Viscoelastic Polyurethane Foam Biocomposites with Enhanced Flame Retardancy
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials for the Manufacture of Polyurethane Foams and Their Composites
2.2. Synthesis of the Polyurethane Foams and Their Composites
2.3. Methods
3. Results and Discussion
3.1. Analysis of the Foaming Process
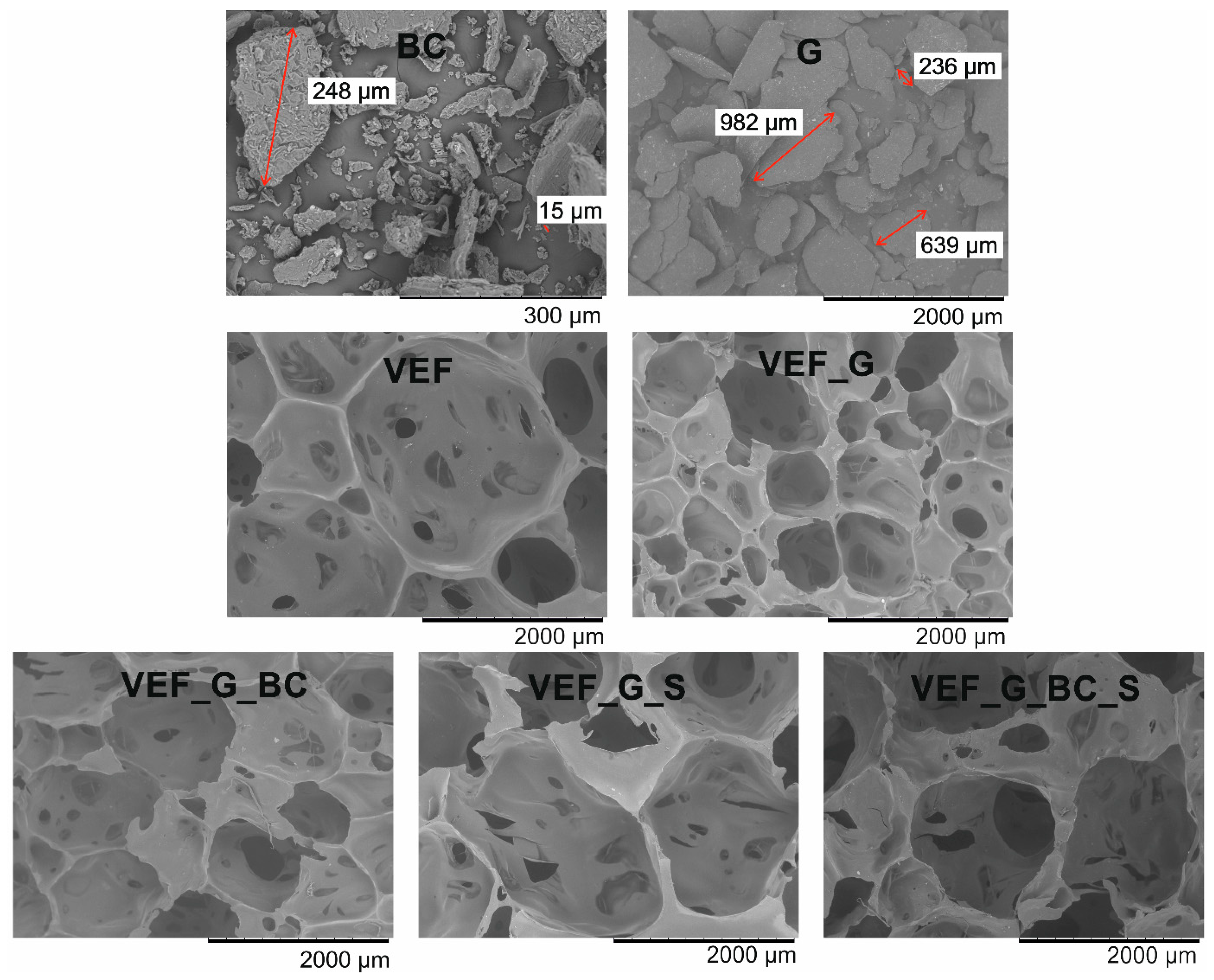
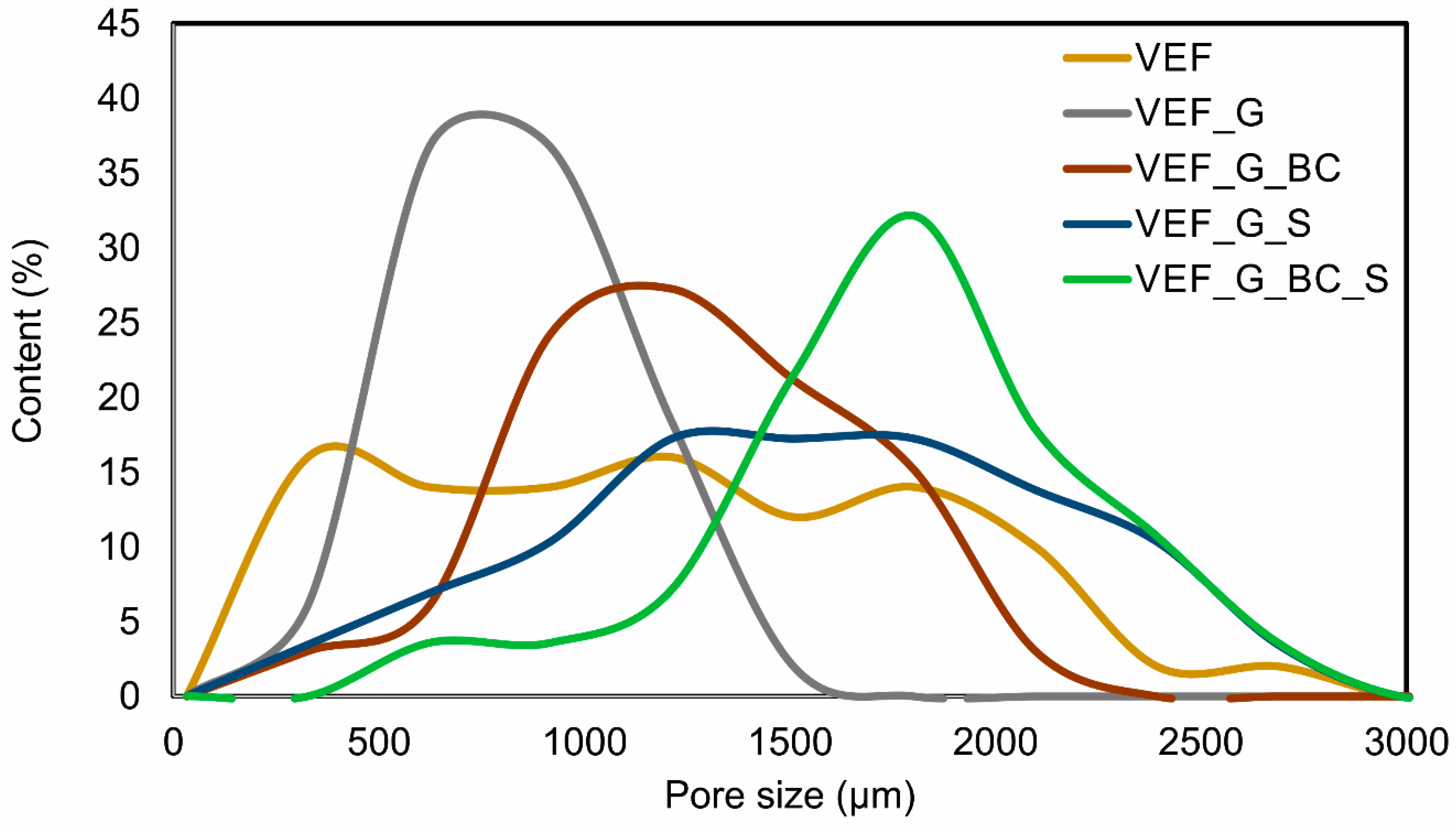
3.2. Microstructural Analysis
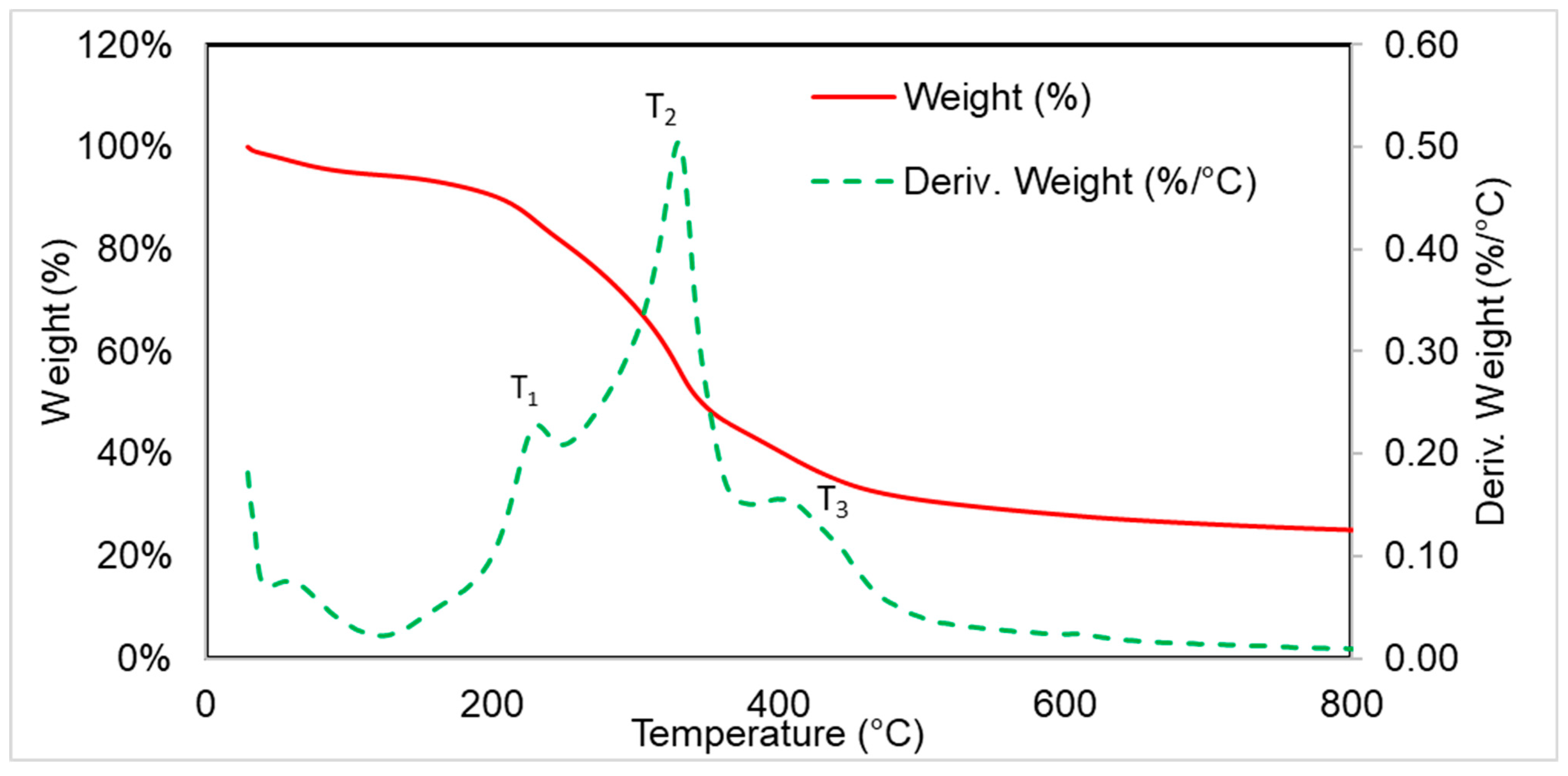
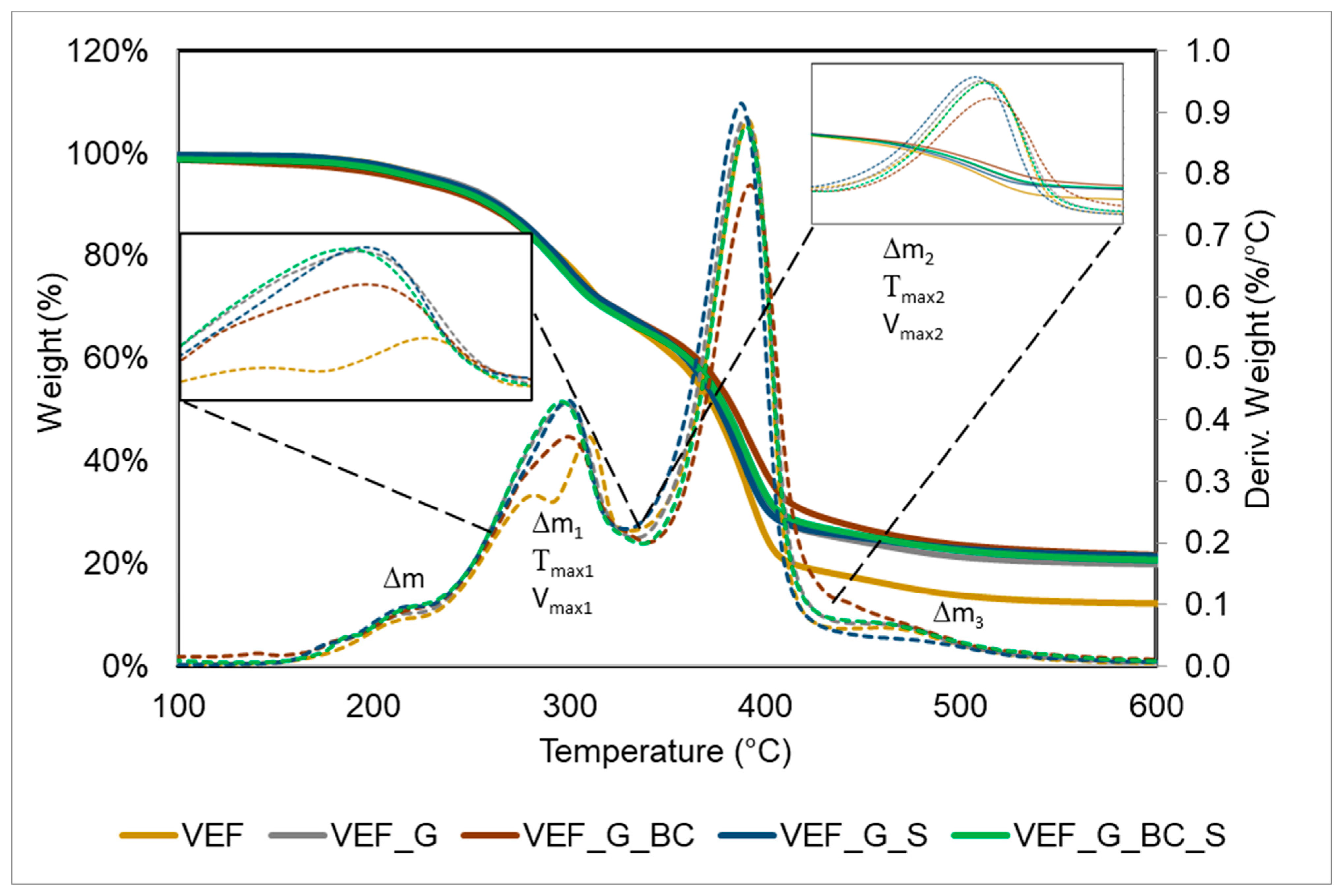
3.3. Thermogravimetric Analysis
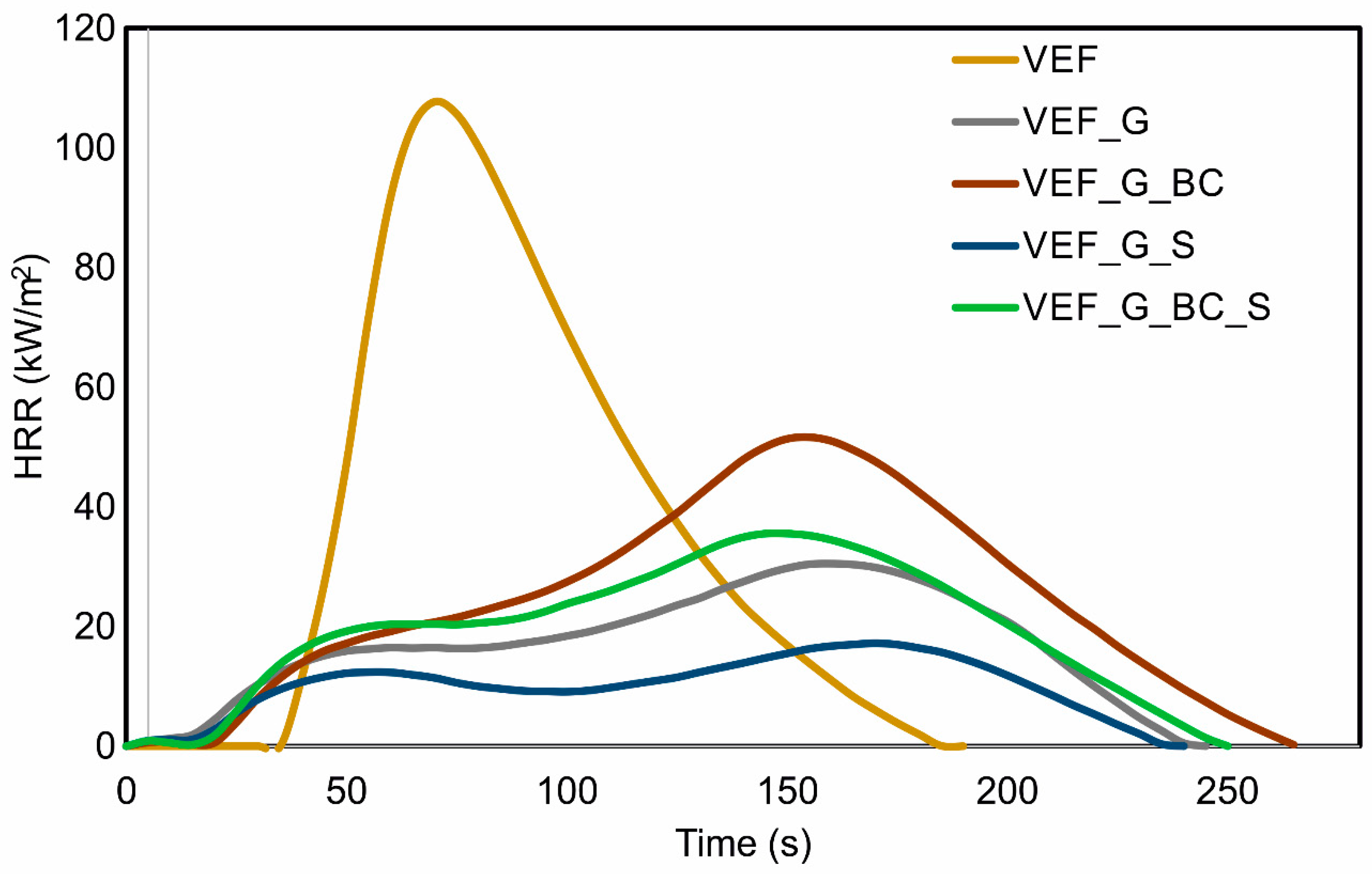
3.4. Flammability Analysis of Materials
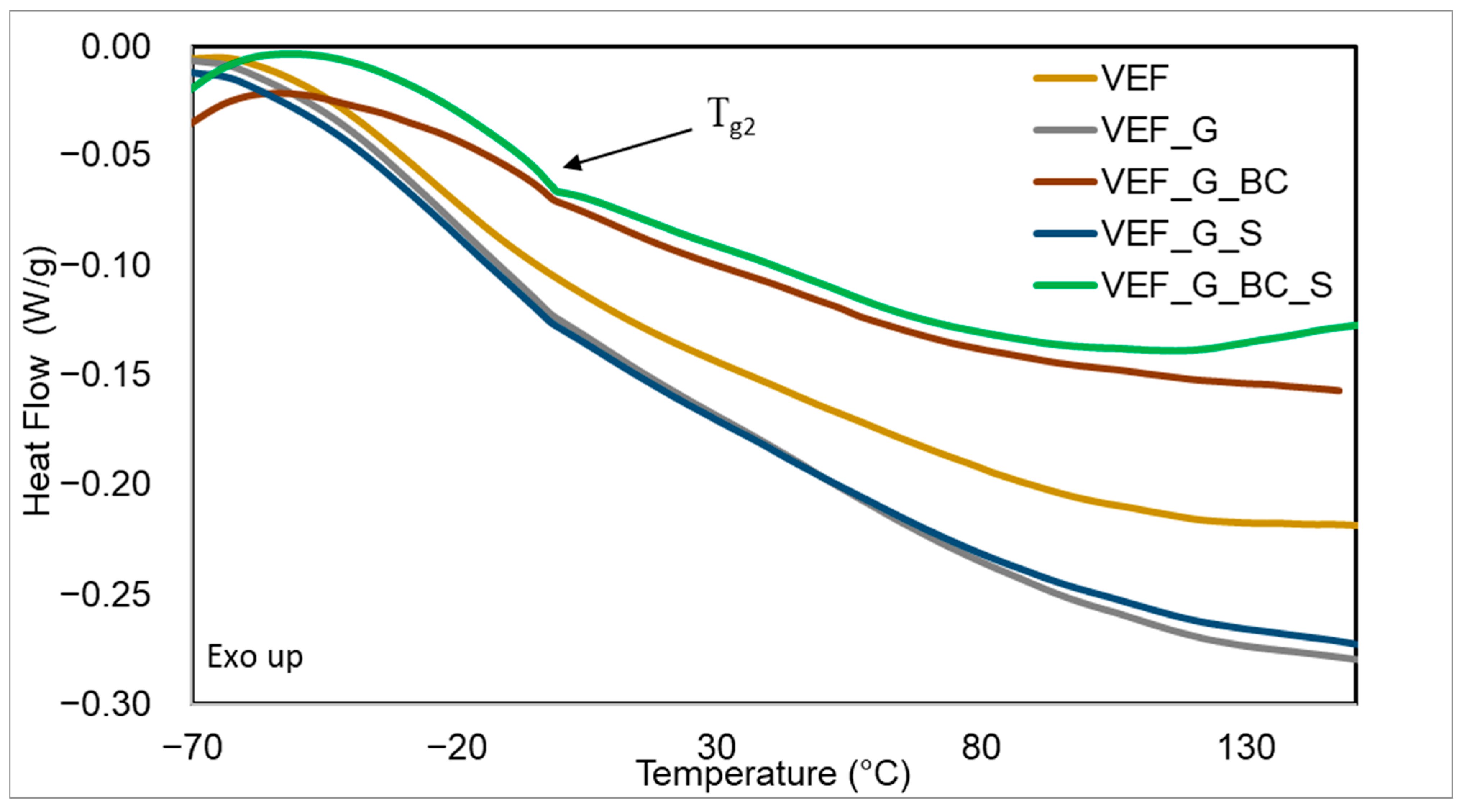
3.5. Differential Scanning Calorimetry Studies of Materials
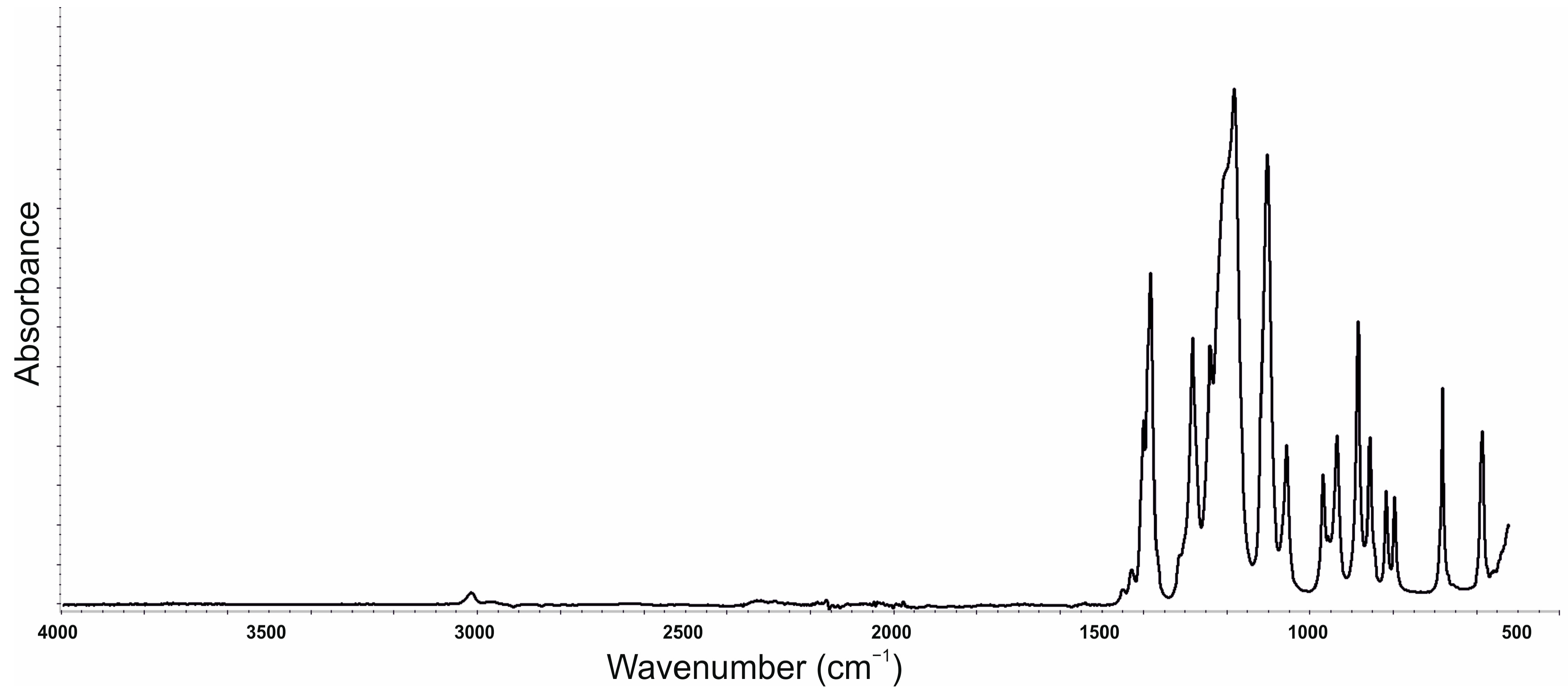
3.6. The Chemical Constitution Analysis of the Materials
3.7. Analysis of the Physico-Mechanical Properties of Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olszewski, A.; Kosmela, P.; Mielewczyk-Gryń, A.; Piszczyk, Ł. Bio-based polyurethane composites and hybrid composites containing a new type of bio-polyol and addition of natural and synthetic fibers. Materials 2020, 13, 2028. [Google Scholar] [CrossRef] [PubMed]
- Gondaliya, A.; Nejad, M. Lignin as a partial polyol replacement in polyurethane flexible foam. Molecules 2021, 26, 2302. [Google Scholar] [CrossRef]
- Park, S.J.; Cho, S.H.; Seo, H.W.; Nam, J.D.; Suhr, J. Natural cork agglomerate enabled mechanically robust rigid polyurethane foams with outstanding viscoelastic damping properties. Polymer 2021, 217, 123437. [Google Scholar] [CrossRef]
- Mo, Y.; Cheng, Z.; Xie, Y.; Chu, J.; Xie, S.; Meng, Y.; Wu, F.; Feng, D.; Mei, Y.; Xie, D. A Bio-Based Polyol with Synergetic Phosphorous and Nitrogenous Effect for Constructing Intrinsic Flame-Retardant Flexible Polyurethane Foam. J. Polym. Environ. 2024, 32, 3142–3158. [Google Scholar] [CrossRef]
- Grand View Research. Polyurethane Market Size, Share & Growth Report, 2030. 2024. Available online: https://www.grandviewresearch.com (accessed on 10 August 2024).
- Zhang, J.; Wu, S.; Zhang, K.; Li, C.; Ling, G.; Ji, C.; Liu, D.; Chen, M.; Zha, Z.; Guo, Y.; et al. Novel polyurethane viscoelastic foam modified with discarded luffa seed oil in accordance with cleaner production. J. Clean. Prod. 2022, 341, 130795. [Google Scholar] [CrossRef]
- Grzęda, D.; Węgrzyk, G.; Leszczyńska, M.; Szczepkowski, L.; Gloc, M.; Ryszkowska, J. Viscoelastic Polyurethane Foams for Use as Auxiliary Materials in Orthopedics. Materials 2021, 15, 133. [Google Scholar] [CrossRef]
- Flôres, C.C.; Rufino, T.D.C.; Oliveira, M.P. Effect of Kraft lignin and palm kernel oil as substitutes of petroleum-based polyols on the properties of viscoelastic polyurethane foams. J. Polym. Res. 2021, 28, 481. [Google Scholar] [CrossRef]
- Lu, W.; Jin, Z. Grafting modification bamboo kraft lignin and its performance in rigid polyurethane foams. J. Appl. Polym. Sci. 2024, 141, e55167. [Google Scholar] [CrossRef]
- Aydoğan, B.; Usta, N. Effects of dolomite and intumescent flame retardant additions on thermal and combustion behaviors of rigid polyurethane foams. J. Appl. Polym. Sci. 2023, 140, e53739. [Google Scholar] [CrossRef]
- Kirpluks, M.; Cabulis, U.; Avots, A. Flammability of Bio-Based Rigid Polyurethane Foam as Sustainable Thermal Insulation Material. In Insulation Materials in Context of Sustainability; Almusaed, A., Almssad, A., Eds.; InTech: Vienna, Austria, 2016. [Google Scholar] [CrossRef]
- Yadav, A.; De Souza, F.M.; Dawsey, T.; Gupta, R.K. Recent Advancements in Flame-Retardant Polyurethane Foams: A Review. Ind. Eng. Chem. Res. 2022, 61, 15046–15065. [Google Scholar] [CrossRef]
- Lin, B.; Yuen, A.C.Y.; Li, A.; Zhang, Y.; Chen, T.B.Y.; Yu, B.; Lee, E.W.M.; Peng, S.; Yang, W.; Lu, H.-D.; et al. MXene/chitosan nanocoating for flexible polyurethane foam towards remarkable fire hazards reductions. J. Hazard. Mater. 2020, 381, 120952. [Google Scholar] [CrossRef] [PubMed]
- Hejna, A.; Haponiuk, J.; Piszczyk, Ł.; Klein, M.; Formela, K. Performance properties of rigid polyurethane-polyisocyanurate/brewers’ spent grain foamed composites as function of isocyanate index. e-Polymers 2017, 17, 427–437. [Google Scholar] [CrossRef]
- Qiu, Y.; Xi, B.; Qian, L.; Liu, A.; Gao, L. Carbonisation-dominated synergistic behaviors of ammonium hypophosphite/ EG composite in improving flame retardancy of flexible polyurethane foam. Polym. Adv. Techs 2022, 33, 3238–3248. [Google Scholar] [CrossRef]
- Akdogan, E.; Erdem, M.; Ureyen, M.E.; Kaya, M. Rigid polyurethane foams with halogen-free flame retardants: Thermal insulation, mechanical, and flame retardant properties. J. Appl. Polym. Sci. 2020, 137, 47611. [Google Scholar] [CrossRef]
- Parcheta-Szwindowska, P.; Habaj, J.; Krzemińska, I.; Datta, J. A Comprehensive Review of Reactive Flame Retardants for Polyurethane Materials: Current Development and Future Opportunities in an Environmentally Friendly Direction. Int. J. Mol. Sci. 2024, 25, 5512. [Google Scholar] [CrossRef]
- Hanzan, N. Effect of methylene diphenyl diisocyanate index on physicomechanical and morphological properties of palm olein-based viscoelastic polyurethane foams. J. Oil Palm Res. 2022, 35, 236–246. [Google Scholar] [CrossRef]
- Jariwala, S.; Desai, Y.N.; Sahu, P.; Gupta, R.K. Hemp Seed Oil Derived Rigid Polyurethane Foams and Their Underlying Flame Retardancy Properties. J. Polym. Environ. 2024, 32, 3822–3834. [Google Scholar] [CrossRef]
- Zulkifli, A.; Amran, U.A.; Armir, N.A.Z.; Zakaria, S. Characterisation of Rigid Polyurethane Foams Produced from Liquefied Kenaf with Different Isocyanate Indexes. J. Polym. Environ. 2024, 32, 1532–1544. [Google Scholar] [CrossRef]
- Członka, S.; Strąkowska, A.; Kairytė, A. Effect of walnut shells and silanised walnut shells on the mechanical and thermal properties of rigid polyurethane foams. Polym. Test. 2020, 87, 106534. [Google Scholar] [CrossRef]
- Auguścik-Królikowska, M.; Ryszkowska, J.; Kurańska, M.; Wantulok, M.; Gloc, M.; Szczepkowski, L.; Dąbkowska-Susfał, K.; Prociak, A. Composites of open-cell viscoelastic foams with blackcurrant pomace. Materials 2021, 14, 934. [Google Scholar] [CrossRef]
- Kairytė, A.; Kizinievič, O.; Kizinievič, V.; Kremensas, A. Synthesis of biomass-derived bottom waste ash based rigid biopolyurethane composite foams: Rheological behaviour, structure and performance characteristics. Compos. Part A Appl. Sci. Manuf. 2019, 117, 193–201. [Google Scholar] [CrossRef]
- Kopik, M. Powstawanie i Zagospodarowanie Odpadów Generowanych w Rolnictwie i Przemyśle Rolno-Spożywczym—Stan Obecny i Perspektywy; Europejska Agencja Rozwoju Sp. J. Kopik i wspólnicy: Kielce, Poland, 2019. (In Polish) [Google Scholar]
- Ryszkowska, J. Materiały Poliuretanowe Wytwarzane z Zastosowaniem Surowców Odnawialnych; Oficyna Wydawnicza Politechniki Warszawskiej: Warszawa, Poland, 2019. (In Polish) [Google Scholar]
- Da Silva, V.R.; Mosiewicki, M.A.; Yoshida, M.I.; da Silva, M.C.; Stefani, P.M.; Marcovich, N.E. Polyurethane foams based on modified tung oil and reinforced with rice husk ash I: Synthesis and physical chemical characterisation. Polym. Test. 2013, 32, 438–445. [Google Scholar] [CrossRef]
- Członka, S.; Bertino, M.F.; Strzelec, K. Rigid polyurethane foams reinforced with industrial potato protein. Polym. Test. 2018, 68, 135–145. [Google Scholar] [CrossRef]
- Oliwa, R.; Ryszkowska, J.; Oleksy, M.; Auguścik-Królikowska, M.; Gzik, M.; Bartoń, J.; Budzik, G. Effects of Various Types of Expandable Graphite and Blackcurrant Pomace on the Properties of Viscoelastic Polyurethane Foams. Materials 2021, 14, 1801. [Google Scholar] [CrossRef] [PubMed]
- Kuranchie, C.; Yaya, A.; Bensah, Y.D. The effect of natural fibre reinforcement on polyurethane composite foams—A review. Sci. Afr. 2021, 11, e00722. [Google Scholar] [CrossRef]
- Wrześniewska-Tosik, K.; Ryszkowska, J.; Mik, T.; Wesołowska, E.; Kowalewski, T.; Pałczyńska, M.; Walisiak, D.; Królikowska, M.A.; Leszczyńska, M.; Niezgoda, K.; et al. Viscoelastic Polyurethane Foam with Keratin and Flame-Retardant Additives. Polymers 2021, 13, 1380. [Google Scholar] [CrossRef]
- Leszczyńska, M.; Ryszkowska, J.; Prządka, M.; Czajka, A.; Szczepkowski, L. Kompozyty Poliuretanowychpianek Sztywnych i Napełniaczy Roślinnych; Tworzywa Sztuczne w Przemyśle: Racibórz, Poland, 2020. [Google Scholar]
- Ahir, M.; Bodhak, C.; Gupta, R.K. Harnessing Enhanced Flame Retardancy in Rigid Polyurethane Composite Foams through Hemp Seed Oil-Derived Natural Fillers. Polymers 2024, 16, 1584. [Google Scholar] [CrossRef]
- Członka, S.; Strąkowska, A.; Kairytė, A. The Impact of Hemp Shives Impregnated with Selected Plant Oils on Mechanical, Thermal, and Insulating Properties of Polyurethane Composite Foams. Materials 2020, 13, 4709. [Google Scholar] [CrossRef]
- Członka, S.; Strąkowska, A.; Kairytė, A.; Kremensas, A. Nutmeg filler as a natural compound for the production of polyurethane composite foams with antibacterial and anti-aging properties. Polym. Test. 2020, 86, 106479. [Google Scholar] [CrossRef]
- Paciorek-Sadowska, J.; Borowicz, M.; Czuprynski, B.; Liszkowska, J. Composites of rigid polyurethane-polyisocyanurate foams with oak bark. Polimery 2017, 62, 666–672. [Google Scholar] [CrossRef]
- Gu, R.; Sain, M.M. Effects of Wood Fiber and Microclay on the Performance of Soy Based Polyurethane Foams. J. Polym. Environ. 2013, 21, 30–38. [Google Scholar] [CrossRef]
- Okrasa, M.; Leszczyńska, M.; Sałasińska, K.; Szczepkowski, L.; Kozikowski, P.; Majchrzycka, K.; Ryszkowska, J. Viscoelastic Polyurethane Foams for Use in Seals of Respiratory Protective Devices. Materials 2021, 14, 1600. [Google Scholar] [CrossRef] [PubMed]
- Leszczyńska, M.; Malewska, E.; Ryszkowska, J.; Kurańska, M.; Gloc, M.; Leszczyński, M.K.; Prociak, A. Vegetable Fillers and Rapeseed Oil-Based Polyol as Natural Raw Materials for the Production of Rigid Polyurethane Foams. Materials 2021, 14, 1772. [Google Scholar] [CrossRef] [PubMed]
- Członka, S.; Strąkowska, A.; Pospiech, P.; Strzelec, K. Effects of Chemically Treated Eucalyptus Fibers on Mechanical, Thermal and Insulating Properties of Polyurethane Composite Foams. Materials 2020, 13, 1781. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability and flame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Bryśkiewicz, A.; Zieleniewska, M.; Przyjemska, K.; Chojnacki, P.; Ryszkowska, J. Modification of flexible polyurethane foams by the addition of natural origin fillers. Polym. Degrad. Stab. 2016, 132, 32–40. [Google Scholar] [CrossRef]
- Mimura, H.; Fujita, H.; Eguchi, H. Fluorine-Containing Phosphate Ester-Amide, and Flame Retardant Resin, Flame Retardant Liquid and Flame Retardant Solvent for Organic Synthesis Containing Same. EP2832737A1, 1 August 2018. [Google Scholar]
- Zhou, J.; Wei, H. Solar Cell Backboard with Fluorine-Containing Flame-Retardant Coating. CN218677169U, 2 December 2015. [Google Scholar]
- Zhou, L.; Chen, T.; Zhou, T.; Zheng, D. Fluorine-Containing Silicone Oil and Fluorine-Containing Flame Retardant Prepared from Fluorine-Containing Silicone Oil. CN115850707A, 12 January 2021. [Google Scholar]
- Prociak, A.; Rokicki, G.; Ryszkowska, J. Materiały Poliuretanowe; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2014. (In Polish) [Google Scholar]
- Günther, M.; Levchik, S.V.; Schartel, B. Bubbles and collapses: Fire phenomena of flame-retarded flexible polyurethane foams. Polym. Adv. Techs 2020, 31, 2185–2198. [Google Scholar] [CrossRef]
- Rao, W.-H.; Hu, Z.-Y.; Xu, H.-X.; Xu, Y.-J.; Qi, M.; Liao, W.; Xu, S.; Wang, Y.-Z. Flame-Retardant Flexible Polyurethane Foams with Highly Efficient Melamine Salt. Ind. Eng. Chem. Res. 2017, 56, 7112–7119. [Google Scholar] [CrossRef]
- Lu, S.; Feng, Y.; Zhang, P.; Hong, W.; Chen, Y.; Fan, H.; Yu, D.; Chen, X. Preparation of Flame-Retardant Polyurethane and Its Applications in the Leather Industry. Polymers 2021, 13, 1730. [Google Scholar] [CrossRef]
- Duquesne, S.; Bras, M.L.; Bourbigot, S.; Delobel, R.; Vezin, H.; Camino, G.; Eling, B.; Lindsay, C.; Roels, T. Expandable graphite: A fire retardant additive for polyurethane coatings. Fire Mater. 2003, 27, 103–117. [Google Scholar] [CrossRef]
- Imiolek, P.; Kasprowicz, K.; Laska, J. Antistatic polyethylene free-standing films modified with expanded graphite—Technological aspects. Polimery 2020, 65, 275–279. [Google Scholar] [CrossRef]
- Barton-Pudlik, J.; Czaja, K.; Grzymek, M.; Lipok, J. Evaluation of wood-polyethylene composites biodegradability caused by filamentous fungi. Int. Biodeterior. Biodegrad. 2017, 118, 10–18. [Google Scholar] [CrossRef]
- Barry, J.; Locke, G.; Scollard, D.; Sidebottom, H.; Treacy, J.; Clerbaux, C.; Colin, R.; Franklin, J. 1,1,1,3,3,-Pentafluorobutane (HFC-365mfc): Atmospheric Degradation and Contribution to Radiative Forcing. In Atmospheric Degradation; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1997; pp. 607–618. [Google Scholar]
- Carriço, C.S.; Fraga, T.; Pasa, V.M.D. Production and characterisation of polyurethane foams from a simple mixture of castor oil, crude glycerol and untreated lignin as bio-based polyols. Eur. Polym. J. 2016, 85, 53–61. [Google Scholar] [CrossRef]
- Gaidukova, G.; Ivdre, A.; Fridrihsone, A.; Verovkins, A.; Cabulis, U.; Gaidukovs, S. Polyurethane rigid foams obtained from polyols containing bio-based and recycled components and functional additives. Ind. Crops Prod. 2017, 102, 133–143. [Google Scholar] [CrossRef]
- El-Kabbany, F.; Taha, S.; Hafez, M. IR spectroscopic analysis of polymorphism in C13H 14N4O. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2011, 78, 981–988. [Google Scholar] [CrossRef]
- Zieleniewska, M.; Leszczyński, M.K.; Szczepkowski, L.; Bryśkiewicz, A.; Krzyżowska, M.; Bień, K.; Ryszkowska, J. Development and applicational evaluation of the rigid polyurethane foam composites with egg shell waste. Polym. Degrad. Stab. 2016, 132, 78–86. [Google Scholar] [CrossRef]
- Maia, L.S.; Zanini, N.C.; Camani, P.H.; Medeiros, S.F.; Rosa, D.S.; Mulinari, D.R. PU Foams Resistance Against Natural Weathering Aging: The Effect of Coffee Husk Residues in Different Contents. J. Polym. Environ. 2023, 31, 2073–2092. [Google Scholar] [CrossRef]






| Sample | Additives in Polyols, php | Additives in Foam Substrates, wt. % | |||||
|---|---|---|---|---|---|---|---|
| BC | G | S | BC | G | S | Polyurethane Substrates | |
| VEF | - | - | - | - | - | - | 100 |
| VEF_G | - | 15 | - | - | 8.7 | - | 91.3 |
| VEF_G_BC | 30 | 15 | - | 14.8 | 7.3 | - | 77.9 |
| VEF_G_S | - | 15 | 10 | - | 8.2 | 5.5 | 86.3 |
| VEF_G_BC_S | 30 | 15 | 10 | 14.1 | 7.1 | 4.7 | 74.1 |
| Sample | Start Time, s | Growth Time, s |
|---|---|---|
| VEF | 25 | 240 |
| VEF_G | 35 | 200 |
| VEF_G_BC | 45 | 220 |
| VEF_G_S | 30 | 190 |
| VEF_G_BC_S | 35 | 240 |
| Size Intervals (µm) | Average Pore Sizes (µm) | |
|---|---|---|
| VEF | 327–2909 | 1354 |
| VEF_G | 411–1562 | 994 |
| VEF_G_BC | 352–2119 | 1407 |
| VEF_G_S | 515–2789 | 1724 |
| VEF_G_BC_S | 753–2980 | 1939 |
| T5% (°C) | Δm (%), 50–230 °C | Tmax1 (°C), (Vmax1, %/°C) | Δm1 (%), 230–335 °C | Tmax2 (°C), Vmax2, %/°C | Δm2 (%), 335–430 °C | Δm3 (%) (430–600 °C) | P600 (%) | |
|---|---|---|---|---|---|---|---|---|
| VEF | 235 | 4.3 | 281 (0.33) 309 (0.46) | 29.7 | 390 (1.06) | 47.7 | 6.3 | 11.9 |
| VEF_G | 234 | 4.3 | 299 (0.43) | 28.4 | 390 (0.89) | 41.5 | 5.8 | 19.8 |
| VEF_G_BC | 216 | 6.0 | 299 (0.37) | 26.4 | 392 (0.78) | 38.4 | 7.3 | 21.6 |
| VEF_G_S | 231 | 4.6 | 299 (0.43) | 27.9 | 387 (0.91) | 41.2 | 4. 6 | 21.5 |
| VEF_G_BC_S | 219 | 5.4 | 300 (0.42) | 26.6 | 391 (0.88) | 39.0 | 6.0 | 21.4 |
| Sample | Ti (s) | pHRR (kW/m2) | Time to pHRR (s) | pHRRmax (kW/m2) | Time to pHRRmax (s) | THR (MJ/m2) | Rc (%) | FIGRA (kW/m2·s) |
|---|---|---|---|---|---|---|---|---|
| VEF | 24 | - | - | 107.7 | 70 | 8.5 | 9.7 | 1.54 |
| VEF_G | 7 | 15.9 | 50 | 30.5 | 160 | 4.1 | 37.8 | 0.19 |
| VEF_G_BC | 13 | 17.2 | 50 | 51.6 | 155 | 8.2 | 29.0 | 0.33 |
| VEF_G_S | 8 | 12.2 | 50 | 17.2 | 170 | 3.2 | 38.6 | 0.10 |
| VEF_G_BC_S | 10 | 19.2 | 50 | 35.6 | 150 | 6.2 | 32.7 | 0.24 |
| Sample | UL-94 Classification | LOI, % |
|---|---|---|
| VEF | - 1 | 20.6 |
| VEF_G | - 2, HB40 | 27.5 |
| VEF_G_BC | V0, HB40 | 27.9 |
| VEF_G_S | V0, HB40 | 27.8 |
| VEF_G_BC_S | - 2, HB40 | 26.8 |
| Tg1 (°C) | ∆HR (J/g) | TR (°C) | Tg2 (°C) | |
|---|---|---|---|---|
| VEF | −7.0 | 18.3 | 110.2 | −8.2 |
| VEF_G | −3.2 | 30.9 | 97.4 | −4.5 |
| VEF_G_BC | −3.3 | 33.1 | 93.9 | −3.4 |
| VEF_G_S | −3.5 | 31.0 | 82.1 | −4.1 |
| VEF_G_BC_S | −3.5 | 36.2 | 85.2 | −3.6 |
| VEF | VEF_G | VEF_G_BC | VEF_G_S | VEF_G_BC_S | Bond (Vibration) |
|---|---|---|---|---|---|
| Wavenumbers [cm−1] | |||||
| 3600–3400 | O-H (stretching) | ||||
| 3400–3200 | N-H (stretching) | ||||
| 2969 | 2968 | 2968 | 2969 | 2968 | C-H (asymmetric stretching) |
| 2868 | 2868 | 2868 | 2868 | 2868 | C-H (symmetric stretching) |
| 1722 | 1722 | 1722 | 1722 | 1722 | C=O (stretching/unbounded) |
| 1708 | 1708 | 1708 | 1708 | 1708 | C=O (stretching/bounded) |
| 1597 | 1597 | 1597 | 1597 | 1597 | C=C (stretching) |
| 1536 | 1536 | 1536 | 1536 | 1536 | N-H (bending) |
| 1509 | 1509 | 1509 | 1509 | 1509 | N-H (bending) |
| 1452 | 1452 | 1452 | 1452 | 1452 | C-H (deformation, asymmetric) |
| 1373 | 1373 | 1373 | 1373 | 1373 | C-H (deformation, symmetric) |
| 1306 | 1306 | 1306 | 1306 | 1306 | C-H (stretching) |
| 1226 | 1226 | 1227 | 1226 | 1227 | C-N (stretching) |
| 1085 | 1084 | 1085 | 1085 | 1083 | C-O (stretching) |
| Sample | Apparent Density (kg/m3) | Compression Set (%), 50%, 22 h, 70 °C 1 | Resilience (%) | Hardness CV40, [kPa] | Comfort Factor—SAG, Units |
|---|---|---|---|---|---|
| VEF | 48 ± 1 | 4.0 ± 0.0 | 7.2 ± 0.8 | 0.94 ± 0.01 | 2.44 ± 0.07 |
| VEF_G | 52 ± 1 | 2.7 ± 1.9 | 7.0 ± 0.9 | 0.90 ± 0.01 | 2.95 ± 0.09 |
| VEF_G_BC | 60 ± 2 | 2.7 ± 1.9 | 6.9 ± 0.8 | 0.64 ± 0.06 | 3.14 ± 0.49 |
| VEF_G_S | 41 ± 1 | 6.7 ± 1.9 | 7.0 ± 0.7 | 0.54 ± 0.04 | 3.02 ± 0.08 |
| VEF_G_BC_S | 58 ± 1 | 2.7 ± 1.9 | 8.3 ± 1.0 | 0.47 ± 0.02 | 6.07 ± 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Węgrzyk, G.; Grzęda, D.; Leszczyńska, M.; Nędza, B.; Bulanda, K.; Oleksy, M.; Ryszkowska, J.; Cabulis, U. Viscoelastic Polyurethane Foam Biocomposites with Enhanced Flame Retardancy. Polymers 2024, 16, 3189. https://doi.org/10.3390/polym16223189
Węgrzyk G, Grzęda D, Leszczyńska M, Nędza B, Bulanda K, Oleksy M, Ryszkowska J, Cabulis U. Viscoelastic Polyurethane Foam Biocomposites with Enhanced Flame Retardancy. Polymers. 2024; 16(22):3189. https://doi.org/10.3390/polym16223189
Chicago/Turabian StyleWęgrzyk, Grzegorz, Dominik Grzęda, Milena Leszczyńska, Bartosz Nędza, Katarzyna Bulanda, Mariusz Oleksy, Joanna Ryszkowska, and Ugis Cabulis. 2024. "Viscoelastic Polyurethane Foam Biocomposites with Enhanced Flame Retardancy" Polymers 16, no. 22: 3189. https://doi.org/10.3390/polym16223189
APA StyleWęgrzyk, G., Grzęda, D., Leszczyńska, M., Nędza, B., Bulanda, K., Oleksy, M., Ryszkowska, J., & Cabulis, U. (2024). Viscoelastic Polyurethane Foam Biocomposites with Enhanced Flame Retardancy. Polymers, 16(22), 3189. https://doi.org/10.3390/polym16223189









