Effects of Particle Shape and Surface Structure on the Adsorption Properties of Polystyrene Microplastics
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification of the Surface Layer of Polystyrene Particles
2.3. Determination of the Surface Area, Average Pore Diameter, and Pore Volume of Polymer Particles
2.4. Study of Rhodamine B Sorption
2.5. Characterization by Optical Microscopy, Transmission Electron Microscopy (TEM), and Atomic Force Microscopy (AFM)
2.6. Analysis of Experimental Data on the Sorption Capacity of Rhodamine B
3. Results
3.1. Characteristics of the Initial Model PS Particles
3.2. Modification of the Structure of the Surface Layer of Model PS Particles
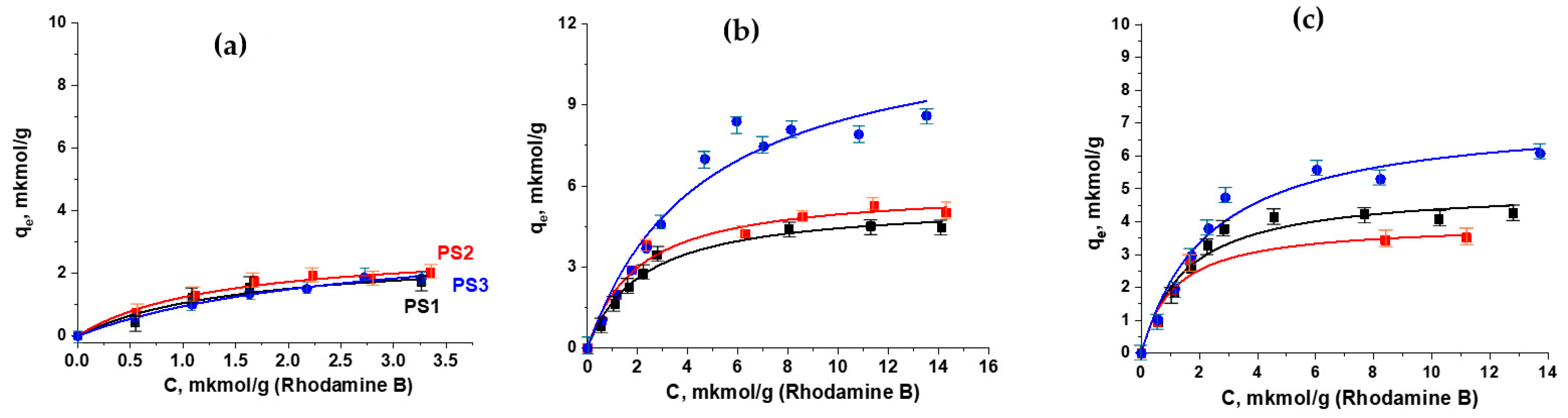
3.3. Study of Rhodamine B Adsorption on Model PS Particles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Cauwenberghe, L.; Janssen, C.R. Microplastics in bivalves cultured for human consumption. Environ. Pollut. 2014, 193, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Shim, W.J.; Jang, M.; Han, G.M.; Hong, S.H. Abundance and characteristics of microplastics in market bivalves from South Korea. Environ. Pollut. 2019, 245, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qu, X.; Su, L.; Zhang, W.; Yang, D.; Kolandhasamy, P.; Li, D.; Shi, H. Microplastics in mussels along the coastal waters of China. Environ. Pollut. 2016, 214, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Akbulut, S.; Akman, P.K.; Tornuk, F.; Yetim, H. Microplastic Release from Single-Use Plastic Beverage Cups. Foods 2024, 13, 1564. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ling, X.; Cheng, J.; Yao, W.; Qian, H.; Ding, D.; Yu, Z.; Xie, Y.; Yang, F. Identification and Visualization of Polystyrene Microplastics/Nanoplastics in Flavored Yogurt by Raman Imaging. Toxics 2024, 12, 330. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, G.; Wang, J.; Wang, M.; Ying, R.; Li, X.; Hu, Z.; Zhang, Y. Disposable plastic materials release microplastics and harmful substances in hot water. Sci. Total Environ. 2022, 818, 151685. [Google Scholar] [CrossRef] [PubMed]
- Demirelli, E.; Tepe, Y.; Oğuz, U.; Aydın, H.; Kodat, M.; Tok, D.S.; Sönmez, M.G.; Öğreden, E. The first reported values of microplastics in prostate. BMC Urol. 2024, 24, 106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Piñon-Colin, T.d.J.; Rodriguez-Jimenez, R.; Pastrana-Corral, M.A.; Rogel-Hernandez, E.; Wakida, F.T. Microplastics on sandy beaches of the Baja California Peninsula, Mexico. Mar. Pollut. Bull. 2018, 131 Pt A, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Goyal, T.; Singh, S.; Das Gupta, G.; Verma, S.K. Microplastics in environment: A comprehension on sources, analytical detection, health concerns, and remediation. Environ. Sci. Pollut. Res. 2023, 30, 114707–114721. [Google Scholar] [CrossRef] [PubMed]
- Coralli, I.; Goßmann, I.; Fabbri, D.; Scholz-Böttcher, B.M. Determination of polyurethanes within microplastics in complex environmental samples by analytical pyrolysis. Anal. Bioanal. Chem. 2023, 415, 2891–2905. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Donchenko, A.; Aubin, S.; Gagné, S.; Spence, M.; Breau, L.; Lesage, J. Development of a method for quantification of toluene diisocyanate and methylenediphenyl diisocyanate migration from polyurethane foam sample surface to artificial sweat by HPLC-UV-MS. J. Chromatogr. B 2020, 1142, 122027. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, K.; Johnson, E.V.; Malmendal, A.; Linse, S.; Hansson, L.-A.; Cedervall, T. Brain damage and behavioural disorders in fish induced by plastic nanoparticles delivered through the food chain. Sci. Rep. 2017, 7, 11452. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mattsson, K.; Ekvall, M.T.; Hansson, L.A.; Linse, S.; Malmendal, A.; Cedervall, T. Altered behavior, physiology, and metabolism in fish exposed to polystyrene nanoparticles. Environ. Sci. Technol. 2015, 49, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Kelpsiene, E.; Ekvall, M.T.; Lundqvist, M.; Torstensson, O.; Hua, J.; Cedervall, T. Review of ecotoxicological studies of widely used polystyrene nanoparticles. Environ. Sci. Process. Impacts 2022, 24, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Hou, J.; You, G.; Liu, Z.; Liu, S.; Li, T.; Mo, Y.; Guo, S.; Qu, H. Acute effects of nanoplastics and microplastics on periphytic biofilms depending on particle size, concentration and surface modification. Environ. Pollut. 2019, 255, 113300. [Google Scholar] [CrossRef] [PubMed]
- Bergami, E.; Pugnalini, S.; Vannuccini, M.; Manfra, L.; Faleri, C.; Savorelli, F.; Dawson, K.; Corsi, I. Long-term toxicity of surface-charged polystyrene nanoplastics to marine planktonic species Dunaliella tertiolecta and Artemia franciscana. Aquat. Toxicol. 2017, 189, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Guo, J.; Yao, Y.; Xu, S. Polystyrene nanoplastics induced cardiomyocyte apoptosis and myocardial inflammation in carp by promoting ROS production. Fish Shellfish. Immunol. 2022, 125, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schröter, L.; Ventura, N. Nanoplastic Toxicity: Insights and Challenges from Experimental Model Systems. Small 2022, 18, e2201680. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Choi, D.; Han, S.; Jung, S.Y.; Choi, J.; Hong, J. Potential toxicity of polystyrene microplastic particles. Sci. Rep. 2020, 10, 7391. [Google Scholar] [CrossRef]
- Lamichhane, G.; Acharya, A.; Marahatha, R.; Modi, B.; Paudel, R.; Adhikari, A.; Raut, B.K.; Aryal, S.; Parajuli, N. Microplastics in environment: Global concern, challenges, and controlling measures. Int. J. Environ. Sci. Technol. 2023, 20, 4673–4694. [Google Scholar] [CrossRef]
- Hwang, J.; Choi, D.; Han, S.; Choi, J.; Hong, J. An assessment of the toxicity of polypropylene microplastics in human derived cells. Sci. Total Environ. 2019, 684, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of polystyrene microplastic on the gut barrier, microbiota and metabolism of mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2016, 6, 620. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hernández, V.A. An overview of surface forces and the DLVO theory. ChemTexts 2023, 9, 1–16. [Google Scholar] [CrossRef]
- Derjaguin, B.; Landau, L. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Prog. Surf. Sci. 1941, 43, 30–59. [Google Scholar] [CrossRef]
- Jönsson, B.; Wennerström, H. Ion–ion correlations in liquid dispersions. J. Adhes. 2004, 80, 339–364. [Google Scholar] [CrossRef]
- Martin, L.; Simpson, K.; Brzezinski, M.; Watt, J.; Xu, W. Cellular response of keratinocytes to the entry and accumulation of nanoplastic particles. Part. Fibre Toxicol. 2024, 21, 1–14. [Google Scholar] [CrossRef]
- Andrews, S.N.; Jeong, E.; Prausnitz, M.R. Transdermal Delivery of Molecules is Limited by Full Epidermis, Not Just Stratum Corneum. Pharm. Res. 2013, 30, 1099–1109. [Google Scholar] [CrossRef]
- Galloway, T.S.; Cole, M.; Lewis, C. Interactions of microplastic debris throughout the marine ecosystem. Nat. Ecol. Evol. 2017, 1, 116. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Niven, S.J.; Galloway, T.S.; Rowland, S.J.; Thompson, R.C. Microplastic moves pollutants and additives to worms, reducing functions linked to health and biodiversity. Curr. Biol. 2013, 23, 2388–2392. [Google Scholar] [CrossRef] [PubMed]
- Ramsperger, A.F.R.M.; Narayana, V.K.B.; Gross, W.; Mohanraj, J.; Thelakkat, M.; Greiner, A.; Schmalz, H.; Kress, H.; Laforsch, C. Environmental exposure enhances the internalization of microplastic particles into cells. Sci. Adv. 2020, 6, eabd1211. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Argun, B.R.; Statt, A. Influence of shape on heteroaggregation of model microplastics: A simulation study. Soft Matter 2023, 19, 8081–8090. [Google Scholar] [CrossRef] [PubMed]
- Harshe, Y.M.; Lattuada, M.; Soos, M. Experimental and modeling study of breakage and restructuring of open and dense colloidal aggregates. Langmuir 2011, 27, 5739–5752. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Shim, W.J.; Hong, L. Methods of analysing chemicals associated with microplastics: A review. Anal. Methods 2017, 9, 1361–1368. [Google Scholar] [CrossRef]
- Rao, W.; Piliouras, P.; Wang, X.; Guido, A.; Kugler, K.; Sieren, B.; Wang, L.; Lv, G.; Li, Z. Zwitterionic dye rhodamine B (RhB) uptake on different types of clay minerals. Appl. Clay Sci. 2020, 197, 105790. [Google Scholar] [CrossRef]
- Al-Gheethi, A.A.; Azhar, Q.M.; Kumar, P.S.; Yusuf, A.A.; Al-Buriahi, A.K.; Mohamed, R.M.S.R.; Al-Shaibani, M.M. Sustainable approaches for removing Rhodamine B dye using agricultural waste adsorbents: A review. Chemosphere 2022, 287, 132080. [Google Scholar] [CrossRef]
- Men’shikova, A.Y.; Bilibin, A.Y.; Shevchenko, N.N.; Shabsel’s, B.M.; Evseeva, T.G.; Bazhenova, A.G.; Sel’kin, A.V. Emulsifier-free emulsion copolymerization of styrene with methacrylic acid as a method of preparing building blocks for photonic crystals. Polym. Sci. Ser. A 2006, 48, 910–917. [Google Scholar] [CrossRef]
- Shevchenko, N.N.; Evseeva, T.G.; Shevaldysheva, D.I.; Shabsel’s, B.M.; Skurkis, Y.O.; Men’shikova, A.Y. The seed heterophase polymerisation as a method of forming crosslinked monodisperse microspheres. Russ. J. Appl. Chem. 2013, 86, 242–252. [Google Scholar] [CrossRef]
- Wang, D.; Dimonie, V.L.; Sudol, E.D.; El-Aasser, M.S. Effect of PVP in dispersion and seeded dispersion polymerizations. J. Appl. Polym. Sci. 2002, 84, 2721–2732. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Sittig, M. Handbook of Toxic and Hazardous Chemicals and Carcinogens; Noyes Publications: Park Ridge, NJ, USA, 1985. [Google Scholar]
- García, M.T.; Gracia, I.; Duque, G.; de Lucas, A.; Rodríguez, J.F. Study of the solubility and stability of polystyrene wastes in a dissolution recycling process. Waste Manag. 2009, 29, 1814–1818. [Google Scholar] [CrossRef] [PubMed]
- Menshikova, A.Y.; Shabsels, B.M.; Shevchenko, N.E.A.; Bazhenova, A.G.; Pevtsov, A.B.; Sel’kin, A.V.; Bilibin, A.Y. Surface modified latex particles: Synthesis and self-assembling into photonic crystals. Colloids Surf. A Physicochem. Eng. Asp. 2007, 298, 27–33. [Google Scholar] [CrossRef]
- Yu, F.; Yang, C.; Huang, G.; Zhou, T.; Zhao, Y.; Ma, J. Interfacial interaction between diverse microplastics and tetracycline by adsorption in an aqueous solution. Sci. Total Environ. 2020, 721, 137729. [Google Scholar] [CrossRef] [PubMed]
- Hailing, D.; Yingshuang, Z.; Hongru, J.; Hui, W. Adsorption of rhodamine B on polyvinyl chloride, polystyrene, and polyethylene terephthalate microplastics in aqueous environments. Environ. Technol. Innov. 2022, 27, 102495. [Google Scholar]
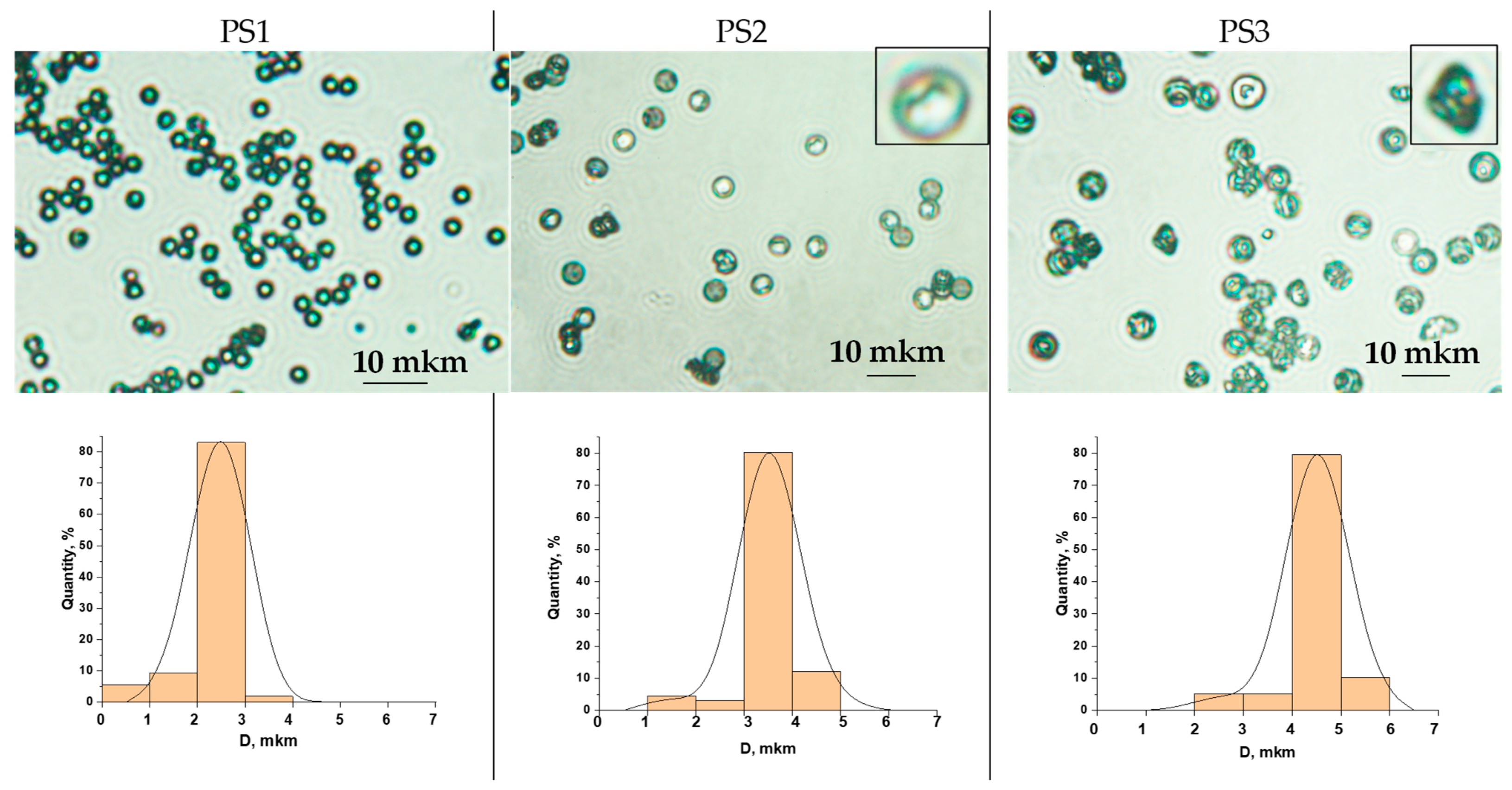

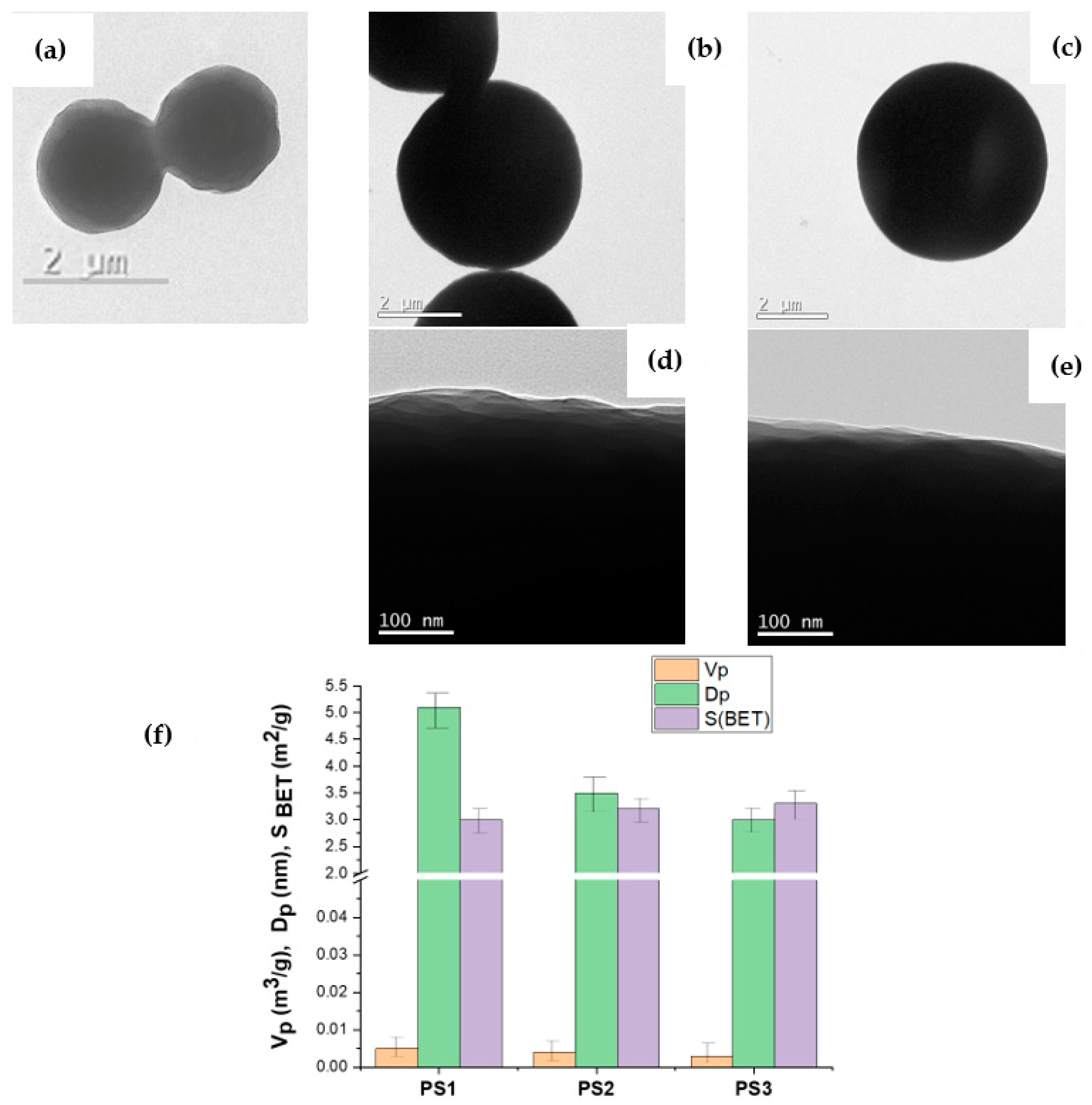


| Sample | D, mkm | CV 1 | Surface Area (S), m2/g | Shape | |
|---|---|---|---|---|---|
| Theoretical | Experimental | ||||
| PS1 | 2–3 | 17 | 2.9–1.9 | 3.0 | spherical |
| PS2 | 4 | 11 | 1.4 | 3.2 | sphere with one dent |
| PS3 | 5 | 15 | 1.1 | 3.3 | non-spherical |
| Parameters of the Langmuir Model | PS1 | PS1/M | PS2 | PS2/M | PS3 | PS3/M | |||
|---|---|---|---|---|---|---|---|---|---|
| 48 h | 96 h | 48 h | 96 h | 48 h | 96 h | ||||
| qexp μmol/g | 1.71 | 4.4 | 4.3 | 2.02 | 5.0 | 3.5 | 1.84 | 8.6 | 6.1 |
| qmax theory, μmol/g | 2.68 | 5.4 | 5.0 | 2.87 | 5.9 | 4.0 | 3.45 | 12.1 | 7.3 |
| R2 | 0.94 | 0.98 | 0.95 | 0.97 | 0.98 | 0.96 | 0.99 | 0.95 | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shevchenko, N.; Iakobson, O.; Isakov, V.; Zorin, I. Effects of Particle Shape and Surface Structure on the Adsorption Properties of Polystyrene Microplastics. Polymers 2024, 16, 3159. https://doi.org/10.3390/polym16223159
Shevchenko N, Iakobson O, Isakov V, Zorin I. Effects of Particle Shape and Surface Structure on the Adsorption Properties of Polystyrene Microplastics. Polymers. 2024; 16(22):3159. https://doi.org/10.3390/polym16223159
Chicago/Turabian StyleShevchenko, Natalia, Olga Iakobson, Vladimir Isakov, and Ivan Zorin. 2024. "Effects of Particle Shape and Surface Structure on the Adsorption Properties of Polystyrene Microplastics" Polymers 16, no. 22: 3159. https://doi.org/10.3390/polym16223159
APA StyleShevchenko, N., Iakobson, O., Isakov, V., & Zorin, I. (2024). Effects of Particle Shape and Surface Structure on the Adsorption Properties of Polystyrene Microplastics. Polymers, 16(22), 3159. https://doi.org/10.3390/polym16223159







