Humidity Resistant Biodegradable Starch Foams Reinforced with Polyvinyl Butyral (PVB) and Chitosan
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
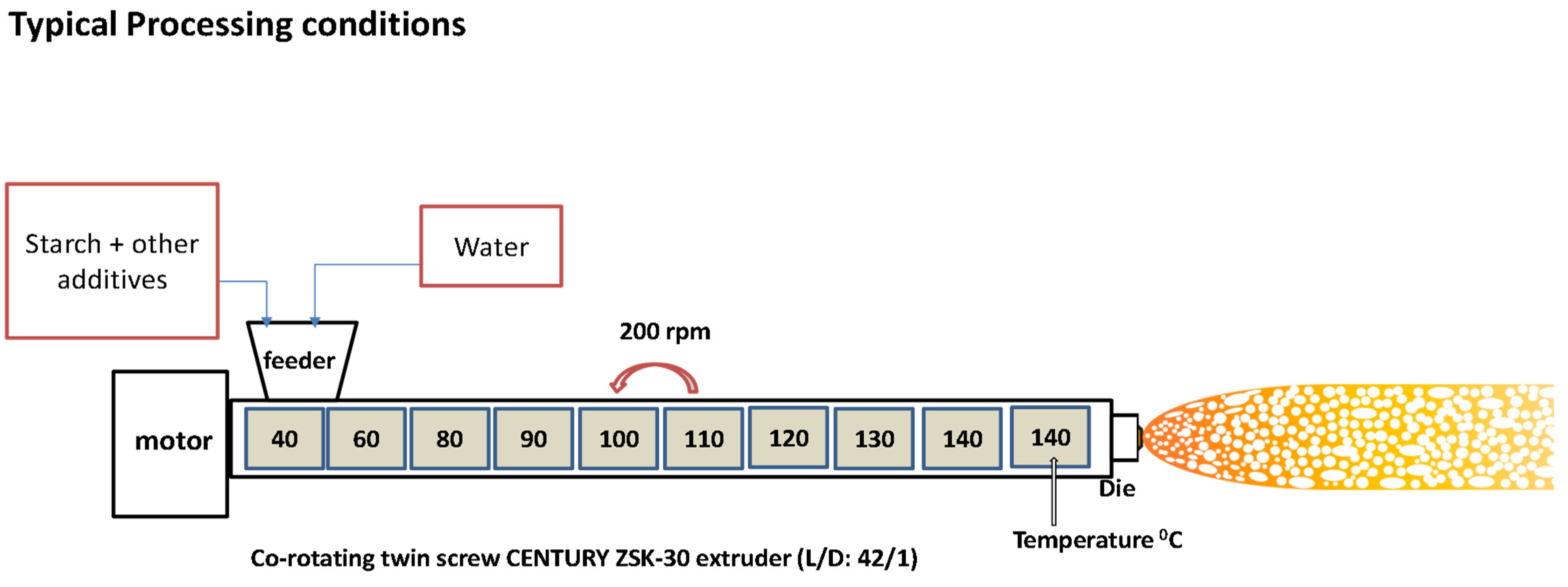
2.2. Preparation of Starch-Based Foams via Foam Extrusion Process
2.3. Characterization and Analysis
3. Results and Discussion
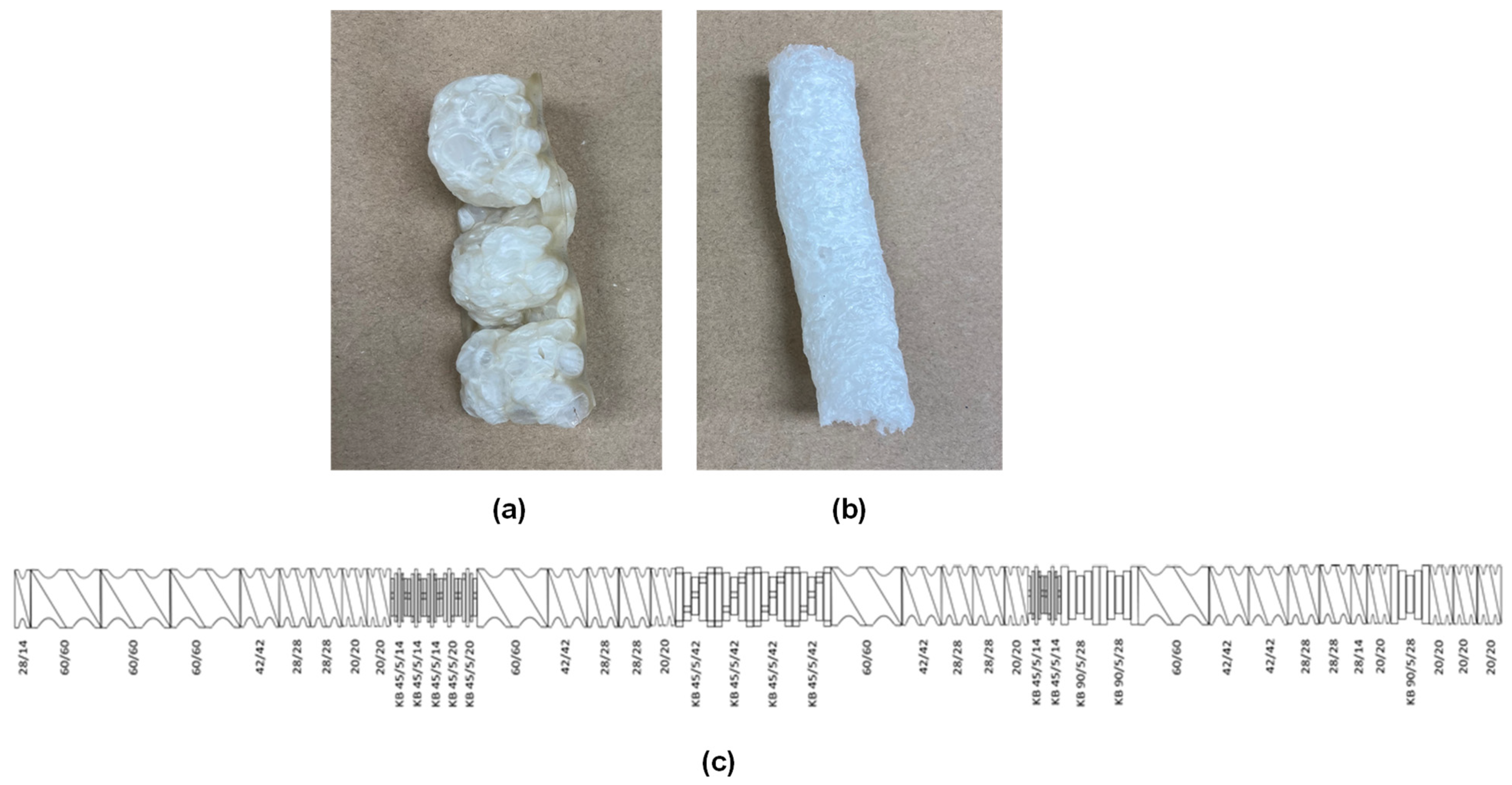
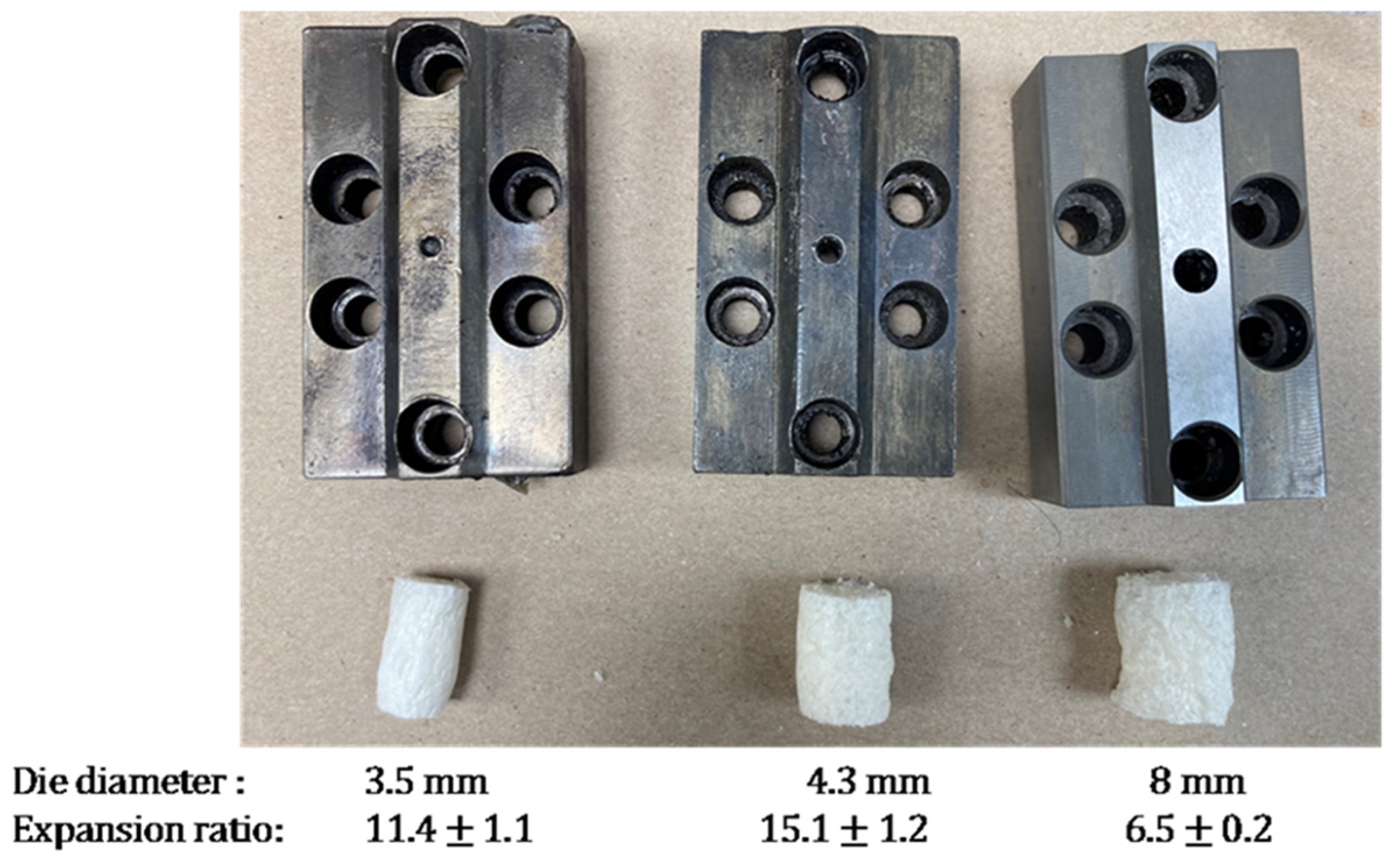
3.1. Foam Extrusion Process Optimization
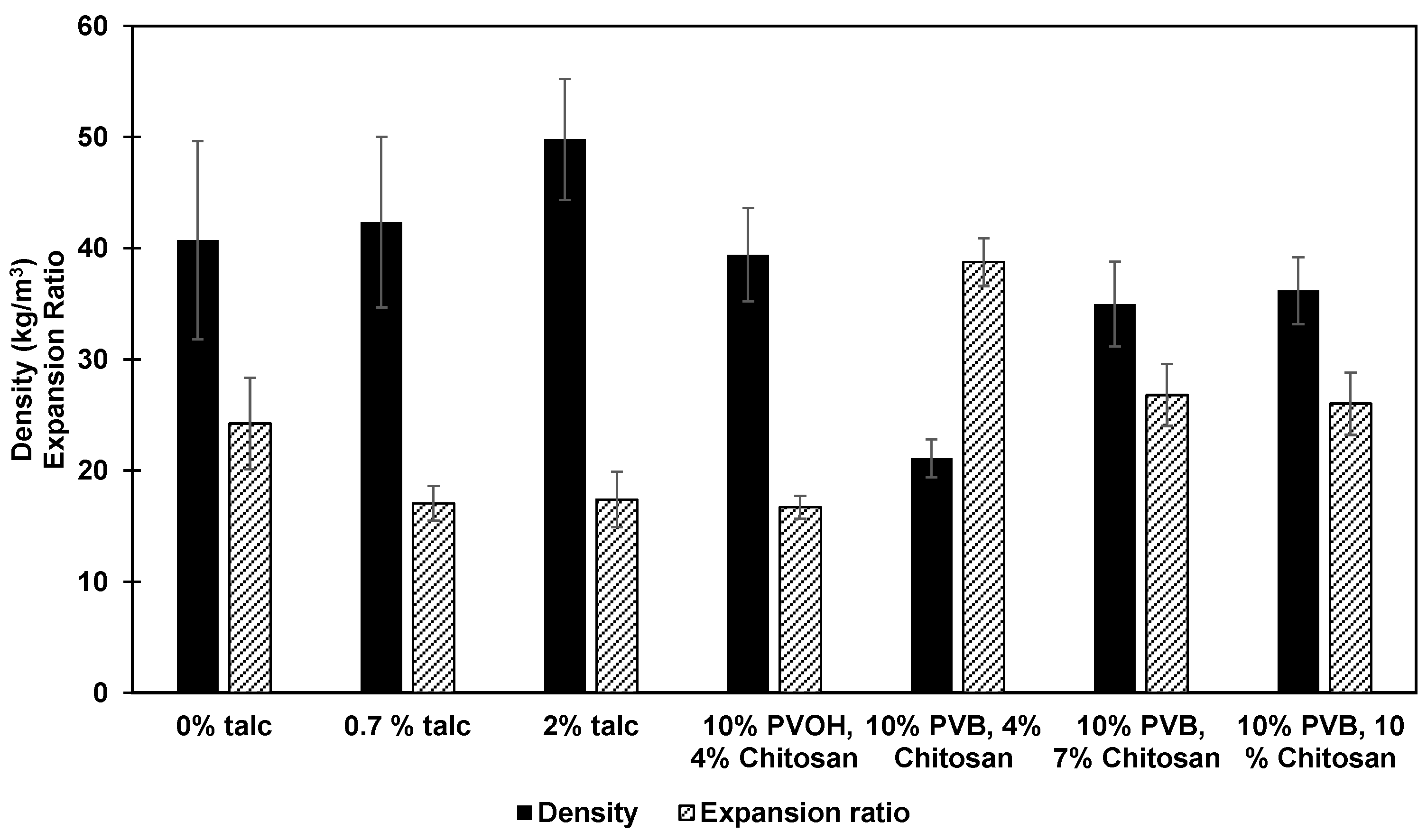
3.2. Effect of Various Additives on Densities and Expansion Ratios of Foams
3.3. Cell Size and Cell Size Distributions
3.4. Evaluating Water Resistance in Starch Foams
3.5. Analysis of Moisture Sensitivity Using Peleg Model
3.6. Effect of Chitosan and PVB on Surface Properties of Starch Foams
3.7. Compressive Strength and Resiliency
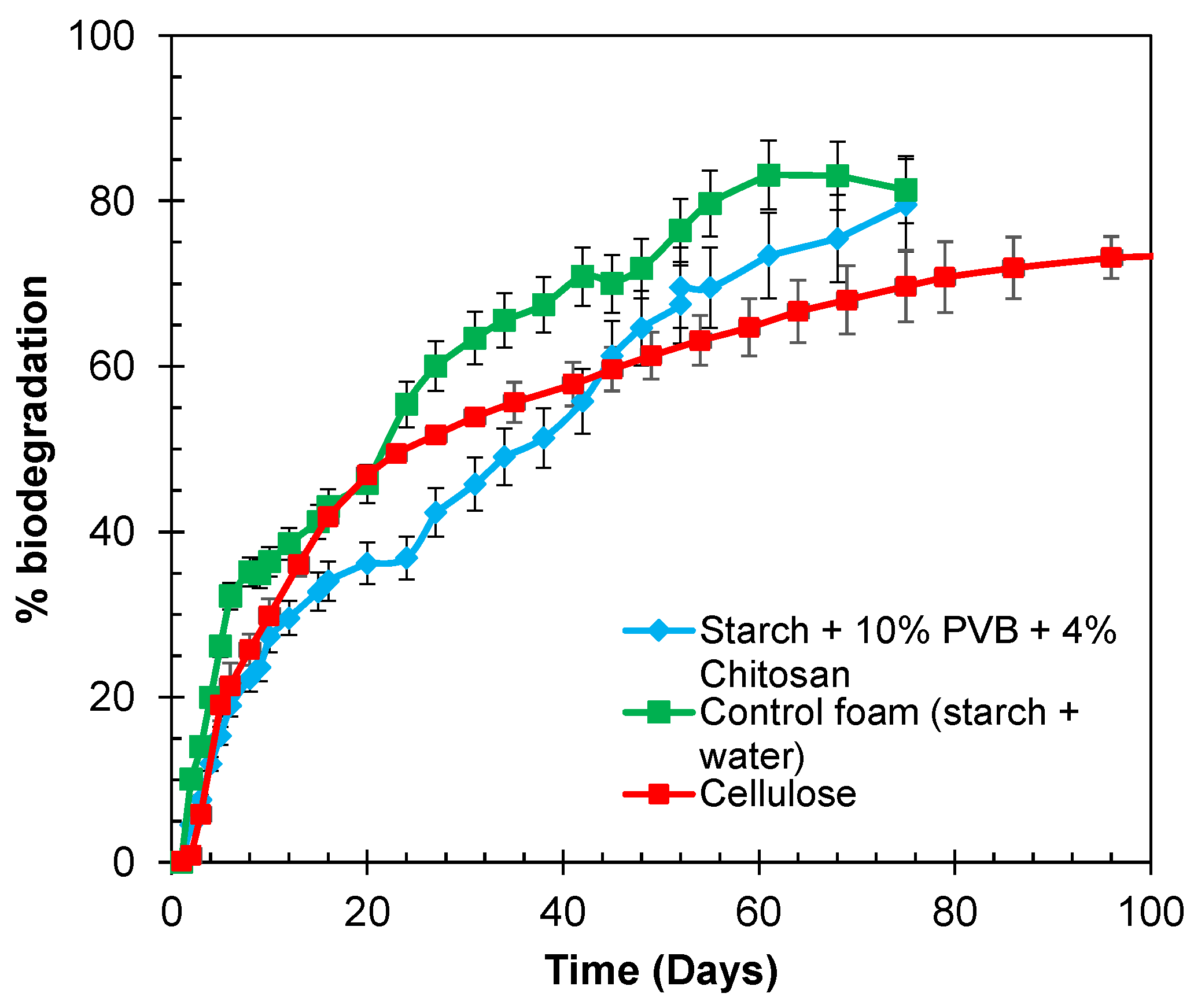
3.8. Biodegradability of the Foams in Aqueous Environment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, X.; Wang, Y.; Chen, X.; Yu, X.; Li, W.; Zhang, S.; Meng, X.; Zhao, Z.M.; Dong, T.; Anderson, A.; et al. Sustainable Bioplastics Derived from Renewable Natural Resources for Food Packaging. Matter 2023, 6, 97–127. [Google Scholar] [CrossRef]
- Mulchandani, N.; Narayan, R. End-of-Life of Plastics/Bioplastics. Bioplastics Biocomposites 2023, 79, 274–290. [Google Scholar] [CrossRef]
- Law, K.L.; Narayan, R. Reducing Environmental Plastic Pollution by Designing Polymer Materials for Managed End-of-Life. Nat. Rev. Mater. 2021, 7, 104–116. [Google Scholar] [CrossRef]
- Yang, Z.; Graiver, D.; Narayan, R. Extrusion of Humidity-Resistant Starch Foam Sheets. Polym. Eng. Sci. 2013, 53, 857–867. [Google Scholar] [CrossRef]
- Starch Medical|Starch Medical Launches Novel Plant Sourced Surgical Hemostatic Pad. Available online: https://starchmedical.com/starch-medical-launches-novel-plant-sourced-surgical-hemostatic-pad/ (accessed on 9 November 2020).
- Soykeabkaew, N.; Thanomsilp, C.; Suwantong, O. A Review: Starch-Based Composite Foams. Compos. Part A: Appl. Sci. Manuf. 2015, 78, 246–263. [Google Scholar] [CrossRef]
- Kulkarni, A.; Narayan, R. Effects of Modified Thermoplastic Starch on Crystallization Kinetics and Barrier Properties of PLA. Polymers 2021, 13, 4125. [Google Scholar] [CrossRef]
- Li, H.; Huneault, M.A. Comparison of Sorbitol and Glycerol as Plasticizers for Thermoplastic Starch in TPS/PLA Blends. J. Appl. Polym. Sci. 2011, 119, 2439–2448. [Google Scholar] [CrossRef]
- Pushpadass, H.A.; Babu, G.S.; Weber, R.W.; Hanna, M.A. Extrusion of Starch-Based Loose-Fill Packaging Foams: Effects of Temperature, Moisture and Talc on Physical Properties. Packag. Technol. Sci. 2008, 21, 171–183. [Google Scholar] [CrossRef]
- Giri, P.; Tambe, C.; Narayan, R.; Junction, M.; States, U. Greener Products: From Laboratory Fundamentals to Commercial Scale. In Biomass Extrusion and Reaction Technologies: Principles to Practices and Future Potential; American Chemical Society: Washington, DC, USA, 2018; Volume 1304, pp. 1–23. [Google Scholar] [CrossRef]
- Duan, Q.; Zhu, Z.; Chen, Y.; Liu, H.; Yang, M.; Chen, L.; Yu, L. Starch-Based Foams Nucleated and Reinforced by Polysaccharide-Based Crystals. ACS Sustain. Chem. Eng. 2022, 10, 2169–2179. [Google Scholar] [CrossRef]
- Fang, Q.; Hanna, M.A. Mechanical Properties of Starch Based Foams as Affected by Ingredient Formulations and Foam Physical Characteristics. Am. Soc. Agric. Eng. 2000, 43, 1715–1723. [Google Scholar] [CrossRef]
- Nabar, Y.U.; Draybuck, D.; Narayan, R. Physicomechanical and Hydrophobic Properties of Starch Foams Extruded with Different Biodegradable Polymers; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; Volume 102, pp. 58–68. [Google Scholar] [CrossRef]
- Hassan, M.M.; Tucker, N.; Le Guen, M.J. Thermal, Mechanical and Viscoelastic Properties of Citric Acid-Crosslinked Starch/Cellulose Composite Foams. Carbohydr. Polym. 2020, 230, 115675. [Google Scholar] [CrossRef] [PubMed]
- Uslu, M.K.; Polat, S. Effects of Glyoxal Cross-Linking on Baked Starch Foam. Carbohydr. Polym. 2012, 87, 1994–1999. [Google Scholar] [CrossRef]
- Rubin, B.S. Bisphenol A: An Endocrine Disruptor with Widespread Exposure and Multiple Effects. J. Steroid Biochem. Mol. Biol. 2011, 127, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Products, S. Scientific Committee on Consumer Products: Opinion on Glyoxal; Committee on Consumer, 2005; Available online: https://ec.europa.eu/health/ph_risk/committees/04_sccp/sccp_opinions_en.htm (accessed on 9 November 2020).
- Xu, Y.; Hanna, M.A. Physical, Mechanical, and Morphological Characteristics of Extruded Starch Acetate Foams. J. Polym. Environ. 2005, 13, 221–230. [Google Scholar] [CrossRef]
- Guan, J.; Hanna, M.A. Extruding Foams from Corn Starch Acetate and Native Corn Starch. Biomacromolecules 2004, 5, 2329–2339. [Google Scholar] [CrossRef]
- Srisuwan, Y.; Baimark, Y. Improvement of Water Resistance of Thermoplastic Starch Foams by Dip-Coating with Biodegradable Polylactide-b-Polyethylene Glycol-b-Polylactide Copolymer and Its Blend with Poly(D-Lactide). Prog. Org. Coat. 2021, 151, 106074. [Google Scholar] [CrossRef]
- PVB Films. Polymer Properties Database. Available online: http://polymerdatabase.com/Films/PVBFilms.html (accessed on 9 November 2020).
- Zhang, X.; Cao, C.; Xiao, B.; Yan, L.; Zhang, Q.; Jiang, B. Preparation and Characterization of Polyvinyl Butyral/Silica Hybrid Antireflective Coating: Effect of PVB on Moisture-Resistance and Hydrophobicity. J. Solgel. Sci. Technol. 2010, 53, 79–84. [Google Scholar] [CrossRef]
- Syaqira, S.S.N.; Leman, Z.; Sapuan, S.M.; Dele-Afolabi, T.T.; Azmah Hanim, M.A.; Budati, S. Tensile Strength and Moisture Absorption of Sugar Palm-Polyvinyl Butyral Laminated Composites. Polymers 2020, 12, 1923. [Google Scholar] [CrossRef]
- Dang, K.M.; Yoksan, R. Morphological Characteristics and Barrier Properties of Thermoplastic Starch/Chitosan Blown Film. Carbohydr. Polym. 2016, 150, 40–47. [Google Scholar] [CrossRef]
- Nabar, Y.; Raquez, J.M.; Dubois, P.; Narayan, R. Production of Starch Foams by Twin-Screw Extrusion: Effect of Maleated Poly(Butylene Adipate-Co-Terephthalate) as a Compatibilizer. Biomacromolecules 2005, 6, 807–817. [Google Scholar] [CrossRef]
- Standard Test Method for Compressive Properties of Rigid Cellular Plastics (2023) D1621. Available online: https://www.astm.org/standards/d1621 (accessed on 24 November 2024).
- ISO 14852:2021; Determination of the Ultimate Aerobic Biodegradability of Plastic Materials in an Aqueous Medium—Method by Analysis of Evolved Carbon Dioxide. International Organization for Standardization: Geneva, Switzerland, 2021. Available online: https://www.iso.org/standard/80303.html (accessed on 24 November 2024).
- Kahvand, F.; Fasihi, M. Microstructure and Physical Properties of Thermoplastic Corn Starch Foams as Influenced by Polyvinyl Alcohol and Plasticizer Contents. Int. J. Biol. Macromol. 2020, 157, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Sita, C.; Burns, M.; Häβler, R.; Focke, W.W. Tensile Properties of Thermoplastic Starch-PVB Blends. J. Appl. Polym. Sci. 2006, 101, 1751–1755. [Google Scholar] [CrossRef]
- McClurg, R.B. Design Criteria for Ideal Foam Nucleating Agents. Chem. Eng. Sci. 2004, 59, 5779–5786. [Google Scholar] [CrossRef]
- Leung, S.N.; Wong, A.; Park, C.B.; Zong, J.H. Ideal Surface Geometries of Nucleating Agents to Enhance Cell Nucleation in Polymeric Foaming Processes. J. Appl. Polym. Sci. 2008, 108, 3997–4003. [Google Scholar] [CrossRef]
- González-Núñez, R.; Vázquez, M.O.; Bernache, J.; Gómez, C.; Robledo-Ortiz, J.R.; Ramírez-Arreola, D.E.; Rodrigue, D. Using Chitosan as a Nucleation Agent in Thermoplastic Foams for Heavy Metal Adsorption. Macromol. Symp. 2009, 283–284, 152–158. [Google Scholar] [CrossRef]
- Turhan, M.; Sayar, S.; Gunasekaran, S. Application of Peleg Model to Study Water Absorption in Chickpea during Soaking. J. Food Eng. 2002, 53, 153–159. [Google Scholar] [CrossRef]
- Combrzyński, M.; Mościcki, L.; Kwaśniewska, A.; Oniszczuk, T.; Wójtowicz, A.; Sołowiej, B.; Gładyszewska, B.; Muszyński, S. Moisture Sorption Characteristics of Extrusion-Cooked Starch Protective Loose-Fill Cushioning Foams. Int. Agrophys. 2017, 31, 457–463. [Google Scholar] [CrossRef]
- Cunha, A.G.; Fernandes, S.; Freire, C.S.R.; Silvestre, A.J.D. Why, After All, Are Chitosan Films Hydrophobic? Biomacromolecules 2008, 9, 610–614. [Google Scholar] [CrossRef]
- Santacruz, S.; Rivadeneira, C.; Castro, M. Edible Films Based on Starch and Chitosan. Effect of Starch Source Andconcentration, Plasticizer, Surfactant’s Hydrophobic Tail Andmechanical Treatment. Food Hydrocoll. 2015, 49, 89–94. [Google Scholar] [CrossRef]
- Fabi, I. Talc Filled PLA. Bioplastics Magazine, 6 December 2024; 24–26. [Google Scholar]
- Machado, C.M.; Benelli, P.; Tessaro, I.C. Study of Interactions between Cassava Starch and Peanut Skin on Biodegradable Foams. Int. J. Biol. Macromol. 2020, 147, 1343–1353. [Google Scholar] [CrossRef]
- Onishi, H.; Machida, Y. Biodegradation and Distribution of Water-Soluble Chitosan in Mice. Biomaterials 1999, 20, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Ma, O.; Lavertu, M.; Sun, J.; Nguyen, S.; Buschmann, M.D.; Winnik, F.M.; Hoemann, C.D. Precise Derivatization of Structurally Distinct Chitosans with Rhodamine B Isothiocyanate. Carbohydr. Polym. 2008, 72, 616–624. [Google Scholar] [CrossRef]
- Ferri, J.M.; Fombuena Borràs, V.; Fernando, M.; Carrasco, A.; Tan, S.X.; Chyuan Ong, H.; Andriyana, A.; Lim, S.; Pang, Y.L.; Kusumo, F.; et al. Characterization and Parametric Study on Mechanical Properties Enhancement in Biodegradable Chitosan-Reinforced Starch-Based Bioplastic Film. Polymers 2022, 14, 278. [Google Scholar] [CrossRef] [PubMed]

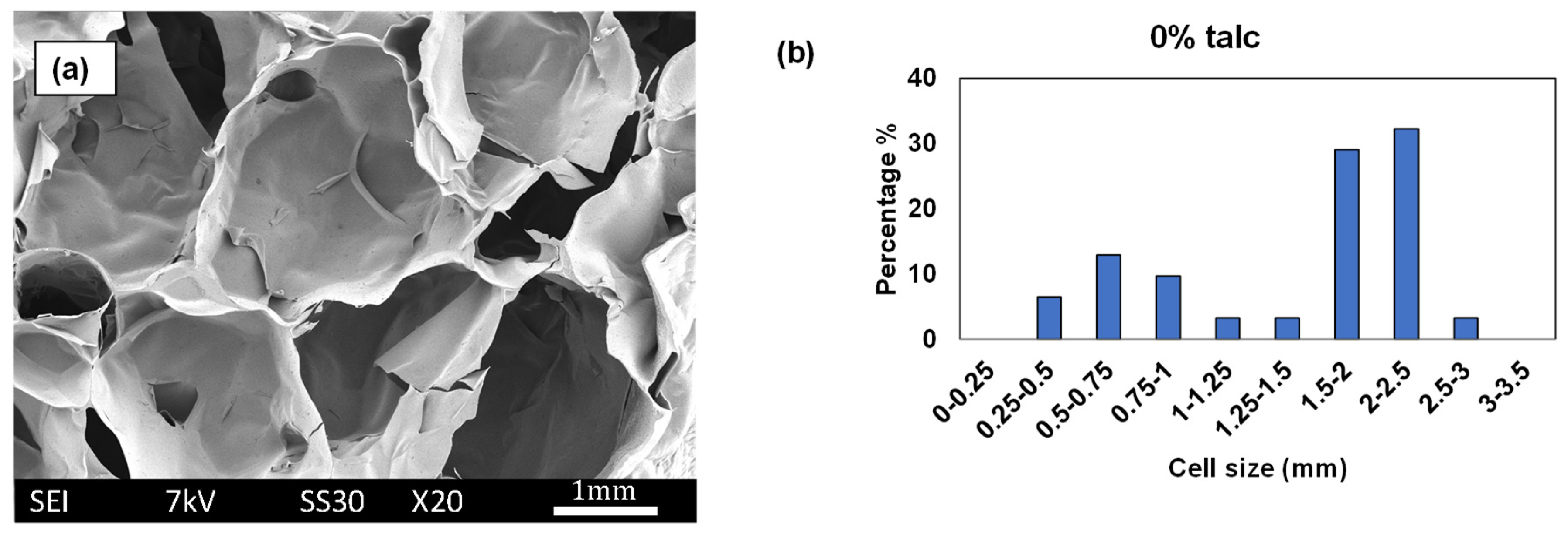
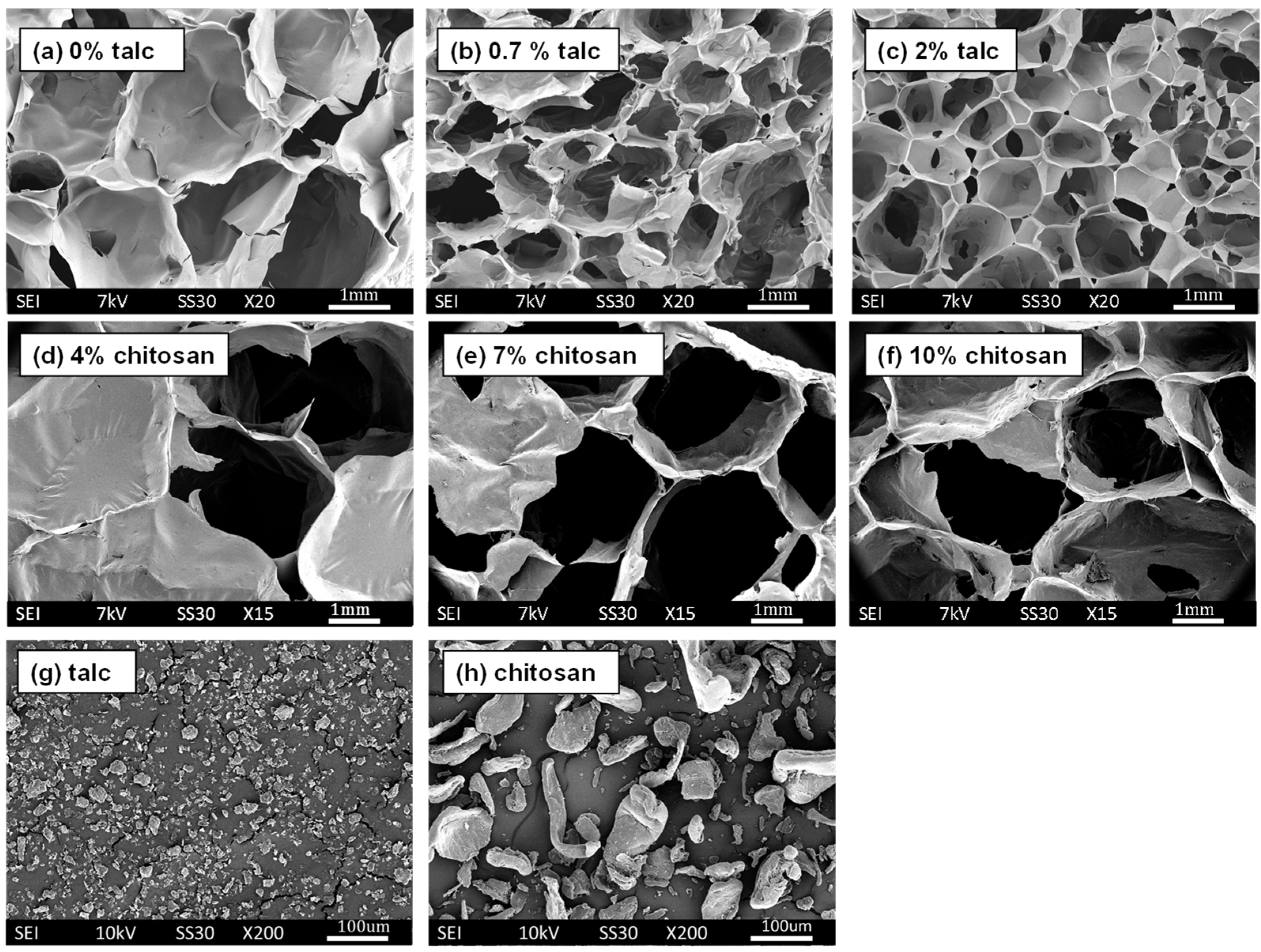
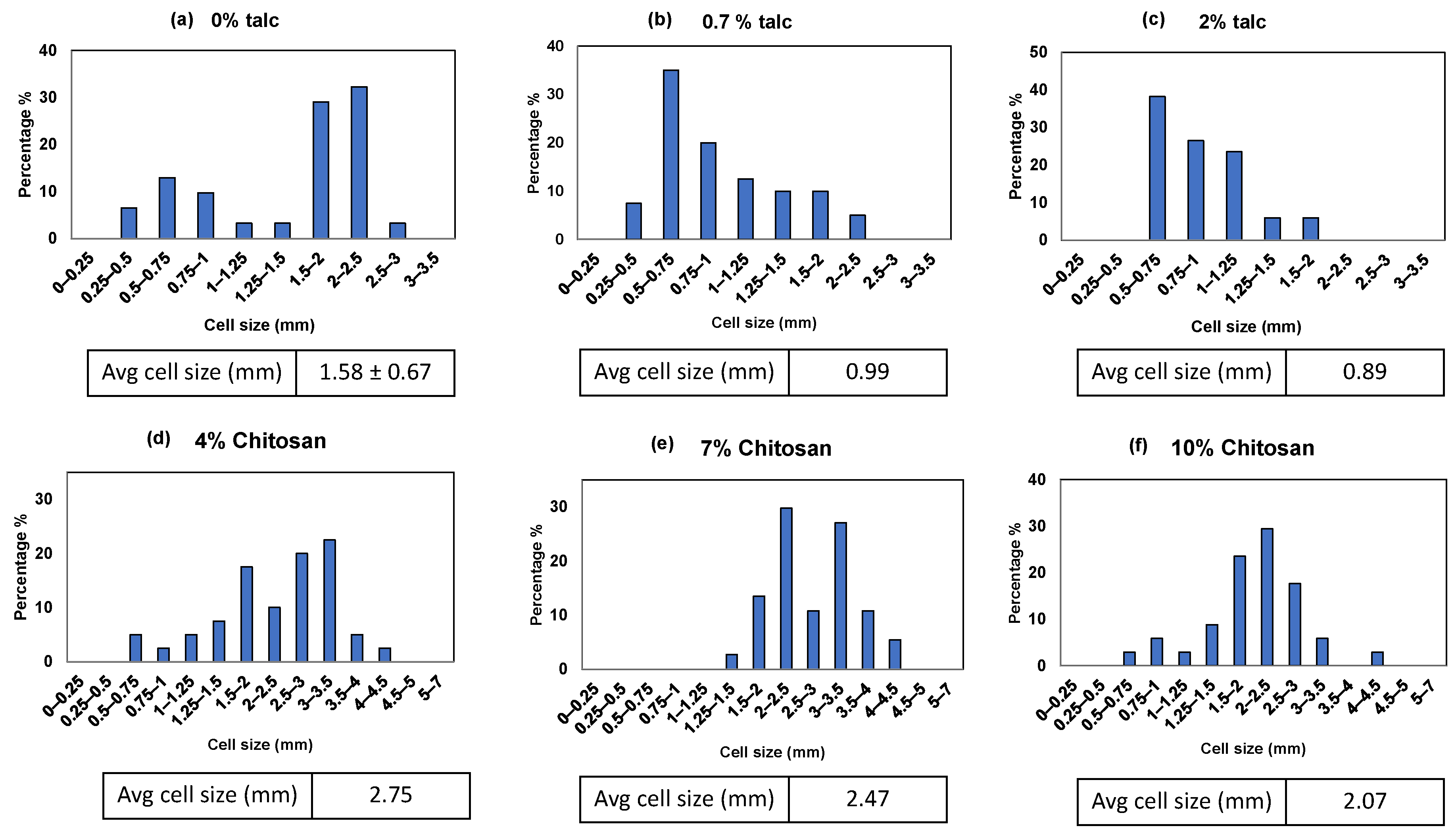

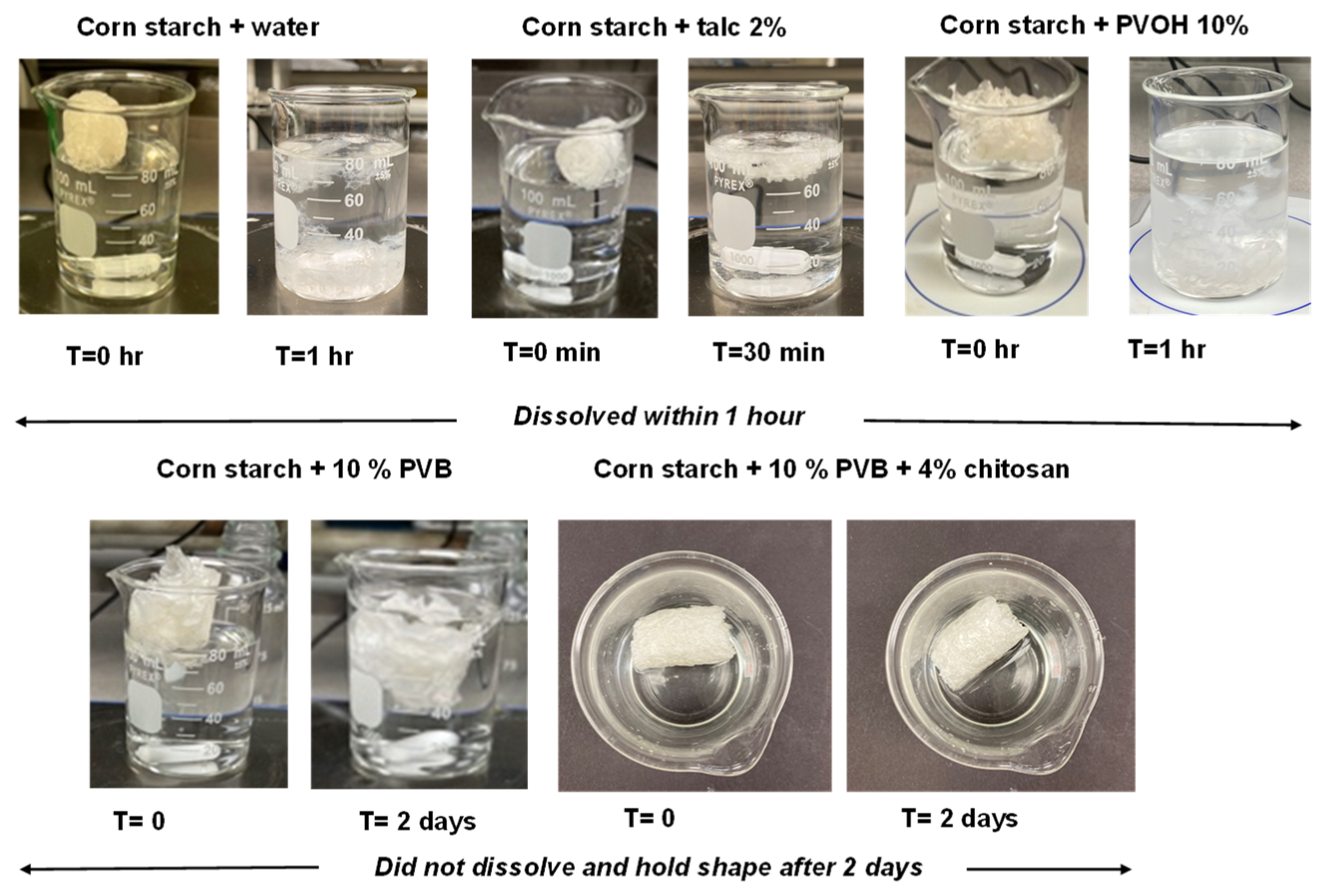
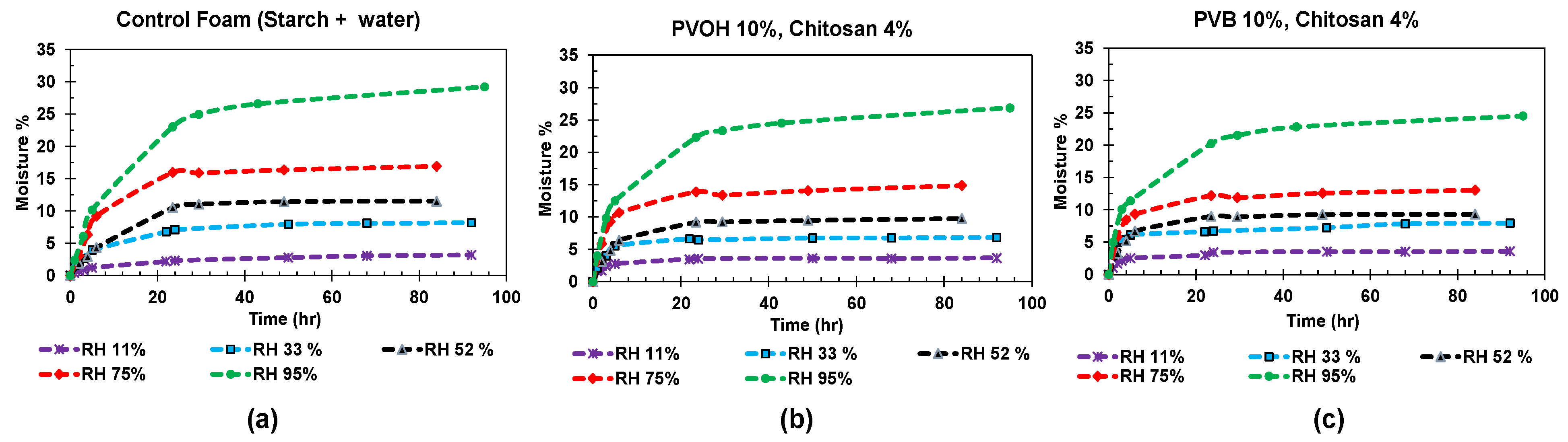
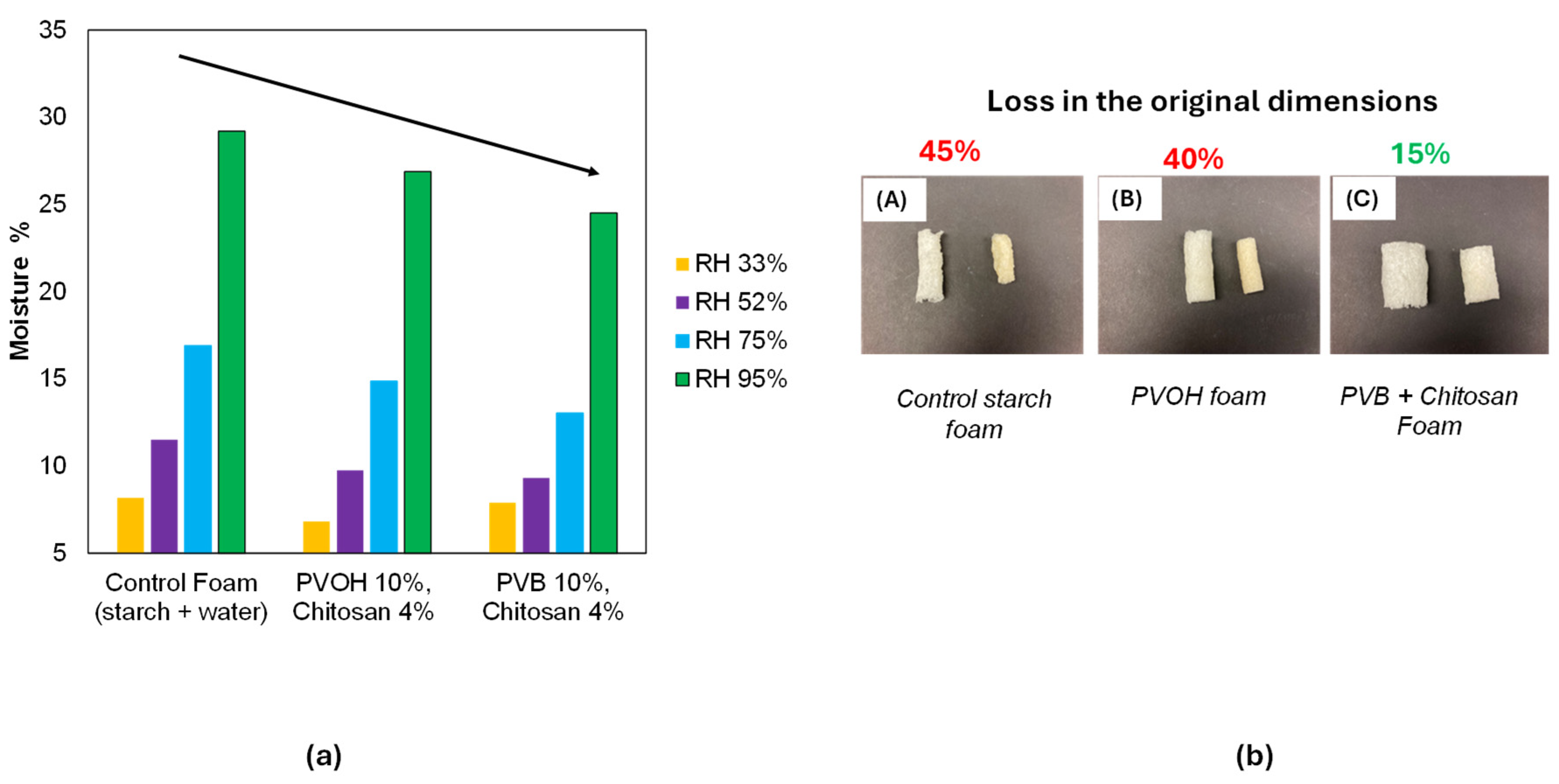
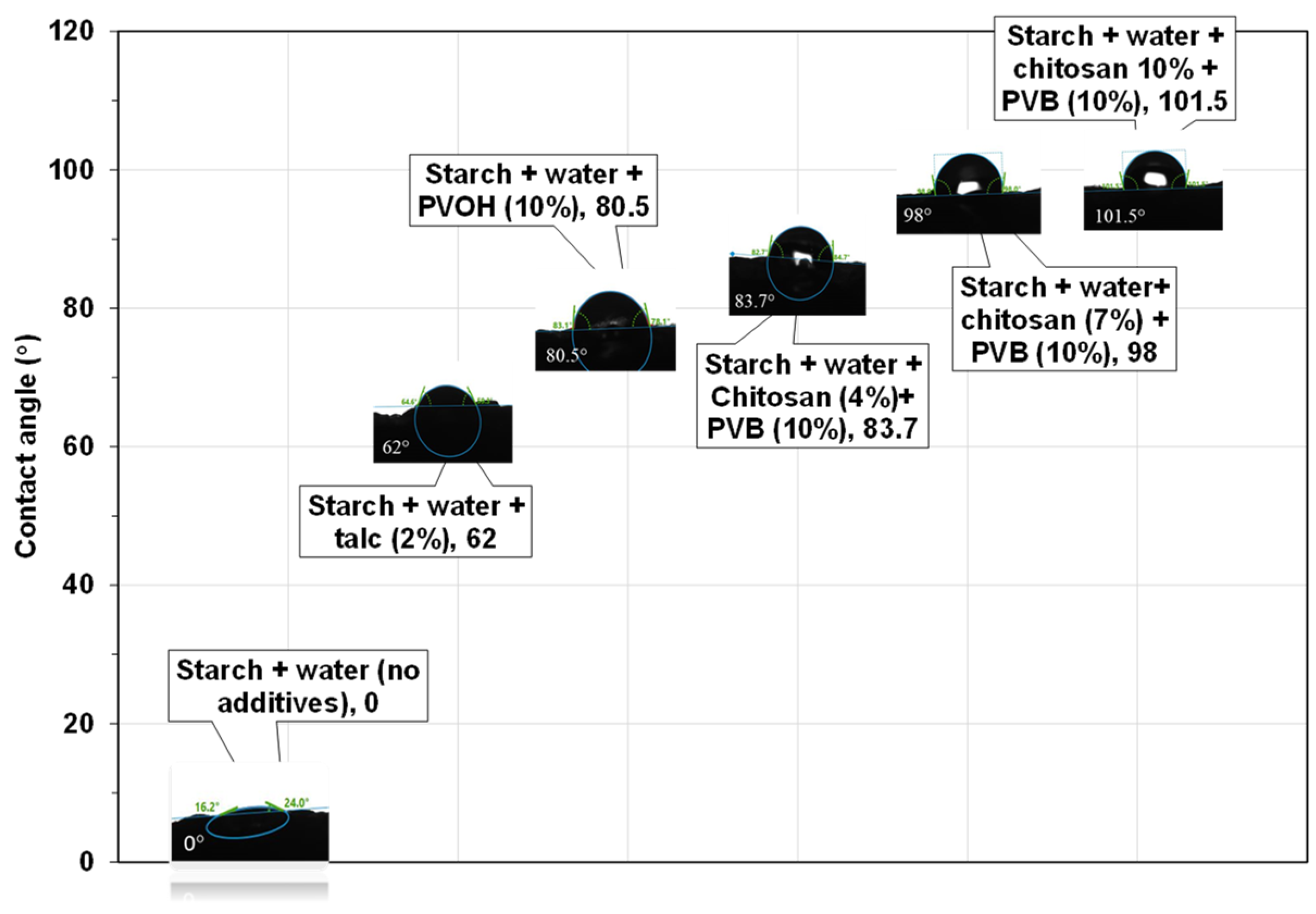
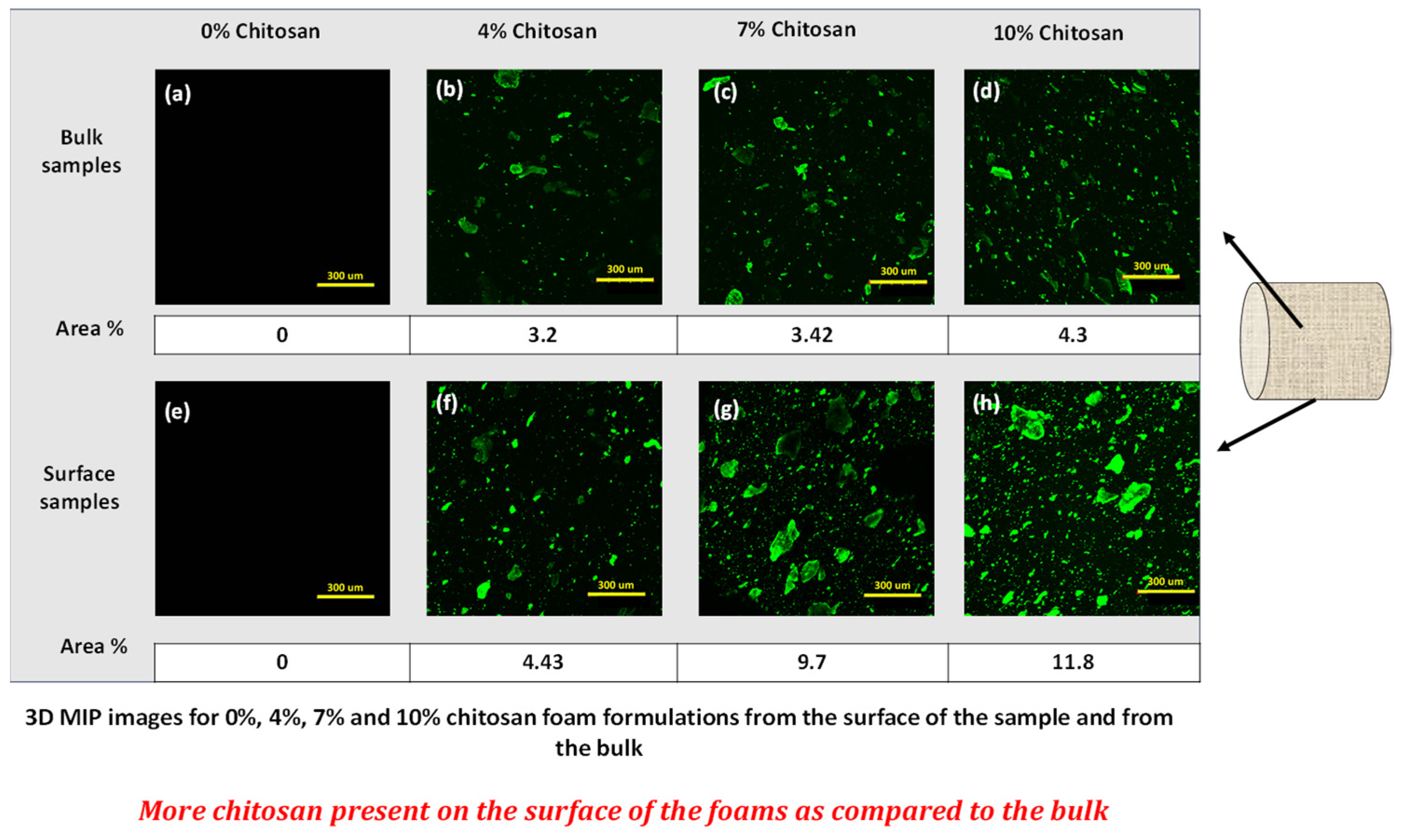

| Formulation # | Starch Type | talc (%) | Chitosan (%) | PVOH (%) | PVB (%) | Die Diameter (mm) |
|---|---|---|---|---|---|---|
| Variation in talc content | ||||||
| 1 | High amylose cornstarch | 0 | - | - | - | 4.3 |
| 2 | High amylose cornstarch | 0.7 | - | - | - | 4.3 |
| 3 | High amylose cornstarch | 2 | - | - | - | 4.3 |
| Using PVOH and chitosan (variation in die diameter) | ||||||
| 4 | High amylose cornstarch | - | - | 10 | - | 4.3 |
| 5 | High amylose cornstarch | - | 4 | 10 | - | 4.3 |
| 6 | High amylose cornstarch | - | 4 | 10 | - | 3.5 |
| 7 | High amylose cornstarch | - | 4 | 10 | - | 8 |
| Using PVB and Variation in chitosan content | ||||||
| 8 | High amylose cornstarch | - | 0 | - | 10 | 4.3 |
| 9 | High amylose cornstarch | - | 4 | - | 10 | 4.3 |
| 10 | High amylose cornstarch | - | 7 | - | 10 | 4.3 |
| 11 | High amylose cornstarch | - | 10 | - | 10 | 4.3 |
| Salt | RH |
|---|---|
| Lithium chloride | 11% |
| Magnesium chloride | 33% |
| Magnesium nitrate | 52% |
| Sodium chloride | 75% |
| Potassium Nitrate | 95% |
| Zone | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Die |
|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 40 | 60 | 80 | 90 | 100 | 110 | 120 | 130 | 140 | 140 |
| Sample | RH | k1 | k2 | R2 |
|---|---|---|---|---|
| Control foam | LiCl = 11% | 0.444 | 0.249 | 0.984 |
| PvOH 10%, chitosan 4% | 0.460 | 0.270 | 0.988 | |
| PVB 10%, chitosan 4% | 0.615 | 0.279 | 0.987 | |
| Control foam | MgCl2 = 33% | 0.114 | 0.131 | 0.998 |
| PVOH 10%, chitosan 4% | 0.302 | 0.141 | 0.991 | |
| PVB 10%, chitosan 4% | 0.147 | 0.136 | 0.994 | |
| Control foam | MgNO3 = 52% | 0.100 | 0.093 | 0.997 |
| PVOH 10%, chitosan 4% | 0.410 | 0.095 | 0.996 | |
| PVB 10%, chitosan 4% | 0.352 | 0.100 | 0.994 | |
| Control foam | NaCl = 75% | 0.129 | 0.050 | 0.994 |
| PVOH 10%, chitosan 4% | 0.181 | 0.066 | 0.884 | |
| PVB 10%, chitosan 4% | 0.185 | 0.075 | 0.998 | |
| Control foam | KNO3 = 95% | 0.070 | 0.037 | 0.988 |
| PVOH 10%, chitosan 4% | 0.215 | 0.035 | 0.999 | |
| PVB 10%, chitosan 4% | 0.206 | 0.039 | 0.995 |
| Sample | Density (kg/m3) | Expansion Ratio | Compressive Strength (MPa) | Resiliency (%) |
|---|---|---|---|---|
| Control foam | 40.7 ± 8.9 | 46.6 ± 7.9 | 0.076 ± 0.01 | 64.5 ± 3.7 |
| PVOH 10%, chitosan 4% | 45.7 ± 2.3 | 27.5 ± 0.9 | 0.114 ± 0.01 | 54.8 ± 1.5 |
| PVB 10%, chitosan 4% | 21.1 ± 1.7 | 74.5 ± 3.9 | 0.040 ± 0.005 | 64.8 ± 2.3 |
| PVB 10%, chitosan 7% | 35.0 ± 3.8 | 51.1 ± 5.5 | 0.0614 ± 0.008 | 62.4 ± 1.8 |
| PVB 10%, chitosan 10% | 35.7 ± 3.0 | 50.1 ± 5.7 | 0.0800 ± 0.005 | 60.9 ± 2.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulkarni, A.; Emrich, J.; Narayan, R. Humidity Resistant Biodegradable Starch Foams Reinforced with Polyvinyl Butyral (PVB) and Chitosan. Polymers 2024, 16, 3402. https://doi.org/10.3390/polym16233402
Kulkarni A, Emrich J, Narayan R. Humidity Resistant Biodegradable Starch Foams Reinforced with Polyvinyl Butyral (PVB) and Chitosan. Polymers. 2024; 16(23):3402. https://doi.org/10.3390/polym16233402
Chicago/Turabian StyleKulkarni, Apoorva, Jakob Emrich, and Ramani Narayan. 2024. "Humidity Resistant Biodegradable Starch Foams Reinforced with Polyvinyl Butyral (PVB) and Chitosan" Polymers 16, no. 23: 3402. https://doi.org/10.3390/polym16233402
APA StyleKulkarni, A., Emrich, J., & Narayan, R. (2024). Humidity Resistant Biodegradable Starch Foams Reinforced with Polyvinyl Butyral (PVB) and Chitosan. Polymers, 16(23), 3402. https://doi.org/10.3390/polym16233402









