Preparation of Thermal Conductivity-Enhanced, Microencapsulated Phase Change Materials Using Cellulose-Assisted Graphene Dispersion for Thermal Regulation in Textiles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GNs/MF MPCMs
2.3. Preparation of MPCMs/Textile Composite
2.4. Characterization
2.5. Theoretical Calculations
3. Results and Discussion
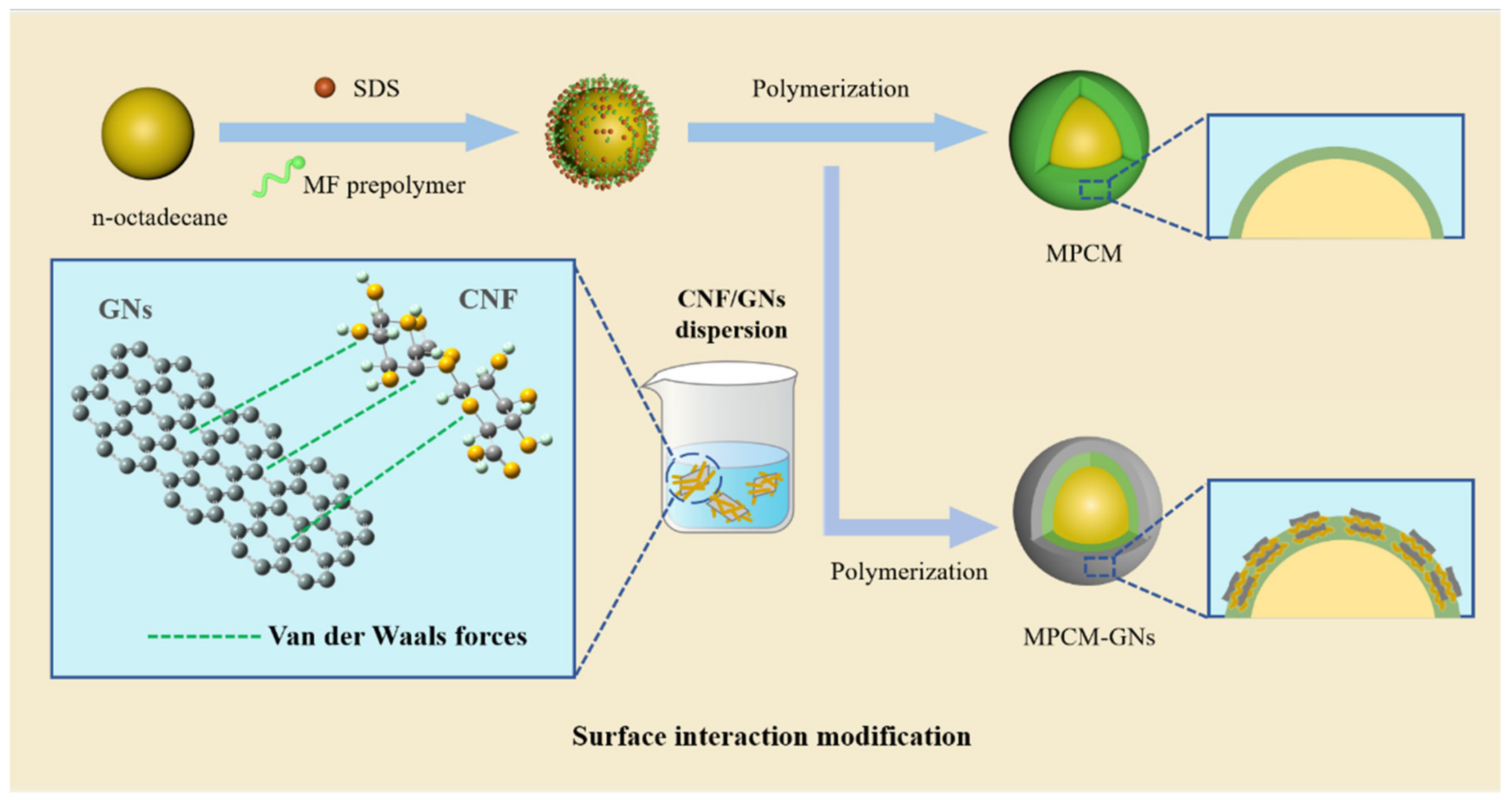
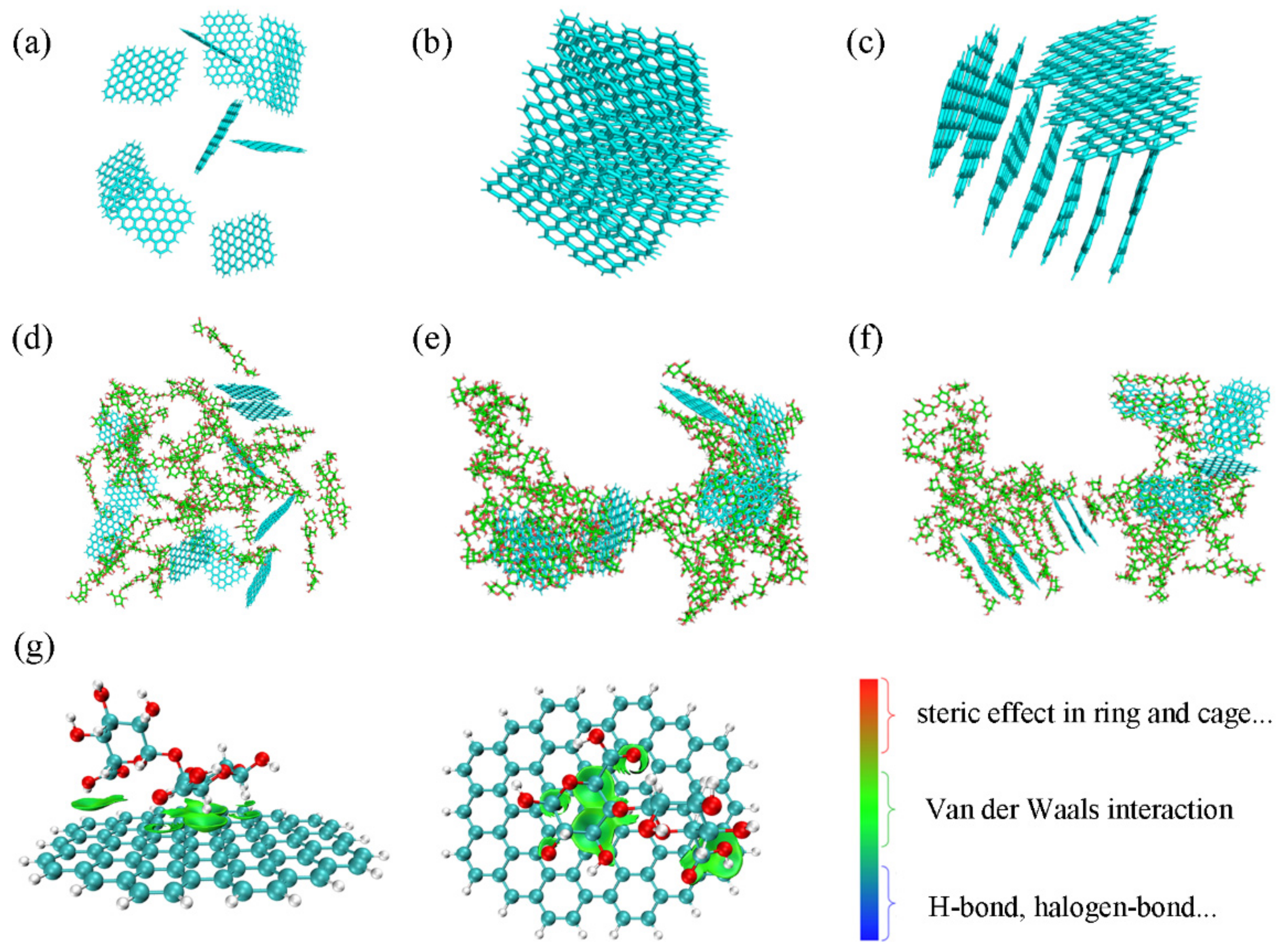
3.1. The Designed Strategy of MPCMs
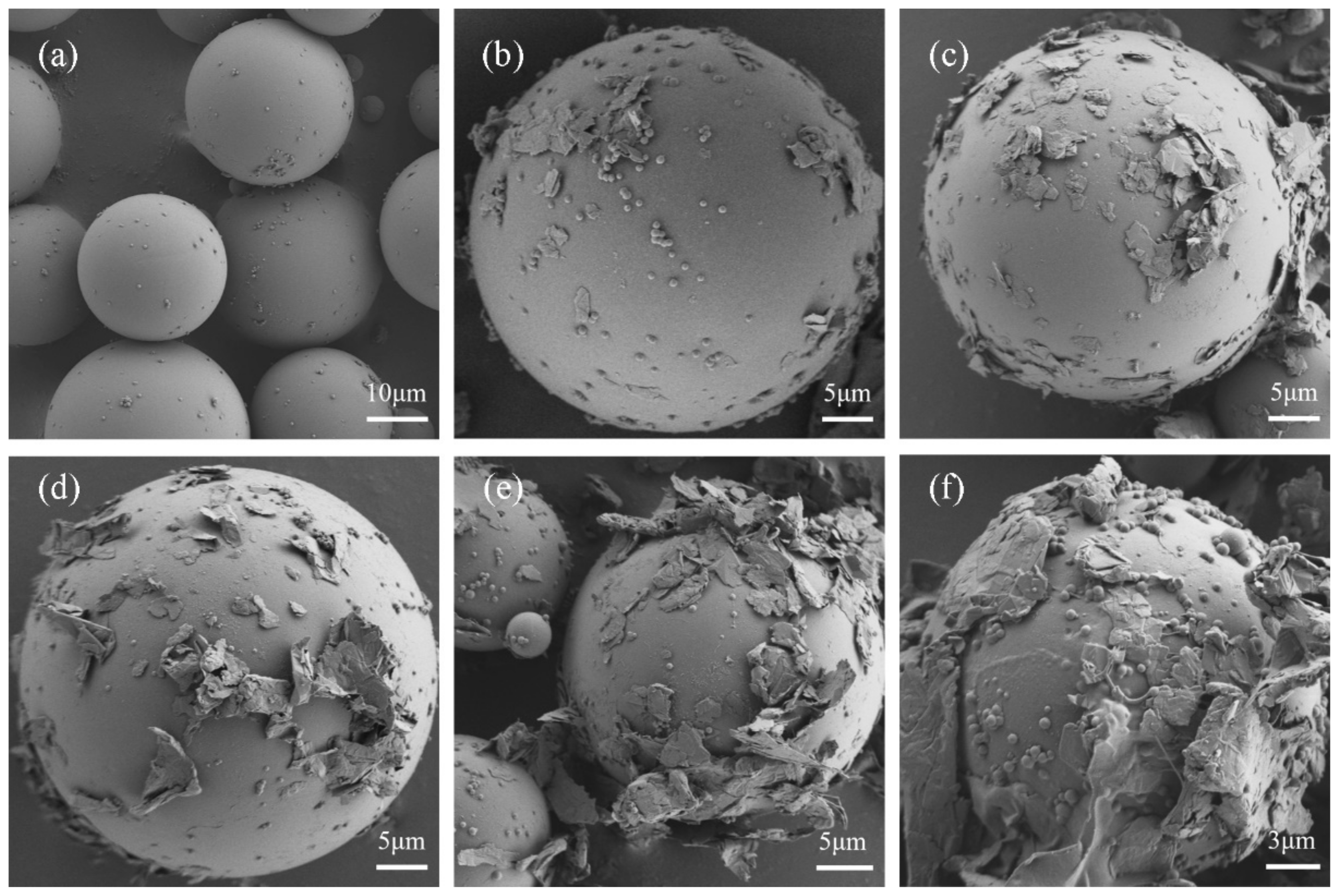
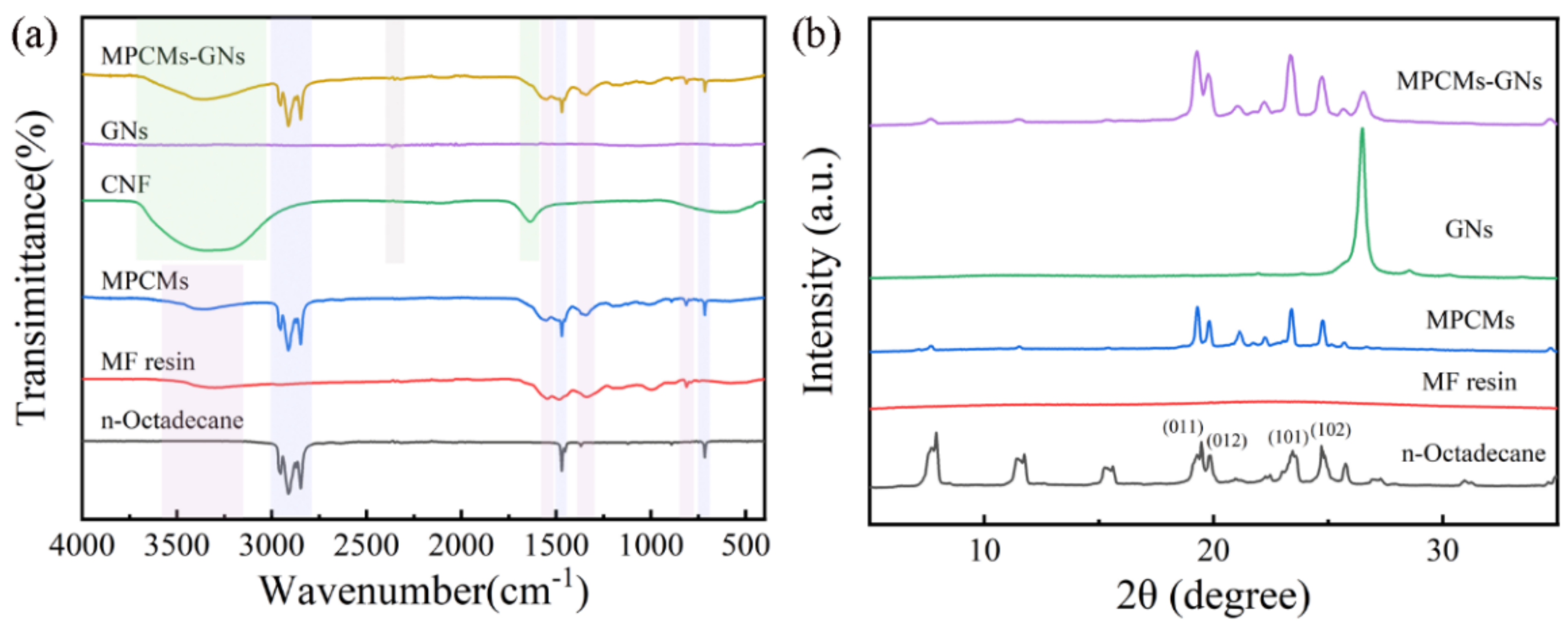
3.2. Chemical Structure and Morphology of MPCMs
3.3. Thermal Properties of MPCMs
3.3.1. Phase-Change Behavior
3.3.2. Thermal Conductivity of MPCMs–GNs
3.3.3. Thermal Stability of MPCMs
3.3.4. Thermal Cycling Property of MPCMs
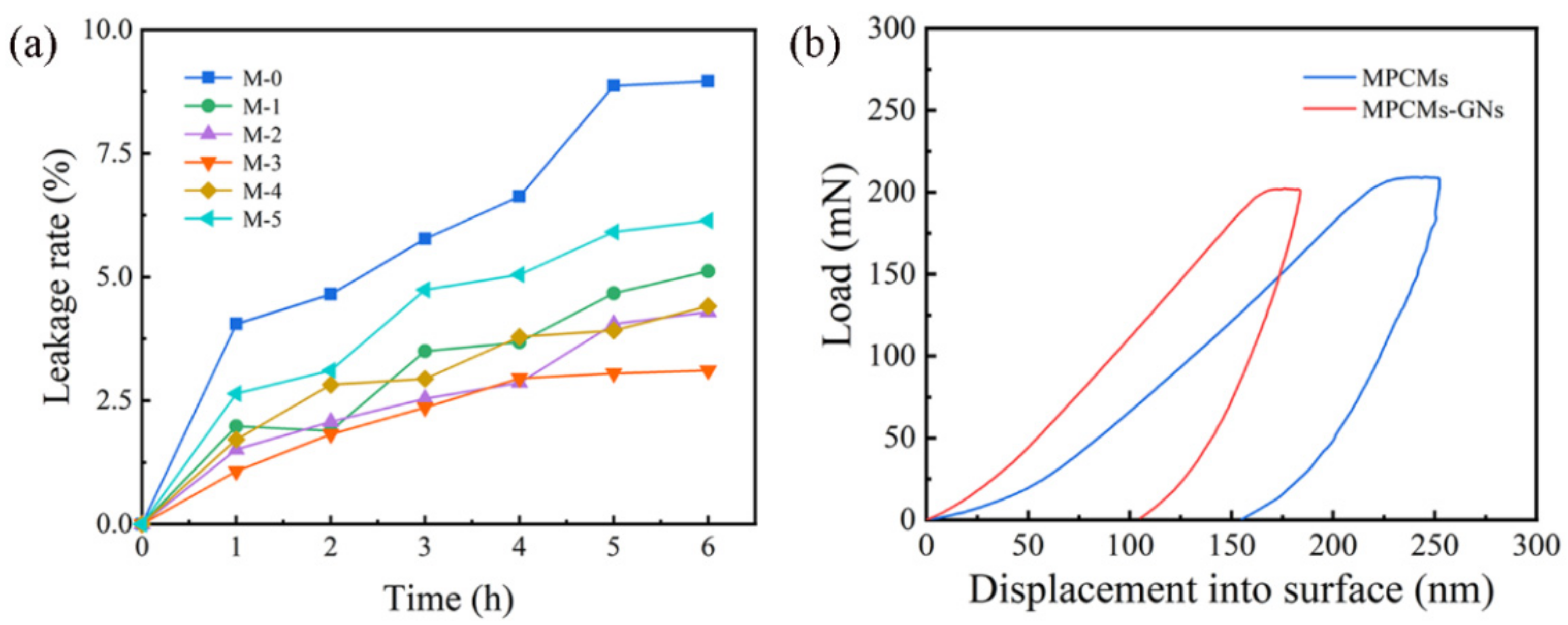
3.4. Leakage Rate of MPCMs
3.5. Mechanical Properties of MPCMs
3.6. Temperature Regulating Performance of MPCMs/Textile Composite
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Han, L.; Zhang, X.; Ji, J.; Ma, K. Research progress on the influence of nano-additives on phase change materials. J. Energy Storage 2022, 55, 105807. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, J.; Li, C.; He, Z.; Zhou, P.; Zheng, X.; You, S.; Zhang, H.; Li, Y.; Zhang, Y.; et al. Performance analysis of the phase-change heat-storage tank based on numerical simulation. Appl. Therm. Eng. 2023, 235, 121370. [Google Scholar] [CrossRef]
- Jiang, B.; Mu, M.; Zhou, Y.; Zhang, J.; Li, W. Nanoparticle-Empowered Core-Shell Microcapsules: From Architecture Design to Fabrication and Functions. Small 2024, 20, e2311897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xing, F.; Cui, H.; Chen, D.; Ouyang, X.; Xu, S.; Wang, J.; Huang, Y.; Zuo, J.; Tang, J. A novel phase-change cement composite for thermal energy storage: Fabrication, thermal and mechanical properties. Appl. Energy 2016, 170, 130–139. [Google Scholar] [CrossRef]
- Liu, Y.; Kang, M.; Lin, W.; Liang, C.; Qu, W.; Wang, Y.; Guan, Y.; Cheng, J. Enhanced thermal performance of phase change material microcapsules using halloysite nanotubes. Appl. Therm. Eng. 2023, 231, 120965. [Google Scholar] [CrossRef]
- Yang, D.; Tu, S.; Chen, J.; Zhang, H.; Chen, W.; Hu, D.; Lin, J. Phase Change Composite Microcapsules with Low-Dimensional Thermally Conductive Nanofillers: Preparation, Performance, and Applications. Polymers 2023, 15, 1562. [Google Scholar] [CrossRef]
- Niu, S.S.; Cheng, J.J.; Zhao, Y.Q.; Kang, M.Y.; Liu, Y.Q. Preparation and characterization of multifunctional phase change material microcapsules with modified carbon nanotubes for improving the thermal comfort level of buildings. Constr. Build. Mater. 2022, 347, 128628. [Google Scholar] [CrossRef]
- Li, Q.; Tan, Y.; Lin, C.H.; Ning, Y.H.; Yu, L.P.; Cao, Z.; Sun, L.X.; Zeng, J.L. Preparation and properties of erythritol/exfoliated graphite nanoplatelets @ polyaniline microencapsulated phase change materials with improved photothermal conversion efficiency. J. Energy Storage 2023, 72, 108553. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, H.; Pan, R.; Yang, J.H.; Gong, Y.; Gan, Z.Y.; Hu, R.; Ding, J.J.; Chen, L.; Zhang, X.; et al. Phase Change Microcapsules with a Polystyrene/Boron Nitride Nanosheet Hybrid Shell for Enhanced Thermal Management of Electronics. Langmuir 2022, 51, 16055–16066. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, S.; He, J.; Xu, D.; Abdou, S.N.; Ibrahim, M.M.; Sun, S.; Chen, Y.; Li, H.; Xu, B.B.; et al. Experimental design of paraffin/methylated melamine-formaldehyde microencapsulated composite phase change material and the application in battery thermal management system. J. Mater. Sci. Technol. 2024, 169, 124–136. [Google Scholar] [CrossRef]
- Alam, T.; Dhau, J.; Goswami, D.; Stefanakos, E. Macroencapsulation and characterization of phase change materials for latent heat thermal energy storage systems. Appl. Energy 2015, 154, 92–101. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Chen, X.-H.; Wang, F.-Q.; Chen, R.-S.; Han, L. Dodecane/Silica Phase Change Microcapsules: Fabrication, Structure and Stability. Sci. Adv. Mater. 2023, 15, 887–893. [Google Scholar] [CrossRef]
- Umair, M.; Zhang, Y.; Iqbal, K.; Zhang, S.; Tang, B. Novel strategies and supporting materials applied to shape-stabilize organic phase change materials for thermal energy storage—A review. Appl. Energy 2019, 235, 846–873. [Google Scholar] [CrossRef]
- Meng, X.; Qin, S.; Fan, H.; Huang, Z.; Hong, J.; Xu, X.; Ouyang, X.; Chen, D.-Z. Long alkyl chain-grafted carbon nanotube-decorated binary-core phase-change microcapsules for heat energy storage: Synthesis and thermal properties. Sol. Energy Mater. Sol. Cells 2020, 212, 110589. [Google Scholar] [CrossRef]
- Aftab, W.; Huang, X.; Wu, W. Nanoconfined phase change materials for thermal energy applications. Energy Environ. Sci. 2018, 11, 1392–1424. [Google Scholar] [CrossRef]
- Kang, M.; Lin, W.; Liang, C.; Zeng, J.; Wang, Y.; Guan, Y.; Cheng, J. Construction of ammonium polyphosphate-modified halloysite nanotubes on phase change material microcapsules for the enhancement of thermophysical performance and flame retardant properties. Appl. Therm. Eng. 2024, 239, 122160. [Google Scholar] [CrossRef]
- Maithya, O.M.; Zhu, X.; Li, X.; Korir, S.J.; Feng, X.; Sui, X.; Wang, B. High-energy storage graphene oxide modified phase change microcapsules from regenerated chitin Pickering Emulsion for photothermal conversion. Sol. Energy Mater. Sol. Cells 2021, 222, 110924. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, J.; Mu, J. ZrC nanoparticle-modified microencapsulated phase change materials with enhanced thermal conductivity and photo-thermal conversion performance. Int. J. Energy Res. 2020, 44, 7283–7298. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, Y.; Rong, M.; Zhang, M. Microencapsulation of styrene with melamine-formaldehyde resin. Colloid Polym. Sci. 2009, 287, 1089–1097. [Google Scholar] [CrossRef]
- Seitz, S.; Ajiro, H. Self-assembling weak polyelectrolytes for the layer-by-layer encapsulation of paraffin-type phase change material icosane. Sol. Energy Mater. Sol. Cells 2019, 190, 57–64. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Qin, Y.S.; Liang, S.E.; Chen, K.P.; Tian, C.R.; Wang, J.H.; Luo, X.; Zhang, L. Graphene/SiO2/n-octadecane nanoencapsulated phase change material with flower like morphology, high thermal conductivity, and suppressed supercooling. Appl. Energy 2019, 250, 98–108. [Google Scholar] [CrossRef]
- Tan, C.; He, Y.; Luo, B.; Liu, M. Microencapsulated phase change material with chitin nanocrystals stabilized Pickering emulsion for thermal energy storage. Int. J. Biol. Macromol. 2023, 240, 124374. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, G.; Chen, L.; Wang, B.; Feng, X.; Mao, Z.; Sui, X.; Xu, H. Phase change microcapsules with graphene nanoplates embedded polyurea shell for enhanced thermal conductivity. Mater. Lett. 2023, 330, 133223. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Yu, F.; Zhang, Z.; Gao, X. Preparation and properties of graphene oxide-modified poly(melamine-formaldehyde) microcapsules containing phase change material n-dodecanol for thermal energy storage. J. Mater. Chem. 2015, 21, 11624–11630. [Google Scholar] [CrossRef]
- Fu, L.; Wu, Z.; Wu, K.; Chen, W.; Zhang, M.; Huang, X.; Ma, C.; Shao, Y.; Ran, J.; Chua, K.J. A thermally induced flexible composite phase change material with boron nitride nanosheets/carbon nanotubes modified skeleton for battery thermal management. Appl. Energy 2024, 373, 123899. [Google Scholar] [CrossRef]
- Al-Shannaq, R.; Kurdi, J.; Al-Muhtaseb, S.; Farid, M. Innovative method of metal coating of microcapsules containing phase change materials. Sol. Energy 2016, 129, 54–64. [Google Scholar] [CrossRef]
- Yuan, H.M.; Liu, S.T.; Hao, S.Y.; Zhang, Z.F.; An, H.F.; Tian, W.J.; Chan, M.S.; Bai, H. Fabrication and properties of nanoencapsulated stearic acid phase change materials with Ag shell for solar energy storage. Sol. Energy Mater. Sol. Cells 2022, 239, 111653. [Google Scholar] [CrossRef]
- Li, S.S.; Lin, Z.K.; Zhou, Z.Z.; Zhao, Y.; Ling, Z.Y.; Zhang, Z.G.; Fang, X.M. Incorporating paraffin@SiO2 nanocapsules with abundant surface hydroxyl groups into polydimethylsiloxane to develop composites with enhanced interfacial heat conductance for chip heat dissipation. Nanoscale 2023, 15, 3419–3429. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, L.; Song, G.L.; Tang, G.Y.; Shi, X.H. Organic-inorganic hybrid shell microencapsulated phase change materials prepared from SiO2/TiC-stabilized pickering emulsion polymerization. Sol. Energy Mater. Sol. Cells 2018, 175, 102–110. [Google Scholar] [CrossRef]
- Wang, X.G.; Zhang, C.Y.; Wang, K.; Huang, Y.Q.; Chen, Z.F. Highly efficient photothermal conversion capric acid phase change microcapsule: Silicon carbide modified melamine urea formaldehyde. J. Colloid Interface Sci. 2021, 582, 30–40. [Google Scholar] [CrossRef]
- Li, C.; Yu, H.; Song, Y.; Liang, H.; Yan, X. Preparation and characterization of PMMA/TiO2 hybrid shell microencapsulated PCMs for thermal energy storage. Energy 2019, 167, 1031–1039. [Google Scholar] [CrossRef]
- Wei, S.; Duan, Z.; Xia, Y.; Huang, C.; Ji, R.; Zhang, H.; Xu, F.; Sun, L.; Sun, Y. Preparation and thermal performances of microencapsulated phase change materials with a nano-Al2O3-doped shell. J. Therm. Anal. Calorim. 2019, 138, 233–241. [Google Scholar] [CrossRef]
- Sun, W.; Wang, X.; Zhang, X. Design and Synthesis of Microencapsulated Phase-Change Materials with a Poly(divinylbenzene)/Dioxide Titanium Hybrid Shell for Energy Storage and Formaldehyde Photodegradation. J. Phys. Chem. C 2020, 124, 20806–20815. [Google Scholar] [CrossRef]
- Kang, L.; Niu, H.; Ren, L.; Lv, R.; Bai, S. Phase change composites with ultra-high through-plane thermal conductivity achieved by vertically-aligned graphite film and double-shelled microcapsules. Compos. Part A Appl. Sci. Manuf. 2024, 182, 108162. [Google Scholar] [CrossRef]
- Liu, X.; Wen, N.; Wang, X.; Zheng, Y. A high-performance hierarchical graphene@ polyaniline@ graphene sandwich containing hollow structures for supercapacitor electrodes. ACS Sustain. Chem. Eng. 2015, 3, 475–482. [Google Scholar] [CrossRef]
- Teweldebrhan, D.; Calizo, I.; Miao, F.; Ghosh, S.; Bao, W.; Pokatilov, E.P.; Nika, D.L.; Lau, C.N.; Balandin, A.A. Extremely high thermal conductivity of graphene: Prospects for thermal management applications in nanoelectronic circuits. Appl. Phys. Lett 2008, 92, 151911. [Google Scholar] [CrossRef]
- Zhao, Q.H.; Zhang, F.; Fan, Q.; He, J.; Zhang, R.; Yan, K.; Yang, H.; Wenbin, Y. Microencapsulated phase change materials based on graphene Pickering emulsion for light-to-thermal energy conversion and management. Sol. Energy Mater. Sol. Cells 2019, 203, 110204. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, Y.; Liu, T.; Huang, R.; Chai, S.; Chen, F.; Fu, Q. Completely Green Approach for the Preparation of Strong and Highly Conductive Graphene Composite Film by Using Nanocellulose as Dispersing Agent and Mechanical Compression. ACS Sustain. Chem. Eng. 2017, 5, 9102–9113. [Google Scholar] [CrossRef]
- Hajian, A.; Lindström, S.B.; Pettersson, T.; Hamedi, M.M. Understanding the dispersive action of nanocellulose for carbon nanomaterials. Nano Lett. 2017, 17, 1439–1447. [Google Scholar] [CrossRef]
- Brakat, A.; Zhu, H. Nanocellulose-graphene derivative hybrids: Advanced structure-based functionality from top-down synthesis to bottom-up assembly. ACS Appl. Bio Mater. 2021, 4, 7366–7401. [Google Scholar] [CrossRef]
- Fu, F.; Zhou, J.; Zhou, X.; Zhang, L.; Li, D.; Kondo, T. Green method for production of cellulose multifilament from cellulose carbamate on a pilot scale. ACS Sustain. Chem. Eng. 2014, 2, 2363–2370. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, N.; Zhang, X.; Xu, L.; Wu, J.; Yu, J.; He, J.; Zhang, J. All-Cellulose Nanocomposites Reinforced with in Situ Retained Cellulose Nanocrystals during Selective Dissolution of Cellulose in an Ionic Liquid. ACS Sustain. Chem. Eng. 2016, 4, 4417–4423. [Google Scholar] [CrossRef]
- Frisch, M.J.T.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 [Software], Gaussian Inc.: Wallingford, CT, USA, 2016.
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Lefebvre, C.; Rubez, G.; Khartabil, H.; Boisson, J.-C.; Contreras-Garcia, J.; He, E. Accurately extracting the signature of intermolecular interactions present in the NCI plot of the reduced density gradient versus electron density. Phys. Chem. Chem. Phys. 2017, 19, 17928–17936. [Google Scholar] [CrossRef]
- Lu, Q.C.T. Independent gradient model based on Hirshfeld partition: A new method for visual study of interactions in chemical systems. J. Comput. Chem. 2022, 43, 539–555. [Google Scholar] [CrossRef]
- Coon, E.; Svyatsky, D.; Jan, A.; Kikinzon, E.; Berndt, M.; Atchley, A.; Harp, D.; Manzini, G.; Shelef, E.; Lipnikov, K. Advanced Terrestrial Simulator (ATS); Zenodo: Geneva, Switzerland, 2020. [Google Scholar] [CrossRef]
- Cygan, R.T.; Greathouse, J.A.; Kalinichev, A.G. Advances in clayff molecular simulation of layered and nanoporous materials and their aqueous interfaces. J. Phys. Chem. C 2021, 125, 17573–17589. [Google Scholar] [CrossRef]
- Liang, S.; Li, Q.; Zhu, Y.; Chen, K.; Tian, C.; Wang, J.; Bai, R. Nanoencapsulation of n-octadecane phase change material with silica shell through interfacial hydrolysis and polycondensation in miniemulsion. Energy 2015, 93, 1684–1692. [Google Scholar] [CrossRef]
- Yin, D.; Ma, L.; Geng, W.; Zhang, B.; Zhang, Q. Microencapsulation of n-hexadecanol by in situ polymerization of melamine-formaldehyde resin in emulsion stabilized by styrene-maleic anhydride copolymer. Int. J. Energy Res. 2015, 39, 661–667. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X. Fabrication and performances of microencapsulated phase change materials based on n-octadecane core and resorcinol-modified melamine-formaldehyde shell. Colloids Surf. A Physicochem. Eng. Asp. 2009, 332, 129–138. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, W.; He, F.; Xie, C.; Fan, J.; Wu, J.; Zhang, K. Electrostatic interaction-based self-assembly of paraffin@graphene microcapsules with remarkable thermal conductivity for thermal energy storage. Fuller. Nanotub. Carbon Nanostruct. 2019, 27, 120–127. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X. Synthesis and properties of microencapsulated n-octadecane with polyurea shells containing different soft segments for heat energy storage and thermal regulation. Sol. Energy Mater. Sol. Cells 2009, 93, 1366–1376. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, H.; Liu, S.; Zhang, X.; Li, W. Preparation and crystallization behavior of sensitive thermochromic microencapsulated phase change materials. Appl. Energy 2024, 362, 122993. [Google Scholar] [CrossRef]
- Gao, F.; Wang, X.; Wu, D. Design and fabrication of bifunctional microcapsules for solar thermal energy storage and solar photocatalysis by encapsulating paraffin phase change material into cuprous oxide. Sol. Energy Mater. Sol. Cells 2017, 168, 146–164. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Wu, D. Tailoring of bifunctional microencapsulated phase change materials with CdS/SiO2 double-layered shell for solar photocatalysis and solar thermal energy storage. Appl. Therm. Eng. 2018, 134, 603–614. [Google Scholar] [CrossRef]
- Huang, X.; Zhi, C.; Jiang, P.; Golberg, D.; Bando, Y.; Tanaka, T. Polyhedral Oligosilsesquioxane-Modified Boron Nitride Nanotube Based Epoxy Nanocomposites: An Ideal Dielectric Material with High Thermal Conductivity. Adv. Funct. Mater. 2013, 23, 1824–1831. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, H.; Wan, X.; Chen, D.; Chen, X.; Ye, X.; Ouyang, X.; Qin, S.; Wen, H.; Tang, J. Carbon nanotube-enhanced double-walled phase-change microcapsules for thermal energy storage. J. Mater. Chem. 2017, 5, 7482–7493. [Google Scholar] [CrossRef]
- Han, R.; Wang, X.; Zhu, G.; Han, N.; Xing, F. Investigation on viscoelastic properties of urea-formaldehyde microcapsules by using nanoindentation. Polym. Test. 2019, 80, 106146. [Google Scholar] [CrossRef]
- Ji, X.; Wang, S.; Yao, B.; Si, W.; Wang, C.; Wu, T.; Zhang, X. Preparation and properties of nano-SiO2 modified microcapsules for asphalt pavement. Mater. Des. 2023, 229, 111871. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, A.-S.; Jiang, Z.; He, F.; Li, Y.; Li, X.; Chen, Z.; Yang, W. Paraffin@silica@poly(dopamine)/Silver Phase Change Microcapsules with Efficient Photothermal Conversion Performance. Energy Fuels 2023, 37, 16951–16961. [Google Scholar] [CrossRef]
- Zhao, K.; Guo, Z.; Wang, J.; Xie, H. Enhancing solar photothermal conversion and energy storage with titanium carbide (Ti3C2) MXene nanosheets in phase-change microcapsules. J. Colloid Interface Sci. 2023, 650, 1591–1604. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, Q.; Wu, M.; Du, P.; Liu, C.; Rao, Z. Thermal properties optimization of lauric acid as phase change material with modified boron nitride nanosheets-sodium sulfate for thermal energy storage. J. Energy Storage 2023, 61, 106781. [Google Scholar] [CrossRef]
- Liao, H.; Guo, S.; Liu, Y.; Wang, Q. Form-stable phase change composites with high thermal conductivity and adjustable thermal management capacity. Sol. Energy Mater. Sol. Cells 2021, 221, 110881. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Liang, Z.; Li, F.; Yong, Y.; Li, Z. High thermal storage ability and photothermal conversion capacity phase change capsule with graphene oxide covalently grafted silica shell. Colloids Surf. A Physicochem. Eng. Asp. 2023, 657, 130594. [Google Scholar] [CrossRef]




| Samples | Melting | Crystallization | Ees | ||||
|---|---|---|---|---|---|---|---|
| Tms (°C) | Tm (°C) | ΔHm (J/g) | Tcs (°C) | Tc (°C) | ΔHc (J/g) | ||
| n-Octadecane | 27.1 | 31.49 | 270.8 | 27.02 | 24.37 | 269.0 | - |
| M-0 | 27.38 | 35.92 | 196.5 | 26.72 | 18.35 | 196.2 | 72.75 |
| M-1 | 28.03 | 33.78 | 187.6 | 26.58 | 21.67 | 187.2 | 69.43 |
| M-2 | 27.58 | 33.61 | 207.2 | 26.58 | 17.46 | 206.7 | 76.68 |
| M-3 | 27.51 | 33.34 | 222.0 | 26.73 | 17.47 | 221.3 | 82.12 |
| M-4 | 27.31 | 32.68 | 187.2 | 26.79 | 23.35 | 184.9 | 68.93 |
| M-5 | 27.99 | 31.25 | 162.4 | 26.67 | 24.97 | 159.2 | 59.58 |
| Samples | Thermal Conductivity (W/m·k) | Increase Percentage (%) |
|---|---|---|
| MPCMs | 0.256 | - |
| MPCMs-4 wt.% | 0.415 | 621% |
| MPCMs-6 wt.% | 0.521 | 1035% |
| MPCMs-8 wt.% | 0.666 | 1601% |
| MPCMs-10 wt.% | 1.214 | 3741% |
| MPCMs-12 wt.% | 1.416 | 4527% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, F.; Li, X.; Zhang, M.; Zhao, Y.; Li, Z.; Zhang, S.; Li, H. Preparation of Thermal Conductivity-Enhanced, Microencapsulated Phase Change Materials Using Cellulose-Assisted Graphene Dispersion for Thermal Regulation in Textiles. Polymers 2024, 16, 3291. https://doi.org/10.3390/polym16233291
Meng F, Li X, Zhang M, Zhao Y, Li Z, Zhang S, Li H. Preparation of Thermal Conductivity-Enhanced, Microencapsulated Phase Change Materials Using Cellulose-Assisted Graphene Dispersion for Thermal Regulation in Textiles. Polymers. 2024; 16(23):3291. https://doi.org/10.3390/polym16233291
Chicago/Turabian StyleMeng, Fanfan, Xiaopeng Li, Min Zhang, Yue Zhao, Zenghe Li, Shouxin Zhang, and Heguo Li. 2024. "Preparation of Thermal Conductivity-Enhanced, Microencapsulated Phase Change Materials Using Cellulose-Assisted Graphene Dispersion for Thermal Regulation in Textiles" Polymers 16, no. 23: 3291. https://doi.org/10.3390/polym16233291
APA StyleMeng, F., Li, X., Zhang, M., Zhao, Y., Li, Z., Zhang, S., & Li, H. (2024). Preparation of Thermal Conductivity-Enhanced, Microencapsulated Phase Change Materials Using Cellulose-Assisted Graphene Dispersion for Thermal Regulation in Textiles. Polymers, 16(23), 3291. https://doi.org/10.3390/polym16233291






