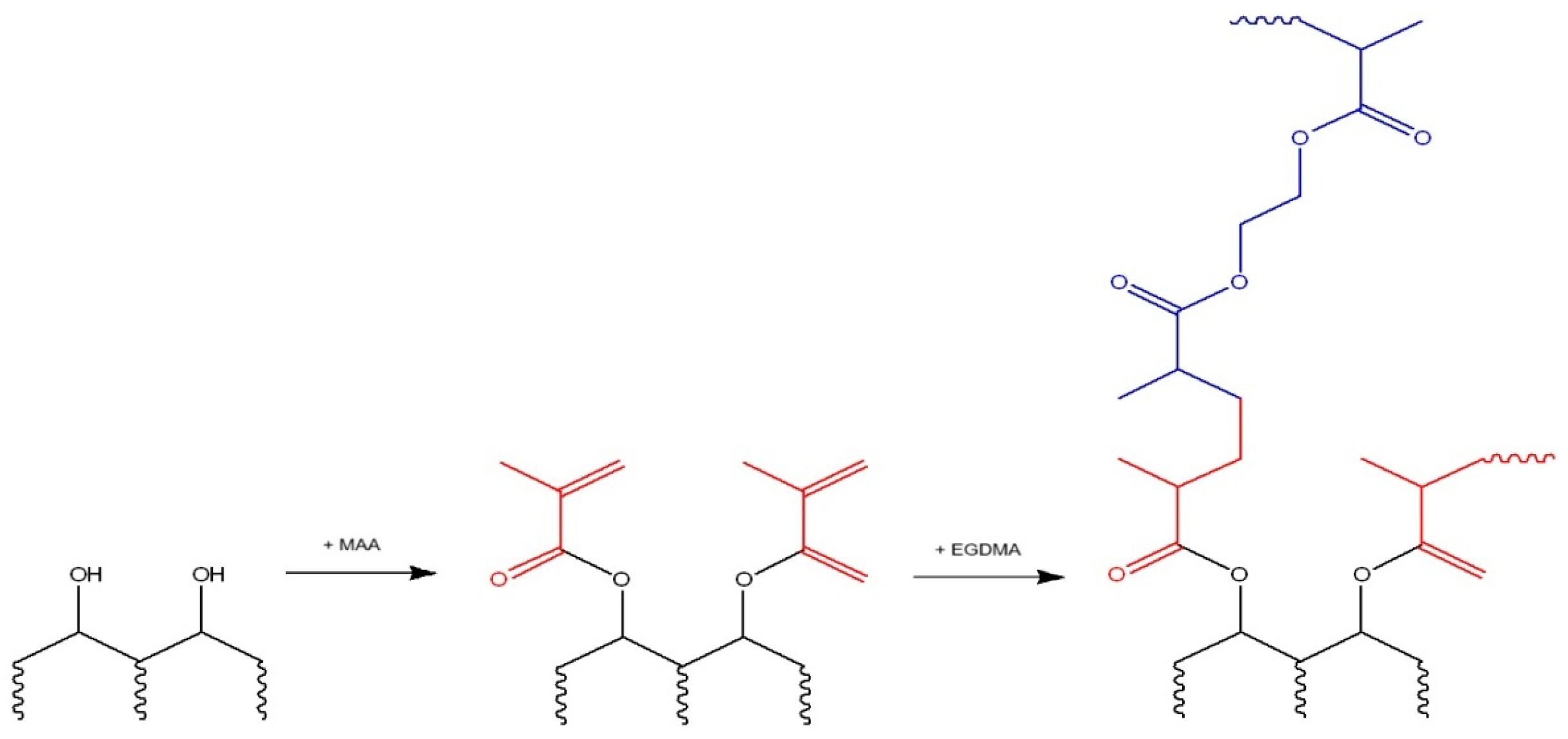
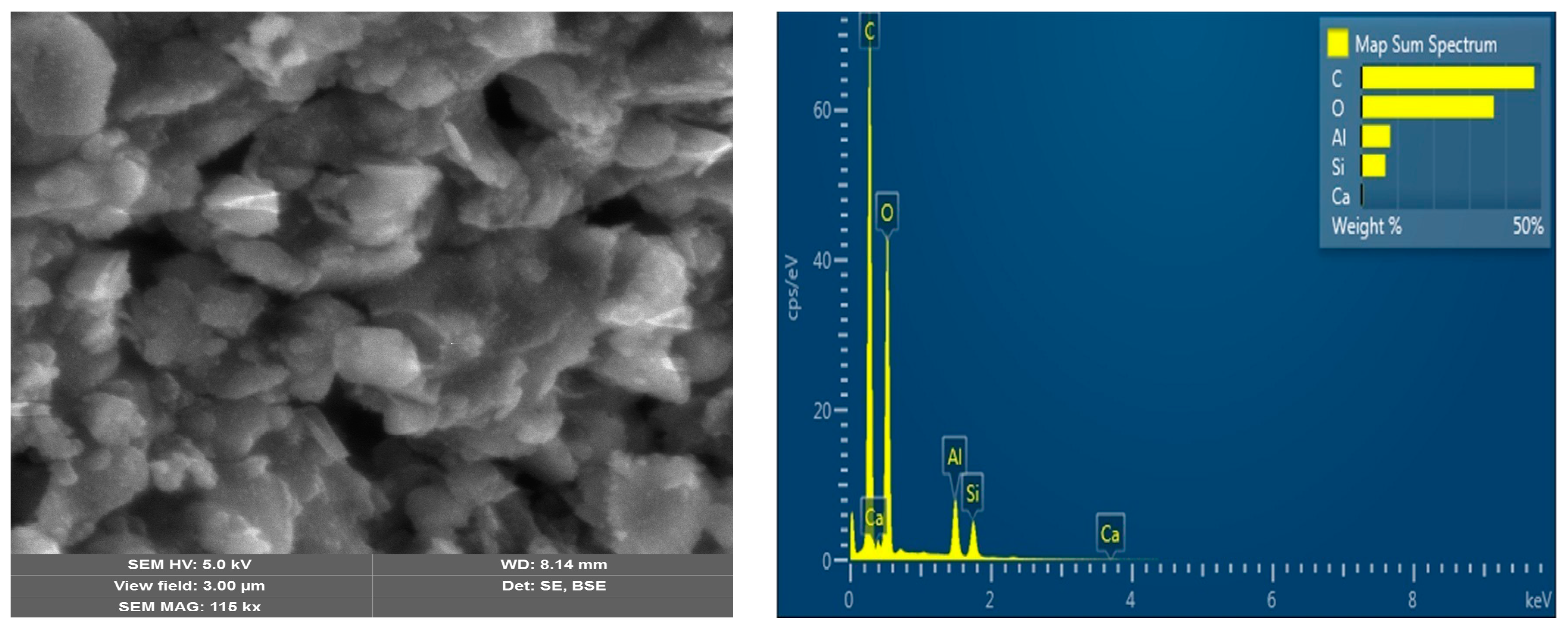
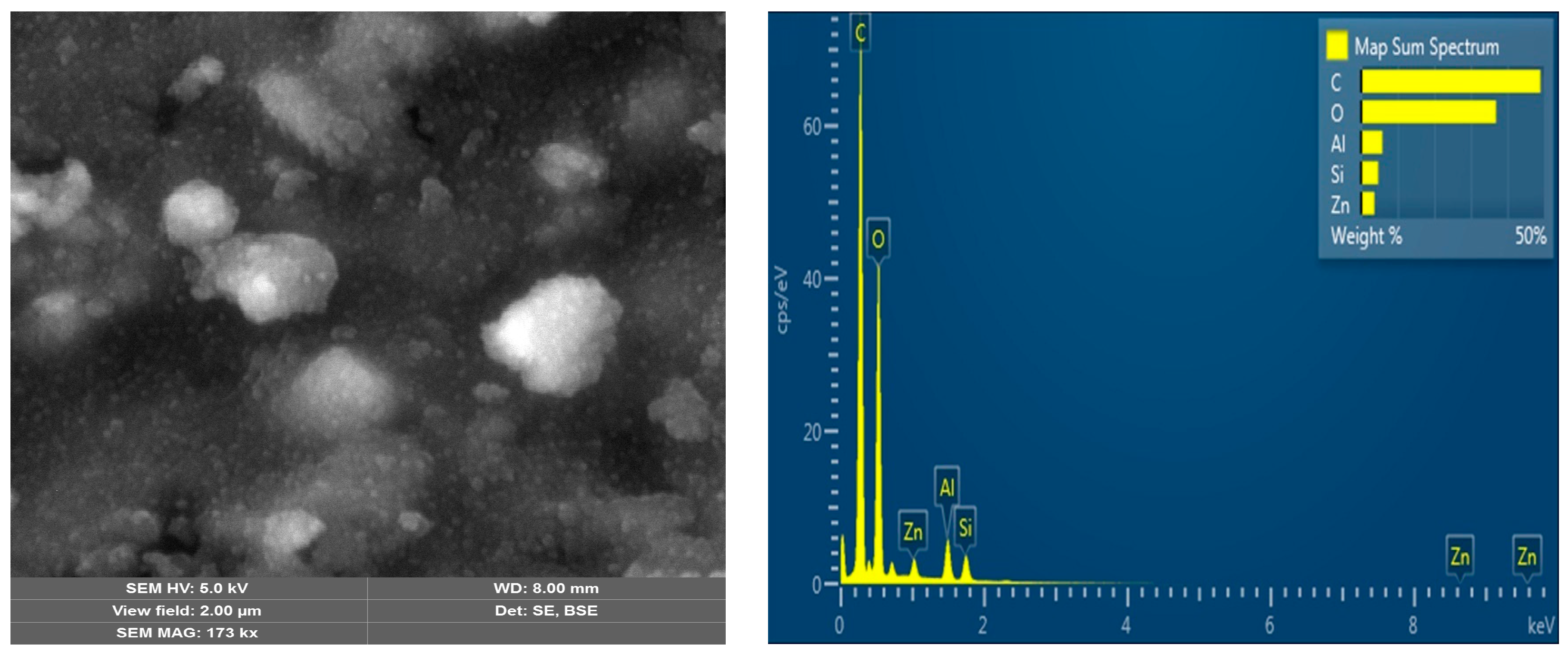
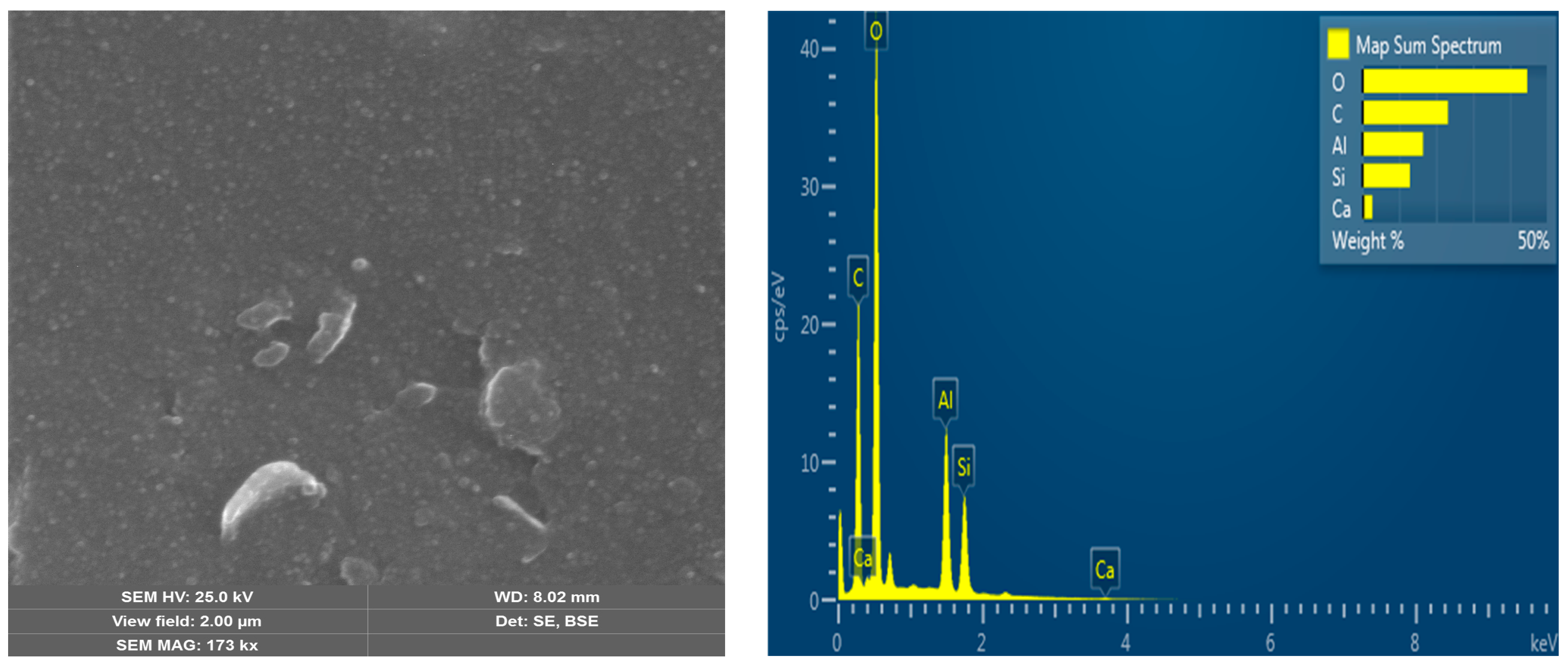
| Polymer | HA, (mmol) | MAA, (mmol) | Zn(CH3COO)2, (mmol) | EGDMA, (mmol) | BP, (mmol) |
|---|---|---|---|---|---|
| ZnIP | 1 | 1 | 1.00 | 10 | 0.1 |
| NIP | 1 | 1 | – | 10 | 0.1 |
| Polymer | Cg, % | Hg, % | Ng, % | Og, % | Σ(COOH+OH) mg-eq/g | Yield, % |
|---|---|---|---|---|---|---|
| ZnIP | 61.32 ± 0.2 | 3.97 ± 0.1 | 0.67 ± 0.1 | 34.04 ± 0.3 | 4.43 ± 0.2 | 76.54 |
| NIP | 57.32 ± 0.2 | 3.85 ± 0.1 | 0.67 ± 0.1 | 38.16 ± 0.3 | 4.95 ± 0.2 | 77.98 |
| The Pseudo-Order of the Reaction | Sorbed Ion Zn2+ Zinc-Imprinted Polymer | ||
|---|---|---|---|
| First | lnC = 1.4083 − 0.0118t | r = 0.9973 | k1 = 0.0118 |
| Second | 1/C = 0.1665 + 0.0067t | r = 0.9813 | k2 = 0.0067 |
| Third | 1/C2 = 0.1001 + 0.0085t | r = 0.9341 | k3 = 0.0085 |
| The Pseudo-Order of the Reaction | Sorbed Ion Zn2+ by a Non-Imprinted Polymer | ||
| First | lnC = 1.9653 − 0.0079t | r = 0.9970 | k1 = 0.0079 |
| Second | 1/C = 0.1234 + 0.0019t | r = 0.9863 | k2 = 0.0019 |
| Third | 1/C2 = 0.0047 + 0.001t | r = 0.9571 | k3 = 0.0010 |
| R, % | D·10−2 | IF | ||
|---|---|---|---|---|
| ZnIP | NIP | ZnIP | NIP | |
| 83.33 | 50.00 | 12.50 | 2.50 | 5.00 |
| 70.00 | 50.00 | 5.83 | 2.50 | 2.33 |
| 44.26 | 32.79 | 1.99 | 1.22 | 1.63 |
| 26.23 | 18.03 | 0.89 | 0.55 | 1.62 |
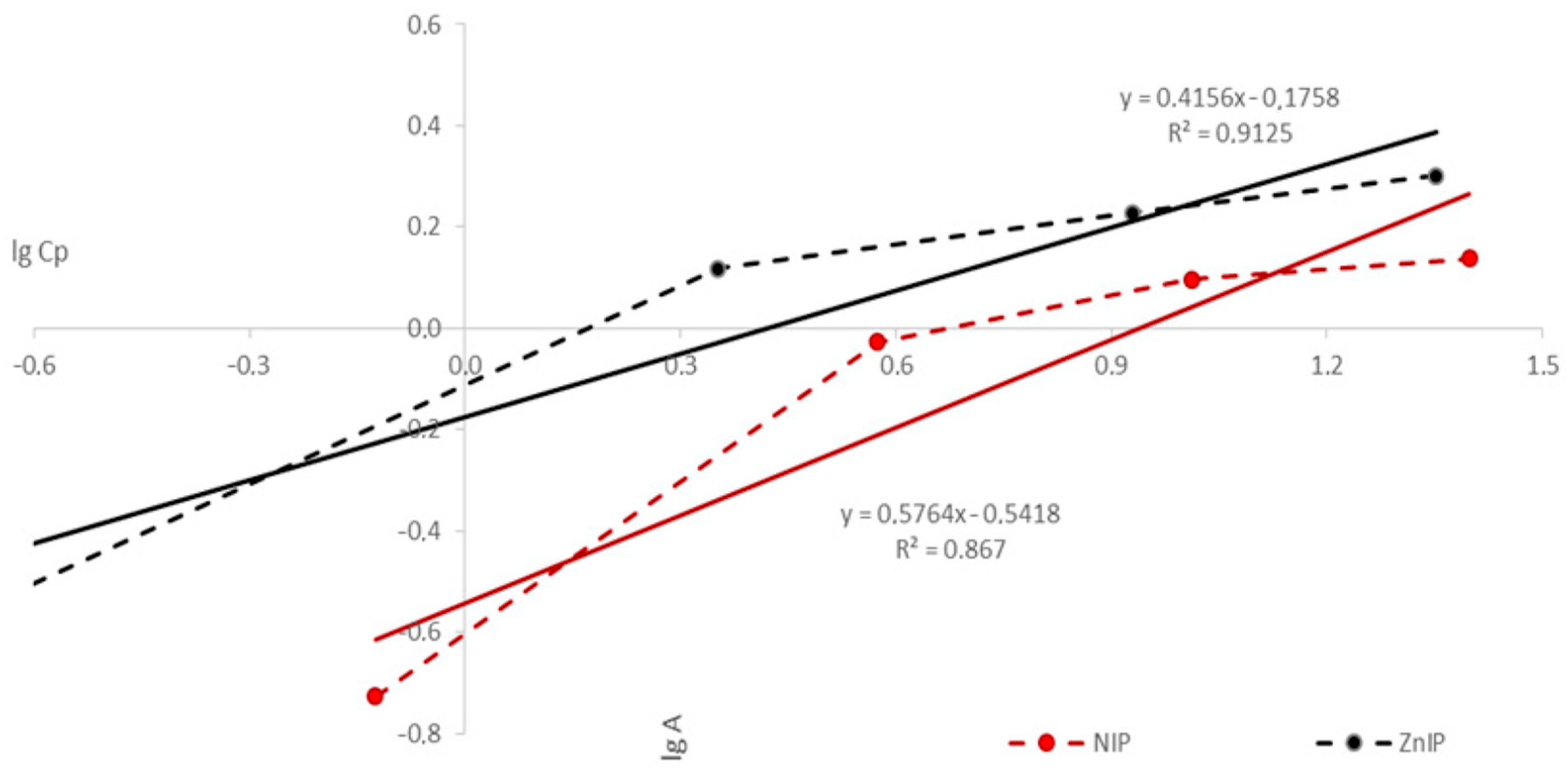
| Polymer | k | b | KFr | n | r |
|---|---|---|---|---|---|
| ZnIP | 0.4156 | −0.1758 | 1.1832 | 2.4060 | 0.9125 |
| NIP | 0.5764 | −0.5418 | 2.0526 | 1.7348 | 0.8670 |
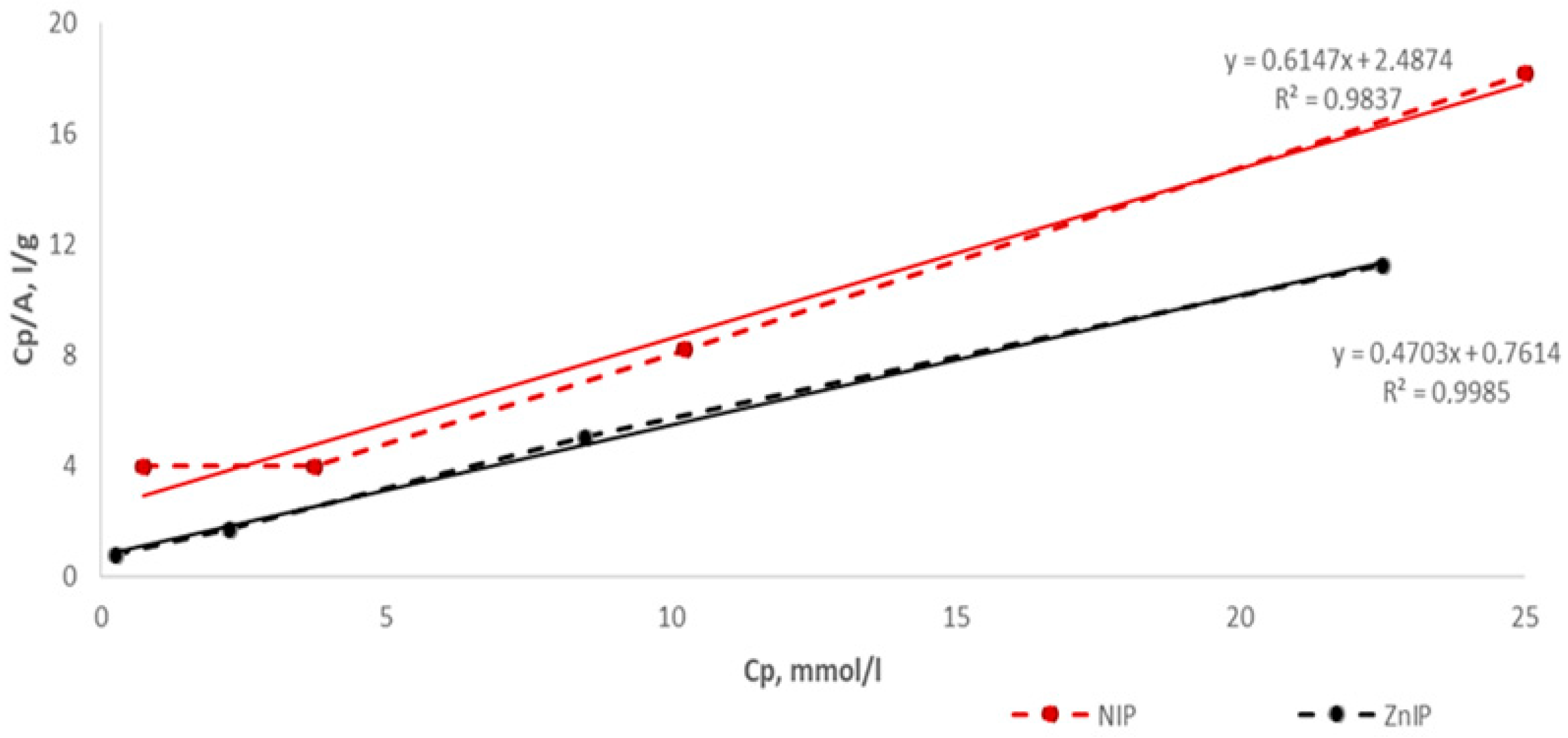
| Polymer | k | b | Maximum Specific Adsorption, A∞, mmol/g | Adsorption Equilibrium Constant, KL, L/mmol | r |
|---|---|---|---|---|---|
| ZnIP | 0.4703 | 0.7614 | 2.1262 | 0.6177 | 0.9985 |
| NIP | 0.6147 | 2.4874 | 1.6269 | 0.2471 | 0.9837 |
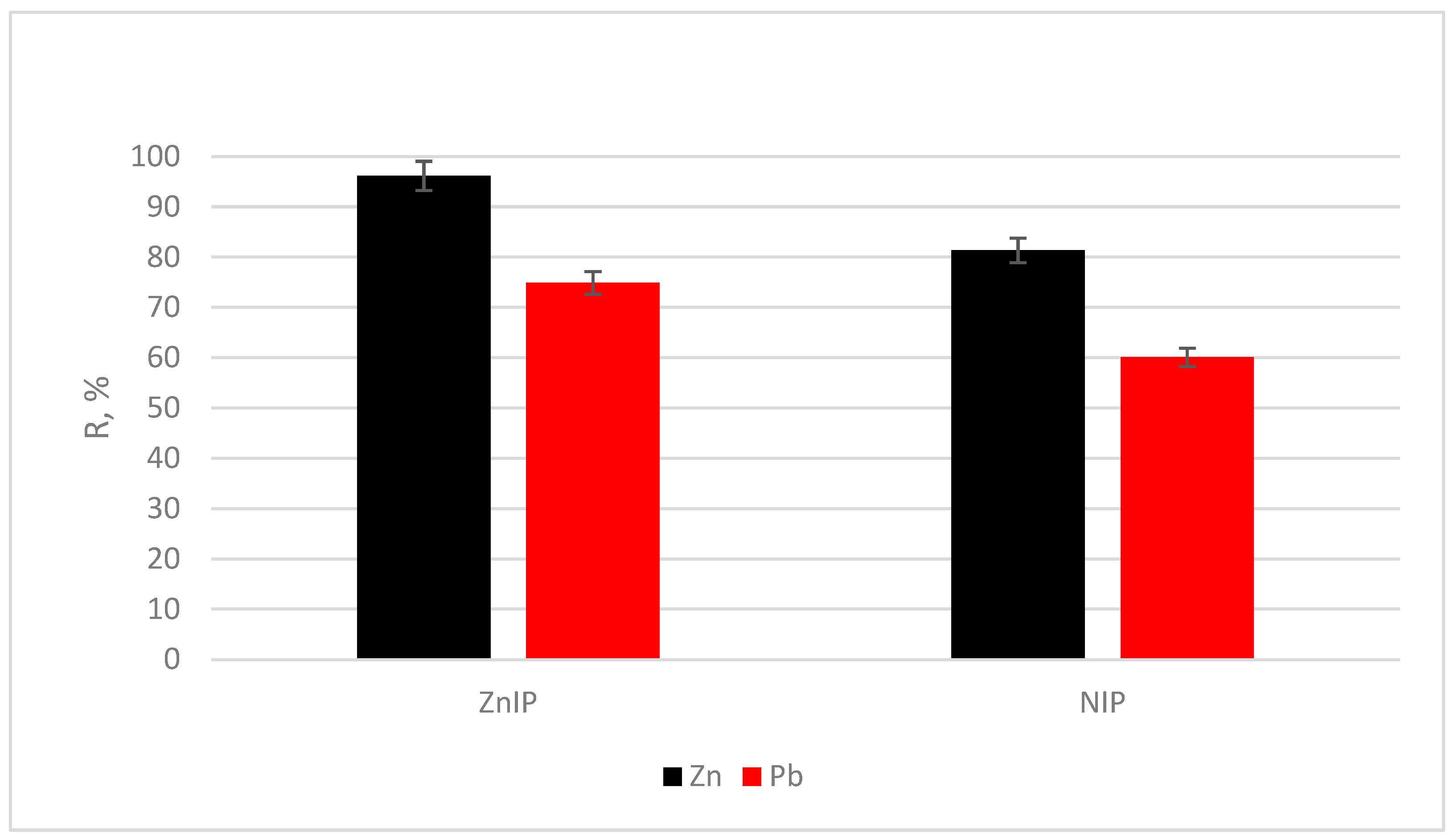
| Ions | D·10−2 | IF | k | k’ | |||
|---|---|---|---|---|---|---|---|
| ZnIP | NIP | ZnIP | NIP | ZnIP | NIP | ||
| Zn2+ | 2.50 | 0.44 | 5.73 | – | – | – | – |
| Pb2+ | 0.30 | 0.15 | 1.98 | – | 8.38 | 2.89 | 2.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhakina, A.K.; Vassilets, Y.P.; Arnt, O.V.; Zhakin, A.M. Synthesis and Study of Sorption Properties of Zinc-Imprinted Polymer. Polymers 2024, 16, 3545. https://doi.org/10.3390/polym16243545
Zhakina AK, Vassilets YP, Arnt OV, Zhakin AM. Synthesis and Study of Sorption Properties of Zinc-Imprinted Polymer. Polymers. 2024; 16(24):3545. https://doi.org/10.3390/polym16243545
Chicago/Turabian StyleZhakina, Alma Khassenovna, Yevgeniy Petrovich Vassilets, Oxana Vasilievna Arnt, and Almat Maulenuly Zhakin. 2024. "Synthesis and Study of Sorption Properties of Zinc-Imprinted Polymer" Polymers 16, no. 24: 3545. https://doi.org/10.3390/polym16243545
APA StyleZhakina, A. K., Vassilets, Y. P., Arnt, O. V., & Zhakin, A. M. (2024). Synthesis and Study of Sorption Properties of Zinc-Imprinted Polymer. Polymers, 16(24), 3545. https://doi.org/10.3390/polym16243545









