Exploring Rhamnus alaternus Polysaccharides: Extraction, Characterization, and Analysis of Antioxidant and Antimicrobial Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Raw Materials
2.3. Bacterial Strains
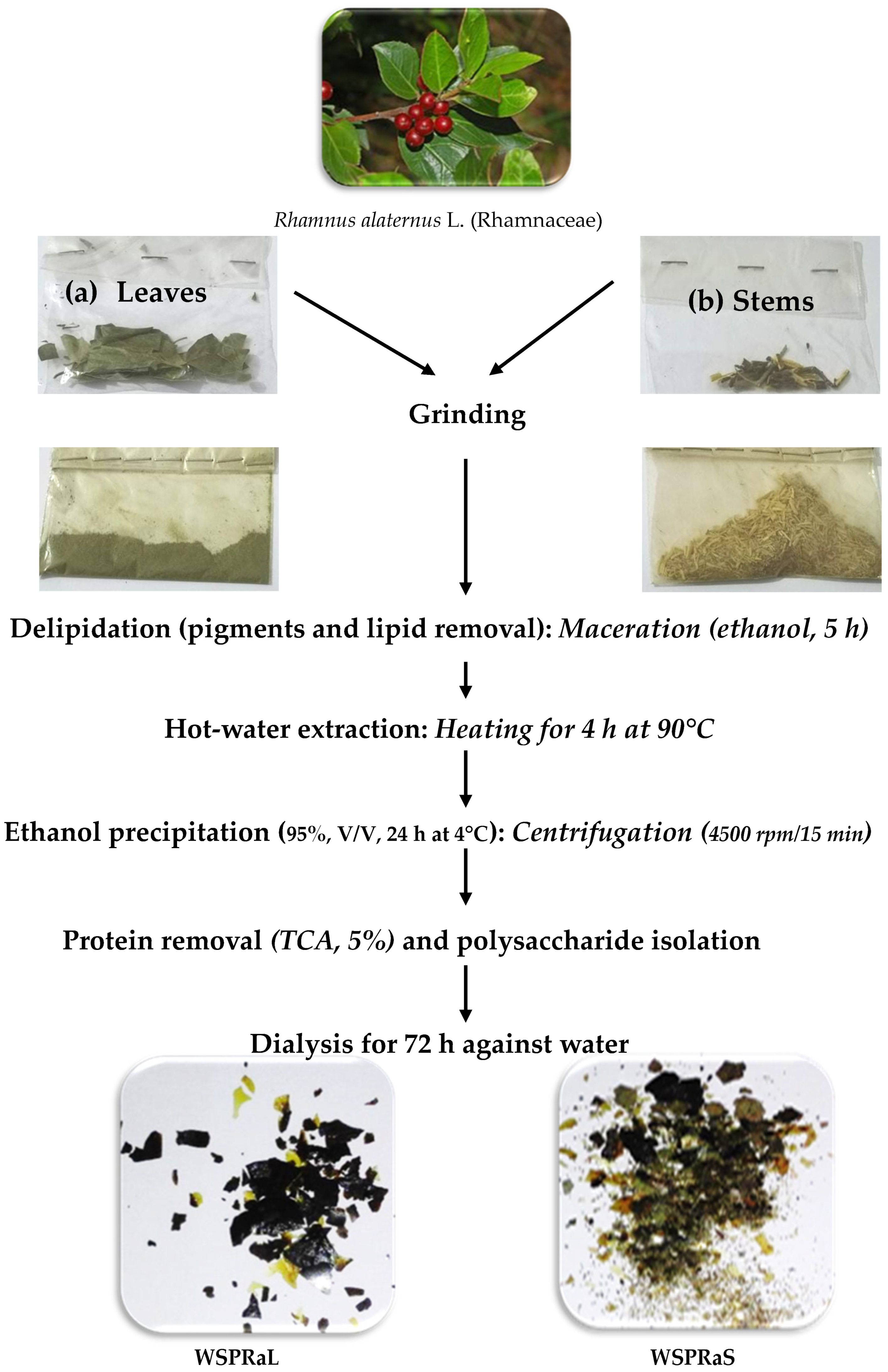
2.4. Extraction of Water-Soluble Polysaccharides
2.5. Spectral Characterization of Water-Soluble Polysaccharides
2.6. Biochemical Polysaccharide Composition
2.7. Determination of Monosaccharide Composition
2.8. High-Performance Liquid Chromatography (HPLC) Conditions
2.9. Antioxidant and Antibacterial Activities of WSP from the Stems and Leaves of Rhamnus alaternus
2.9.1. In Vitro Antioxidant Activities
2.9.2. Antibacterial Activities
2.10. Antibiofilm Activity
2.11. Statistical Analyses
3. Results
3.1. Extraction and Yield of Water-Soluble Polysaccharides from Rhamnus alaternus
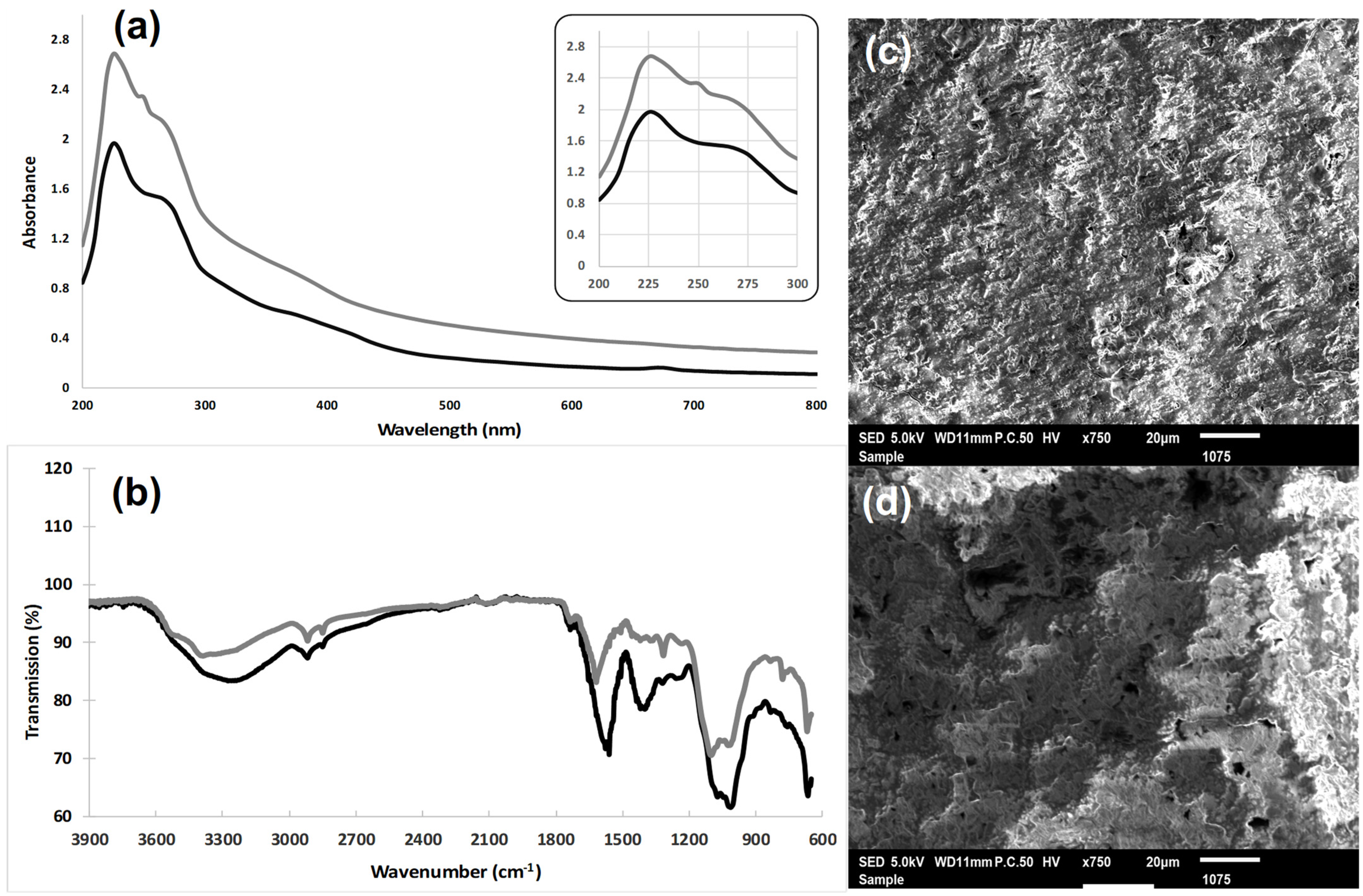
3.2. Spectral Analysis of Extracted Water Polysaccharides
3.2.1. Ultraviolet Absorption (UV) Spectroscopy
3.2.2. Fourier Transform Infrared (FT-IR) Analysis
3.2.3. Microstructure Analysis
3.3. Determination of Biochemical Polysaccharide Composition
3.4. Determination of the Monosaccharide Composition
3.4.1. Thin-Layer Chromatography (TLC)
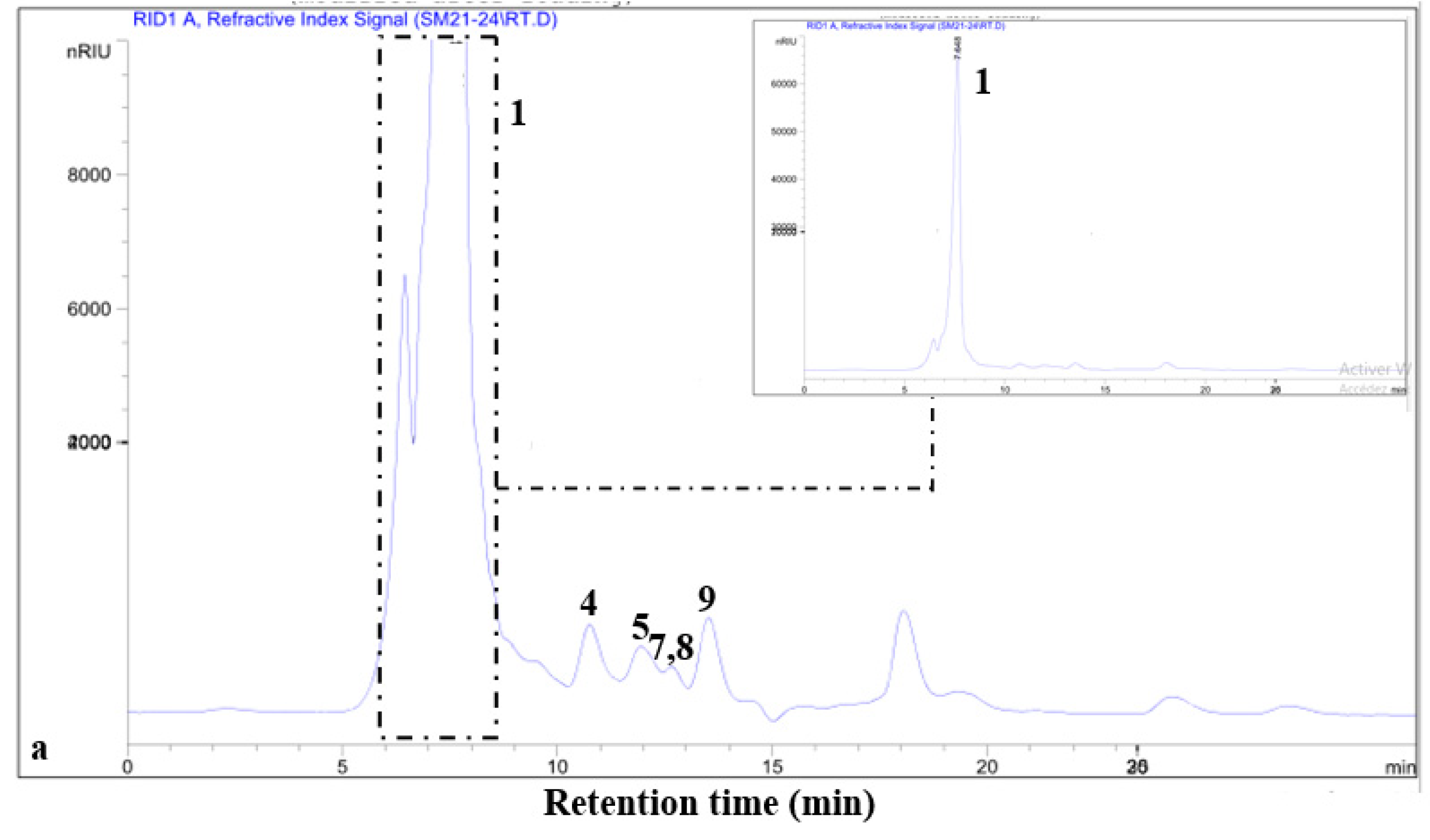
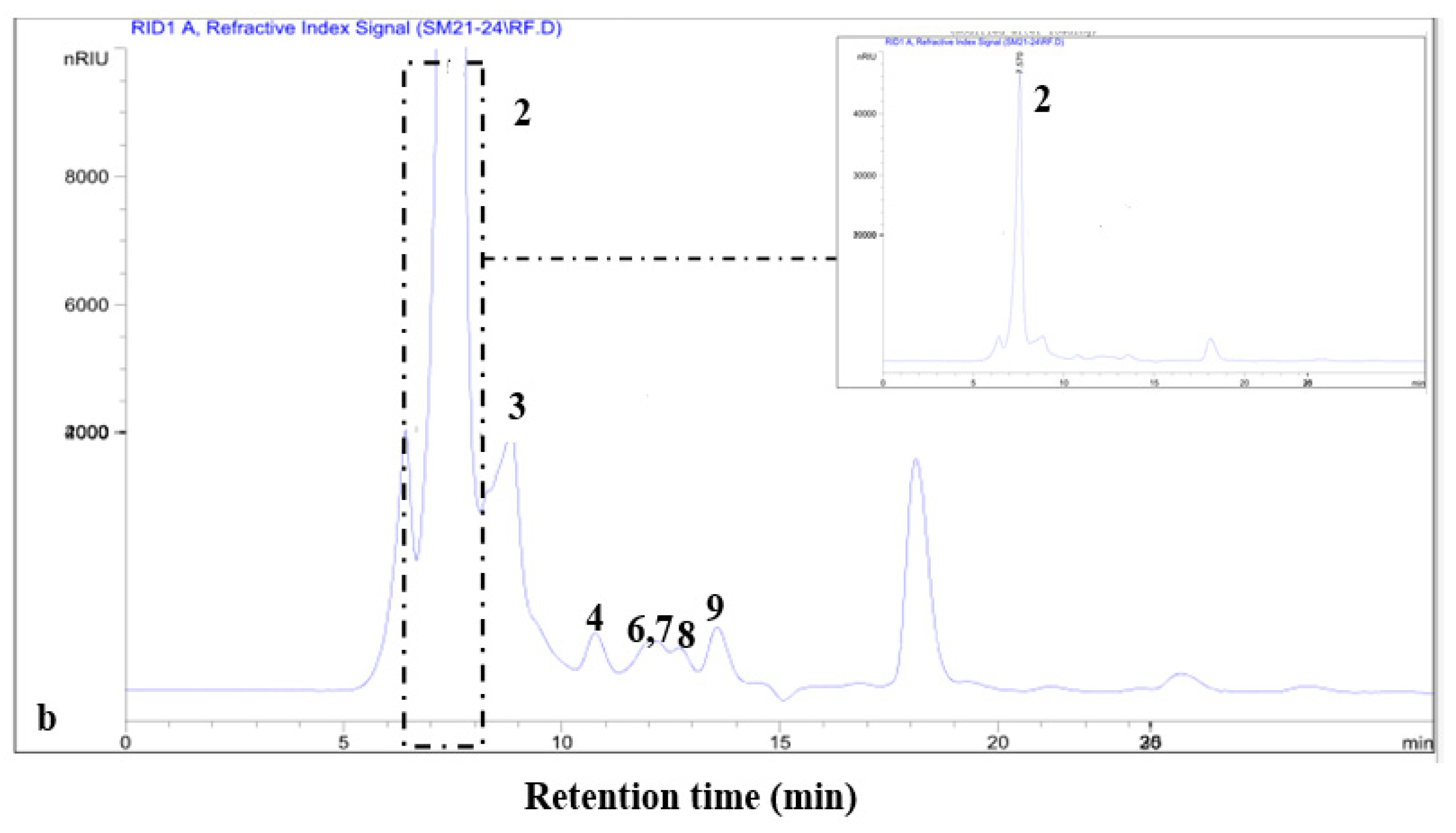
3.4.2. HPLC Separation
3.5. Antioxidant Activity
3.5.1. DPPH Free Radical Scavenging Activity
3.5.2. ABTS Radical Scavenging Activity
3.5.3. Reducing Power Assay
3.5.4. Ferrous (Fe2+) Ion Chelating Activity
3.6. Antibacterial Activities
3.6.1. The Minimum Inhibitory Concentration (MIC) and Bactericidal Concentration (MBC) Determined by the Microdilution Method
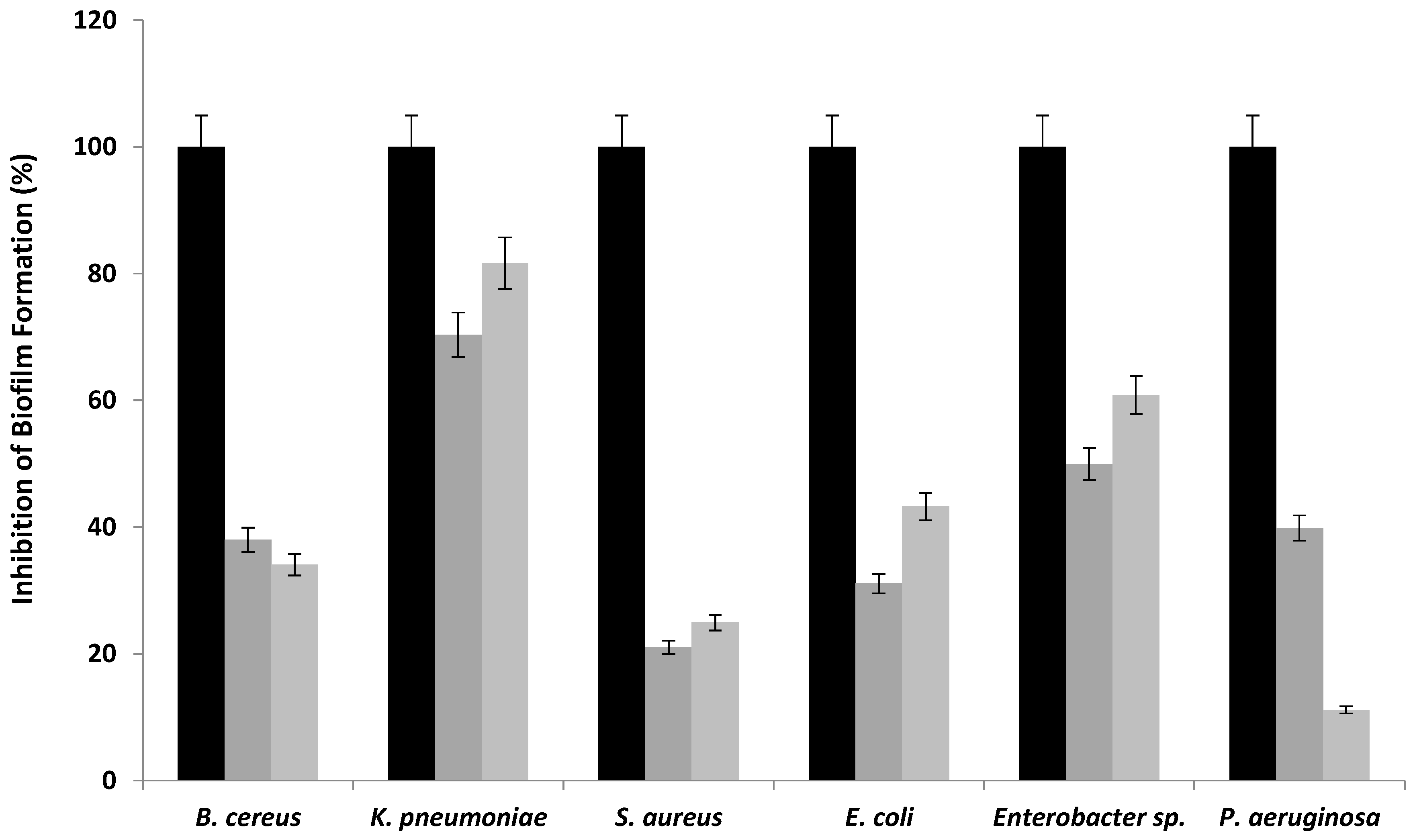
3.6.2. Biofilm Inhibition Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aguilera-Carbo, A.; Augur, C.; Prado-Barragan, L.A.; Favela-Torres, E.; Aguilar, C.N. Microbial production of ellagic acid and biodegradation of ellagitannins. Appl. Microbiol. Biotechnol. 2008, 78, 189–199. [Google Scholar] [CrossRef]
- Merghni, A.; Noumi, E.; Hadded, O.; Dridi, N.; Panwar, H.; Ceylan, O.; Mastouri, M.; Snoussi, M. Assessment of the antibiofilm and antiquorum sensing activities of Eucalyptus globulus essential oil and its main component 1,8-cineole against methicillin-resistant Staphylococcus aureus strains. Microb. Pathog. 2018, 118, 74–80. [Google Scholar] [CrossRef]
- Gurib-Fakim, A. Plantago lanceolata L. Record from Protabase; Schmelzer, G.H., Gurib-Fakim, A., Eds.; PROTA (Plant Resources of Tropical Africa/Ressources Végétales de L’AFRIQUE Tropicale): Wageningen, The Netherlands, 2006. [Google Scholar]
- Jardak, M.; Elloumi-Mseddi, J.; Aifa, S.; Mnif, S. Chemical composition, anti-biofilm activity, and potential cytotoxic effect on cancer cells of Rosmarinus officinalis L. essential oil from Tunisia. Lipids Health Dis. 2017, 16, 190. [Google Scholar] [CrossRef]
- Worowounga, X.; Ernest, L.-Y.; Armel-Frederic, N.; Firmin, B.P.; Marinette, S.; Jean-Laurent, S.-M.; Boniface, K. Activité antibactérienne de l’extrait méthanolique des écorces de Manilkara mabokeensis. Int. J. Innov. Appl. Stud. 2019, 25, 785. [Google Scholar]
- Hamed, M.; Bougatef, H.; Karoud, W.; Krichen, F.; Haddar, A.; Bougatef, A.; Sila, A. Polysaccharides extracted from pistachio external hull: Characterization, antioxidant activity, and potential application on meat as preservative. Ind. Crops Prod. 2020, 148, 112315. [Google Scholar] [CrossRef]
- Liu, S.; Yang, H.; Li, S.; Zhang, J.; Li, T.; Zhu, B.; Zhang, B. Polyphenolic compositions and chromatic characteristics of bog bilberry syrup wines. Molecules 2015, 20, 19865–19877. [Google Scholar] [CrossRef]
- Xie, J.H.; Shen, M.Y.; Nie, S.P.; Zhao, Q.; Li, C.; Xie, M.Y. Separation of water-soluble polysaccharides from Cyclocarya paliurus by ultrafiltration process. Carbohydr. Polym. 2015, 101, 479–483. [Google Scholar] [CrossRef]
- Murphy, E.J.; Fehrenbach, G.W.; Abidin, I.Z.; Buckley, C.; Montgomery, T.; Pogue, R.; Murray, P.; Major, I.; Rezoagli, E. Polysaccharides—Naturally occurring immune modulators. Polymers 2023, 15, 2373. [Google Scholar] [CrossRef]
- Bouaziz, F.; Koubaa, M.; Ellouz Ghorbel, R.; Ellouz Chaabouni, S. Biological properties of water-soluble polysaccharides and hemicelluloses from almond gum. Int. J. Biol. Macromol. 2017, 95, 667–674. [Google Scholar] [CrossRef]
- Feriani, A.; Tir, M.; Hamed, M.; Sila, A.; Nahdi, S.; Alwasel, S.; Harrath, A.H.; Tlili, N. Multidirectional insights on polysaccharides from Schinus terebinthifolius and Schinus molle fruits: Physicochemical and functional profiles, in vitro antioxidant, anti-genotoxicity, antidiabetic, and antihemolytic capacities, and in vivo anti-inflammatory and anti-nociceptive properties. Molecules 2020, 25, 2115. [Google Scholar]
- Hammami, N.; Ben Gara, A.; Bargougui, K.; Ayedi, H.; Ben Abdalleh, F.; Belghith, K. Improved in vitro antioxidant and antimicrobial capacities of polysaccharides isolated from Salicornia arabica. Int. J. Biol. Macromol. 2018, 120, 2123–2130. [Google Scholar] [CrossRef]
- Ben Ammar, R.; Ben Sghaier, M.; Boubaker, J.; Bhouri, W.; Naffeti, A.; Skandrani, I.; Bouhlel, I.; Kilani, S.; Ghedira, K.; Chekir-Ghedira, L. Antioxidant activity and inhibition of aflatoxin B1-, nifuroxazide-, and sodium azide-induced mutagenicity by extracts from Rhamnus alaternus L. Chem.-Biol. Interact. 2008, 174, 1–10. [Google Scholar] [CrossRef]
- Li, S.G.; Zhang, Y.Q. Characterization and renal protective effect of a polysaccharide from Astragalus membranaceus. Carbohydr. Polym. 2009, 78, 343–348. [Google Scholar] [CrossRef]
- Ben Younes, S.; Dallali, C.; Ellafi, A.; Bouslama, L.; Feriani, A.; Sayadi, S. Extracellular enzymatic activities of bacterial strains isolated from Tunisian biotopes: Decolorization and detoxification of indigo carmine. Catal. Lett. 2021, 151, 1248–1261. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, X.; Jiang, W.; Tan, J.; Chen, G. Preparation, structural characterization, and in vitro activity of ginger polysaccharide. Chem. Biol. Technol. Agric. 2023, 10, 126. [Google Scholar] [CrossRef]
- Al-Kindy, S.M.Z.; Abdulnour, A.O.; Al-Rasbi, M.M. Determination of sugar and mineral contents in some Omani fruits. Sci. Technol. 2001, 6, 39–44. [Google Scholar]
- Ben Younes, S.; Cherif, I.; Dhouib, A.; Sayadi, S. Trametes trogii: A biologically powerful tool for dyes decolorization and detoxification. Catal. Lett. 2016, 146, 204–211. [Google Scholar] [CrossRef]
- Karoud, W.; Sila, A.; Krichen, F.; Martinez Alvarez, O.; Bougatef, A. Characterization, surface properties and biological activities of protein hydrolysates obtained from hake (Merluccius merluccius) heads. Waste Biomass Valorization 2019, 10, 287–297. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.; Lu, Y.; Feng, X.; Tian, G.; Liu, Q. Antioxidative and hepatoprotective activities of a novel polysaccharide (LSAP) from Lepista sordida mycelia. Food Sci. Hum. Wellness 2021, 10, 536–544. [Google Scholar] [CrossRef]
- Haney, E.F.; Trimble, M.J.; Cheng, J.T.; Vallé, Q.; Hancock, R.E.W. Critical assessment of methods to quantify biofilm growth and evaluate antibiofilm activity of host defence peptides. Biomolecules 2018, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Ebringerova, A.; Hromadkova, Z.; Machova, E.; Naran, R.; Hríbalova, V. Isolation and characterization of mitogenic pectic polysaccharides from Cistanche deserticola Y.C. Ma. Chem. Pap. 1997, 57, 289–294. [Google Scholar]
- Dong, Q.; Yao, J.; Fang, J.N.; Ding, K. Structural characterization and immunological activity of two cold-water extractable polysaccharides from Cistanche deserticola Y.C. Ma. Carbohydr. Res. 2007, 342, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.M.; Gao, X.M.; Tsim, K.W.K.; Tu, P.F. An arabinogalactan isolated from the stems of Cistanche deserticola induces the proliferation of cultured lymphocytes. Int. J. Biol. Macromol. 2005, 37, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Boual, Z. Caractérisation Physico-Chimique des Polysaccharides de Quelques Plantes Spontanées à Caractère Médicinal Récoltées dans la Région de Ghardaïa (Sahara Septentrional Est Algérien): Activité Biologique. Ph.D. Thesis, Université de Ouargla, Ouargla, Algeria, 2014; 219p. [Google Scholar]
- Kiyohara, H.; Uchida, T.; Takakiwa, M.; Matsuzaki, T.; Hada, N.; Takeda, T.; Shibata, T.; Yamada, H. Different contributions of side-chains in β-D-(1→3,6)-galactans on intestinal Peyer’s patch immunomodulation by polysaccharides from Astragalus mongholicus Bunge. Phytochemistry 2010, 71, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Ebringerova, A.; Hromadkova, Z. An overview on the application of ultrasound in extraction, separation and purification of plant polysaccharides. Cent. Eur. J. Chem. 2010, 8, 243–257. [Google Scholar] [CrossRef]
- Kaijanen, L.; Paakkunainen, M.; Pietarinen, S.; Jernstrom, E.; Reinikainen, S.-P. Ultraviolet detection of monosaccharides: Multiple wavelength strategy to evaluate results after capillary zone electrophoretic separation. Int. J. Electrochem. Sci. 2015, 10, 2950–2961. [Google Scholar] [CrossRef]
- Guan, H.; Ling, X.; Xu, J.; Zhu, Y.; Zhang, J.; Liu, X. Structural characterization of polysaccharide derived from Gastrodia elata and its immunostimulatory effect on RAW264.7 cells. Molecules 2022, 27, 8059. [Google Scholar] [CrossRef]
- Venyaminov, S.Y.; Kalnin, N.N. Quantitative IR spectrophotometry of peptide compounds in water (H2O) solutions. I. Spectr. Parameters Amino Acid. Residue Absorpt. Bands. Biopolymers 1990, 30, 1243–1257. [Google Scholar] [CrossRef]
- Teka, N.; Alminderej, F.M.; Souid, G.; El-Ghoul, Y.; Le Cerf, D.; Majdoub, H. Characterization of polysaccharides sequentially extracted from Allium roseum leaves and their hepatoprotective effects against cadmium-induced toxicity in mouse liver. Antioxidants 2022, 11, 1866. [Google Scholar] [CrossRef]
- Moreira, T.A.; Costa, B.B.; Celestino, R.C.A.; Faria, C.N.; Gianelli, J.L.D.; Santos, G.R.C.d.; Glauser, B.F.; Soares, A.R.; Mourao, P.A.S.; Rodrigues, C.R.; et al. Isolation, fractionation and anticoagulant activity of a sulfated galactan extracted from the green algae Penicillus capitatus. Ciênc. Rural 2021, 51, e20200138. [Google Scholar] [CrossRef]
- Kacurakova, M.; Capek, P.; Sasinkova, V.; Wellner, N.; Ebringerova, A. FT-IR Study of Plant Cell Wall Model Compounds: Pectic Polysaccharides and Hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Castellane, T.C.L.; Otoboni, A.M.M.B.; Lemos, E.G.M. Characterization of exopolysaccharides produced by rhizobia species. Rev. Bras. Cienc. Solo 2015, 39, 1566–1575. [Google Scholar] [CrossRef]
- Delattre, C. Stratégie D’obtention D’oligosaccharides Anioniques par Dégradation Enzymatique de Gluconate. Ph.D. Thesis, Universite Kasdi Merbah Ouargla, Ouargla, Algeria, 2005; pp. 4–11. [Google Scholar]
- Krichen, F.; Karaoud, W.; Sayari, N.; Sila, A.; Kallel, F.; Ellouz-Chaabouni, S.; Bougatef, A. Sulfated polysaccharides from Tunisian fish skins: Antioxidant, DNA damage protective effect and antihypertensive activities. Polym. Environ. 2016, 24, 166–175. [Google Scholar] [CrossRef]
- Qiu, X.; Ou, Y.; Lu, S.; Liang, Y.; Zhang, Y.; Li, M.; Li, G.; Ma, H.; Wu, Y.; He, Z.; et al. Study of the structure and bioactivity of polysaccharides from different parts of Stemona tuberosa Lour. Molecules 2024, 29, 1347. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Yu, Z.; Chen, Y.; Zhu, X.; Ai, Z.; Liu, S.; Ni, D. Rapid determination of the monosaccharide composition and contents in tea polysaccharides from Yingshuang green tea by pre-column derivatization HPLC. J. Chem. 2016, 2016, 6065813. [Google Scholar] [CrossRef]
- Thambiraj, S.R.; Phillips, M.; Koyyalamudi, S.R.; Reddy, N. Antioxidant activities and characterization of polysaccharides isolated from the seeds of Lupinus angustifolius. Ind. Crops Prod. 2015, 74, 950–956. [Google Scholar] [CrossRef]
- Ben Jeddou, F.; Chaari, S.; Maktouf, O.; Nouri-Ellouz, C.; Boisset Helbert, R.; Ellouz Ghorbel, R. Structural, functional, and antioxidant properties of water-soluble polysaccharides from potato peels. Food Chem. 2016, 205, 97–105. [Google Scholar] [CrossRef]
- Ben Abdallah Kolsi, R.; Bkhairia, I.; Gargouri, L.; Ktari, N.; Chaaben, R.; El Feki, A.; Nasri, M.; Jamoussi, K.; Fki, L.; Belghith, K. Protective effect of Sargassum vulgare sulfated polysaccharide against molecular, biochemical, and histopathological damage caused by alloxan in experimental diabetic rats. Int. J. Biol. Macromol. 2017, 105, 1598–1607. [Google Scholar] [CrossRef]
- Abbou, A.; Kadri, N.; Debbache, N.; Dairi, S.; Remini, H.; Dahmoune, F.; Berkani, F.; Adel, K.; Belbahi, A.; Madani, K. Effect of precipitation solvent on some biological activities of polysaccharides from Pinus halepensis Mill. seeds. Int. J. Biol. Macromol. 2019, 141, 663–670. [Google Scholar] [CrossRef]
- Liang, L.; Su, Q.; Ma, Y.; Zhao, S.; Zhang, H.; Gao, X. Research progress on the polysaccharide extraction and antibacterial activity. Ann. Microbiol. 2024, 74, 17. [Google Scholar] [CrossRef]
- Veerabhadra Raju, D.; Nagarajan, A.; Pandit, S.; Nag, M.; Lahiri, D.; Upadhye, V. Effect of bacterial quorum sensing and mechanism of antimicrobial resistance. Biocatal. Agric. Biotechnol. 2022, 43, 102409. [Google Scholar]
- Rendueles, O.; Kaplan, J.B.; Ghigo, J.M. Antibiofilm polysaccharides. Environ. Microbiol. 2013, 15, 334–346. [Google Scholar] [CrossRef] [PubMed]
| WSPRaL | WSPRaS | |
|---|---|---|
| Chemical Composition | ||
| Proteins (µg/mg) | 260.740 ± 0.98 | 269.629 ± 1.48 |
| Lipids (µg/mg) | 53.34 ± 2.38 | 13.33 ± 0.28 |
| Total sugar (µg/mg) | 482.716 ± 3.02 | 569.135 ± 3.82 |
| Reducing sugar (µg/mg) | 420.240 ± 1.68 | 531.732 ± 2.59 |
| Saccharide composition (%) | ||
| Glucuronic acid | - | 89.865 ± 2.42 |
| Galacturonic acid | 77.373 ± 2.34 | - |
| Sucrose | 13.131 ± 1.9 | - |
| Glucose | 2.336 ± 0.45 | 2.765 ± 0.66 |
| Galactose | - | 2.162 ± 0.52 |
| Rhamnose | 2.936 ± 0.24 | - |
| Mannose | 0.742 ± 0.08 | 0.503 ± 0.04 |
| Xylose | 0.740 ± 0.06 | 0.5 ± 0.02 |
| Arabinose | 2.740 ± 0.48 | 2.662 ± 0.76 |
| Monosaccharide | Rf | |
|---|---|---|
| WSPRaL | WSPRaS | |
| Rhamnose | 0.56 | - |
| Xylose | 0.45 | 0.45 |
| Arabinose | 0.34 | 0.34 |
| Glucose | 0.27 | 0.27 |
| Galactose | - | 0.23 |
| Galacturonic acid | 0.14 | - |
| Glucuronic acid | - | 0.11 |
| IC50 (DPPH) | IC50 (ABTS) | IC50 (FRAP) | IC50 (Chelating Power) | |
|---|---|---|---|---|
| RaWSPL | 615 ± 2.05 µg/mL | 470 ± 5.78 µg/mL | 203.89 ± 1.07 µg/mL | 219 ± 2.51 µg/mL |
| RaWSPS | 628 ± 2.38 µg/mL | 559 ± 4.32 µg/mL | 141.76 ± 3.16 µg/mL | 225 ± 1.75 µg/mL |
| Strains | MIC (mg/mL) | MBC (mg/mL) | MBC/MIC | |||
|---|---|---|---|---|---|---|
| WSPRaL | WSPRaS | WSPRaL | WSPRaS | WSPRaL | WSPRaS | |
| Bacillus cereus | 9 | 10 | 11 | 11 | 1.22 | 1.1 |
| Klebsiella pneumoniae | 5 | 3 | 7 | 5 | 1.4 | 1.66 |
| Staphylococcus aureus | 4 | 5 | 6 | 7 | 1.5 | 1.4 |
| Escherichia coli | 2 | 3 | 4 | 6 | 2 | 2 |
| Enterobacter sp. | 3 | 4 | 5 | 7 | 1.66 | 1.75 |
| Pseudomonas aeruginosa | 2 | 4 | 4 | 6 | 2 | 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chokri, S.; Ben Younes, S.; Ellafi, A.; Mnif, S.; López-Maldonado, E.A.; Slaheddine Masmoudi, A. Exploring Rhamnus alaternus Polysaccharides: Extraction, Characterization, and Analysis of Antioxidant and Antimicrobial Properties. Polymers 2024, 16, 3180. https://doi.org/10.3390/polym16223180
Chokri S, Ben Younes S, Ellafi A, Mnif S, López-Maldonado EA, Slaheddine Masmoudi A. Exploring Rhamnus alaternus Polysaccharides: Extraction, Characterization, and Analysis of Antioxidant and Antimicrobial Properties. Polymers. 2024; 16(22):3180. https://doi.org/10.3390/polym16223180
Chicago/Turabian StyleChokri, Souha, Sonia Ben Younes, Ali Ellafi, Sami Mnif, Eduardo Alberto López-Maldonado, and Ahmed Slaheddine Masmoudi. 2024. "Exploring Rhamnus alaternus Polysaccharides: Extraction, Characterization, and Analysis of Antioxidant and Antimicrobial Properties" Polymers 16, no. 22: 3180. https://doi.org/10.3390/polym16223180
APA StyleChokri, S., Ben Younes, S., Ellafi, A., Mnif, S., López-Maldonado, E. A., & Slaheddine Masmoudi, A. (2024). Exploring Rhamnus alaternus Polysaccharides: Extraction, Characterization, and Analysis of Antioxidant and Antimicrobial Properties. Polymers, 16(22), 3180. https://doi.org/10.3390/polym16223180







