Research Progress of Application and Interaction Mechanism of Polymers in Mineral Flotation: A Review
Abstract
1. Introduction
2. Natural Polymers
2.1. Plant Polysaccharide Polymers
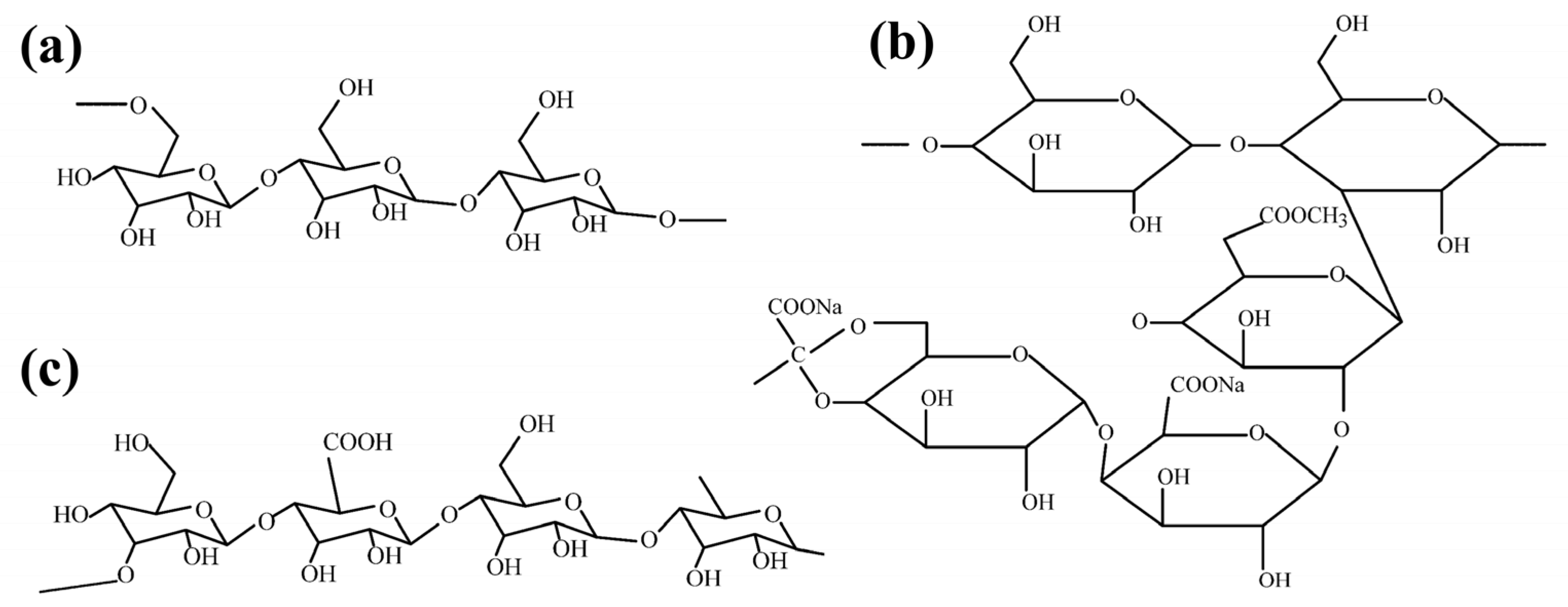
2.1.1. Starch

2.1.2. Guar Gum
2.1.3. Locust Bean Gum
2.1.4. Sodium Alginate
2.2. Animal Polysaccharide Polymers
2.2.1. Chitosan
2.2.2. Hyaluronic Acid
2.3. Microbial Polysaccharide Polymers
2.3.1. Pullulan
2.3.2. Xanthan Gum
2.3.3. Gellan Gum
3. Modified Polymers
3.1. Modified Starch
3.2. Modified Cellulose
3.2.1. Nanocrystal Cellulose
3.2.2. Carboxymethyl Cellulose
3.3. Modified Chitosan
3.4. Modified Lignin
4. Synthesized Polymers
4.1. Polyacrylic Acid
4.2. Polyether Polyol
4.3. Polyacrylamide
4.4. Polyethylene Oxide
4.5. Thermoresponsive Polymers
5. Discussion
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.; Qiu, Y.; Zhang, Z.; Wang, W.; Peng, W.; Cao, Y. A perspective on molecular recognition technology for recovering critical metals from minerals and processing wastes. Sep. Purif. Technol. 2024, 347, 127734. [Google Scholar] [CrossRef]
- Qin, P.; Gu, X.; Xuan, G.; Song, W.; Wu, H.; Li, S. Insights on the additive formulation for the energy-efficient production of fused calcium magnesium phosphate fertilizer from waste sludge. J. Clean. Prod. 2023, 423, 138804. [Google Scholar] [CrossRef]
- Yu, Y.; Zuo, H.; Wang, Y.; Kang, H.; Guo, H.; Rong, T.; Wang, J. Thermal extraction of coal and derivatives to prepare hot-pressed coal briquette for COREX application. Fuel 2024, 357, 129773. [Google Scholar] [CrossRef]
- Anzoom, S.J.; Bournival, G.; Ata, S. Coarse particle flotation: A review. Miner. Eng. 2024, 206, 108499. [Google Scholar] [CrossRef]
- Kang, Y.; Zhang, C.; Wang, H.; Xu, L.; Li, P.; Li, J.; Li, G.; Peng, W.; Zhang, F.; Fan, G.; et al. A novel sodium trans-2-nonene hydroxamate for the flotation separation of ilmenite and forsterite: Superior collecting and selectivity. Sep. Purif. Technol. 2024, 333, 125830. [Google Scholar] [CrossRef]
- Zhai, Q.; Dong, W.; Liu, R.; Xie, Z.; Cao, Z.; Sun, W. Green separation of galena from molybdenite by flotation using DL-dithiothreitol as a depressant. Sep. Purif. Technol. 2024, 347, 127676. [Google Scholar] [CrossRef]
- Vasileiou, A.V.; Korfia, S.T.; Sarigiannidou, M.; Maniar, D.; Loos, K. Macromolecular design for biobased polymers. Polymer 2024, 312, 127652. [Google Scholar] [CrossRef]
- Liu, P.; Du, J.; Ma, Y.; Wang, Q.; Lim, K.H.; Li, B.-G. Progress of polymer reaction engineering: From process engineering to product engineering. Chin. J. Chem. Eng. 2022, 50, 3–11. [Google Scholar] [CrossRef]
- Satchanska, G.; Davidova, S.; Petrov, P.D. Natural and Synthetic Polymers for Biomedical and Environmental Applications. Polymers 2024, 16, 1159. [Google Scholar] [CrossRef]
- Schultz, B.J.; Snow, E.D.; Walker, S. Mechanism of d-alanine transfer to teichoic acids shows how bacteria acylate cell envelope polymers. Nat. Microbiol. 2023, 8, 1318–1329. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, X.; Cheng, Z. Research on the Application of Synthetic Polymer Materials in Contemporary Public Art. Polymers 2022, 14, 1208. [Google Scholar] [CrossRef] [PubMed]
- Ben Amara, F.; Bouzid, M.; Sahnoun, M.; Ben Nasr, Y.; Jaouadi, B.; Bejar, S.; Jemli, S. Valorization of Potato Peels Starch for Efficient β-Cyclodextrin Production and Purification through an Eco-Friendly Process. Starch Stärke 2022, 74, 2200037. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Aravamudhan, A.; Ramos, D.M.; Nada, A.A.; Kumbar, S.G. Chapter 4—Natural Polymers: Polysaccharides and Their Derivatives for Biomedical Applications. In Natural and Synthetic Biomedical Polymers; Kumbar, S.G., Laurencin, C.T., Deng, M., Eds.; Elsevier: Oxford, UK, 2014; pp. 67–89. [Google Scholar]
- Albuquerque, P.B.S.; de Oliveira, W.F.; dos Santos Silva, P.M.; dos Santos Correia, M.T.; Kennedy, J.F.; Coelho, L.C.B.B. Skincare application of medicinal plant polysaccharides—A review. Carbohydr. Polym. 2022, 277, 118824. [Google Scholar] [CrossRef]
- Adhikary, N.D.; Bains, A.; Sridhar, K.; Kaushik, R.; Chawla, P.; Sharma, M. Recent advances in plant-based polysaccharide ternary complexes for biodegradable packaging. Int. J. Biol. Macromol. 2023, 253, 126725. [Google Scholar] [CrossRef]
- Zhu, J.; Gilbert, R.G. Starch molecular structure and diabetes. Carbohydr. Polym. 2024, 344, 122525. [Google Scholar] [CrossRef]
- Peres, A.E.C.; Correa, M.I. Depression of iron oxides with corn starches. Miner. Eng. 1996, 9, 1227–1234. [Google Scholar] [CrossRef]
- Zuo, R.; Kong, X.; Wang, Y.; He, Y.; Deng, S.; Zhuang, X.; Qiu, D. Isolation and characterization of natural nano starch from amaranth starch. Int. J. Biol. Macromol. 2024, 260, 129525. [Google Scholar] [CrossRef]
- Zhang, C.; Tan, Y.; Yin, F.; Zhao, J.; Gao, Z.; Sun, W.; McFadzean, B.; Cao, J. Utilization of phosphorylated starch as a selective depressant for serpentine in the flotation of nickel sulfide ore. Miner. Eng. 2024, 217, 108906. [Google Scholar] [CrossRef]
- Filippov, L.O.; Severov, V.V.; Filippova, I.V. An overview of the beneficiation of iron ores via reverse cationic flotation. Int. J. Miner. Process. 2014, 127, 62–69. [Google Scholar] [CrossRef]
- Tang, M.; Tong, X.; Wen, S. Investigation on the adsorption behaviour of heating-treated starch on hematite. Sep. Sci. Technol. 2016, 51, 1280–1286. [Google Scholar] [CrossRef]
- Yang, S.; Li, C.; Wang, L. Dissolution of starch and its role in the flotation separation of quartz from hematite. Powder Technol. 2017, 320, 346–357. [Google Scholar] [CrossRef]
- Pinto, C.L.L.; de Araujo, A.C.; Peres, A.E.C. The effect of starch, amylose and amylopectin on the depression of oxi-minerals. Miner. Eng. 1992, 5, 469–478. [Google Scholar] [CrossRef]
- Kar, B.; Sahoo, H.; Rath, S.S.; Das, B. Investigations on different starches as depressants for iron ore flotation. Miner. Eng. 2013, 49, 1–6. [Google Scholar] [CrossRef]
- Yang, S.; Wang, L. Structural and functional insights into starches as depressant for hematite flotation. Miner. Eng. 2018, 124, 149–157. [Google Scholar] [CrossRef]
- Wei, M.; Lv, J.; Kong, L.; Tong, X. Differential depression and mechanism of copper ion-activated sphalerite with wheat starch and rice starch as flotation depressants. Adv. Powder Technol. 2024, 35, 104446. [Google Scholar] [CrossRef]
- Han, G.; Wen, S.; Wang, H.; Feng, Q. Effect of starch on surface properties of pyrite and chalcopyrite and its response to flotation separation at low alkalinity. Miner. Eng. 2019, 143, 106015. [Google Scholar] [CrossRef]
- Shrimali, K.; Atluri, V.; Wang, Y.; Bacchuwar, S.; Wang, X.; Miller, J.D. The nature of hematite depression with corn starch in the reverse flotation of iron ore. J. Colloid Interf. Sci. 2018, 524, 337–349. [Google Scholar] [CrossRef]
- Tang, M.; Wang, Y.; Niu, X.; Liu, D. Morphological characteristics of starch sol-gel and its influences on flocculation of fine particles. Miner. Eng. 2022, 186, 107745. [Google Scholar] [CrossRef]
- Hao, H.; Fan, G.; Yu, J.; Cao, Y.; Liu, J.; Das, S. Adsorption changes of starch on minerals in carbonate-containing iron ore flotation by introducing amino radicals. J. Mol. Liq. 2021, 343, 117511. [Google Scholar] [CrossRef]
- Mu, Y.; Peng, Y.; Lauten, R.A. Electrochemistry aspects of pyrite in the presence of potassium amyl xanthate and a lignosulfonate-based biopolymer depressant. Electrochim. Acta 2015, 174, 133–142. [Google Scholar] [CrossRef]
- Yu, M.; Zhu, S.; Li, Y.; Zhong, F.; Huang, D.; Chen, X. Role of phenolic acids with different functional groups in the regulation of starch digestion in simulated dietary intake patterns. Int. J. Biol. Macromol. 2023, 235, 123815. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Li, Y.; Zhao, X.; Jiang, M.; Gao, E. Utilization of soluble starch as the depressant to flotation separation of pyrite from arsenopyrite. Sep. Purif. Technol. 2023, 310, 123155. [Google Scholar] [CrossRef]
- Sun, W.; Han, H.; Hu, Y.; Sun, W.; Zhu, Y.; Gui, X.; Cao, X.; Xing, Y.; Li, C.; Wei, Z. Flotation theory and research progress of metal ion coordination regulation molecule assembly. Chin. J. Nonferrous Met. 2020, 30, 927–941. [Google Scholar] [CrossRef]
- Hao, H.; Li, L.; Somasundaran, P.; Yuan, Z. Adsorption of Pregelatinized Starch for Selective Flocculation and Flotation of Fine Siderite. Langmuir 2019, 35, 6878–6887. [Google Scholar] [CrossRef]
- Laskowski, J.S.; Liu, Q.; O’Connor, C.T. Current understanding of the mechanism of polysaccharide adsorption at the mineral/aqueous solution interface. Int. J. Miner. Process. 2007, 84, 59–68. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.; Laskowski, J.S. The adsorption of polysaccharides onto mineral surfaces: An acid/base interaction. Int. J. Miner. Process. 2000, 60, 229–245. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, Y.; Zawała, J.; Liu, C.; Xiao, W.; Yang, S. A novel interaction theory for the starch adsorption onto hematite surface. Adv. Powder Technol. 2024, 35, 104607. [Google Scholar] [CrossRef]
- Thombare, N.; Jha, U.; Mishra, S.; Siddiqui, M.Z. Guar gum as a promising starting material for diverse applications: A review. Int. J. Biol. Macromol. 2016, 88, 361–372. [Google Scholar] [CrossRef]
- Bicak, O.; Ekmekci, Z.; Bradshaw, D.J.; Harris, P.J. Adsorption of guar gum and CMC on pyrite. Miner. Eng. 2007, 20, 996–1002. [Google Scholar] [CrossRef]
- Guo, W.; Feng, B.; Peng, J.; Zhang, W.; Zhu, X. Depressant behavior of tragacanth gum and its role in the flotation separation of chalcopyrite from talc. J. Mater. Res. Technol. 2019, 8, 697–702. [Google Scholar] [CrossRef]
- Vidal, C.A.G.; Pawlik, M. Molecular weight effects in interactions of guar gum with talc. Int. J. Miner. Process. 2015, 138, 38–43. [Google Scholar] [CrossRef]
- Long, T.; Xiao, W.; Yang, W. The effect of molecular assembly between collectors and inhibitors on the flotation of pyrite and talc. R. Soc. Open Sci. 2019, 6, 191133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, G.; Wu, X.; Zhao, K. Selective depression behavior of guar gum on talc-type scheelite flotation. Int. J. Miner. Metall. Mater. 2017, 24, 857–862. [Google Scholar] [CrossRef]
- Qiu, X.; Sun, C. Influence of the addition orders of guar gum and tannic acid on sulfide flotation. J. Univ. Sci. Technol. Beijing 2014, 36, 283–288. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Li, Y.; Han, Y.; Gu, X.; Wang, S. Adsorption mechanism of a new depressant on pyrite surfaces and its application to the selective separation of chalcopyrite from pyrite. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126892. [Google Scholar] [CrossRef]
- Gao, Z.; Song, S.; Sun, W.; Hu, Y.; Zhong, H. Depressant behavior and mechanism of guar and xanthan gums on calcite flotation. J. Cent. South Univ. Sci.Technol. 2016, 47, 1459–1464. [Google Scholar]
- Xue, M.; Yin, W. Efffeet of Low-Temperature Combined Reagent on Flotation Separation of Magnesite and Dolomite. Nonferrous Met. Mieral Process. Sect. 2024, 124–135. [Google Scholar] [CrossRef]
- Chen, X.; Gu, G.; Li, L.; Zhu, R. The selective effect of food-grade guar gum on chalcopyrite–monoclinic pyrrhotite separation using mixed aerofloat (CSU11) as collector. Int. J. Miner. Metall. Mater. 2018, 25, 1123–1131. [Google Scholar] [CrossRef]
- Jain, V.; Tammishetti, V.; Joshi, K.; Kumar, D.; Pradip; Rai, B. Guar gum as a selective flocculant for the beneficiation of alumina rich iron ore slimes: Density functional theory and experimental studies. Miner. Eng. 2017, 109, 144–152. [Google Scholar] [CrossRef]
- Di Guardo, M.; Scollo, F.; Ninot, A.; Rovira, M.; Hermoso, J.F.; Distefano, G.; La Malfa, S.; Batlle, I. Genetic structure analysis and selection of a core collection for carob tree germplasm conservation and management. Tree Genet. Genomes 2019, 15, 41. [Google Scholar] [CrossRef]
- Barak, S.; Mudgil, D. Locust bean gum: Processing, properties and food applications—A review. Int. J. Biol. Macromol. 2014, 66, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, T.; Cai, X.; Liu, Y.; Huang, C.; He, J.; Tian, D.; Yang, G.; Shen, F.; Zhang, Y. Eco-friendly hydrogel based on locust bean gum for water retaining in sandy soil. Int. J. Biol. Macromol. 2024, 275, 133490. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Guo, Y.; Zhang, W.; Peng, J.; Wang, H.; Huang, Z.; Zhou, X. Flotation separation behavior of chalcopyrite and sphalerite in the presence of locust bean gum. Miner. Eng. 2019, 143, 105940. [Google Scholar] [CrossRef]
- Feng, B.; Zhong, C.; Zhang, L.; Guo, Y.; Wang, T.; Huang, Z. Effect of surface oxidation on the depression of sphalerite by locust bean gum. Miner. Eng. 2020, 146, 106142. [Google Scholar] [CrossRef]
- Shen, Z.; Wen, S.; Han, G.; Zhou, Y.; Bai, X.; Feng, Q. Selective depression mechanism of locust bean gum in the flotation separation of chalcopyrite from pyrite in a low-alkalinity media. Miner. Eng. 2021, 170, 107044. [Google Scholar] [CrossRef]
- Miao, Y.; Wen, S.; Shen, Z.; Feng, Q.; Zhang, Q. Flotation separation of chalcopyrite from galena using locust bean gum as a selective and eco-friendly depressant. Sep. Purif. Technol. 2022, 283, 120173. [Google Scholar] [CrossRef]
- Kordloo, M.; Khodadadmahmoudi, G.; Ebrahimi, E.; Rezaei, A.; Tohry, A.; Chelgani, S.C. Green hematite depression for reverse selective flotation separation from quartz by locust bean gum. Sci. Rep. 2023, 13, 8980. [Google Scholar] [CrossRef]
- Feng, B.; Peng, J.; Zhang, W.; Ning, X.; Guo, Y.; Zhang, W. Use of locust bean gum in flotation separation of chalcopyrite and talc. Miner. Eng. 2018, 122, 79–83. [Google Scholar] [CrossRef]
- Ziming, W.; Bo, F.; Yuangan, C. The separation behavior and mechanism of scheelite and dolomite using locust bean gum as depressant. Miner. Eng. 2023, 202, 108280. [Google Scholar] [CrossRef]
- Yan, P.; Lan, W.; Xie, J. Modification on sodium alginate for food preservation: A review. Trends Food Sci. Technol. 2024, 143, 104217. [Google Scholar] [CrossRef]
- Zheng, D.; Wang, K.; Bai, B. A critical review of sodium alginate-based composites in water treatment. Carbohydr. Polym. 2024, 331, 121850. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Feng, Q.; Zhang, G.; Yang, Q.; Zhang, C. The effect of sodium alginate on the flotation separation of scheelite from calcite and fluorite. Miner. Eng. 2017, 113, 1–7. [Google Scholar] [CrossRef]
- Wang, L.; Lyu, W.; Li, F.; Liu, J.; Zhang, H. Discrepant adsorption behavior of sodium alginate onto apatite and calcite surfaces: Implications for their selective flotation separation. Miner. Eng. 2022, 181, 107553. [Google Scholar] [CrossRef]
- Zhong, C.; Feng, B.; Zhang, W.; Zhang, L.; Guo, Y.; Wang, T.; Wang, H. The role of sodium alginate in the flotation separation of apatite and dolomite. Powder Technol. 2020, 373, 620–626. [Google Scholar] [CrossRef]
- Chen, W.; Chen, F.; Zhang, Z.; Tian, X.; Bu, X.; Feng, Q. Investigations on the depressant effect of sodium alginate on galena flotation in different sulfide ore collector systems. Miner. Eng. 2021, 160, 106705. [Google Scholar] [CrossRef]
- Qiu, H.; Wu, B.; Chen, J.; Deng, J.; Sun, X.; Hu, M.; Cai, J.; Chen, Z.; Zheng, C. Selective depression of marmatite by sodium alginate in flotation separation of galena and marmatite. Miner. Eng. 2023, 201, 108229. [Google Scholar] [CrossRef]
- Wang, C.; Liu, R.; Xie, F.; Zhai, Q.; Sun, W.; Wen, X.; Li, J. Separation of sphalerite and dolomite using sodium alginate as an environmentally friendly depressant in a carbonate-hosted Pb-Zn ore system. J. Clean. Prod. 2022, 380, 135107. [Google Scholar] [CrossRef]
- Wang, W.; Xue, C.; Mao, X. Radioprotective effects and mechanisms of animal, plant and microbial polysaccharides. Int. J. Biol. Macromol. 2020, 153, 373–384. [Google Scholar] [CrossRef]
- Hu, W.-B.; Ouyang, K.-H.; Wu, G.-Q.; Chen, H.; Xiong, L.; Liu, X.; Wang, N.; Wang, W.-J. Hepatoprotective effect of flavonoid-enriched fraction from Cyclocarya paliurus leaves on LPS/D-GalN-induced acute liver failure. J. Funct. Foods 2018, 48, 337–350. [Google Scholar] [CrossRef]
- Li, H.; Xie, W.; Qiao, X.; Cui, H.; Yang, X.; Xue, C. Structural characterization of arabinogalactan extracted from Ixeris chinensis (Thunb.) Nakai and its immunomodulatory effect on RAW264.7 macrophages. Int. J. Biol. Macromol. 2020, 143, 977–983. [Google Scholar] [CrossRef]
- Wiranowska, M. Advances in the use of chitosan and chlorotoxin- functionalized chitosan polymers in drug delivery and detection of glioma—A review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100427. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Dong, Q.; Xu, C.; Deng, S.; Kang, Y.; Fan, M.; Li, L. Application of functionalized chitosan in food: A review. Int. J. Biol. Macromol. 2023, 235, 123716. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Peng, J.; Guo, W.; Zhang, W.; Ai, G.; Wang, H. The effect of changes in pH on the depression of talc by chitosan and the associated mechanisms. Powder Technol. 2018, 325, 58–63. [Google Scholar] [CrossRef]
- Feng, B.; Peng, J.; Guo, W.; Zhu, X.; Huang, W. The stimulus response of chitosan and its depression effect on talc flotation. Miner. Process. Extr. Metall. 2018, 127, 56–61. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Hu, Y.; Gao, X.; Yuan, Q.; Zhao, F. Investigation of the specularite/chlorite separation using chitosan as a novel depressant by direct flotation. Carbohydr. Polym. 2020, 240, 116334. [Google Scholar] [CrossRef]
- Huang, P.; Cao, M.; Liu, Q. Selective depression of pyrite with chitosan in Pb–Fe sulfide flotation. Miner. Eng. 2013, 46–47, 45–51. [Google Scholar] [CrossRef]
- Li, M.; Wei, D.; Liu, Q.; Liu, W.; Zheng, J.; Sun, H. Flotation separation of copper–molybdenum sulfides using chitosan as a selective depressant. Miner. Eng. 2015, 83, 217–222. [Google Scholar] [CrossRef]
- Feng, B.; Peng, J.; Zhu, X.; Huang, W. The settling behavior of quartz using chitosan as flocculant. J. Mater. Res. Technol. 2017, 6, 71–76. [Google Scholar] [CrossRef]
- El-Batal, A.I.; Nasser, H.A.; Mosallam, F.M. Fabrication and characterization of cobalt hyaluronic acid nanostructure via gamma irradiation for improving biomedical applications. Int. J. Biol. Macromol. 2020, 147, 1328–1342. [Google Scholar] [CrossRef]
- Perera, G.G.G.; Argenta, D.F.; Caon, T. The rheology of injectable hyaluronic acid hydrogels used as facial fillers: A review. Int. J. Biol. Macromol. 2024, 268, 131880. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan as an Immune Regulator in Human Diseases. Physiol. Rev. 2011, 91, 221–264. [Google Scholar] [CrossRef]
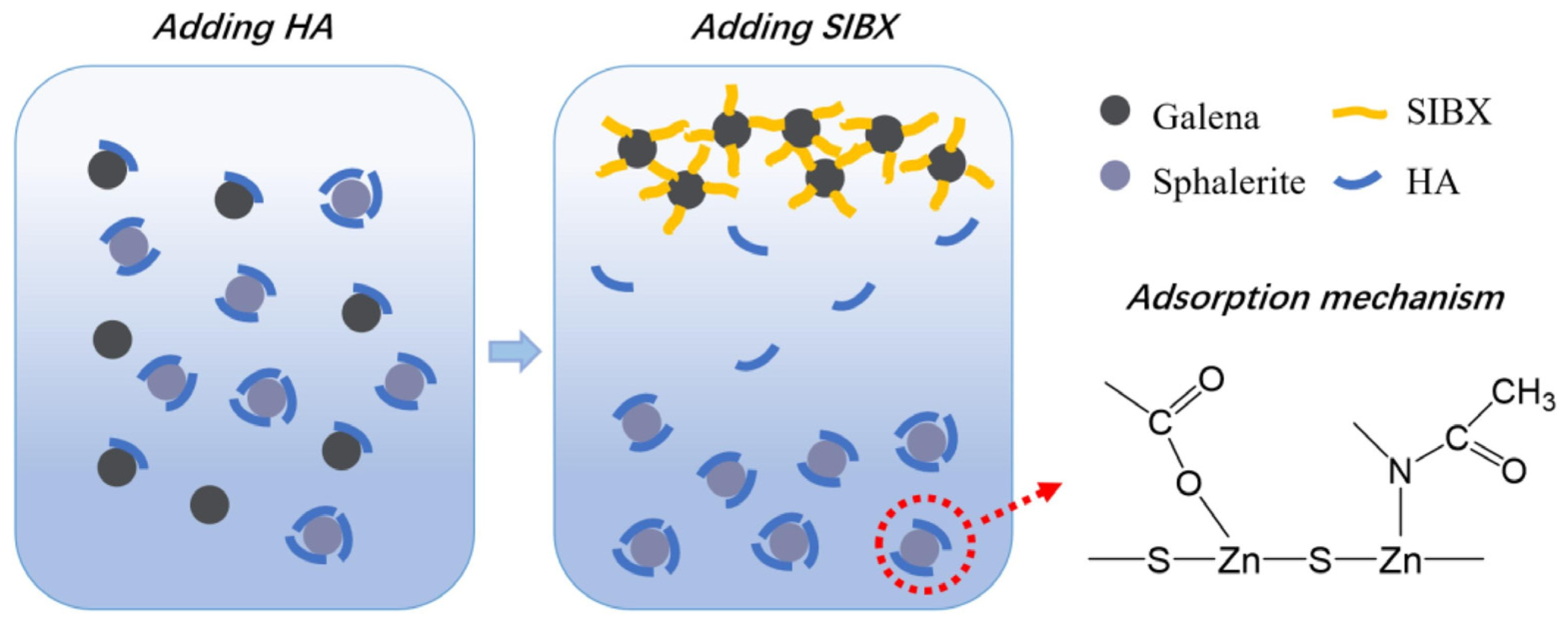
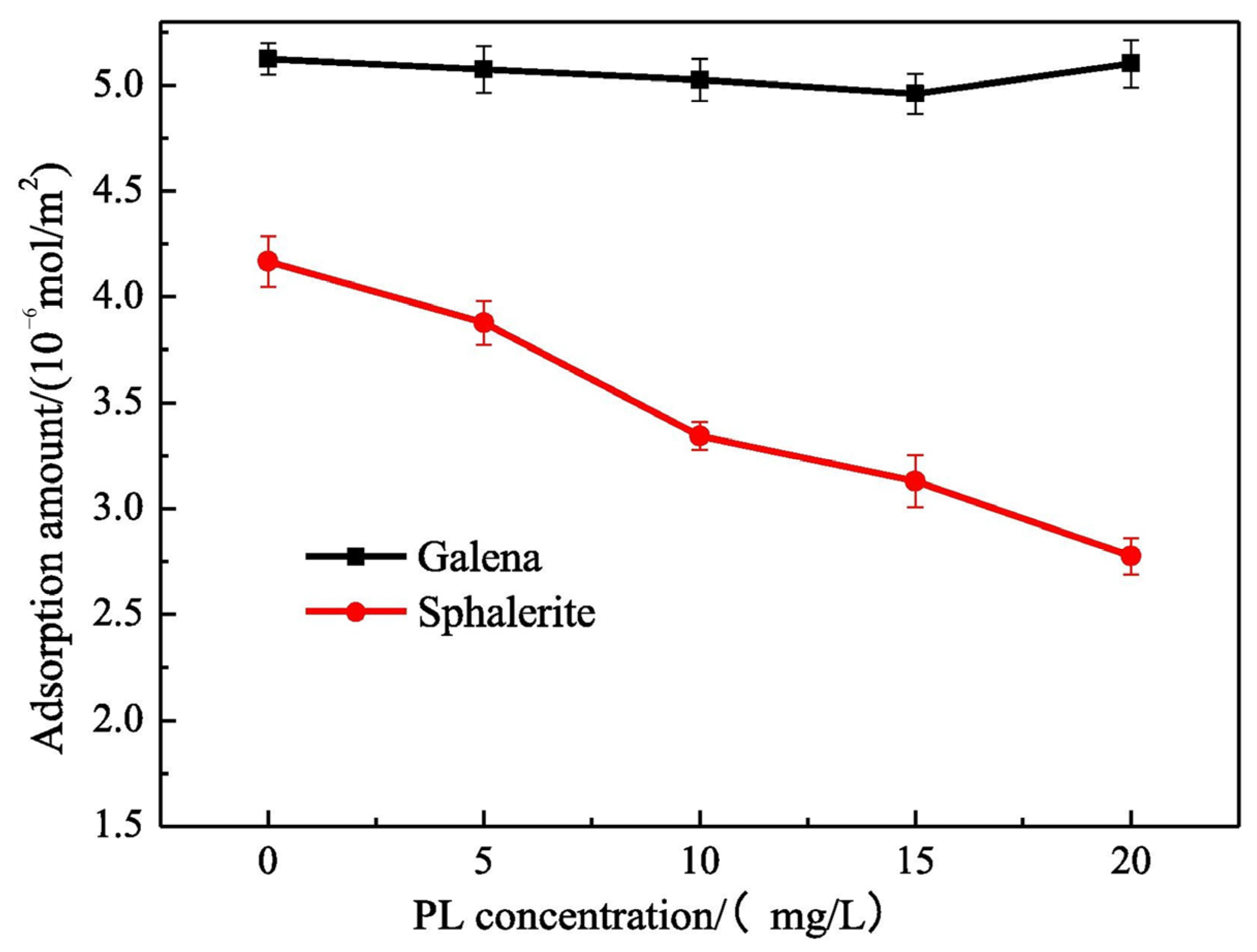
- Zhu, H.; Yang, B.; Martin, R.; Zhang, H.; He, D.; Luo, H. Flotation separation of galena from sphalerite using hyaluronic acid (HA) as an environmental-friendly sphalerite depressant. Miner. Eng. 2022, 187, 107771. [Google Scholar] [CrossRef]
- Song, Y.; Li, S.; Gong, H.; Yip, R.C.S.; Chen, H. Biopharmaceutical applications of microbial polysaccharides as materials: A Review. Int. J. Biol. Macromol. 2023, 239, 124259. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Fu, Y.; Jiao, H.; Wang, X.; Wang, Q.; Zhou, M.; Yong, Y.-c.; Liu, J. A structure-functionality insight into the bioactivity of microbial polysaccharides toward biomedical applications: A review. Carbohydr. Polym. 2024, 335, 122078. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Singh, D.; Purewal, S.S.; Kennedy, J.F. Pullulan in pharmaceutical and cosmeceutical formulations: A review. Int. J. Biol. Macromol. 2023, 231, 123353. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Hassan, M.; Kennedy, J.F. Pullulan in biomedical research and development—A review. Int. J. Biol. Macromol. 2021, 166, 694–706. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, G.; Chen, Y.; Huang, G.; Gao, Y. Investigations on the utilization of konjac glucomannan in the flotation separation of chalcopyrite from pyrite. Miner. Eng. 2020, 145, 106098. [Google Scholar] [CrossRef]
- Ning, S.; Li, G.; Shen, P.; Zhang, X.; Li, J.; Liu, R.; Liu, D. Selective separation of chalcopyrite and talc using pullulan as a new depressant. Colloids Surf. A Physicochem. Eng. Asp. 2021, 623, 126764. [Google Scholar] [CrossRef]
- Zhang, W.; Tao, L.; Xun, L.; Qi, Z.; Pooley, S.; Sun, W.; Cao, J.; Gao, Z. Improved flotation of molybdenite from talc using a selective reagent scheme. Miner. Eng. 2022, 176, 107324. [Google Scholar] [CrossRef]
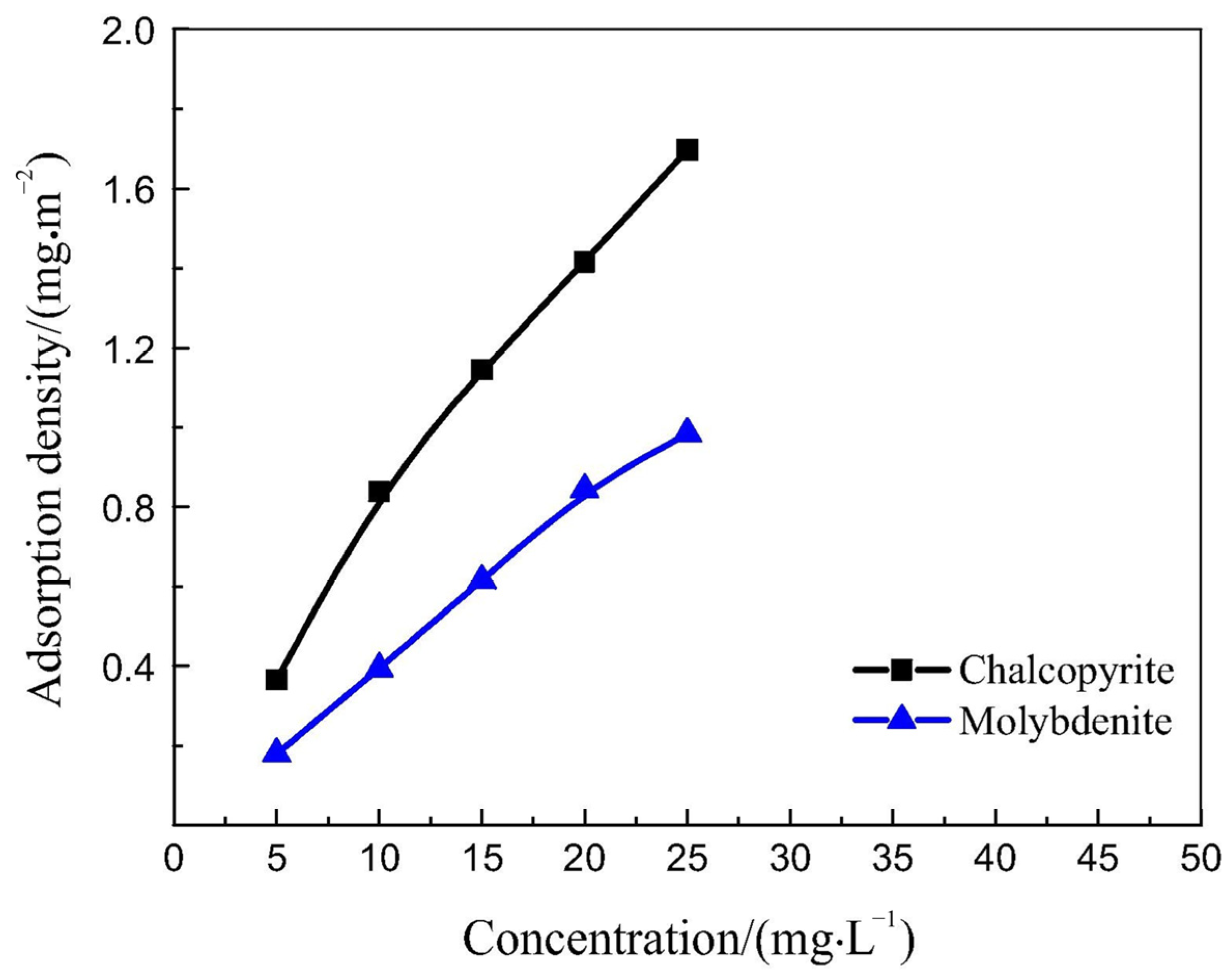
- Yang, W.; Qiu, T.; Qiu, X.; Yan, H.; Jiao, Q.; Ding, K.; Zhao, G. Pullulan Polysaccharide as an Eco-Friendly Depressant for Flotation Separation of Chalcopyrite and Molybdenite. ACS Omega 2024, 9, 29557–29565. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Jiao, F.; Qin, W.; Wang, C.; Li, X. Flotation separation of sphalerite from galena using eco-friendly and efficient depressant pullulan. Sep. Purif. Technol. 2022, 295, 121013. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Application of xanthan gum as polysaccharide in tissue engineering: A review. Carbohydr. Polym. 2018, 180, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Wang, X.; Yan, W.; Gu, G.; Wang, C.; Wang, Z.; Xu, L.; Peng, T. Depression mechanism of pyrophyllite by a novel polysaccharide xanthan gum. Miner. Eng. 2019, 132, 134–141. [Google Scholar] [CrossRef]
- Zhong, C.; Wang, H.; Zhang, L.; Guo, M.; Feng, B. Flotation separation of molybdenite and talc by xanthan gum. Powder Technol. 2021, 388, 158–165. [Google Scholar] [CrossRef]
- Pan, G.; Shi, Q.; Zhang, G.; Huang, G. Selective depression of talc in chalcopyrite flotation by xanthan gum: Flotation response and adsorption mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124902. [Google Scholar] [CrossRef]
- Ming, P.; Xie, Z.; Guan, Y.; Wang, Z.; Li, F.; Xing, Q. The effect of polysaccharide depressant xanthan gum on the flotation of arsenopyrite from chlorite. Miner. Eng. 2020, 157, 106551. [Google Scholar] [CrossRef]
- Dong, L.; Jiao, F.; Qin, W.; Liu, W. Selective flotation of scheelite from calcite using xanthan gum as depressant. Miner. Eng. 2019, 138, 14–23. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, H.; Yang, J.; Tang, Z.; Luo, L.; Shu, K.; Xu, Y.; Xu, L. Selective flotation separation of bastnaesite from calcite using xanthan gum as a depressant. Appl. Surf. Sci. 2020, 512, 145714. [Google Scholar] [CrossRef]
- Nambaje, C.; Mweene, L.; Subramanian, S.; Sajeev, K.; Santosh, M. Xanthan gum based investigations into the surface chemistry of cassiterite and beneficiation of cassiterite tailings. Miner. Process. Extr. Metall. Rev. 2022, 43, 150–164. [Google Scholar] [CrossRef]
- Cai, Z.; Guo, Y.; Ma, A.; Zhang, H. NMR analysis of the side-group substituents in welan gum in comparison to gellan gum. Int. J. Biol. Macromol. 2024, 254, 127847. [Google Scholar] [CrossRef] [PubMed]
- Sahu, N.; Mahanty, B.; Haldar, D. Challenges and opportunities in bioprocessing of gellan gum: A review. Int. J. Biol. Macromol. 2024, 276, 133912. [Google Scholar] [CrossRef] [PubMed]
- Lalebeigi, F.; Alimohamadi, A.; Afarin, S.; Aliabadi, H.A.M.; Mahdavi, M.; Farahbakhshpour, F.; Hashemiaval, N.; Khandani, K.K.; Eivazzadeh-Keihan, R.; Maleki, A. Recent advances on biomedical applications of gellan gum: A review. Carbohydr. Polym. 2024, 334, 122008. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lyu, W.; Huang, L.; Li, F.; Zhang, H. Utilization of gellan gum as a novel eco-friendly depressant in the flotation separation of fluorite from barite. Miner. Eng. 2022, 184, 107640. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Zhang, H.; Lyu, W.; Zhu, Y.; Ma, Y.; Li, F. The role of gellan gum in the selective flotation separation of fluorite from calcite: An experimental and molecular dynamics simulation study. Powder Technol. 2024, 432, 119156. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, J.; Shen, M.; Nie, S.; Xie, M. Sulfated modification of polysaccharides: Synthesis, characterization and bioactivities. Trends Food Sci. Technol. 2018, 74, 147–157. [Google Scholar] [CrossRef]
- Siroha, A.K.; Sandhu, K.S.; Punia, S. Impact of octenyl succinic anhydride on rheological properties of sorghum starch. Qual. Assur. Saf. Crop. Foods 2019, 11, 221–229. [Google Scholar] [CrossRef]
- Sweedman, M.C.; Tizzotti, M.J.; Schäfer, C.; Gilbert, R.G. Structure and physicochemical properties of octenyl succinic anhydride modified starches: A review. Carbohydr. Polym. 2013, 92, 905–920. [Google Scholar] [CrossRef]
- Deka, D.; Sit, N. Dual modification of taro starch by microwave and other heat moisture treatments. Int. J. Biol. Macromol. 2016, 92, 416–422. [Google Scholar] [CrossRef]
- EI Halal, S.L.M.; Colussi, R.; Pinto, V.Z.; Bartz, J.; Radunz, M.; Carreño, N.L.V.; Dias, A.R.G.; Zavareze, E.d.R. Structure, morphology and functionality of acetylated and oxidised barley starches. Food Chem. 2015, 168, 247–256. [Google Scholar] [CrossRef]
- Stahl, J.A.; Lobato, L.P.; Bochi, V.C.; Kubota, E.H.; Gutkoski, L.C.; Emanuelli, T. Physicochemical properties of Pinhão (Araucaria angustifolia, Bert, O. Ktze) starch phosphates. LWT-Food Sci. Technol. 2007, 40, 1206–1214. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, H.; Xu, Y.; Bao, S.; Liu, C.; Yang, S. The molecular structure effects of starches and starch phosphates in the reverse flotation of quartz from hematite. Carbohydr. Polym. 2023, 303, 120484. [Google Scholar] [CrossRef] [PubMed]
- Yue, T.; Wu, X. Depressing Iron Mineral by Metallic-Starch Complex (MSC) in Reverse Flotation and Its Mechanism. Minerals 2018, 8, 85. [Google Scholar] [CrossRef]
- Huangfu, Z.; Sun, W.; Zhu, H.; Liu, R.; Chen, C. Selective depression of biodegradable talc depressant sodium carboxymethyl starch on molybdenite flotation with sodium diethyldithiocarbamate. Sep. Purif. Technol. 2025, 352, 127950. [Google Scholar] [CrossRef]
- Khoso, S.A.; Lyu, F.; Meng, X.; Hu, Y.; Sun, W. Selective separation of chalcopyrite and pyrite with a novel and non-hazardous depressant reagent scheme. Chem. Eng. Sci. 2019, 209, 115204. [Google Scholar] [CrossRef]
- Khoso, S.A.; Hu, Y.; Tian, M.; Gao, Z.; Sun, W. Evaluation of green synthetic depressants for sulfide flotation: Synthesis, characterization and floatation performance to pyrite and chalcopyrite. Sep. Purif. Technol. 2021, 259, 118138. [Google Scholar] [CrossRef]
- Chapagai, M.K.; Fletcher, B.; Gidley, M.J. Adsorption and depression effects of native starch, oxidized starch, and dextrin on graphite. Miner. Eng. 2022, 181, 107549. [Google Scholar] [CrossRef]
- Li, W.; Cheng, S.; Zhou, L.; Han, Y. Enhanced iron recovery from magnetic separation of ultrafine specularite through polymer-bridging flocculation: A study of flocculation performance and mechanism. Sep. Purif. Technol. 2023, 308, 122882. [Google Scholar] [CrossRef]
- Nanda, D.; Mandre, N.R. Performance Evaluation of Process Variables for Selective Flocculation of Iron Fines Using Modified Amphoteric Starch Through Full Factorial Statistical Analysis. J. Sustain. Metall. 2023, 9, 123–131. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Q.; Lei, X.; Liu, H.; Liu, J.; He, X. Peptides-modified cellulose microspheres for adsorption of ochratoxin A: Performance and mechanism. Sep. Purif. Technol. 2024, 350, 127764. [Google Scholar] [CrossRef]
- Qiu, X.; Yang, H.; Chen, G.; Zhong, S.; Cai, C.; Lan, B. Inhibited mechanism of carboxymethyl cellulose as a galena depressant in chalcopyrite and galena separation flotation. Miner. Eng. 2020, 150, 106273. [Google Scholar] [CrossRef]
- Liu, A.; Wu, H.; Naeem, A.; Du, Q.; Ni, B.; Liu, H.; Li, Z.; Ming, L. Cellulose nanocrystalline from biomass wastes: An overview of extraction, functionalization and applications in drug delivery. Int. J. Biol. Macromol. 2023, 241, 124557. [Google Scholar] [CrossRef] [PubMed]
- Habibullah, S.; Swain, R.; Nandi, S.; Das, M.; Rout, T.; Mohanty, B.; Mallick, S. Nanocrystalline cellulose as a reinforcing agent for poly (vinyl alcohol)/ gellan-gum-based composite film for moxifloxacin ocular delivery. Int. J. Biol. Macromol. 2024, 270, 132302. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, R.; Kinnunen, P.; Illikainen, M. Cellulose-mineral interactions based on the DLVO theory and their correlation with flotability. Miner. Eng. 2018, 122, 44–52. [Google Scholar] [CrossRef]
- Hartmann, R.; Rudolph, M.; Ämmälä, A.; Illikainen, M. The action of cellulose-based and conventional flotation reagents under dry and wet conditions correlating inverse gas chromatography to microflotation studies. Miner. Eng. 2017, 114, 17–25. [Google Scholar] [CrossRef]
- Lopéz, R.; Jordão, H.; Hartmann, R.; Ämmälä, A.; Carvalho, M.T. Study of butyl-amine nanocrystal cellulose in the flotation of complex sulphide ores. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123655. [Google Scholar] [CrossRef]
- Du, W.; Li, X. Insight into the inhibition mechanism of carboxymethyl cellulose for flotation of dolomite and fluorapatite: Experimental and DFT studies. Colloids Surf. A Physicochem. Eng. Asp. 2023, 674, 131957. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, L.; Hu, X.; Zhang, X.; Zheng, G. Flotation separation of quartz from magnesite using carboxymethyl cellulose as depressant. Trans. Nonferrous Met. Soc. China 2022, 32, 1623–1637. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Song, S.; Li, H. A novel method to improve carboxymethyl cellulose performance in the flotation of talc. Miner. Eng. 2019, 131, 23–27. [Google Scholar] [CrossRef]
- Jin, S.; Shi, Q.; Li, Q.; Ou, L.; Ouyang, K. Effect of calcium ionic concentrations on the adsorption of carboxymethyl cellulose onto talc surface: Flotation, adsorption and AFM imaging study. Powder Technol. 2018, 331, 155–161. [Google Scholar] [CrossRef]
- Liu, Q.; Wannas, D.; Peng, Y. Exploiting the dual functions of polymer depressants in fine particle flotation. Int. J. Miner. Process. 2006, 80, 244–254. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Zhang, L. Effect of the Molecular Weight of Carboxymethyl Cellulose on the Flotation of Chlorite. Materials 2023, 26, 3356. [Google Scholar] [CrossRef] [PubMed]
- Shete, A.; Chavan, A.; Potekar, P.; Yadav, G.; Shah, N. Modification of physicochemical properties of chitosan to improve its pharmaceutical and agrochemical potential applications. Int. J. Biol. Macromol. 2024, 267, 131404. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, L.; Yang, S.; Liu, C.; Xu, Y. Investigations on the reverse flotation of quartz from hematite using carboxymethyl chitosan as a depressant. Powder Technol. 2021, 393, 109–115. [Google Scholar] [CrossRef]
- Wang, T.; Feng, B.; Guo, Y.; Zhang, W.; Rao, Y.; Zhong, C.; Zhang, L.; Cheng, C.; Wang, H.; Luo, X. The flotation separation behavior of apatite from calcite using carboxymethyl chitosan as depressant. Miner. Eng. 2020, 159, 106635. [Google Scholar] [CrossRef]
- Yuan, D.; Cadien, K.; Liu, Q.; Zeng, H. Flotation separation of Cu-Mo sulfides by O-Carboxymethyl chitosan. Miner. Eng. 2019, 134, 202–205. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, B.; Peng, J.; Wang, Z. Selective flotation of galena from sphalerite using a combination of KMnO4 and carboxylated chitosan. Appl. Surf. Sci. 2022, 602, 154412. [Google Scholar] [CrossRef]
- Liu, C.; Feng, Q.; Shi, Q.; Zhang, W.; Song, S. Utilization of N-carboxymethyl chitosan as a selective depressant for talc in flotation of chalcopyrite. Physicochem. Probl. Miner. Process. 2019, 55, 108–115. [Google Scholar]
- Ma, X.; Chen, J.; Zhu, J.; Yan, N. Lignin-Based Polyurethane: Recent Advances and Future Perspectives. Macromol. Rapid Commun. 2021, 42, 2000492. [Google Scholar] [CrossRef]
- Feng, B.; Zhang, L.; Zhang, W.; Wang, H.; Gao, Z. Mechanism of calcium lignosulfonate in apatite and dolomite flotation system. Int. J. Miner. Metall. Mater. 2022, 29, 1697–1704. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.; Zhang, G.; Liu, D.; Li, L. Selective flotation of scheelite from calcite using calcium lignosulphonate as depressant. Miner. Eng. 2018, 119, 73–75. [Google Scholar] [CrossRef]
- Sun, H.; Niu, F.; Zhang, J. Investigation on the flotation separation of smithsonite from calcite using calcium lignosulphonate as depressant. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127571. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, C.; Hu, B.; Sun, Z.; Xu, Q.; Wen, J.; Xiao, J.; Dong, Y.; Gan, M.; Sun, W.; et al. Enhancing flotation separation of chalcopyrite and galena by the surface synergism between sodium sulfite and sodium lignosulfonate. Appl. Surf. Sci. 2020, 507, 145042. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, B.; Guo, Y.; Wang, T.; Zhang, L.; Zhong, C.; Wang, H. The role of oxidizer in the flotation separation of chalcopyrite and galena using sodium lignosulfonate as a depressant. Miner. Eng. 2021, 172, 107160. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Farokh, A.; Rahmani, E.; Eshaghi, M.M.; Aslani, A.; Rahdar, A.; Ferreira, L.F.R. Polyacrylic acid mediated targeted drug delivery nano-systems: A review. J. Drug Deliv. Sci. Technol. 2023, 80, 104169. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, Z.; Hu, Y.; Sun, W.; Tang, H.; Yin, Z.; He, J.; Guan, Q.; Zhu, Y. The effect of polyacrylic acid on the surface properties of calcite and fluorite aiming at their selective flotation. Physicochem. Probl. Miner. Process. 2018, 54, 868–877. [Google Scholar]
- Cheng, K.; Wu, X.; Tang, H.; Zeng, Y. The flotation of fine hematite by selective flocculation using sodium polyacrylate. Miner. Eng. 2022, 176, 107273. [Google Scholar] [CrossRef]
- Dong, L.; Wei, Q.; Qin, W.; Jiao, F. Selective adsorption of sodium polyacrylate on calcite surface: Implications for flotation separation of apatite from calcite. Sep. Purif. Technol. 2020, 241, 116415. [Google Scholar] [CrossRef]
- Cai, J.; Jia, X.; Ma, Y.; Ibrahim, A.M.; Su, C.; Yu, X.; Shen, P.; Liu, D. Effect of pre-oxidation on copper-lead bulk concentrate flotation separation with sodium polyacrylate as galena depressant. Sep. Purif. Technol. 2023, 304, 122276. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y. Removal of aluminosilicates from diasporic-bauxite by selective flocculation using sodium polyacrylate. Sep. Purif. Technol. 2008, 59, 299–303. [Google Scholar] [CrossRef]
- Shu, J.; Yu, L.; Ding, R.; Zhang, L. Efficient synthesis of polyether polyols in simple microreactors. React. Chem. Eng. 2021, 6, 685–693. [Google Scholar] [CrossRef]
- Zhou, X.; Feng, B. The effect of polyether on the separation of pentlandite and serpentine. J. Mater. Res. Technol. 2015, 4, 429–433. [Google Scholar] [CrossRef]
- Qian, Y.; Qin, X.; Peng, Y. Mitigating the coating of fine quartz in fluorite flotation using a triblock copolymer. Miner. Eng. 2019, 136, 81–88. [Google Scholar] [CrossRef]
- Yao, J.; Xue, F.; Yin, W.; Xie, Y.; Yin, X.; Gong, X.; Ban, X. Effective flotation separation of brucite and serpentine using eco-friendly PCE-11 as a novel regulator. Sep. Sci. Technol. 2024, 59, 837–847. [Google Scholar] [CrossRef]
- Zhou, G.; Li, J.; Ye, Y.; Piao, Y.; Wang, L.; Chen, X. Study on flotation behaviors of the frothers with similar formation in the flotation of sulfide ores. Conserv. Util. Miner. Resour. 2019, 39, 1–5. [Google Scholar] [CrossRef]
- Feng, B.; Zhang, W.; Guo, W.; Peng, J.; Luo, G.; Wang, H. Role and mechanism of combined collector and sodium alginate in flotation separation of scheelite and calcite. Chin. J. Nonferrous Met. 2019, 29, 203–210. [Google Scholar] [CrossRef]
- Awasthi, S.; Gaur, J.K.; Bobji, M.S.; Srivastava, C. Nanoparticle-reinforced polyacrylamide hydrogel composites for clinical applications: A review. J. Mater. Sci. 2022, 57, 8041–8063. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, W.; Liu, W.; Chen, X. Flotation separation of molybdenite from chalcopyrite using mechanically degraded polyacrylamide as a novel depressant. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129897. [Google Scholar] [CrossRef]
- Huang, P.; Wang, L.; Liu, Q. Depressant function of high molecular weight polyacrylamide in the xanthate flotation of chalcopyrite and galena. Int. J. Miner. Process. 2014, 128, 6–15. [Google Scholar] [CrossRef]
- Wang, K.; Wang, L.; Cao, M.; Liu, Q. Xanthation-modified polyacrylamide and spectroscopic investigation of its adsorption onto mineral surfaces. Miner. Eng. 2012, 39, 1–8. [Google Scholar] [CrossRef]
- Yin, Z.G.; Khoso, S.A.; Sun, W.; Hu, Y.H.; Zhai, J.H.; Gao, Y.S.; Zhang, C.H.; Liu, R.Q. Flocculation of flotation tailings in presence of silicate gel and polyme. J. Cent. South Univ. Sci.Technol. 2018, 25, 1928–1937. [Google Scholar] [CrossRef]
- Zou, W.; Gong, L.; Huang, J.; Zhang, Z.; Sun, C.; Zeng, H. Adsorption of hydrophobically modified polyacrylamide P(AM-NaAA-C16DMAAC) on model coal and clay surfaces and the effect on selective flocculation of fine coal. Miner. Eng. 2019, 142, 105887. [Google Scholar] [CrossRef]
- Wu, H.; Wang, M.; Li, M.; Jin, G. Longitudinal ultrasound measurements of polyethylene oxide across dilute to concentrated aqueous solutions. Polymer 2024, 310, 127470. [Google Scholar] [CrossRef]
- Gong, J.; Peng, Y.; Bouajila, A.; Ourriban, M.; Yeung, A.; Liu, Q. Reducing quartz gangue entrainment in sulphide ore flotation by high molecular weight polyethylene oxide. Int. J. Miner. Process. 2010, 97, 44–51. [Google Scholar] [CrossRef]
- Alvarez, A.; Gutierrez, L.; Laskowski, J.S. Use of polyethylene oxide to improve flotation of fine molybdenite. Miner. Eng. 2018, 127, 232–237. [Google Scholar] [CrossRef]
- Li, S.; Ma, X.; Wang, J.; Xing, Y.; Gui, X.; Cao, Y. Effect of polyethylene oxide on flotation of molybdenite fines. Miner. Eng. 2020, 146, 106146. [Google Scholar] [CrossRef]
- Liang, L.; Tan, J.; Li, Z.; Peng, Y.; Xie, G. Coal Flotation Improvement Through Hydrophobic Flocculation Induced by Polyethylene Oxide. Int. J. Coal Prep. Util. 2016, 36, 139–150. [Google Scholar] [CrossRef]
- Pugh, R.J. Non-ionic polyethylene oxide frothers in graphite flotation. Miner. Eng. 2000, 13, 151–162. [Google Scholar] [CrossRef]
- Liu, M.; Yi, K.; Zhang, Y.; Long, F.; Hu, X.; Jia, G.; Xiao, T.; Xu, X.; Duan, Y.; Shi, H.; et al. Enhanced exosome capture in urine using aptamer-modified temperature-responsive polymer for sensitive early detection of bladder cancer. Chem. Eng. J. 2024, 489, 151304. [Google Scholar] [CrossRef]
- Ow, V.; Loh, X.J. Recent developments of temperature-responsive polymers for ophthalmic applications. J. Polym. Sci. 2022, 60, 1429–1447. [Google Scholar] [CrossRef]
- Burdukova, E.; Franks, G.V. Flotation of Fine and Ultrafine Quartz Using Poly(N-Isopropylacrylamide) as a Collector. In Chemeca 2010: Engineering at the Edge; 26–29 September 2010, Hilton Adelaide, South Australia; Engineers Australia: Barton, Australia, 2010; pp. 507–518. [Google Scholar]
- Ng, W.S.; Sonsie, R.; Forbes, E.; Franks, G.V. Flocculation/flotation of hematite fines with anionic temperature-responsive polymer acting as a selective flocculant and collector. Miner. Eng. 2015, 77, 64–71. [Google Scholar] [CrossRef]
- Ng, W.S.; Cooper, L.; Connal, L.A.; Forbes, E.; Jameson, G.J.; Franks, G.V. Tuneable collector/depressant behaviour of xanthate-functional temperature-responsive polymers in the flotation of copper sulfide: Effect of shear and temperature. Miner. Eng. 2018, 117, 91–99. [Google Scholar] [CrossRef]
- Neisiani, A.A.; Saneie, R.; Mohammadzadeh, A.; Wonyen, D.G.; Chelgani, S.C. Polysaccharides-based pyrite depressants for green flotation separation: An overview. Int. J. Min. Sci. Technol. 2023, 33, 1229–1241. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, H.; Chi, R.; Bao, S.; Xu, Y.; Liu, C. A critical review on the application of green polymer-type scale inhibitors in mineral flotation. Miner. Eng. 2023, 204, 108436. [Google Scholar] [CrossRef]
- Feng, Q.; Yang, W.; Chang, M.; Wen, S.; Liu, D.; Han, G. Advances in depressants for flotation separation of Cu-Fe sulfide minerals at low alkalinity: A critical review. Int. J. Miner. Metall. Mater. 2024, 31, 1–17. [Google Scholar] [CrossRef]
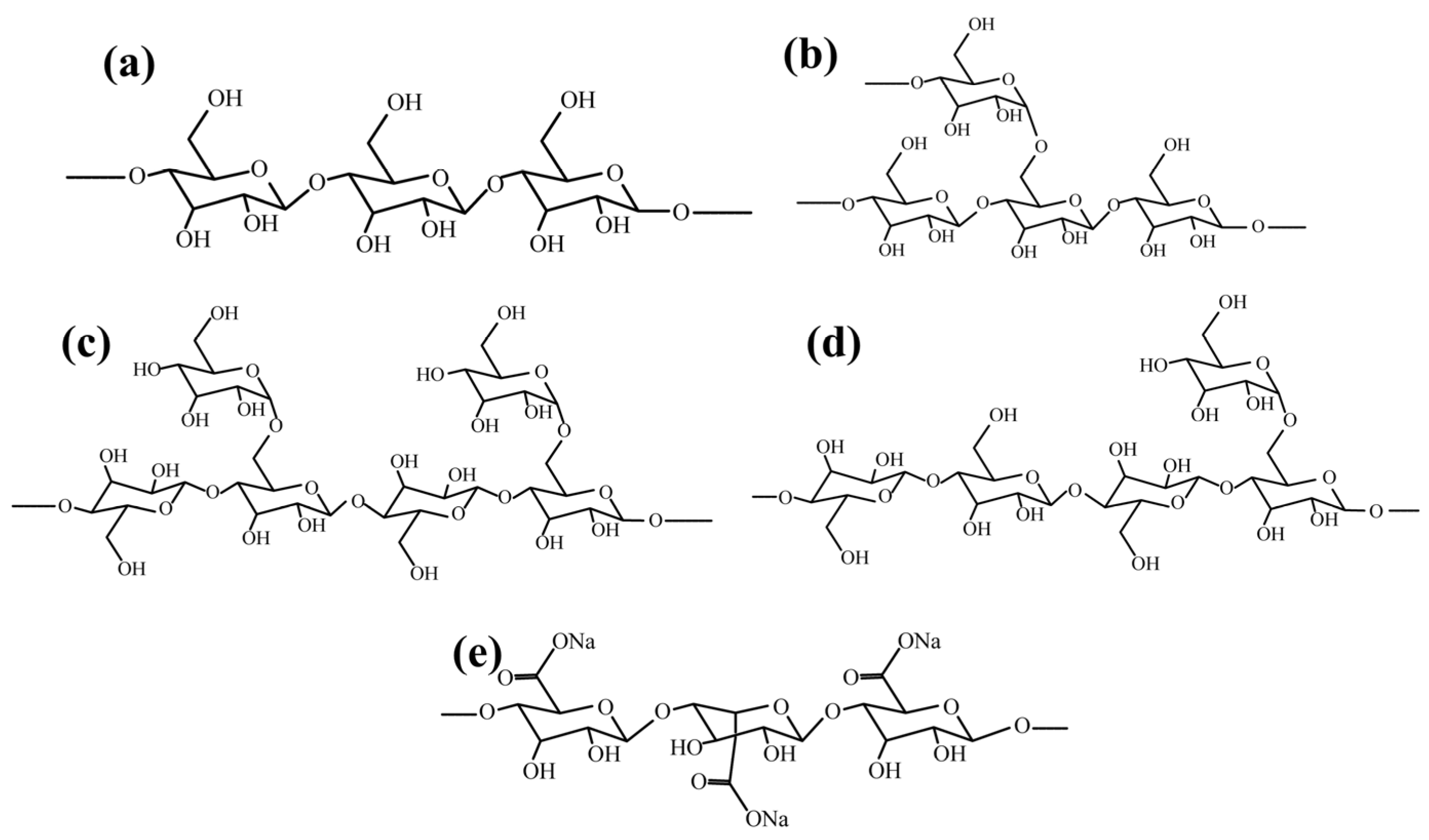

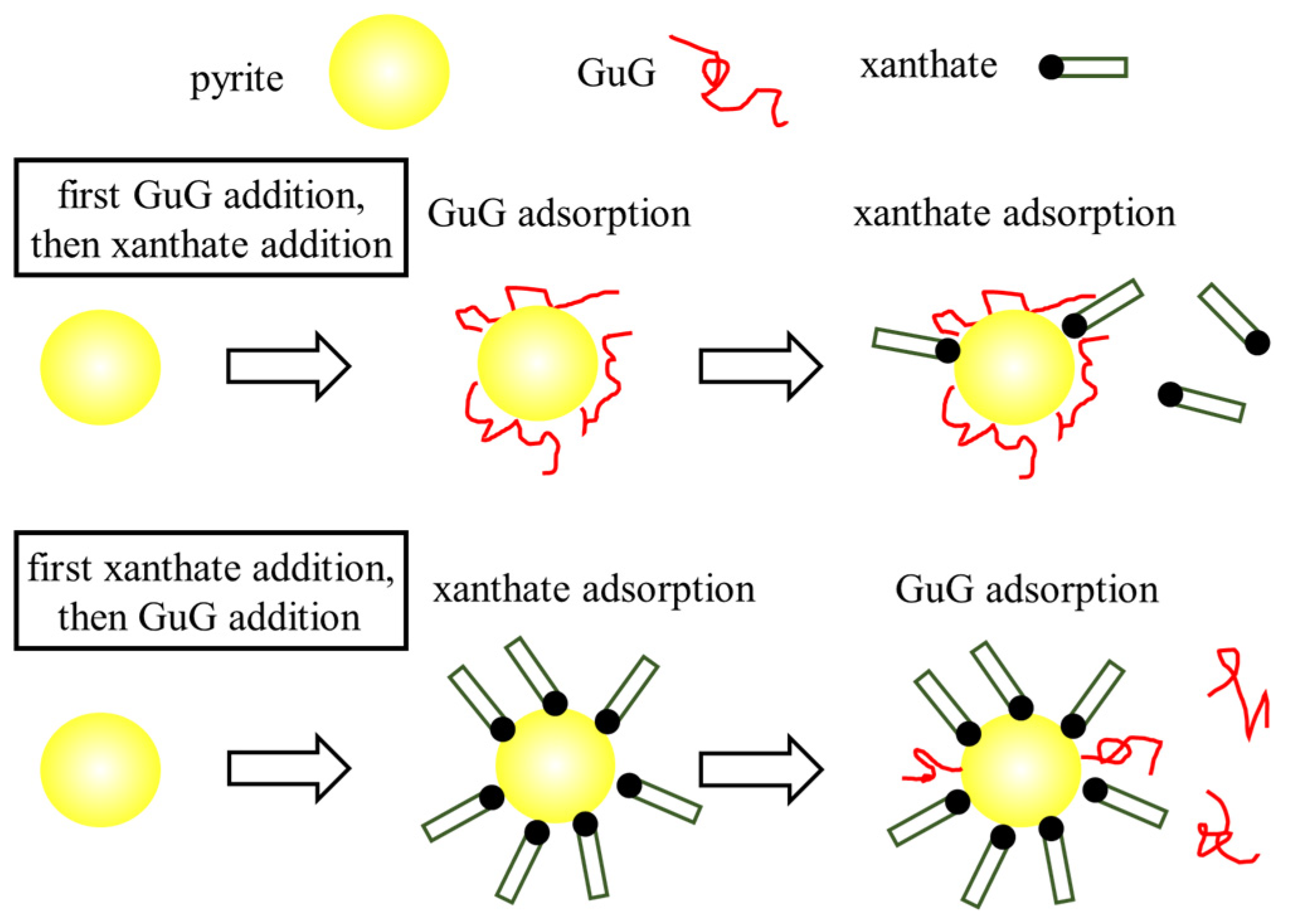
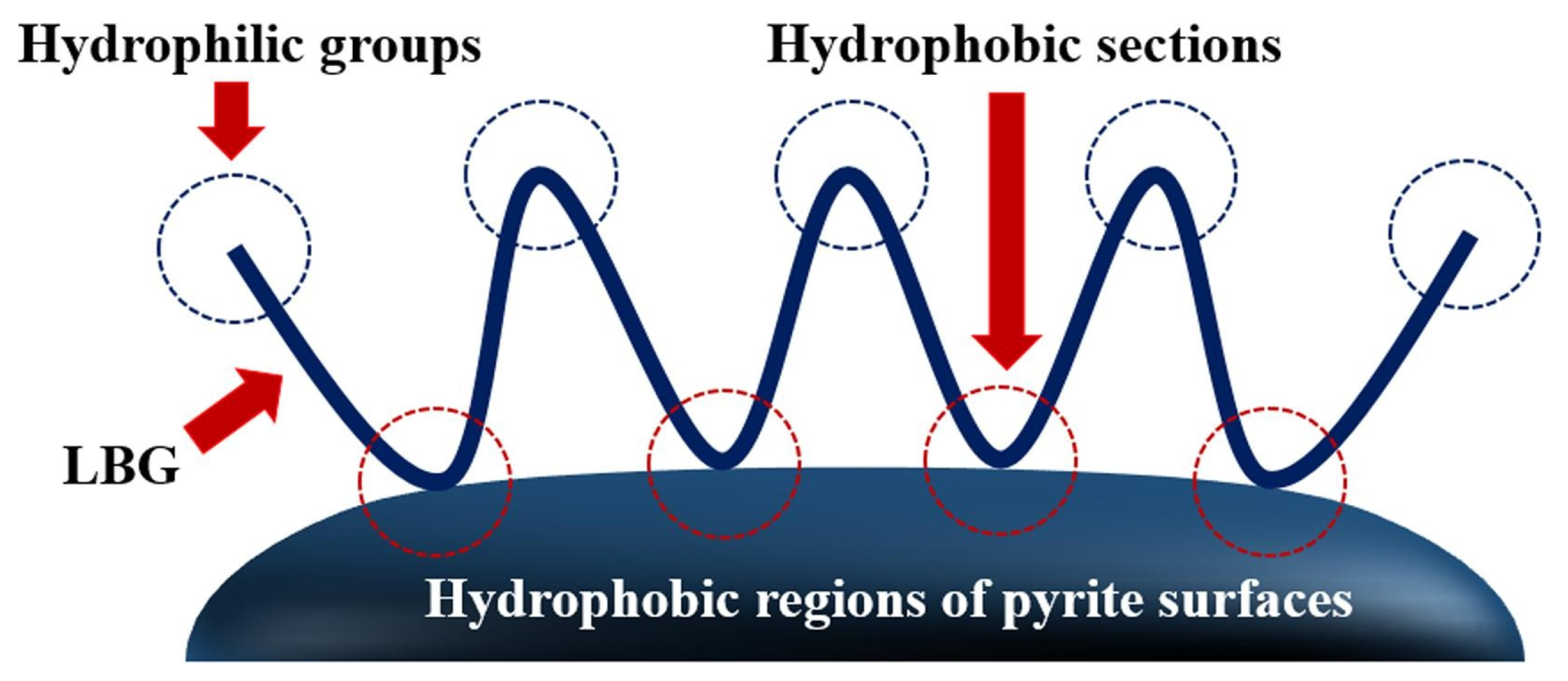
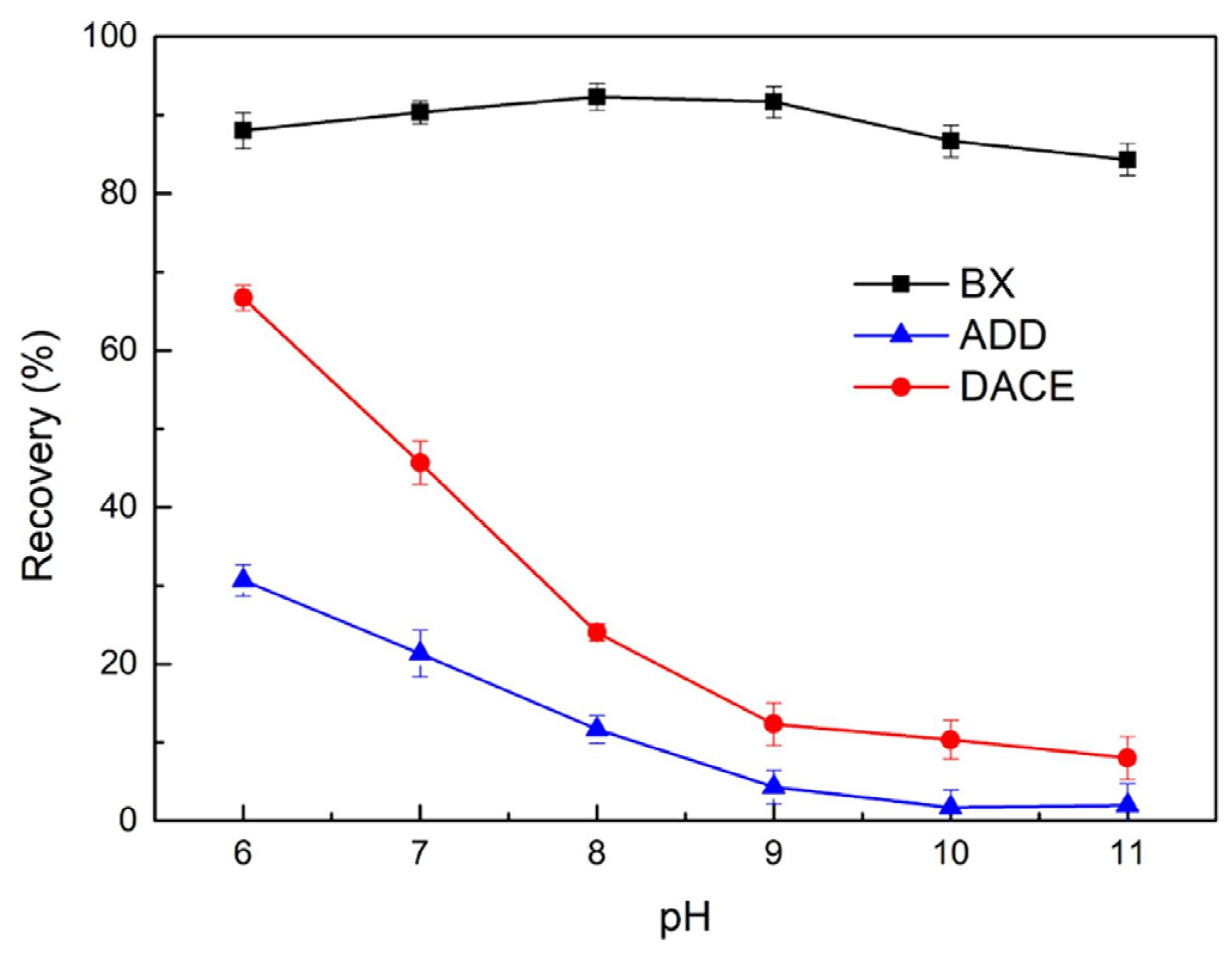

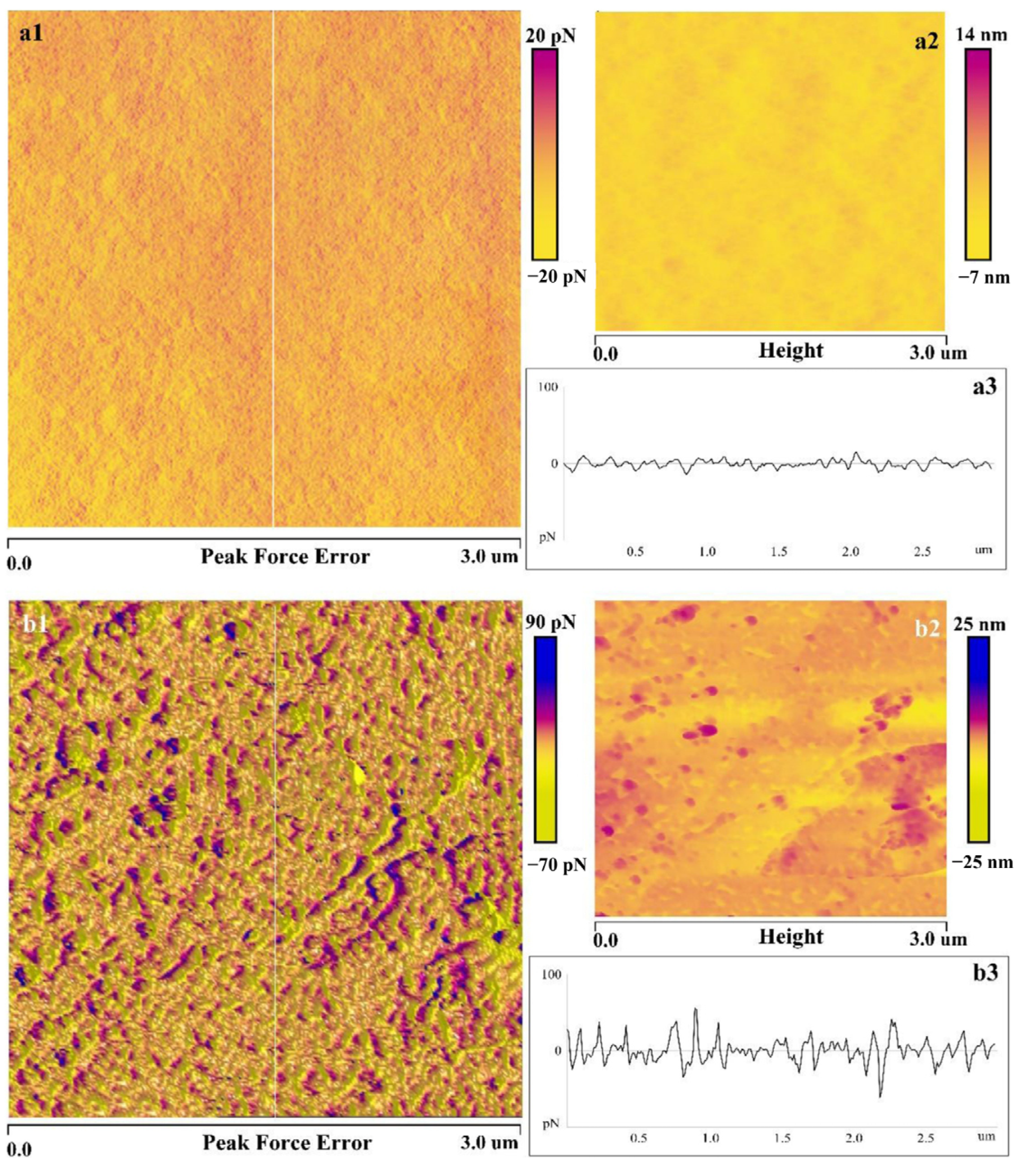
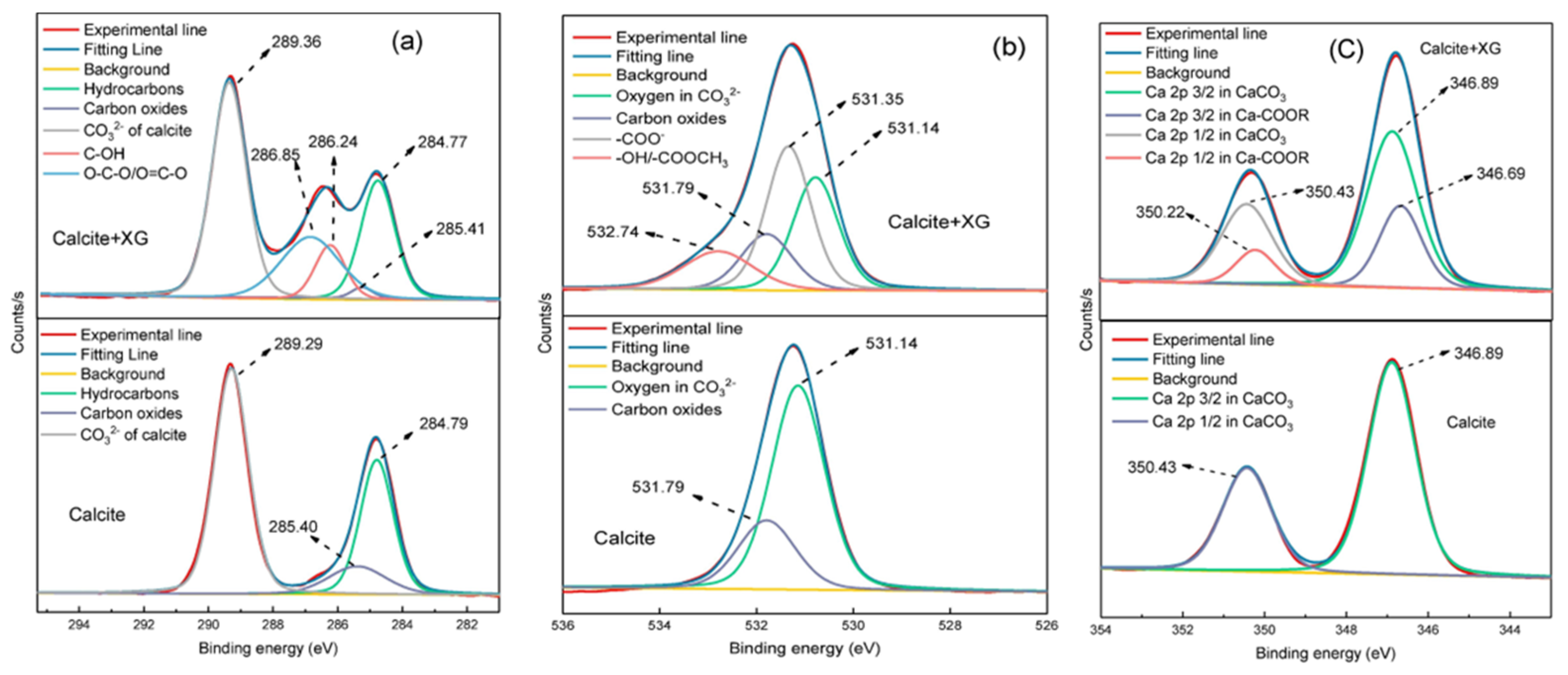
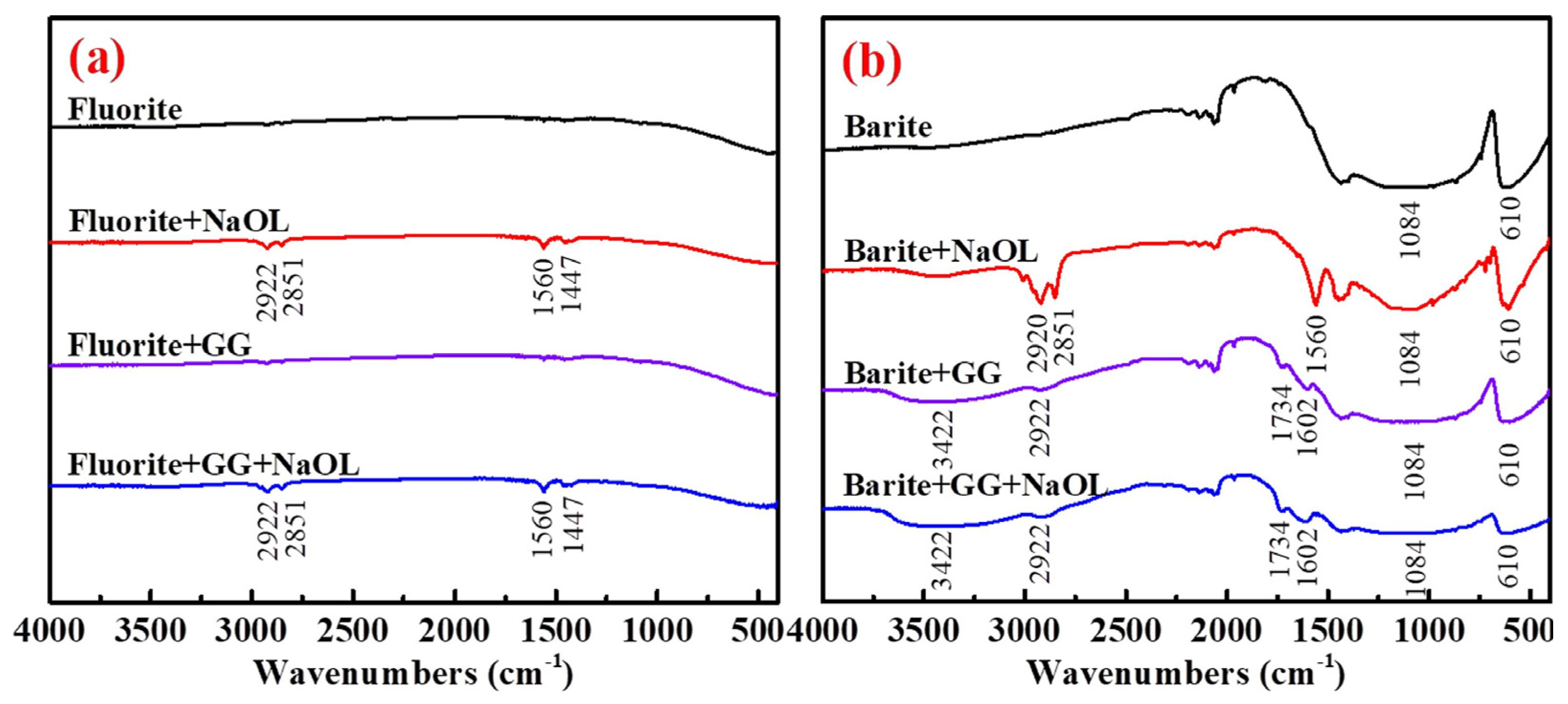

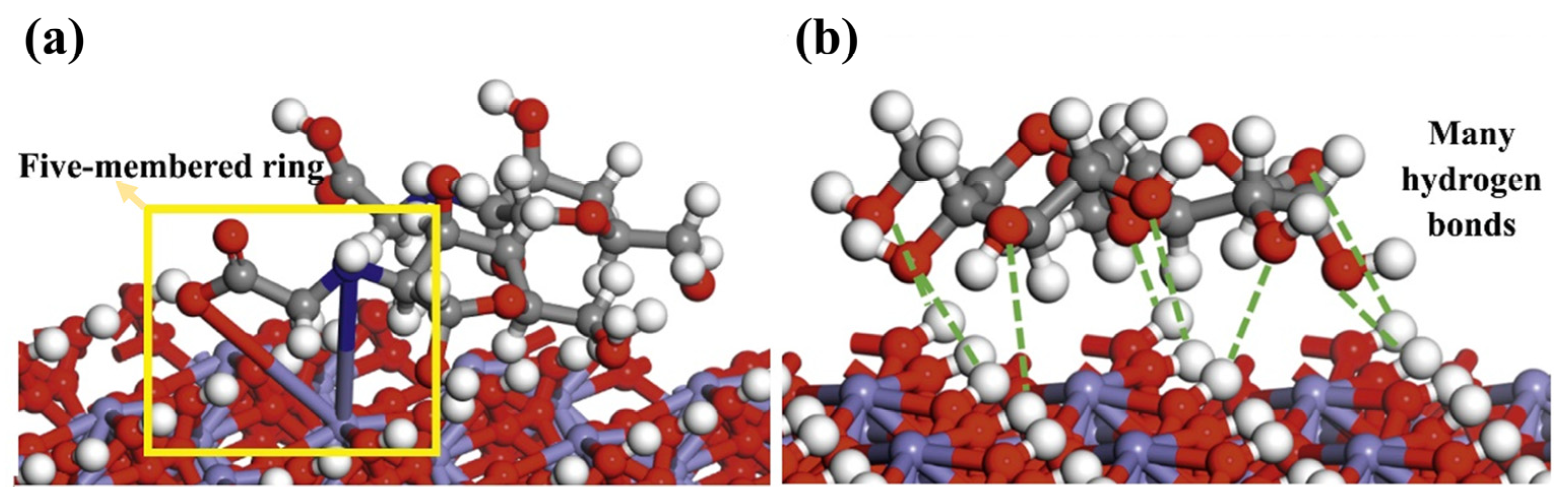

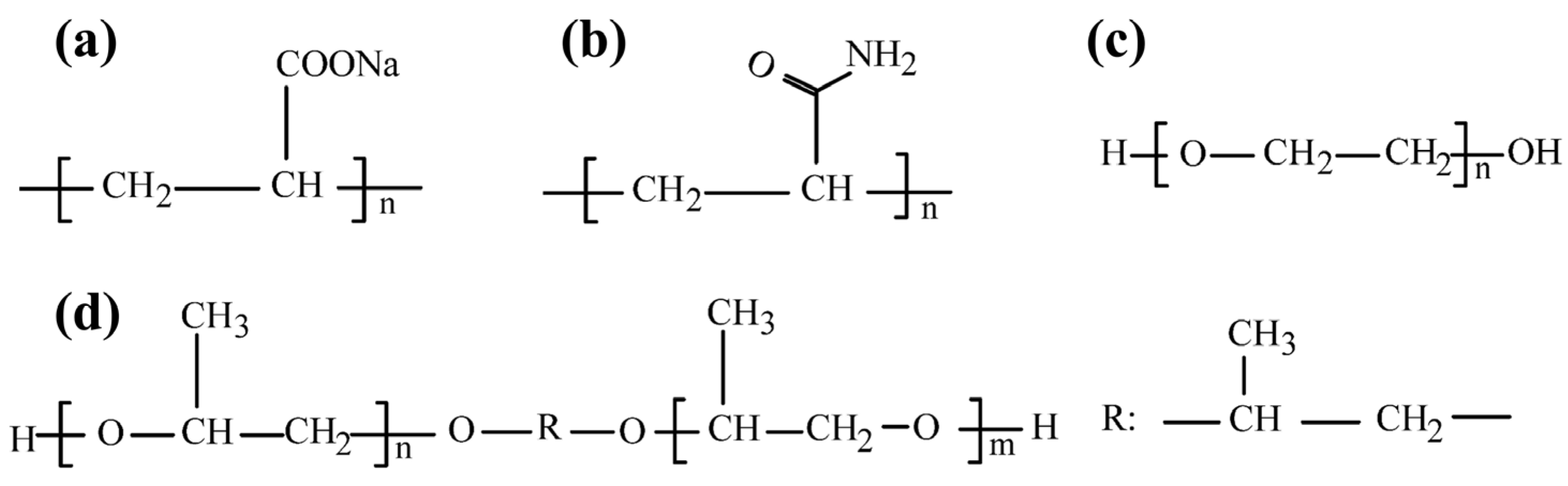
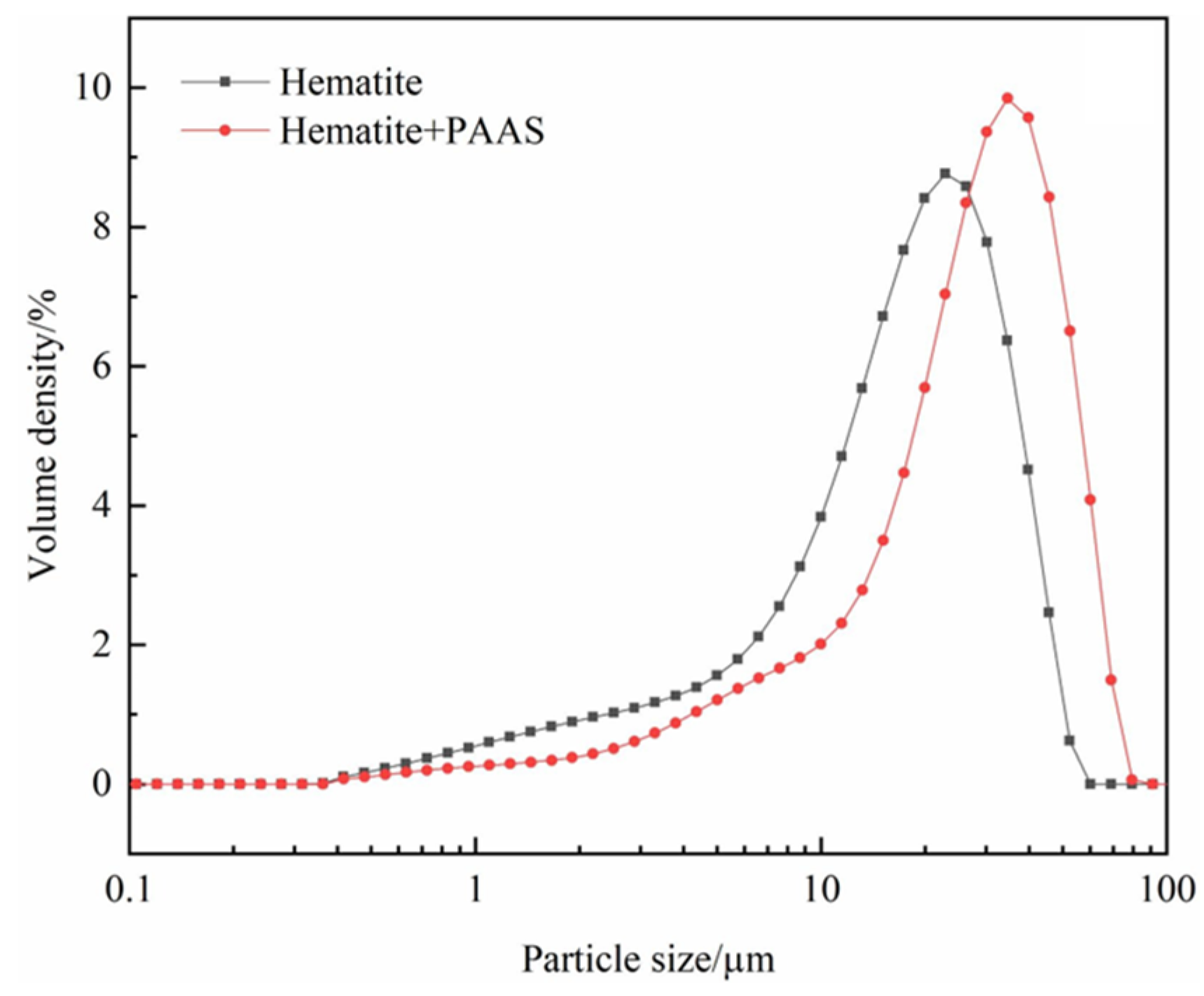
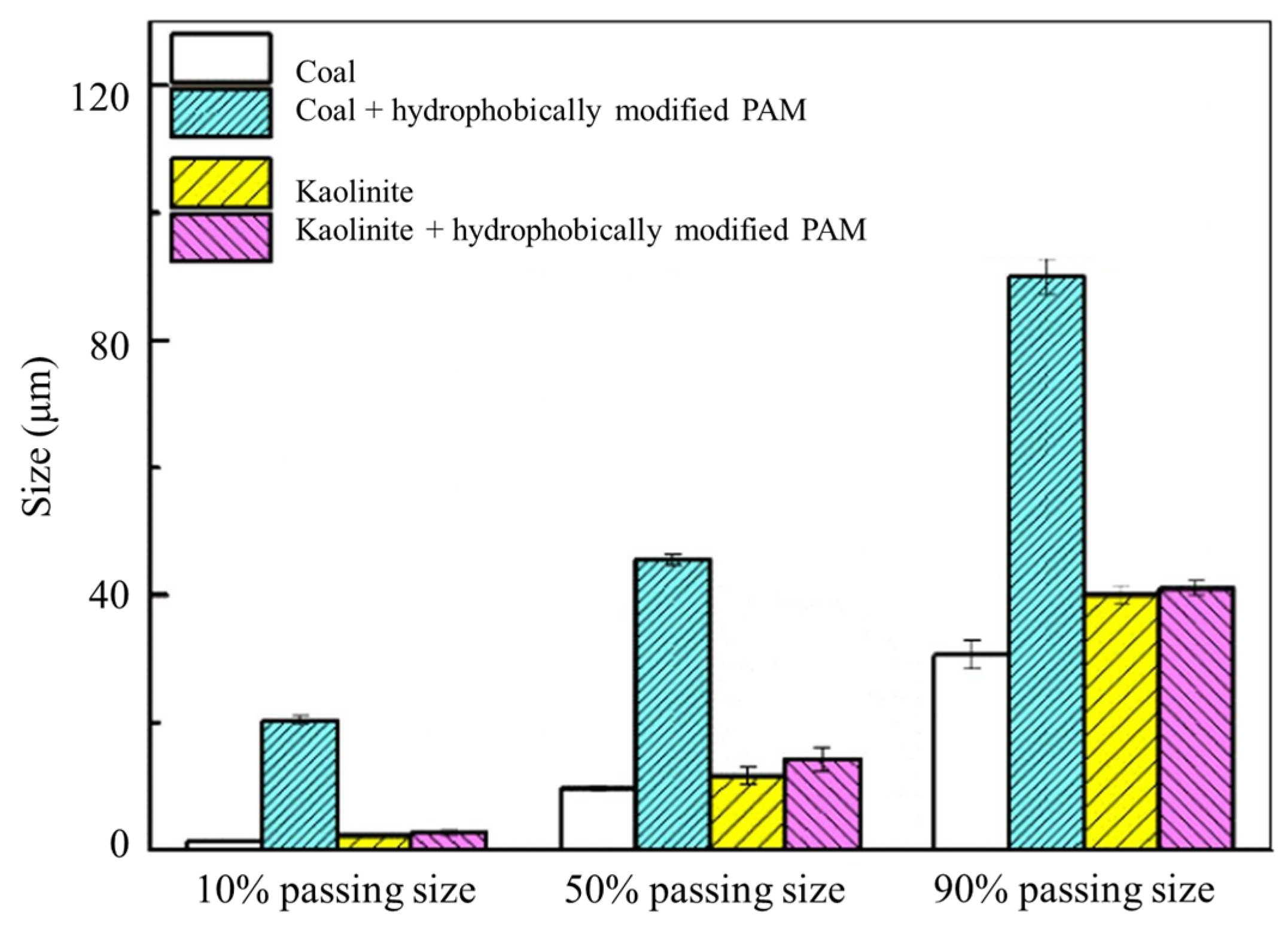
| Reagents | Role | Application Scope of Flotation | Advantages |
|---|---|---|---|
| starch phosphate | Depressant | hematite | a lower dosage, superior depression capacity toward iron minerals compared to native starch |
| cationic starch | Depressant | hematite | superior depression capacity toward iron minerals compared to causticized starch |
| carboxymethyl starch | Depressant | talc | selectively depresses talc over a broad pH range |
| pyrite | reduces the adsorption of collector on pyrite surface; carboxymethyl starch with low substitution is more inhibitory than carboxymethyl starch with high substitution | ||
| oxidized starch | Depressant | pyrite | superior depression capacity compared to native starch |
| cross-linked starch | Flocculant | specularite | reduces the content of particles smaller than 20 microns |
| amphoteric starch | Flocculant | iron ore | improves concentrate grade and recovery |
| modified starch containing amino radicals | Flocculant | iron ore | improves concentrate grade |
| Classification | Reagent | Role | Application Scope of Flotation |
|---|---|---|---|
| Plant Polysaccharide Polymer | Starch | Depressant | quartz/hematite chalcopyrite/sphalerite chalcopyrite/pyrite |
| Flocculant | hematite | ||
| Guar Gum | Depressant | chalcopyrite/talc pyrite calcite magnesite/dolomite chalcopyrite/monoclinic pyrrhotite | |
| Flocculant | hematite, talc | ||
| Locust Bean Gum | Depressant | chalcopyrite/sphalerite chalcopyrite/pyrite chalcopyrite/galena quartz/hematite chalcopyrite/talc scheelite/dolomite | |
| Sodium Alginate | Depressant | scheelite/calcite, fluorite apatite/dolomite marmatite/galena | |
| Flocculant | dolomite | ||
| Animal Polysaccharide Polymer | Chitosan | Depressant | talc specularite/chlorite galena/pyrite molybdenite/chalcopyrite |
| Flocculant | quartz | ||
| Hyaluronic Acid | Depressant | galena/sphalerite | |
| Microbial Polysaccharide Polymer | Pullulan | Depressant | chalcopyrite/talc galena/sphalerite |
| Xanthan Gum | Depressant | chalcopyrite/talc arsenopyrite/chlorite scheelite/calcite apatite/dolomite | |
| Flocculant | cassiterite | ||
| Gellan Gum | Depressant | fluorite/barite fluorite/calcite |
| Classification | Reagent | Role | Application Scope of Flotation |
|---|---|---|---|
| Modified Polymers | Modified Starch | Depressant | quartz/hematite molybdenite/talc chalcopyrite/pyrite graphite |
| Flocculant | specularite, siderite | ||
| Modified Cellulose | Depressant | fluorapatite/dolomite quartz/magnesite chalcopyrite/talc | |
| Flocculant | chlorite, iron oxide, hydroxyapatite | ||
| Modified Chitosan | Depressant | quartz/hematite apatite/calcite chalcopyrite/molybdenite | |
| Modified Lignin | Depressant | apatite/dolomite scheelite/calcite chalcopyrite/galena | |
| Synthesized Polymers | Polyacrylic acid | Depressant | chalcopyrite/talc galena/sphalerite chalcopyrite/galena |
| Flocculant | hematite, diaspore | ||
| Polyether polyol | Depressant | pentlandite/serpentine fluorite/quartz brucite/serpentine | |
| Collector | sulfide ores | ||
| Frother | scheelite | ||
| Polyacrylamide | Depressant | molybdenite/chalcopyrite galena/chalcopyrite sphalerite/galena | |
| Flocculant | tungsten tailings, coal | ||
| Polyethylene oxide | Collector | molybdenite | |
| Frother | graphite | ||
| Depressant | quartz, coal | ||
| Thermoresponsive polymers | Frother | quartz, hematite | |
| Depressant | sulphide ore |
| Classification | Advantages | Disadvantages |
|---|---|---|
| Natural polymers | wide sources, low cost, no pollution | poor water solubility, weak selectivity |
| Modified polymers | high water solubility, high selectivity | by-product interference, high cost |
| Synthetic polymers | small dosage, design structure | derivative flotation application unknown |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Yang, S.; Huang, L.; Liu, S.; Liu, C.; Xu, J. Research Progress of Application and Interaction Mechanism of Polymers in Mineral Flotation: A Review. Polymers 2024, 16, 3335. https://doi.org/10.3390/polym16233335
Wang Q, Yang S, Huang L, Liu S, Liu C, Xu J. Research Progress of Application and Interaction Mechanism of Polymers in Mineral Flotation: A Review. Polymers. 2024; 16(23):3335. https://doi.org/10.3390/polym16233335
Chicago/Turabian StyleWang, Qianqian, Siyuan Yang, Lingyun Huang, Shuo Liu, Cheng Liu, and Jinyue Xu. 2024. "Research Progress of Application and Interaction Mechanism of Polymers in Mineral Flotation: A Review" Polymers 16, no. 23: 3335. https://doi.org/10.3390/polym16233335
APA StyleWang, Q., Yang, S., Huang, L., Liu, S., Liu, C., & Xu, J. (2024). Research Progress of Application and Interaction Mechanism of Polymers in Mineral Flotation: A Review. Polymers, 16(23), 3335. https://doi.org/10.3390/polym16233335








