Scalable Engineering of 3D Printing Filaments Derived from Recycling of Plastic Drinking Water Bottle and Glass Waste
Abstract
1. Introduction
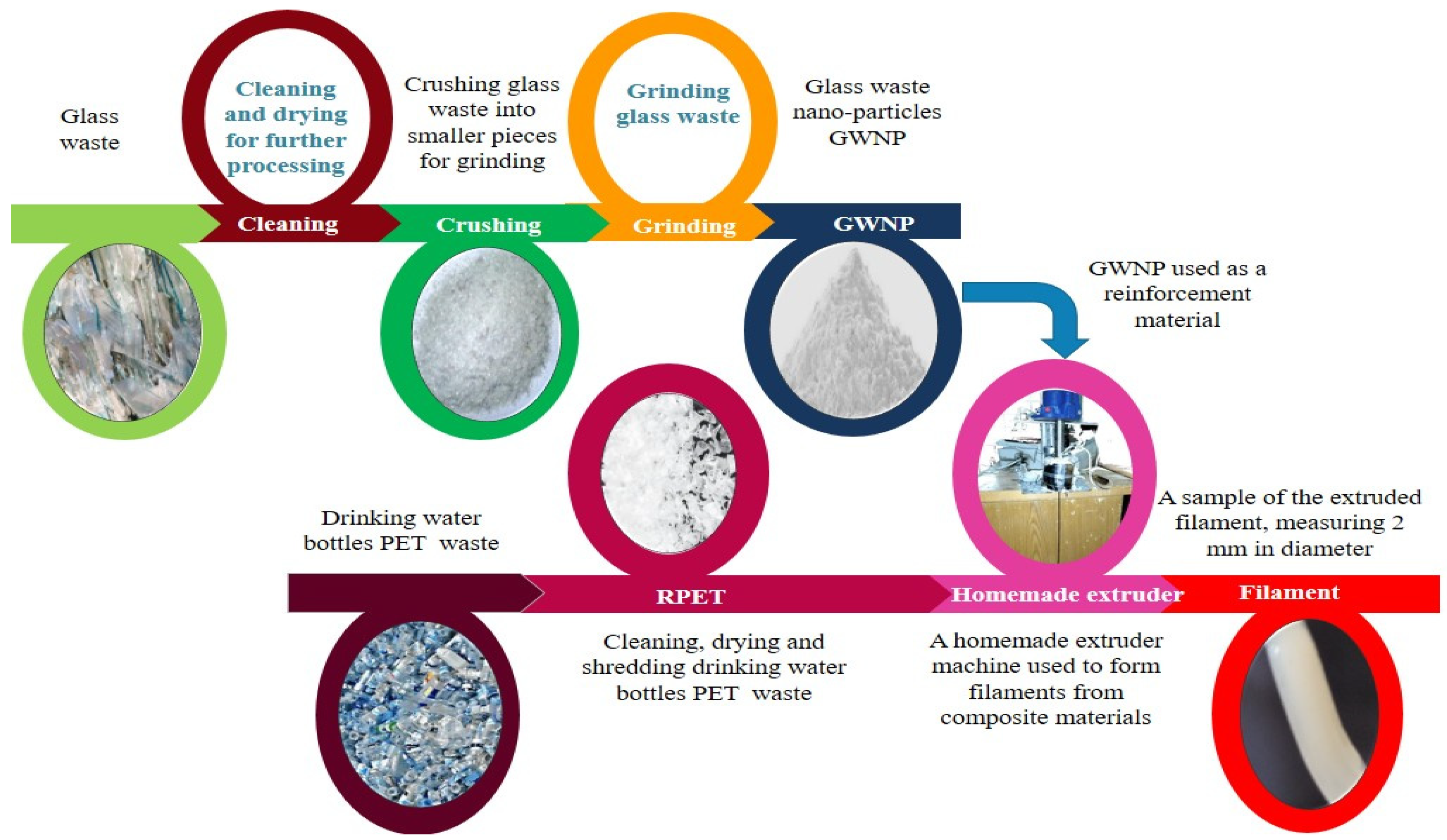
2. Materials and Methods
2.1. Fabrication of the Filament
2.2. Characterizations
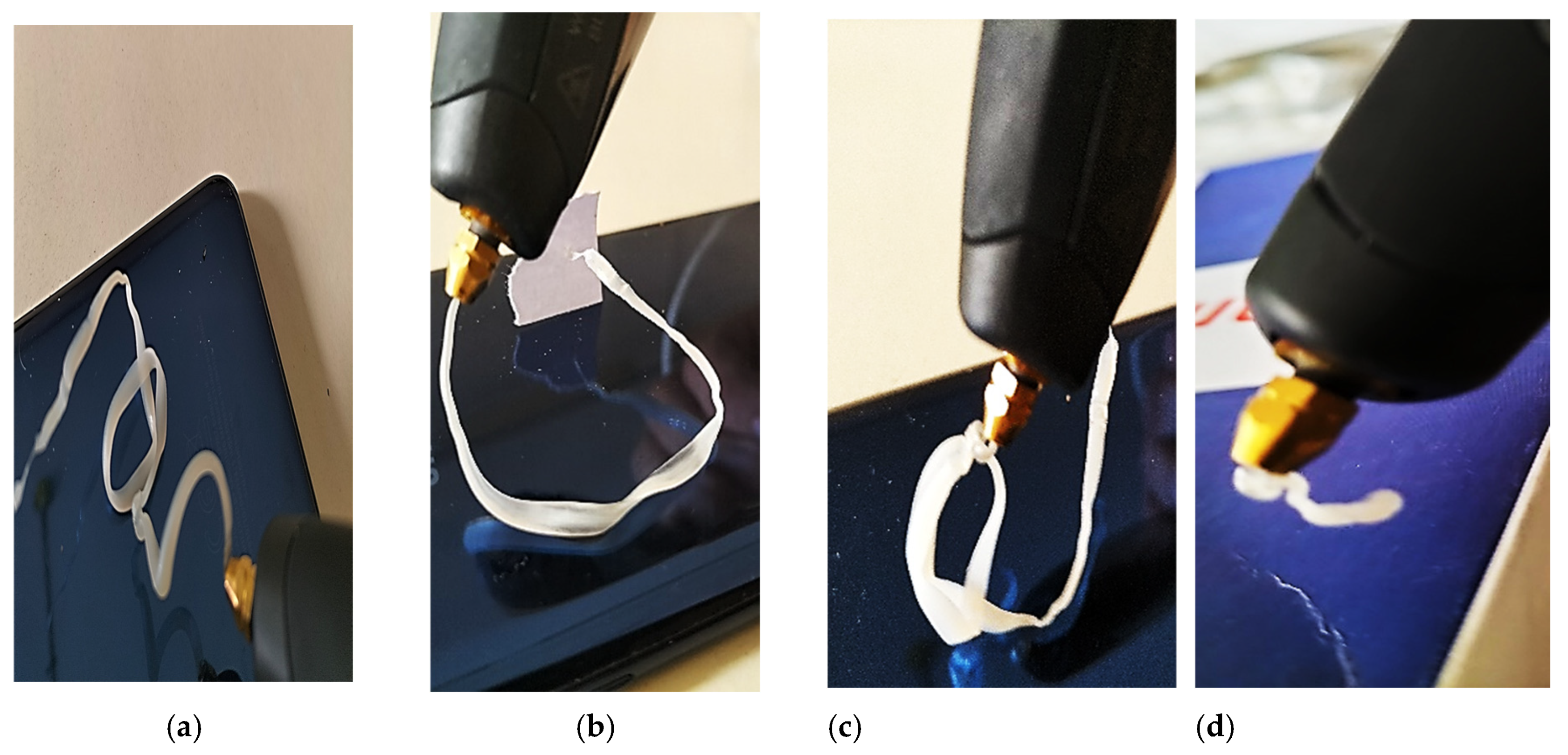
2.3. Printing 3D Pen
3. Results and Discussion
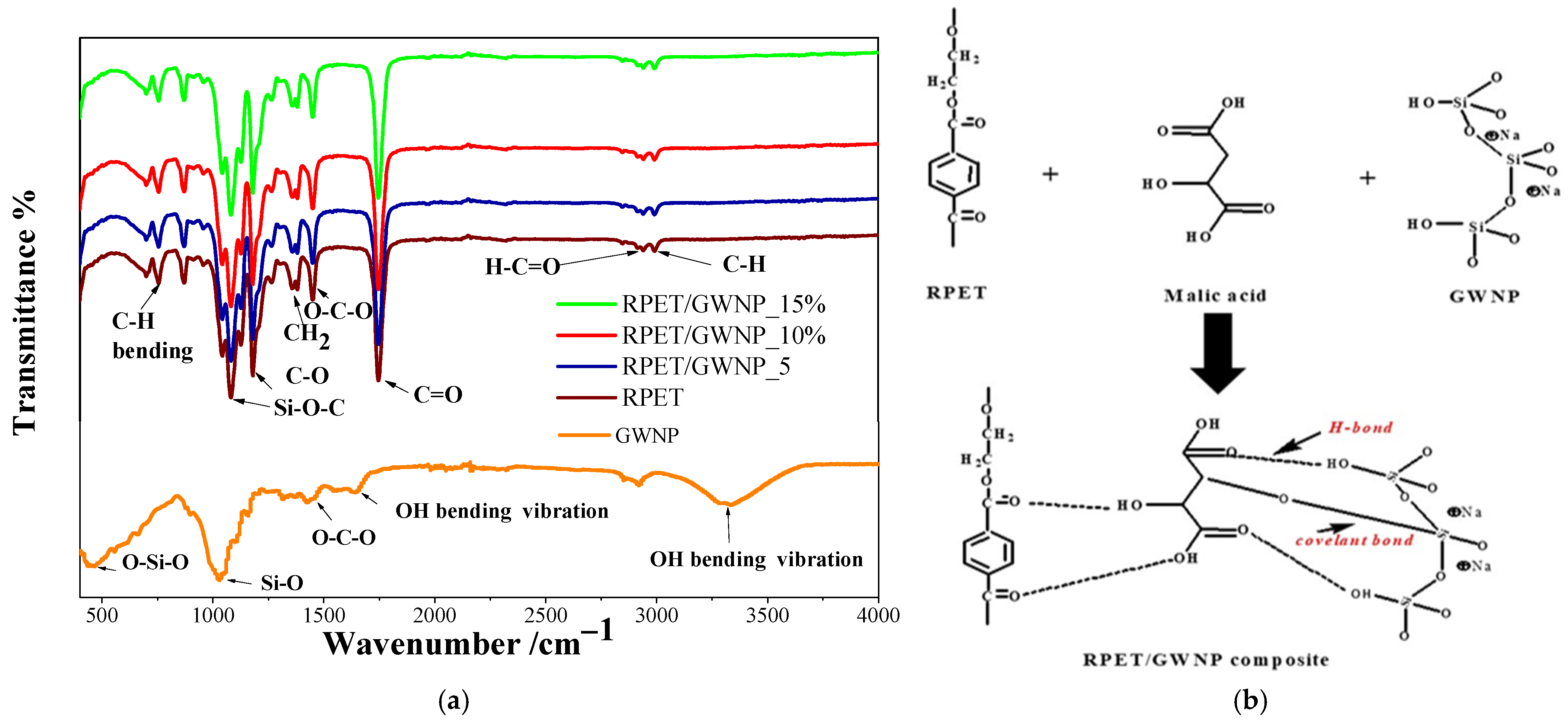
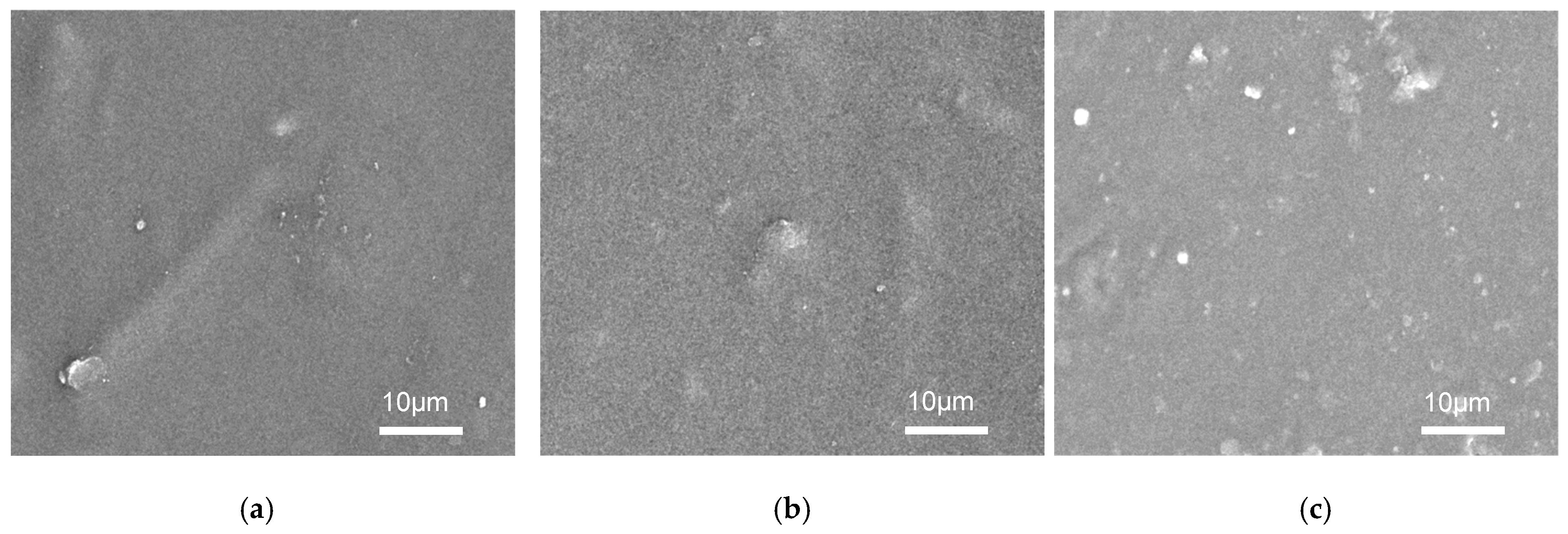
3.1. Characterization of GWNP and Filaments
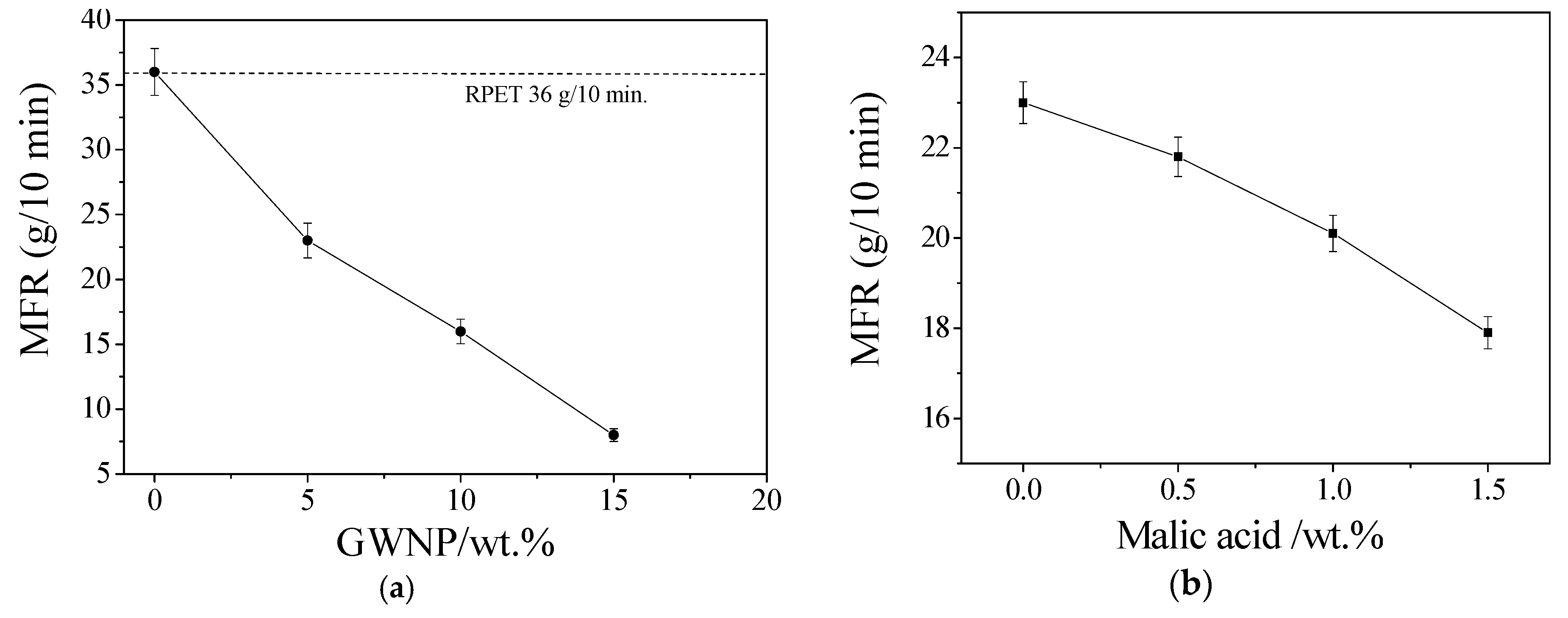
3.2. Optimizing the Filament Content to Enhance the Mechanical Properties
3.3. 3D Printing Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ngo, T.; Kashani, A.; Imbalzano, G.; Nguyen, K.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Jiang, Z.; Diggle, B.; Tan, M.; Viktorova, J.; Bennett, C.W.; Connal, L. Extrusion 3D printing of polymeric materials with advanced properties. Adv. Sci. 2020, 7, 2001379. [Google Scholar] [CrossRef] [PubMed]
- d’Ambrières, W. Plastics recycling worldwide: Current overview and desirable changes. Field Actions Science Reports. J. Field Actions 2019, 12–21. [Google Scholar]
- El Essawy, N.A.; Konsowa, A.H.; Elnouby, M.; Farag, H.A. A novel one-step synthesis for carbon-based nanomaterials from polyethylene terephthalate (PET) bottles waste. J. Air Waste Manag. Assoc. 2017, 67, 358–370. [Google Scholar] [CrossRef]
- Awaja, F.; Pavel, D. Recycling of PET. Eur. Polym. J. 2005, 41, 1453–1477. [Google Scholar] [CrossRef]
- Oussai, A.; Bártfai, Z.; Kátai, L. Development of 3D Printing Raw Materials from Plastic Waste. A Case Study on Recycled Polyethylene Terephthalate. Appl. Sci. 2021, 11, 7338. [Google Scholar] [CrossRef]
- Seibert, M.B.; Capote, G.A.; Gruber, M.; Volk, W.; Osswald, T.A. Manufacturing of a PET Filament from Recycled Material for Material Extrusion (MEX). Recycling 2022, 7, 69. [Google Scholar] [CrossRef]
- Zaidi, N.A.; Rosli, M.F.; Effendi, M.S.M.; Abdullah, M.H. Analysis of polyethylene terephthalate (PET) plastic bottle jointing system using finite element method (FEM). AIP Conf. Proc. 2017, 1885, 020005. [Google Scholar] [CrossRef]
- Djonga, N.D.; Tom, A.; Gomdje Valery, H.; Nkeng, G.E. Mechanical properties and chemical stability of bathroom wall composites manufactured from recycle polyethylene terephthalate (PET) mixed with cocoa hull powder. In Fiber-Reinforced Plastics; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- da Silva Cazella, P.H.; de Souza, M.V.; Rodrigues, F.R.; da Silva, S.A.M.; Bispo, R.A.; De Araujo, V.A.; Christoforo, A.L. Polyethylene terephthalate (PET) as a recycled raw material for particleboards produced from pinus wood and biopolymer resin. J. Clean. Prod. 2024, 447, 141460. [Google Scholar] [CrossRef]
- Exconde, M.K.J.E.; Co, J.A.A.; Manapat, J.Z.; Magdaluyo, E.R. Materials selection of 3D printing filament and utilization of recycled polyethylene terephthalate (PET) in a redesigned breadboard. Procedia CIRP 2019, 84, 28–32. [Google Scholar] [CrossRef]
- Daramola, O.O.; Oladele, I.O.; Adewuyi, B.O.; Sadiku, R.; Agwuncha, S.C. Thermal, structural and morphological properties of High Density Polyethylene matrix composites reinforced with submicron agro silica particles and Titania particles. J. Taibah Univ. Sci. 2017, 11, 645–653. [Google Scholar] [CrossRef]
- Taşdemir, M.; Ersoy, S. Mechanical, morphological and thermal properties of HDPE polymer composites filled with talc, calcium carbonate and glass spheres. Rom. J. Mater. 2015, 45, 147–154. [Google Scholar]
- Głogowska, K.; Sikora, J.W.; Blasé, J. The Use of Untreated Neuburg Siliceous Earth as Filler for High-Density Polyethylene. Tehnički Vjesnik 2018, 25, 1581–1586. [Google Scholar]
- Buitrago-Suescún, O.; Monroy, M.M. Maleated polyethylene as a compatibilizing agent in cannabis indica stem’s flour-reinforced composite materials. Iran. Polym. J. 2018, 27, 819–827. [Google Scholar] [CrossRef]
- Sadik, W.A.; El-Demerdash, A.M.; Abokhateeb, A.E.A.; Elessawy, N.A. Innovative high-density polyethylene/waste glass powder composite with remarkable mechanical, thermal and recyclable properties for technical applications. Heliyon 2021, 7, e06627. [Google Scholar] [CrossRef]
- Odaa, S.A.; Al-Hadithi, A.I.; Mansoor, Y.A. Preparation and Characterization of Nano-waste glass powder. Results Mater. 2023, 20, 100470. [Google Scholar] [CrossRef]
- Park, S.; Thanakkasaranee, S.; Shin, H.; Lee, Y.; Tak, G.; Seo, J. PET/bio-based terpolyester blends with high dimensional thermal stability. Polymers 2021, 13, 728. [Google Scholar] [CrossRef]
- El-Saftawy, A.A.; Elfalaky, A.; Ragheb, M.S.; Zakhary, S.G. Electron beam induced surface modifications of PET film. Radiat. Phys. Chem. 2014, 102, 96–102. [Google Scholar] [CrossRef]
- Chen, Z.; Hay, J.; Jenkins, M. The thermal analysis of poly (ethylene terephthalate) by FTIR spectroscopy. Thermochim. Acta 2014, 552, 123–130. [Google Scholar] [CrossRef]
- Shahrajabian, H.; Sadeghian, F. The investigation of alumina nanoparticles’ effects on the mechanical and thermal properties of HDPE/rPET/MAPE blends. Int. Nano Lett. 2019, 9, 213–219. [Google Scholar] [CrossRef]
- Hristov, V.; Vlachopoulos, J. Influence of Coupling Agents on Melt Flow Behavior of Natural Fiber Composites. Macromol. Mater. Eng. 2007, 292, 608–619. [Google Scholar] [CrossRef]
- Mishra, D.D. Thermal analysis of polyethylene terephthalate (PET)—Coke composites prepared by mechanical alloying technique. Preprints 2016. [Google Scholar] [CrossRef]
- Lendvai, L.; Singh, T.; Ronkay, F. Thermal, thermomechanical and structural properties of recycled polyethylene terephthalate (rPET)/waste marble dust composites. Heliyon 2024, 10, e25015. [Google Scholar] [CrossRef] [PubMed]
- Samsudin, D.; Ismail, H.; Othman, N.; Hamid, Z.A. Comparative study of glut palmitate salt and polyethylene grafted maleic anhydride compatibilizer on the properties of silica filled high-density polyethylene composites. Polym. Test. 2016, 52, 104–110. [Google Scholar] [CrossRef]
- Worku, B.G.; Wubieneh, T.A. Mechanical Properties of Composite Materials from Waste Poly(ethylene terephthalate) Reinforced with Glass Fibers and Waste Window Glass. Int. J. Polym. Sci. 2021, 3320226. [Google Scholar] [CrossRef]
- Koraqi, H.; Aydar, A.Y.; Khalid, W.; Ercisli, S.; Rustagi, S.; Ramniwas, S.; Pandiselvam, R. Ultrasound-assisted extraction with natural deep eutectic solvent for phenolic compounds recovery from Rosa damascene Mill: Experimental design optimization using central composite design. Microchem. J. 2024, 196, 109585. [Google Scholar] [CrossRef]
- Archello. The World’s First 3D Printed, Irrigated Green Wall. Available online: https://archello.com/it/news/the-worlds-first-3d-printed-irrigated-green-wall (accessed on 10 March 2024).
- Nicolau, A.; Pop, M.A.; Coșereanu, C. 3D Printing Application in Wood Furniture Components Assembling. Materials 2022, 15, 2907. [Google Scholar] [CrossRef]
- Idrees, M.; Jeelani, S.; Rangari, V. Three-Dimensional-Printed Sustainable Biochar-Recycled PET Composites. ACS Sustain. Chem. Eng. 2018, 6, 13940–13948. [Google Scholar] [CrossRef]
- Elessawy, N.; El Shakhs, A.; El-Saka, M.; Youssef, M.E.; Youssef, B.A.B.; Ali, M.A.M. Sustainable and eco-friendly 3D printing filament fabricated from different recycled solid wastes and evaluate its impact on interior and furniture design. Results Eng. 2024, 23, 102428. [Google Scholar] [CrossRef]


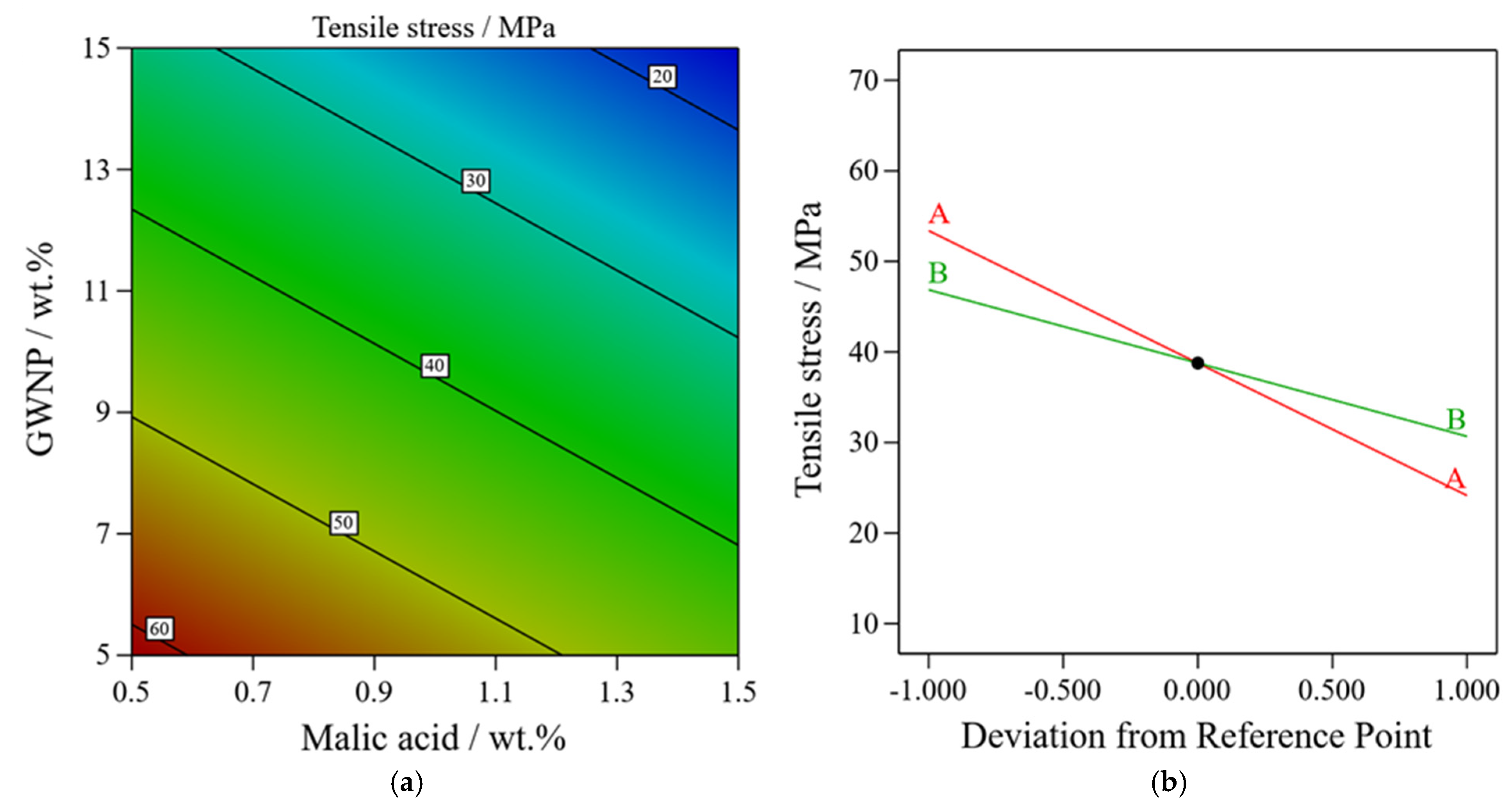
| Trial | GWNP (A; wt.%) | Malic Acid (B; wt.%) | Response Tensile Stress (MPa) |
|---|---|---|---|
| 1 | 15 | 1 | 20 |
| 2 | 10 | 1 | 42 |
| 3 | 5 | 1.5 | 48 |
| 4 | 15 | 0.5 | 24 |
| 5 | 5 | 1 | 61 |
| 6 | 10 | 1 | 42 |
| 7 | 10 | 1 | 42 |
| 8 | 10 | 1 | 42 |
| 9 | 10 | 1 | 42 |
| 10 | 10 | 0.5 | 56 |
| 11 | 5 | 0.5 | 51 |
| 12 | 10 | 1.5 | 18 |
| 13 | 15 | 1.5 | 16 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 2234.53 | 2 | 1117.27 | 30.71 | <0.0001 | significant |
| A-GWNP | 1710.62 | 1 | 1710.62 | 47.02 | <0.0001 | |
| B-MA | 523.91 | 1 | 523.91 | 14.40 | 0.0035 | |
| Residual | 363.78 | 10 | 36.38 | |||
| Lack of Fit | 363.78 | 6 | 60.63 | |||
| Pure Error | 0.0000 | 4 | 0.0000 | |||
| Cor Total | 2598.31 | 12 | ||||
| Std. Dev. | 6.03 | R2 | 0.860 | |||
| Mean | 38.77 | Adjusted R2 | 0.832 | |||
| C.V. % | 15.56 | Predicted R2 | 0.712 | |||
| Adeq Precision | 15.689 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toghan, A.; Alduaij, O.K.; Sanad, M.M.S.; Elessawy, N.A. Scalable Engineering of 3D Printing Filaments Derived from Recycling of Plastic Drinking Water Bottle and Glass Waste. Polymers 2024, 16, 3195. https://doi.org/10.3390/polym16223195
Toghan A, Alduaij OK, Sanad MMS, Elessawy NA. Scalable Engineering of 3D Printing Filaments Derived from Recycling of Plastic Drinking Water Bottle and Glass Waste. Polymers. 2024; 16(22):3195. https://doi.org/10.3390/polym16223195
Chicago/Turabian StyleToghan, Arafat, Omar K. Alduaij, Moustafa M. S. Sanad, and Noha A. Elessawy. 2024. "Scalable Engineering of 3D Printing Filaments Derived from Recycling of Plastic Drinking Water Bottle and Glass Waste" Polymers 16, no. 22: 3195. https://doi.org/10.3390/polym16223195
APA StyleToghan, A., Alduaij, O. K., Sanad, M. M. S., & Elessawy, N. A. (2024). Scalable Engineering of 3D Printing Filaments Derived from Recycling of Plastic Drinking Water Bottle and Glass Waste. Polymers, 16(22), 3195. https://doi.org/10.3390/polym16223195








