Field Crop Evaluation of Polymeric Nanoparticles of Garlic Extract–Chitosan as Biostimulant Seed Nano-Priming in Cereals and Transcriptomic Insights
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Morphological Studies
2.3. Stability Studies
2.4. Evaluation of GE-NPCH as a Seed Coating Agent in a Rainfed Crop Experiment
2.5. Flavonoid, Polyphenol, and Chlorophyll Content in Treated Plants
2.5.1. Determination of Total Polyphenol Content
2.5.2. Determination of Total Flavonoid Content
2.5.3. Determination of Chlorophyll Content
2.6. Transcriptome Analysis
2.6.1. RNA Extraction, Library Preparation, and Sequencing
2.6.2. Data Processing and Analysis
2.7. Statistics
3. Results and Discussion
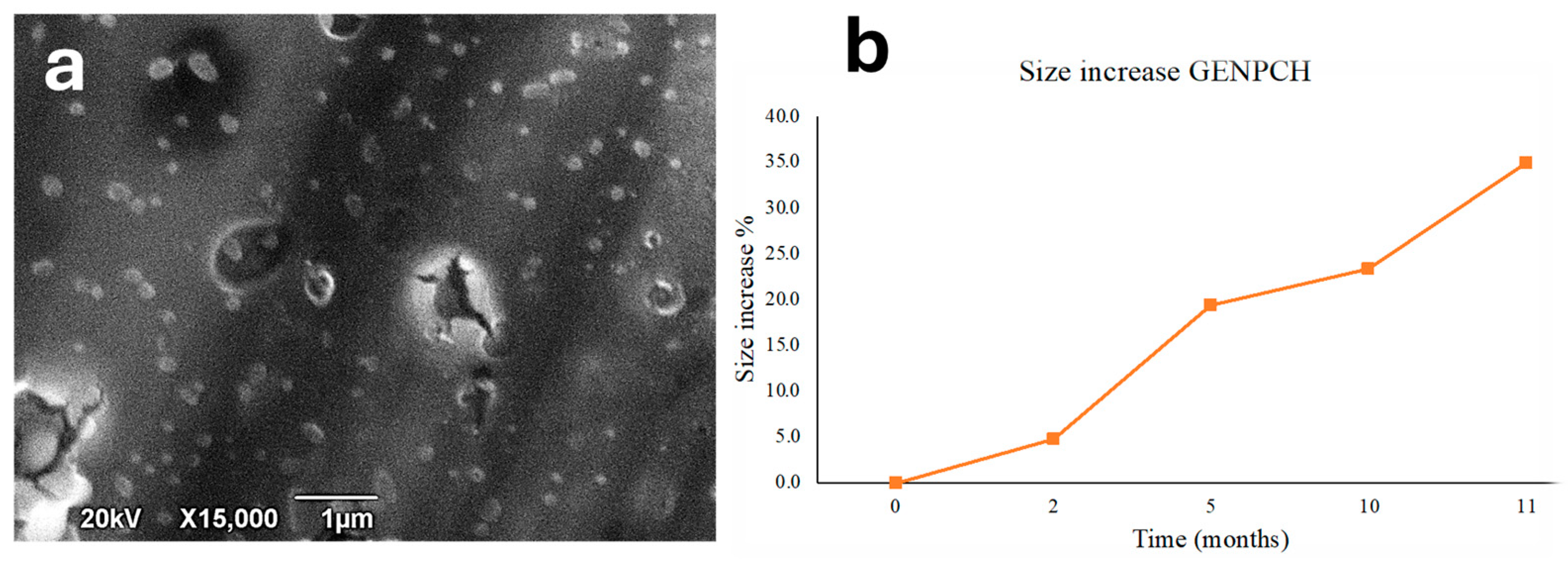
3.1. Morphology Characterization of GE-NPCH
3.2. Stability Properties of GE-NPCH
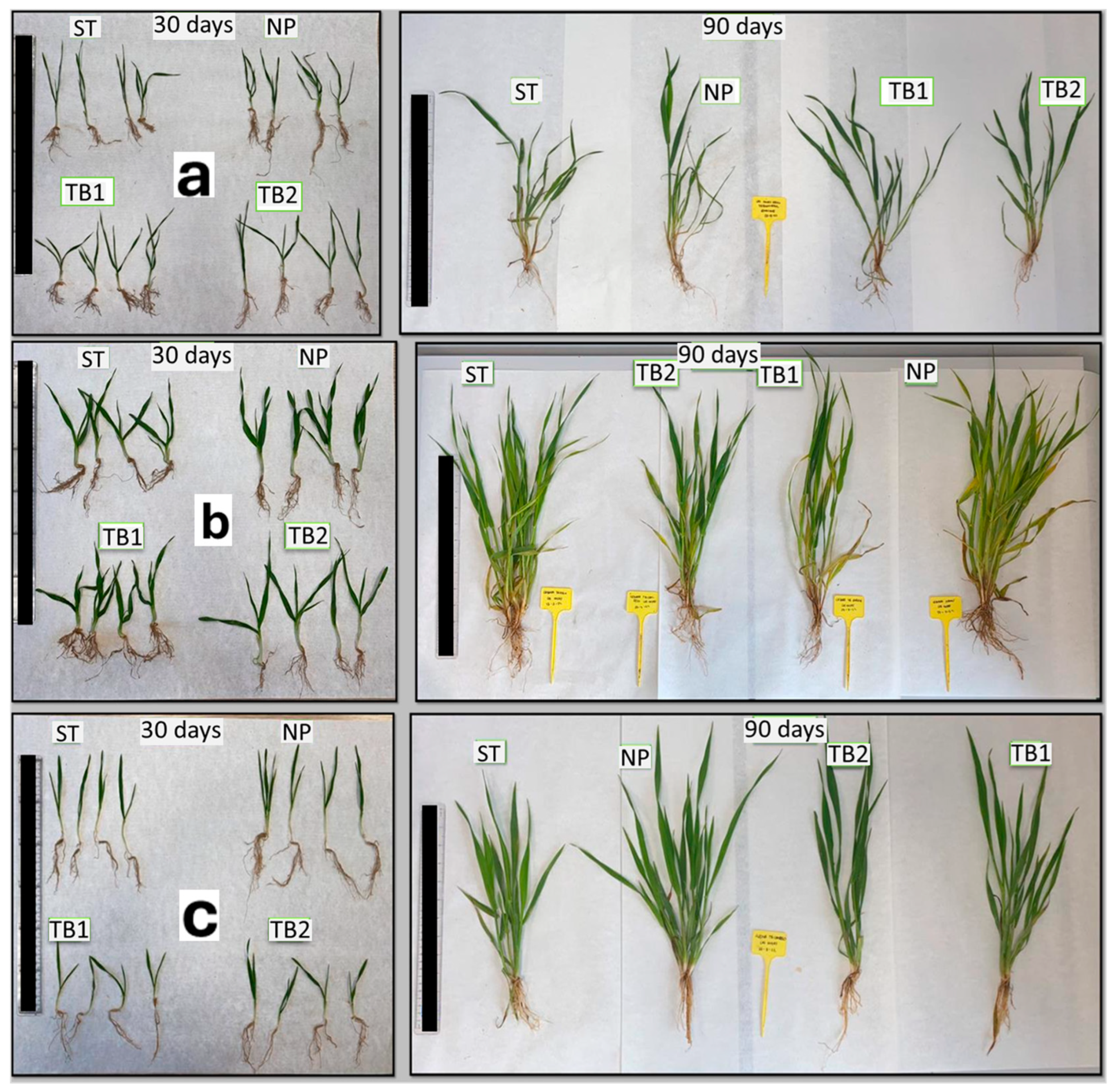
3.3. Evaluation of Seed Treatment with GE-NPCH in Rainfed Crop
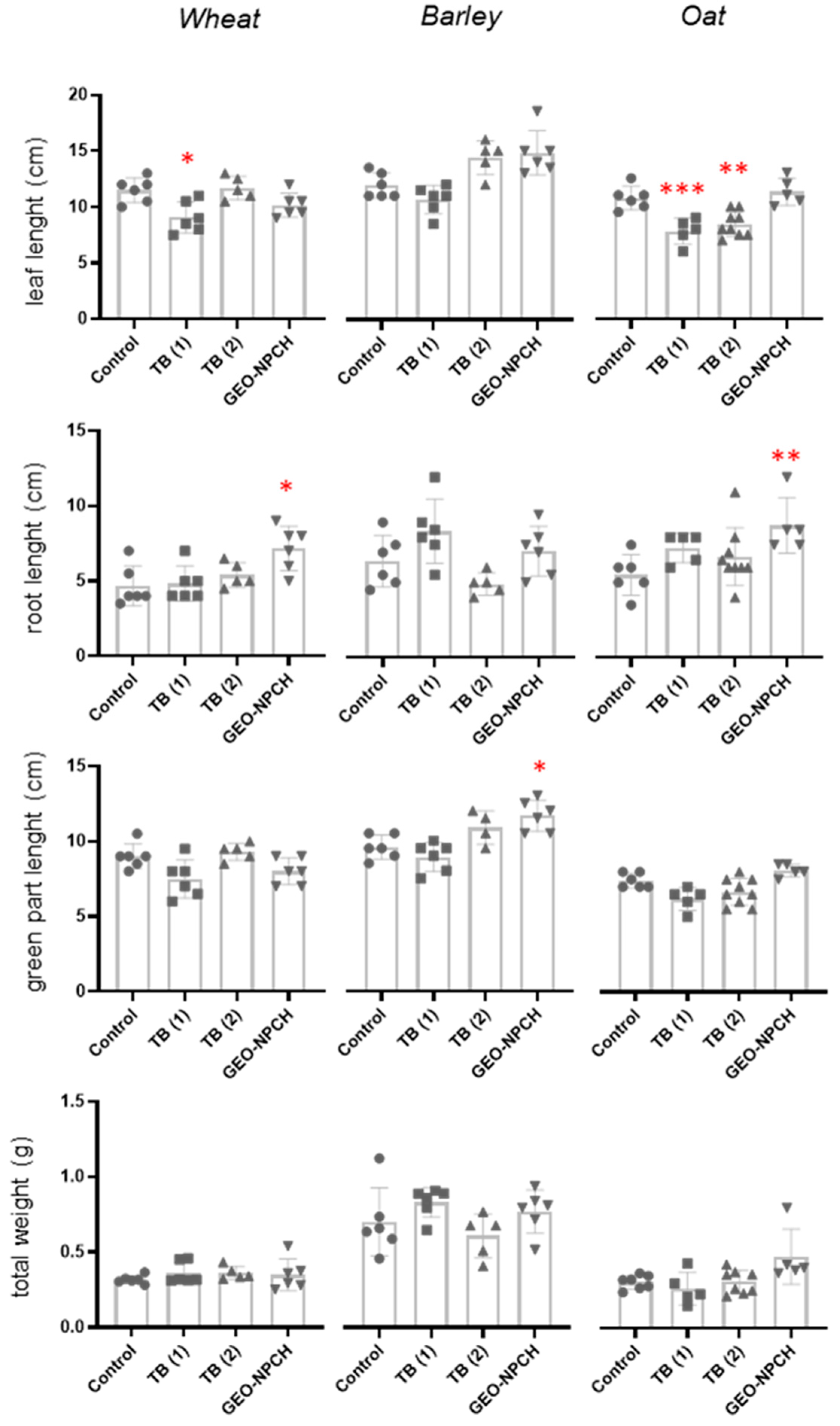
3.3.1. Morphological Evaluation of Wheat
3.3.2. Morphological Evaluation of Barley
3.3.3. Morphological Evaluation of Oat
3.4. Physiological Evaluation in Plants
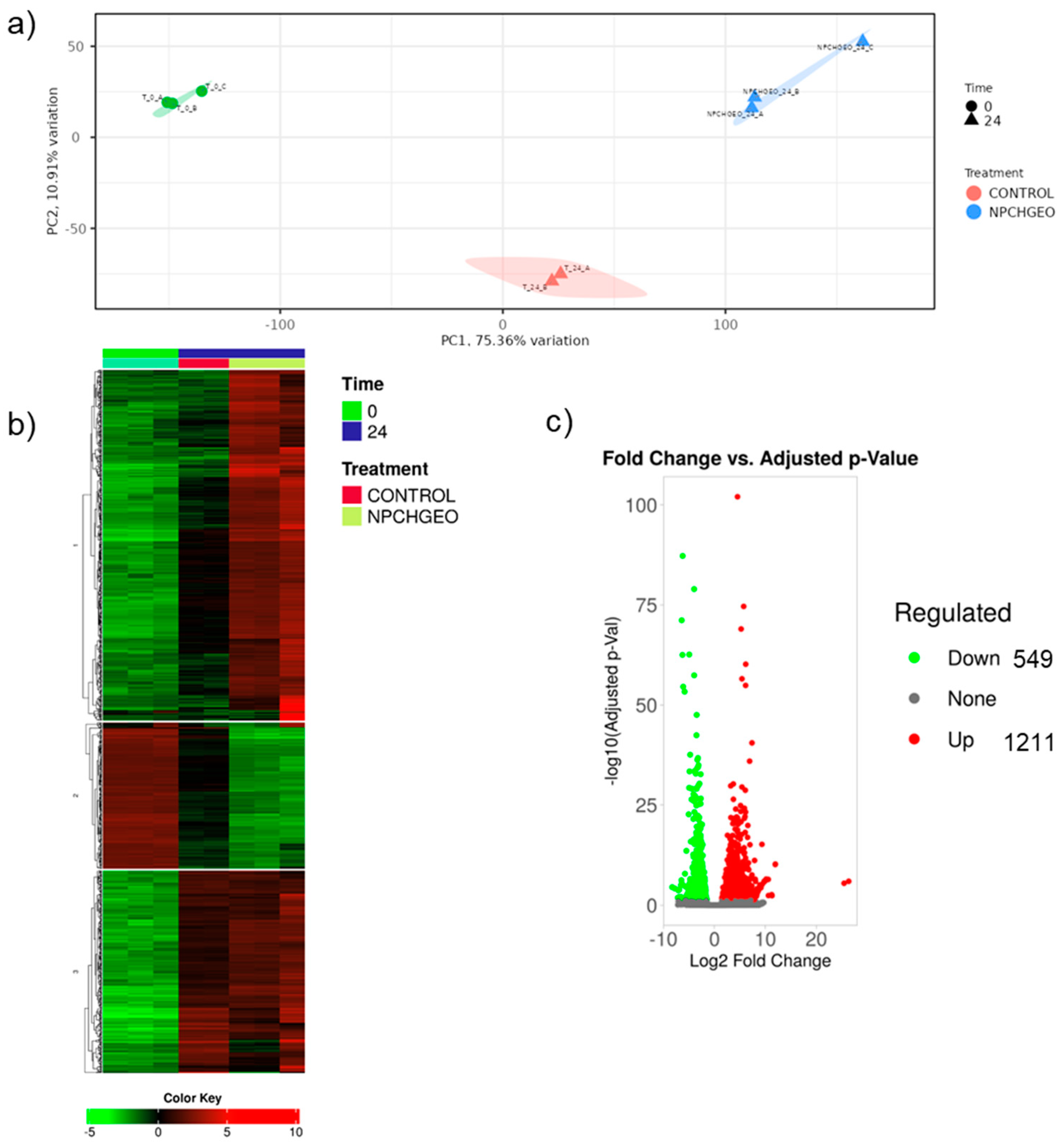
3.5. Transcriptomic Analysis of the Elicitor Effect of GE-NPCH on Germination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopez-Antia, A.; Ortiz-Santaliestra, M.E.; Mougeot, F.; Camarero, P.R.; Mateo, R. Birds feeding on tebuconazole treated seeds have reduced breeding output. Environ. Pollut. 2021, 271, 116292. [Google Scholar] [CrossRef] [PubMed]
- Heuser, D.I. Soil Governance in current European Union Law and in the European Green Deal. Soil Secur. 2022, 6, 100053. [Google Scholar] [CrossRef]
- Mondéjar-López, M.; López-Jimenez, A.J.; Ahrazem, O.; Gómez-Gómez, L.; Niza, E. Chitosan coated—Biogenic silver nanoparticles from wheat residues as green antifungal and nanoprimig in wheat seeds. Int. J. Biol. Macromol. 2023, 225, 964–973. [Google Scholar] [CrossRef] [PubMed]
- de la Paz Salgado-Cruz, M.; Salgado-Cruz, J.; García-Hernández, A.B.; Calderón-Domínguez, G.; Gómez-Viquez, H.; Oliver-Espinoza, R.; Fernández-Martínez, M.C.; Yáñez-Fernández, J. Chitosan as a Coating for Biocontrol in Postharvest Products: A Bibliometric Review. Membranes 2021, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Mahakham, W.; Sarmah, A.K.; Maensiri, S.; Theerakulpisut, P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci. Rep. 2017, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Altabbaa, S.; Mann, N.A.; Chauhan, N.; Utkarsh, K.; Thakur, N.; Mahmoud, G.A.E. Era connecting nanotechnology with agricultural sustainability: Issues and challenges. Nanotechnol. Environ. Eng. 2023, 8, 481–498. [Google Scholar] [CrossRef]
- Kasote, D.M.; Lee, J.H.J.; Jayaprakasha, G.K.; Patil, B.S. Manganese Oxide Nanoparticles as Safer Seed Priming Agent to Improve Chlorophyll and Antioxidant Profiles in Watermelon Seedlings. Nanomaterials 2021, 11, 1016. [Google Scholar] [CrossRef]
- Saritha, G.N.G.; Anju, T.; Kumar, A. Nanotechnology—Big impact: How nanotechnology is changing the future of agriculture? J. Agric. Food Res. 2022, 10, 100457. [Google Scholar] [CrossRef]
- Khan, F.; Shariq, M.; Asif, M.; Siddiqui, M.A.; Malan, P.; Ahmad, F. Green Nanotechnology: Plant-Mediated Nanoparticle Synthesis and Application. Nanomaterials 2022, 12, 673. [Google Scholar] [CrossRef]
- Román-Doval, R.; Torres-Arellanes, S.P.; Tenorio-Barajas, A.Y.; Gómez-Sánchez, A.; Valencia-Lazcano, A.A. Chitosan: Properties and Its Application in Agriculture in Context of Molecular Weight. Polymers 2023, 15, 2867. [Google Scholar] [CrossRef]
- André, W.; Paiba, J.; Cavalcante, G.; Ribeiro, W.; Araújo, J.; Cavalcanti, B.; Morais, S.; Oliveira, L.; Bevilaqua, C.; Abreu, F. Chitosan Nanoparticles Loaded with Carvacrol and Carvacryl Acetate for Improved Anthelmintic Activity. J. Braz. Chem. Soc. 2020, 31, 1614–1622. [Google Scholar] [CrossRef]
- Nile, S.H.; Thiruvengadam, M.; Wang, Y.; Samynathan, R.; Shariati, M.A.; Rebezov, M.; Nile, A.; Sun, M.; Venkidasamy, B.; Xiao, J.; et al. Nano-priming as emerging seed priming technology for sustainable agriculture—Recent developments and future perspectives. J. Nanobiotechnol. 2022, 20, 254. [Google Scholar] [CrossRef] [PubMed]
- Khalaki, M.A.; Moameri, M.; Lajayer, B.A.; Astatkie, T. Influence of nano-priming on seed germination and plant growth of forage and medicinal plants. Plant Growth Regul 2021, 93, 13–28. [Google Scholar] [CrossRef]
- Mondéjar-López, M.; López-Jiménez, A.J.; Abad-Jordá, M.; Rubio-Moraga, A.; Ahrazem, O.; Gómez-Gómez, L.; Niza, E. Biogenic Silver Nanoparticles from Iris tuberosa as Potential Preservative in Cosmetic Products. Molecules 2021, 26, 4696. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Tong, J.; Li, P.; Huang, X.; Dong, P.; Ren, M. Chitosan is an effective inhibitor against potato dry rot caused by Fusarium oxysporum. Physiol. Mol. Plant Pathol. 2021, 113, 101601. [Google Scholar] [CrossRef]
- Zeng, X.; Zhong, B.; Jia, Z.; Zhang, Q.; Chen, Y.; Jia, D. Halloysite nanotubes as nanocarriers for plant herbicide and its controlled release in biodegradable polymers composite film. Appl. Clay. Sci. 2019, 171, 20–28. [Google Scholar] [CrossRef]
- Mondéjar-López, M.; García-Simarro, M.P.; Navarro-Simarro, P.; Gómez-Gómez, L.; Ahrazem, O.; Niza, E. A review on the encapsulation of “eco-friendly” compounds in natural polymer-based nanoparticles as next generation nano-agrochemicals for sustainable agriculture and crop management. Int. J. Biol. Macromol. 2024, 280, 136030. [Google Scholar] [CrossRef]
- Hayat, S.; Ahmad, A.; Ahmad, H.; Hayat, K.; Khan, M.A.; Runan, T. Garlic, from medicinal herb to possible plant bioprotectant: A review. Sci. Hortic. 2022, 304, 111296. [Google Scholar] [CrossRef]
- Mondéjar-López, M.; Rubio-Moraga, A.; López-Jimenez, A.J.; Martínez, J.C.G.; Ahrazem, O.; Gómez-Gómez, L.; Niza, E. Chitosan nanoparticles loaded with garlic essential oil: A new alternative to tebuconazole as seed dressing agent. Carbohydr. Polym. 2022, 277, 118815. [Google Scholar] [CrossRef]
- Raj, S.; Jose, S.; Sumod, U.S.; Sabitha, M. Nanotechnology in cosmetics: Opportunities and challenges. J. Pharm. Bioallied. Sci. 2012, 4, 186–193. [Google Scholar] [CrossRef]
- Alves, H.J.; Gasparrini, L.J.; Silva, F.E.B.; Caciano, L.; de Muniz, G.I.B.; Ballester, E.L.C.; Cremonez, P.A.; Arantes, M.K. Alternative methods for the pilot-scale production and characterization of chitosan nanoparticles. Environ. Sci. Pollut. Res. 2021, 28, 10977–10987. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-Y.; Tang, C.-Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007, 101, 140–147. [Google Scholar] [CrossRef]
- Mondéjar-López, M.; Castillo, R.; Jiménez, A.J.L.; Gómez-Gómez, L.; Ahrazem, O.; Niza, E. Polysaccharide film containing cinnamaldehyde-chitosan nanoparticles, a new eco-packaging material effective in meat preservation. Food Chem. 2024, 437, 137710. [Google Scholar] [CrossRef] [PubMed]
- Choukaife, H.; Doolaanea, A.A.; Alfatama, M. Alginate Nanoformulation: Influence of Process and Selected Variables. Pharmaceuticals 2020, 13, 335. [Google Scholar] [CrossRef] [PubMed]
- Mondéjar-López, M.; López-Jimenez, A.J.; Martínez, J.C.G.; Ahrazem, O.; Gómez-Gómez, L.; Niza, E. Comparative evaluation of carvacrol and eugenol chitosan nanoparticles as eco-friendly preservative agents in cosmetics. Int. J. Biol. Macromol. 2022, 206, 288–297. [Google Scholar] [CrossRef]
- Morris, G.A.; Castile, J.; Smith, A.; Adams, G.G.; Harding, S.E. The effect of prolonged storage at different temperatures on the particle size distribution of tripolyphosphate (TPP)—Chitosan nanoparticles. Carbohydr. Polym. 2011, 84, 1430–1434. [Google Scholar] [CrossRef]
- Jonassen, H.; Kjøniksen, A.-L.; Hiorth, M. Stability of chitosan nanoparticles cross-linked with tripolyphosphate. Biomacromolecules 2012, 13, 3747–3756. [Google Scholar] [CrossRef]
- Wasternack, C. Action of jasmonates in plant stress responses and development–applied aspects. Biotechnol. Adv. 2014, 32, 31–39. [Google Scholar] [CrossRef]
- Balachandran, S.; Hurry, V.M.; Kelley, S.E.; Osmond, C.B.; Robinson, S.A.; Rohozinski, J.; Seaton, G.G.R.; Sims, D.A. Concepts of plant biotic stress. Some insights into the stress physiology of virus-infected plants, from the perspective of photosynthesis. Physiol. Plant 1997, 100, 203–213. [Google Scholar] [CrossRef]
- Tleuova, A.B.; Wielogorska, E.; Talluri, V.S.S.L.P.; Štěpánek, F.; Elliott, C.T.; Grigoriev, D.O. Recent advances and remaining barriers to producing novel formulations of fungicides for safe and sustainable agriculture. J. Control. Release 2020, 326, 468–481. [Google Scholar] [CrossRef]
- Dong, B. A comprehensive review on toxicological mechanisms and transformation products of tebuconazole: Insights on pesticide management. Sci. Total Environ. 2024, 908, 168264. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.K.; Patel, S.; Kushwah, K.S. Green biosynthesis of silver nanoparticles and impact on growth, chlorophyll, yield and phytotoxicity of Phaseolus vulgaris L. Vegetos 2020, 33, 648–657. [Google Scholar] [CrossRef]
- Singh, S.; Kaur, I.; Kariyat, R. The Multifunctional Roles of Polyphenols in Plant-Herbivore Interactions. Int. J. Mol. Sci. 2021, 22, 1442. [Google Scholar] [CrossRef] [PubMed]
- Misra, D.; Dutta, W.; Jha, G.; Ray, P. Interactions and Regulatory Functions of Phenolics in Soil-Plant-Climate Nexus. Agronomy 2023, 13, 280. [Google Scholar] [CrossRef]
- Wallis, C.M.; Galarneau, E.R.-A. Phenolic Compound Induction in Plant-Microbe and Plant-Insect Interactions: A Meta-Analysis. Front. Plant Sci. 2020, 11, 580753. [Google Scholar] [CrossRef]
- Jha, Y.; Mohamed, H.I. Plant Secondary Metabolites as a Tool to Investigate Biotic Stress Tolerance in Plants: A Review. Gesunde Pflanz. 2022, 74, 771–790. [Google Scholar] [CrossRef]
- Balusamy, S.R.; Rahimi, S.; Sukweenadhi, J.; Sunderraj, S.; Shanmugam, R.; Thangavelu, L.; Mijakovic, I.; Perumalsamy, H. Chitosan, chitosan nanoparticles and modified chitosan biomaterials, a potential tool to combat salinity stress in plants. Carbohydr. Polym. 2022, 284, 119189. [Google Scholar] [CrossRef]
- Heimler, D.; Vignolini, P.; Isolani, L.; Arfaioli, P.; Ghiselli, L.; Romani, A. Polyphenol Content of Modern and Old Varieties of Triticum aestivum L. and T. durum Desf. Grains in Two Years of Production. J. Agric. Food Chem. 2010, 58, 7329–7334. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Najafi-Kakavand, S.; Abbas, S.; Shoaib, Y.; Anwar, S.; Sharifi, S.; Lu, G.; Siddique, K.H.M. Role of phytohormones in regulating cold stress tolerance: Physiological and molecular approaches for developing cold-smart crop plants. Plant Stress 2023, 8, 100152. [Google Scholar] [CrossRef]
- Ma, Z.; Bykova, N.V.; Igamberdiev, A.U. Cell Signaling Mechanisms and Metabolic Regulation of Germination and Dormancy in Barley Seeds. Crop J. 2017, 5, 459–477. [Google Scholar] [CrossRef]
- Schopfer, P.; Bajracharya, D.; Plachy, C. Control of Seed Germination by Abscisic Acid: I. Time Course of Action in Sinapis alba L. Plant Physiol. 1979, 64, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Qanmber, G.; Li, F.; Wang, Z. Updated role of ABA in seed maturation, dormancy, and germination. J. Adv. Res. 2022, 35, 199–214. [Google Scholar] [CrossRef] [PubMed]




| Time (Months) | Z-Ave (r.nm) | PDI | Z Potential (mV) |
|---|---|---|---|
| 0 | 251.1 ± 3.3 | 0.3 ±0.0 | +43.6 ± 0.1 |
| 2 | 263.2 ± 6.6 | 0.4 ± 0.0 | +43.7 ± 0.0 |
| 5 | 299.9 ± 2.2 | 0.4 ± 0.0 | +43.3 ± 0.1 |
| 10 | 309.9 ± 1.7 | 0.4 ± 0.0 | +42.5 ± 0.2 |
| 11 | 339.0 ± 54.9 | 0.4 ± 0.1 | +41.8 ± 0.5 |
| Data Obtained from Plants 90 Days After Sowing | |||
|---|---|---|---|
| Sample | Total Chlorophyll mg/g | Total Polyphenols mg/g | Total Flavonoids mg/g |
| WHEAT NT | 8.54 | 11.63 | 13.15 |
| WHEAT NP | 8.24 | 8.66 | 11.88 |
| WHEAT TB1 | 8.25 | 10.41 | 12.89 |
| WHEAT TB2 | 8.46 | 9.38 | 12.49 |
| BARLEY NT | 6.08 | 8.83 | 11.21 |
| BARLEY NP | 3.48 | 12.88 | 12.86 |
| BARLEY TB1 | 3.66 | 11.04 | 15.16 |
| BARLEY TB2 | 3.38 | 14.21 | 11.70 |
| OAT NT | 3.01 | 2.07 | 8.57 |
| OAT NP | 4.21 | 3.54 | 10.35 |
| OAT TB1 | 3.30 | 5.68 | 9.18 |
| OAT TB2 | 4.44 | 3.82 | 9.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mondéjar-López, M.; López-Jiménez, A.J.; Gómez-Gómez, L.; Ahrazem, O.; García-Martínez, J.C.; Niza, E. Field Crop Evaluation of Polymeric Nanoparticles of Garlic Extract–Chitosan as Biostimulant Seed Nano-Priming in Cereals and Transcriptomic Insights. Polymers 2024, 16, 3385. https://doi.org/10.3390/polym16233385
Mondéjar-López M, López-Jiménez AJ, Gómez-Gómez L, Ahrazem O, García-Martínez JC, Niza E. Field Crop Evaluation of Polymeric Nanoparticles of Garlic Extract–Chitosan as Biostimulant Seed Nano-Priming in Cereals and Transcriptomic Insights. Polymers. 2024; 16(23):3385. https://doi.org/10.3390/polym16233385
Chicago/Turabian StyleMondéjar-López, María, Alberto José López-Jiménez, Lourdes Gómez-Gómez, Oussama Ahrazem, Joaquín Calixto García-Martínez, and Enrique Niza. 2024. "Field Crop Evaluation of Polymeric Nanoparticles of Garlic Extract–Chitosan as Biostimulant Seed Nano-Priming in Cereals and Transcriptomic Insights" Polymers 16, no. 23: 3385. https://doi.org/10.3390/polym16233385
APA StyleMondéjar-López, M., López-Jiménez, A. J., Gómez-Gómez, L., Ahrazem, O., García-Martínez, J. C., & Niza, E. (2024). Field Crop Evaluation of Polymeric Nanoparticles of Garlic Extract–Chitosan as Biostimulant Seed Nano-Priming in Cereals and Transcriptomic Insights. Polymers, 16(23), 3385. https://doi.org/10.3390/polym16233385









