Influence of Chitosan Purification on the Immunomodulator Potential of Chitosan Nanoparticles on Human Monocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chitosan Purification
2.2. Experimental Groups
2.3. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.4. Chitosan Nanoparticles (CtsNps) Synthesis
2.5. Size and Potential of Chitosan Nanoparticles
2.6. Scanning Electron Microscopy
2.7. Obtaining Peripheral Blood Mononuclear Cells (PBMCs)
2.8. Confocal Microscopy
2.9. Viability Assay In Vitro
2.10. Analysis of Expression of CD11c, CD14, CD80 and CD86 Markers
2.11. Determination of Phagocytic Activity of Macrophages
2.12. Analysis of Cytokine Secretion
2.13. Arginase and iNOS Detection by Immunofluorescence
2.14. Statistical Analysis
3. Results
3.1. FT-IR Analysis of Chitosan Purified and Unpurified
3.2. Characterization of Chitosan Nanoparticles (CtsNps)
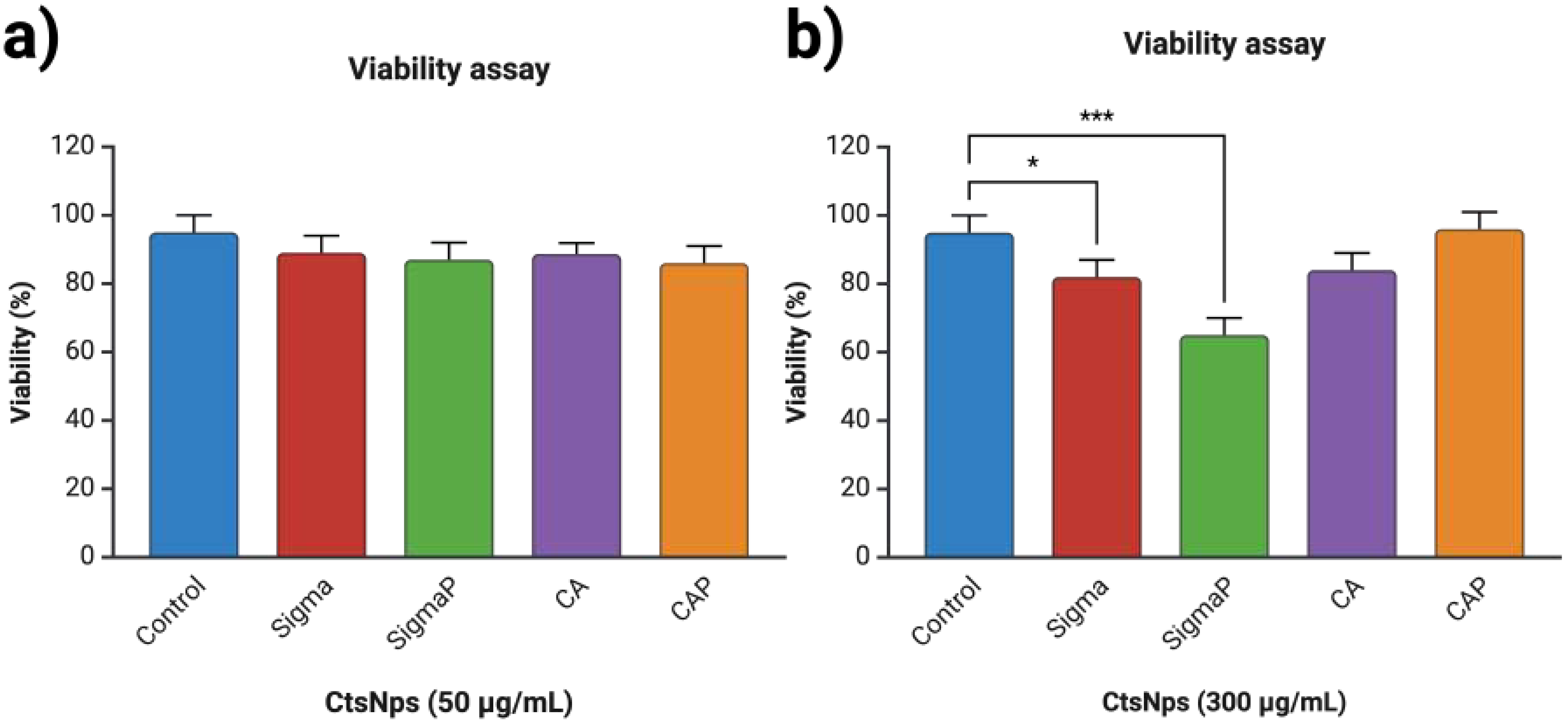
3.3. Viability on Human Macrophages with Chitosan Nanoparticles
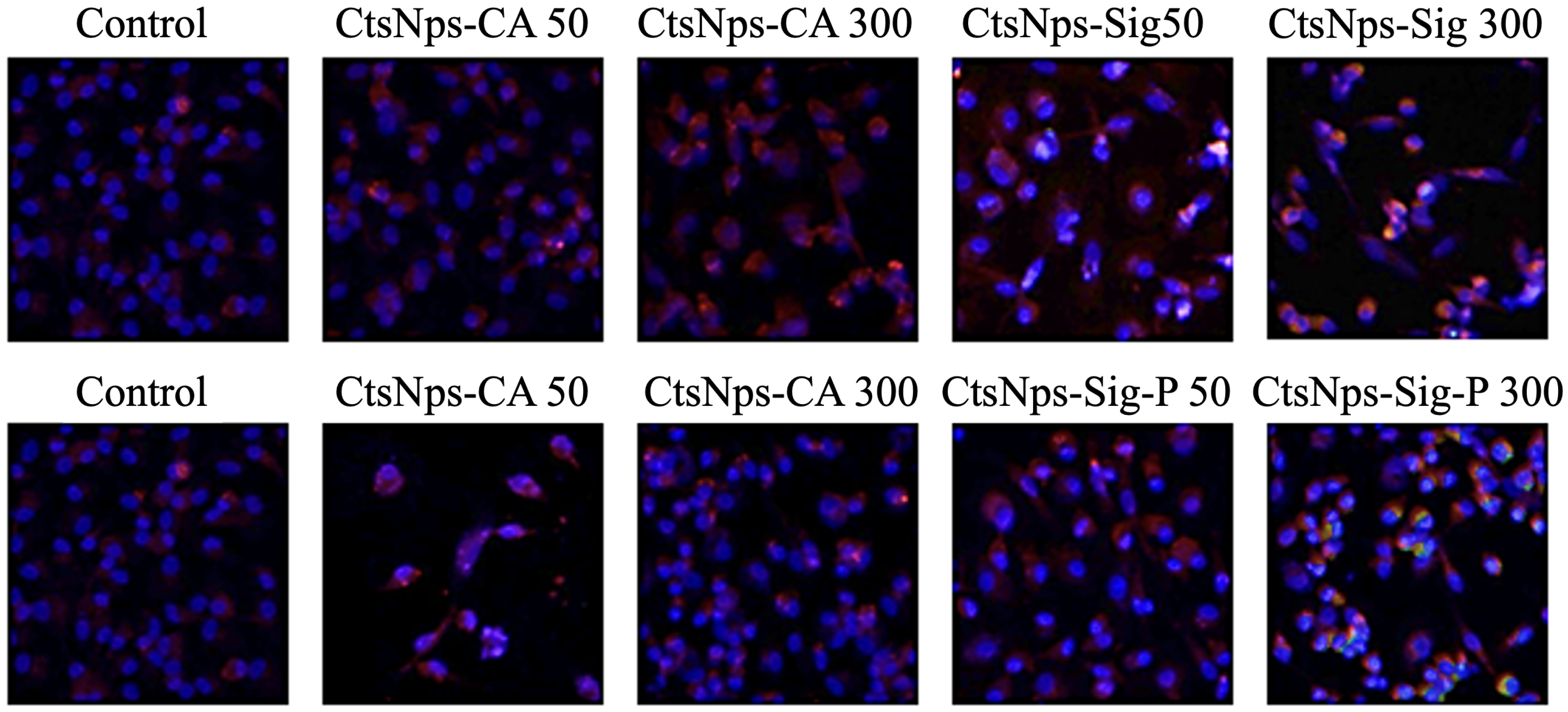
3.4. Confocal Microscopy
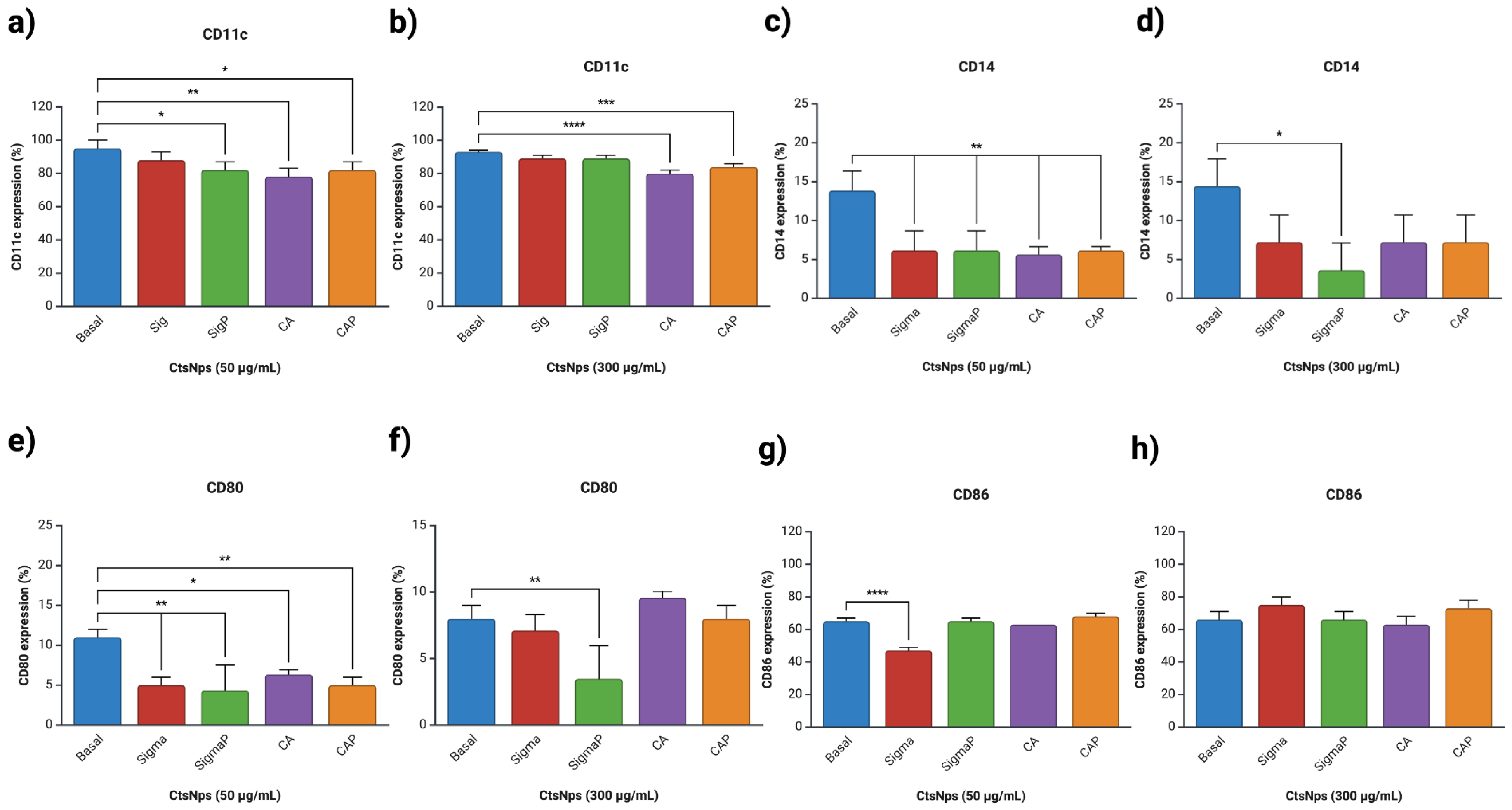
3.5. Expression of Macrophages
3.6. Determination of Phagocytic Activity
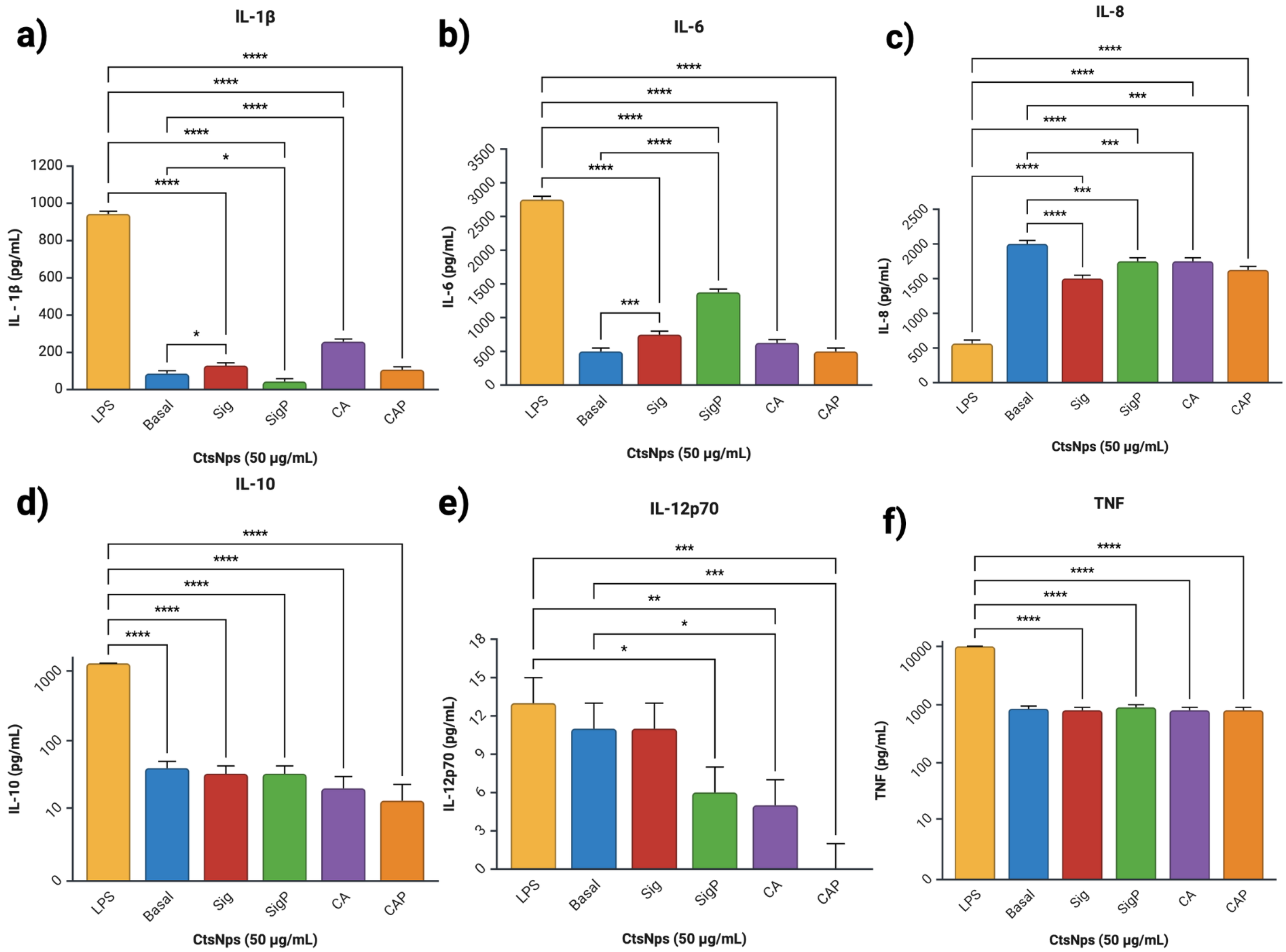
3.7. Cytokine Analysis
3.8. Arginase and iNOS Detection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jafernik, K.; Ładniak, A.; Blicharska, E.; Czarnek, K.; Ekiert, H.; Wiącek, A.E.; Szopa, A. Chitosan-based nanoparticles as effective drug delivery systems-A review. Molecules 2023, 28, 1963. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Mayanovic, R. A review of the preparation, characterization, and applications of chitosan nanoparticles in nanomedicine. Nanomaterials 2023, 13, 1302. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.; Santos, S.; Oliveira, M.; Torres, A.; Barbosa, M. Chitosan drives anti-inflammatory macrophage polarisation and pro-inflammatory dendritic cell stimulation. Eur. Cells Mater. 2012, 24, 133–136. [Google Scholar] [CrossRef]
- Piekarska, K.; Sikora, M.; Owczarek, M.; Jóźwik-Pruska, J.; Wiśniewska-Wrona, M. Chitin and chitosan as polymers of the future—Obtaining, modification, life cycle assessment and main directions of application. Polymers 2023, 15, 793. [Google Scholar] [CrossRef]
- Kim, Y.; Zharkinbekov, Z.; Raziyeva, K.; Tabyldiyeva, L.; Berikova, K.; Zhumagul, D.; Temirkhanova, K.; Saparov, A. Chitosan-Based Biomaterials for Tissue Regeneration. Pharmaceutics 2023, 15, 807. [Google Scholar] [CrossRef] [PubMed]
- Mikušová, V.; Mikuš, P. Advances in chitosan-based nanoparticles for drug delivery. Int. J. Mol. Sci. 2021, 22, 9652. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.; Ng, T.; Wong, J.; Chan, W. Chitosan: An update on potential biomedical and pharmaceutical applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Riva, R.; Ragelle, H.; des Rieux, A.; Duhem, N.; Jérôme, C.; Preat, V. Chitosan and chitosan derivatives in drug delivery and tissue engineering chitosan. In Chitosan for Biomaterials II; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Divya, K.; Jisha, M. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2018, 16, 101–112. [Google Scholar] [CrossRef]
- Álvarez, K.; Rojas, M. Nanoparticles targeting monocytes and macrophages as diagnostic and therapeutic tools for autoimmune diseases. Heliyon 2023, 9, e19861. [Google Scholar] [CrossRef]
- Aibani, N.; Rai, R.; Patel, P.; Cuddihy, G.; Wasan, E.K. Chitosan nanoparticles at the biological interface: Implications for drug delivery. Pharmaceutics 2021, 13, 1686. [Google Scholar] [CrossRef]
- Xu, D.; Hein, S.; Wang, K. Chitosan membrane in separation applications. Mater. Sci. Technol. 2008, 24, 1076–1087. [Google Scholar] [CrossRef]
- Shim, S.; Park, H.E.; Soh, S.H.; Im, Y.B.; Yoo, H.S. Induction of Th2 response through TLR2-mediated MyD88-dependent pathway in human microfold cells stimulated with chitosan nanoparticles loaded with Brucella abortus Mdh. Microb. Pathog. 2020, 142, 104040. [Google Scholar] [CrossRef] [PubMed]
- Portela, L.; Cahú, T.; Bezerra, T.; Santos, D.; Sousa, G.; Portela, R.; Melo, C.; Bezerra, R. Biocompatibility and immunostimulatory properties of fish collagen and shrimp chitosan towards peripheral blood mononuclear cells (PBMCs). Int. J. Biol. Macromol. 2022, 210, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Poupot, R.; Goursat, C.; Fruchon, S. Multivalent nanosystems: Targeting monocytes/macrophages. Int. J. Nanomed. 2018, 13, 5511–5521. [Google Scholar] [CrossRef]
- Chen, S.; Saeed, A.; Liu, Q.; Jiang, Q.; Xu, H.; Xiao, G.; Rao, R.; Duo, Y. Macrophages in immunoregulation and therapeutics. Signal Transduct. Target. Ther. 2023, 8, 207. [Google Scholar] [CrossRef]
- Jayasingam, S.; Citartan, M.; Thang, T.; Mat Zin, A.; Ang Kai, C.; Ch’ng, E. Evaluating the polarization of tumor-associated macrophages into M1 and M2 phenotypes in human cancer tissue: Technicalities and challenges in routine clinical practice. Front. Oncol. 2020, 9, 1512. [Google Scholar] [CrossRef]
- Ravindranathan, S.; Koppolu, B.; Smith, S.; Zaharoff, D. Effect of chitosan properties on immunoreactivity. Mar. Drugs 2016, 14, 91. [Google Scholar] [CrossRef]
- Mima, S.; Miya, M.; Iwamoto, R.; Yoshikawa, S. Highly deacetylated chitosan and its properties. J. Appl. Polym. Sci. 1983, 28, 1909–1917. [Google Scholar] [CrossRef]
- Oudih, S.; Tahtat, D.; Khodja, A.; Mahlous, M.; Hammache, Y.; Guittoum, A.; Gana, S. Chitosan nanoparticles with controlled size and zeta potential. Polym. Eng. Sci. 2023, 53, 1011–1021. [Google Scholar] [CrossRef]
- Calvo, P.; Remuñan-López, C.; Vila-Jato, J.L.; Alonso, M.J. Chitosan and chitosan/ethylene oxide-propylene oxide block copolymer nanoparticles as novel carriers for proteins and vaccines. Pharm. Res. 1997, 10, 1431–1436. [Google Scholar] [CrossRef]
- Kim, H.; Yoon, H.; Lee, M.; Kim, Y.; Lee, C. Impact of UV-C irradiation on bacterial disinfection in a drinking water purification system. J. Microbiol. Biotechnol. 2023, 33, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Ahmed Rather, G.; Patrício, A.; Haq, Z.; Sheikh, A.; Shah, M.; Singh, H.; Khan, A.; Imtiyaz, S.; Ahmad, S. Chitosan nanoparticles: A versatile platform for biomedical applications. Materials 2022, 15, 6521. [Google Scholar] [CrossRef]
- Sontyana, B.; Govatati, S.; Tamanam, R. A quick and easy method for the isolation of peripheral blood mononuclear cells from whole blood by density gradient centrifugation using HISEP LSM medium. Int. J. Adv. Res. 2016, 4, 1774–1778. [Google Scholar] [CrossRef]
- Jiménez-Mejía, G.; Montalvo-Méndez, R.; Hernández-Bautista, C.; Altamirano-Torres, C.; Vázquez, M.; Zurta, M.; Resendez-Perez, D. Trimeric complexes of Antp-TBP with TFIIEβ or Exd modulate transcriptional activity. Hereditas 2022, 159, 23. [Google Scholar] [CrossRef]
- Riss, T.; Moravec, R.; Niles, A.; Sarah, D.; Benik, H.; Worzella, T.; Minor, L. Cell Viability Assays. In Assay Guidance Manual; Markossian, S., Grossman, A., Arkin, M., Auld, D., Austin, C., Baell, J., Brimacombe, K., Chung, T.D.Y., Coussens, N.P., Dahlin, J.L., et al., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2016. [Google Scholar]
- Calmeiro, J.; Mendes, L.; Duarte, I.; Leitão, C.; Tavares, A.; Ferreira, D.; Gomes, C.; Serra, J.; Falcão, A.; Cruz, M.; et al. In-depth analysis of the impact of different serum-free media on the production of clinical grade dendritic cells for cancer immunotherapy. Front. Immunol. 2021, 11, 593363. [Google Scholar] [CrossRef]
- Leuenberger, T.; Pfueller, C.; Luessi, F.; Bendix, I.; Paterka, M.; Prozorovski, T.; Treue, D.; Luenstedt, S.; Herz, J.; Siffrin, V.; et al. Modulation of dendritic cell immunobiology via inhibition of 3-Hydroxy-3-Methylglutaryl-CoA (HMG-CoA) reductase. PLoS ONE 2014, 9, e100871. [Google Scholar] [CrossRef]
- Becton, Dickinson and Company. BD Cytometric Bead Array (CBA) Human Inflammatory Cytokines Kit; Becton, Dickinson and Company: Franklin Lakes, NJ, USA, 2019. [Google Scholar]
- Mytych, J.; Romerowicz-Misielak, M.; Koziorowski, M. Long-term culture with lipopolysaccharide induces dose-dependent cytostatic and cytotoxic effects in THP-1 monocytes. Toxicol. Vitr. 2017, 42, 1–9. [Google Scholar] [CrossRef]
- Durante, W.; Johnson, F.; Johnson, R. Arginase: A critical regulator of nitric oxide synthesis and vascular function. Clin. Exp. Pharmacol. Physiol. 2007, 34, 906–911. [Google Scholar] [CrossRef]
- Wu, J.; Liu, F.; Wang, Z.; Liu, Y.; Zhao, X.; Fang, C.; Leung, F.; Yeung, K.; Wong, T. The development of a magnesium-releasing and long-term mechanically stable calcium phosphate bone cement possessing osteogenic and immunomodulation effects for promoting bone fracture regeneration. Front. Bioeng. Biotechnol. 2022, 9, 803723. [Google Scholar] [CrossRef]
- Ssekatawa, K.; Byarugaba, D.; Wampande, E.; Moja, T.; Nxumalo, E.; Maaza, M.; Sackey, J.; Ejobi, F.; Kirabira, J. Isolation and characterization of chitosan from Ugandan edible mushrooms, Nile perch scales and banana weevils for biomedical applications. Sci. Rep. 2021, 11, 4116. [Google Scholar] [CrossRef]
- Dahmane, E.; Taourirte, M.; Eladlani, N.; Rhazi, M. Extraction and characterization of chitin and chitosan from Parapenaeus longirostris from Moroccan local sources. Int. J. Polym. Anal. Charact. 2014, 19, 342–351. [Google Scholar] [CrossRef]
- Kumari, S.; Kishor, R. Chitin and chitosan: Origin, properties, and applications. In Handbook of Chitin and Chitosan; Gopi, S., Thomas, S., Pius, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Liu, H.; Gao, C. Preparation and properties of ionically cross-linked chitosan nanoparticles. Polym. Adv. Technol. 2009, 20, 613–619. [Google Scholar] [CrossRef]
- da Silva Lucas, A.; Quadro Oreste, E.; Gouveia Costa, H.; Martín López, H.; Medeiros Saad, C.; Prentice, C. Extraction, physicochemical characterization, and morphological properties of chitin and chitosan from cuticles of edible insects. Food Chem. 2021, 343, 128550. [Google Scholar] [CrossRef]
- O’Callaghan, K.; Kerry, J. Preparation of low- and medium-molecular weight chitosan nanoparticles and their antimicrobial evaluation against a panel of microorganisms, including cheese-derived cultures. Food Control 2016, 69, 256–261. [Google Scholar] [CrossRef]
- Shahrooz, S.; Atyabi, F.; Akhlaghi, S.; Ostad, S.; Dinarvand, R. Thiolated chitosan nanoparticles for enhancing oral absorption of docetaxel: Preparation, in vitro and ex vivo evaluation. Int. J. Nanomed. 2011, 6, 119–128. [Google Scholar]
- Aranda-Barradas, M.; Trejo-López, S.; Del Real, A.; Álvarez-Almazán, S.; Méndez-Albores, A.; García-Tovar, C.; González-Díaz, F.; Miranda-Castro, F. Effect of molecular weight of chitosan on the physicochemical, morphological, and biological properties of polyplex nanoparticles intended for gene delivery. Carbohydr. Polym. Technol. Appl. 2022, 4, 100228. [Google Scholar] [CrossRef]
- Zoe, L.; David, S.; Rajabalaya, R. Chitosan nanoparticle toxicity: A comprehensive literature review of in vivo and in vitro assessments for medical applications. Toxicol. Rep. 2023, 11, 83–106. [Google Scholar] [CrossRef]
- Frigaard, J.; Jensen, J.; Galtung, H.; Hiorth, M. The Potential of Chitosan in Nanomedicine: An overview of the cytotoxicity of chitosan based nanoparticles. Front. Pharmacol. 2022, 13, 880377. [Google Scholar] [CrossRef]
- Pellis, A.; Guebitz, G.; Nyanhongo, G. Chitosan: Sources, processing and modification techniques. Gels 2022, 8, 393. [Google Scholar] [CrossRef]
- Chang, S.H.; Lin, Y.Y.; Wu, G.J.; Huang, C.H.; Tsai, G.J. Effect of chitosan molecular weight on anti-inflammatory activity in the RAW 264.7 macrophage model. Int. J. Biol. Macromol. 2019, 131, 167–175. [Google Scholar] [CrossRef]
- Quah, B.; Parish, C. The use of carboxyfluorescein diacetate succinimidyl ester (CFSE) to monitor lymphocyte proliferation. J. Vis. Exp. 2010, 44, 2259. [Google Scholar] [CrossRef]
- Jimenez-Duran, G.; Luque-Martin, R.; Patel, M.; Koppe, E.; Bernard, S.; Sharp, C.; Buchan, N.; Rea, C.; de Winther, M.; Turan, N.; et al. Pharmacological validation of targets regulating CD14 during macrophage differentiation. eBioMedicine 2020, 61, 103039. [Google Scholar] [CrossRef] [PubMed]
- Kiemer, A. Blockade of an innate immune amplifier to fight immune hyperactivation in COVID-19? eBioMedicine 2020, 61, 103086. [Google Scholar] [CrossRef]
- Oates, T.; Moura, P.; Cross, S.; Roberts, K.; Baum, H.; Haydn-Smith, K.; Wilson, M.; Heesom, K.; Severn, C.; Toye, A. Defining the proteomic landscape of cultured macrophages and their polarization continuum. Immunol. Cell Biol. 2023, 101, 947–963. [Google Scholar] [CrossRef]
- Strizova, Z.; Benesova, I.; Bartolini, R.; Novysedlak, R.; Cecrdlova, E.; Foley, L.; Striz, I. M1/M2 macrophages and their overlaps—Myth or reality? Clin. Sci. 2023, 137, 1067–1093. [Google Scholar] [CrossRef]
- Panyam, J.; Labhasetwar, V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, T.; Webster, T.; Duan, H.; Qiu, J.; Zhao, Z.; Yin, X.; Zheng, C. Intracellular disposition of chitosan nanoparticles in macrophages: Intracellular uptake, exocytosis, and intercellular transport. Int. J. Nanomed. 2017, 12, 6383–6398. [Google Scholar] [CrossRef]
- Buchacher, T.; Ohradanova-Repic, A.; Stockinger, H.; Fischer, M.; Weber, V. M2 polarization of human macrophages favors survival of the intracellular pathogen Chlamydia pneumoniae. PLoS ONE 2015, 10, e0143593. [Google Scholar] [CrossRef]
- Nie, J.; Fu, X.; Jiachao, L.; Gao, X. A systematic review of fermented Saccharina japonica: Fermentation conditions, metabolites, potential health benefits and mechanisms. Trends Food Sci. Technol. 2022, 123, 15–27. [Google Scholar] [CrossRef]
- Yang, Z.; Ming, X. Functions of arginase isoforms in macrophage inflammatory responses: Impact on cardiovascular diseases and metabolic disorders. Front. Immunol. 2014, 5, 533. [Google Scholar] [CrossRef]





| Band (cm−1) | Group | Intensity |
|---|---|---|
| 3300 | OH | |
| 3100–2850 * | C-H | Stretching |
| 2800 | C-H | |
| 1650 * | Amide I | Bending |
| 1550 * | NH2 | |
| 1200 | C-O | Stretching |
| 1010 | C-H-C |
| Sample | Size | Polydispersity | Potential |
|---|---|---|---|
| nm | a.a | mV | |
| CtsNps-Sigma | 180 | 0.3 | 31.2 |
| CtsNps-Sigma P | 138 | 0.2 | 16.2 |
| CtsNps-CA | 177 | 0.3 | 3 |
| CtsNps-CAP | 126 | 0.3 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valades-Aguilar, B.A.; Rivera-González, T.I.; Rangel-López, R.; Luna-Barcenas, G.; Franco-Molina, M.Á.; Rodriguez-Padilla, C.; Zárate-Triviño, D.G. Influence of Chitosan Purification on the Immunomodulator Potential of Chitosan Nanoparticles on Human Monocytes. Polymers 2024, 16, 3390. https://doi.org/10.3390/polym16233390
Valades-Aguilar BA, Rivera-González TI, Rangel-López R, Luna-Barcenas G, Franco-Molina MÁ, Rodriguez-Padilla C, Zárate-Triviño DG. Influence of Chitosan Purification on the Immunomodulator Potential of Chitosan Nanoparticles on Human Monocytes. Polymers. 2024; 16(23):3390. https://doi.org/10.3390/polym16233390
Chicago/Turabian StyleValades-Aguilar, Bruno Alejandro, Teodoro Iván Rivera-González, Raúl Rangel-López, Gabriel Luna-Barcenas, Moisés Ármides Franco-Molina, Cristina Rodriguez-Padilla, and Diana Ginette Zárate-Triviño. 2024. "Influence of Chitosan Purification on the Immunomodulator Potential of Chitosan Nanoparticles on Human Monocytes" Polymers 16, no. 23: 3390. https://doi.org/10.3390/polym16233390
APA StyleValades-Aguilar, B. A., Rivera-González, T. I., Rangel-López, R., Luna-Barcenas, G., Franco-Molina, M. Á., Rodriguez-Padilla, C., & Zárate-Triviño, D. G. (2024). Influence of Chitosan Purification on the Immunomodulator Potential of Chitosan Nanoparticles on Human Monocytes. Polymers, 16(23), 3390. https://doi.org/10.3390/polym16233390





