In Vitro Degradation of Collagen-Based Membranes for Guided Bone Regeneration After Zn-Ions or Doxycycline Functionalization
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of the Membranes
2.2. Membranes Functionalizacion
2.3. Degradation Assay
2.4. Statistical Analysis
3. Results
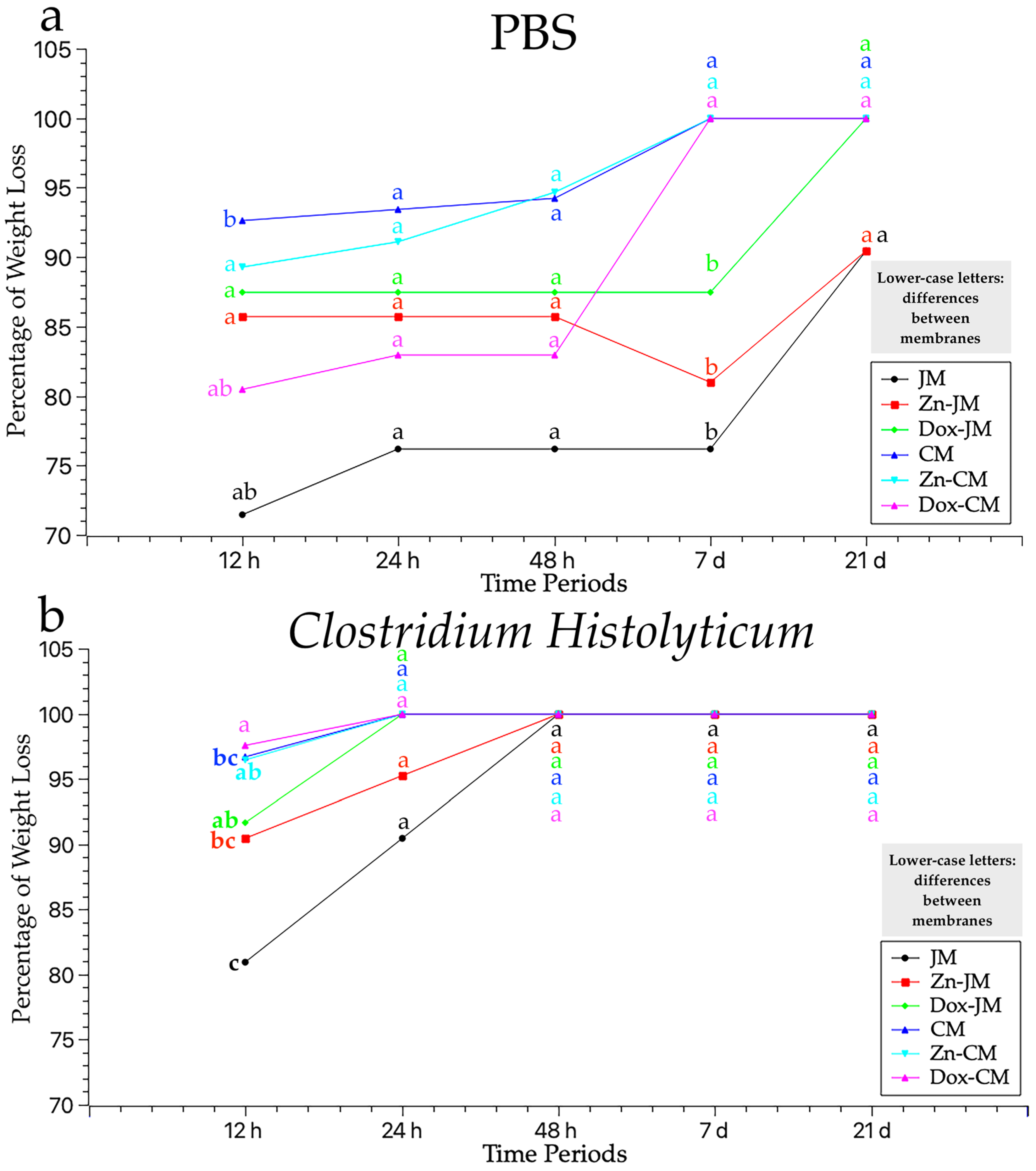
3.1. Thickness Assessment After PBS Degradation Assay
3.2. Thickness Assessment After C. histolyticum Collagenase Degradation Assay
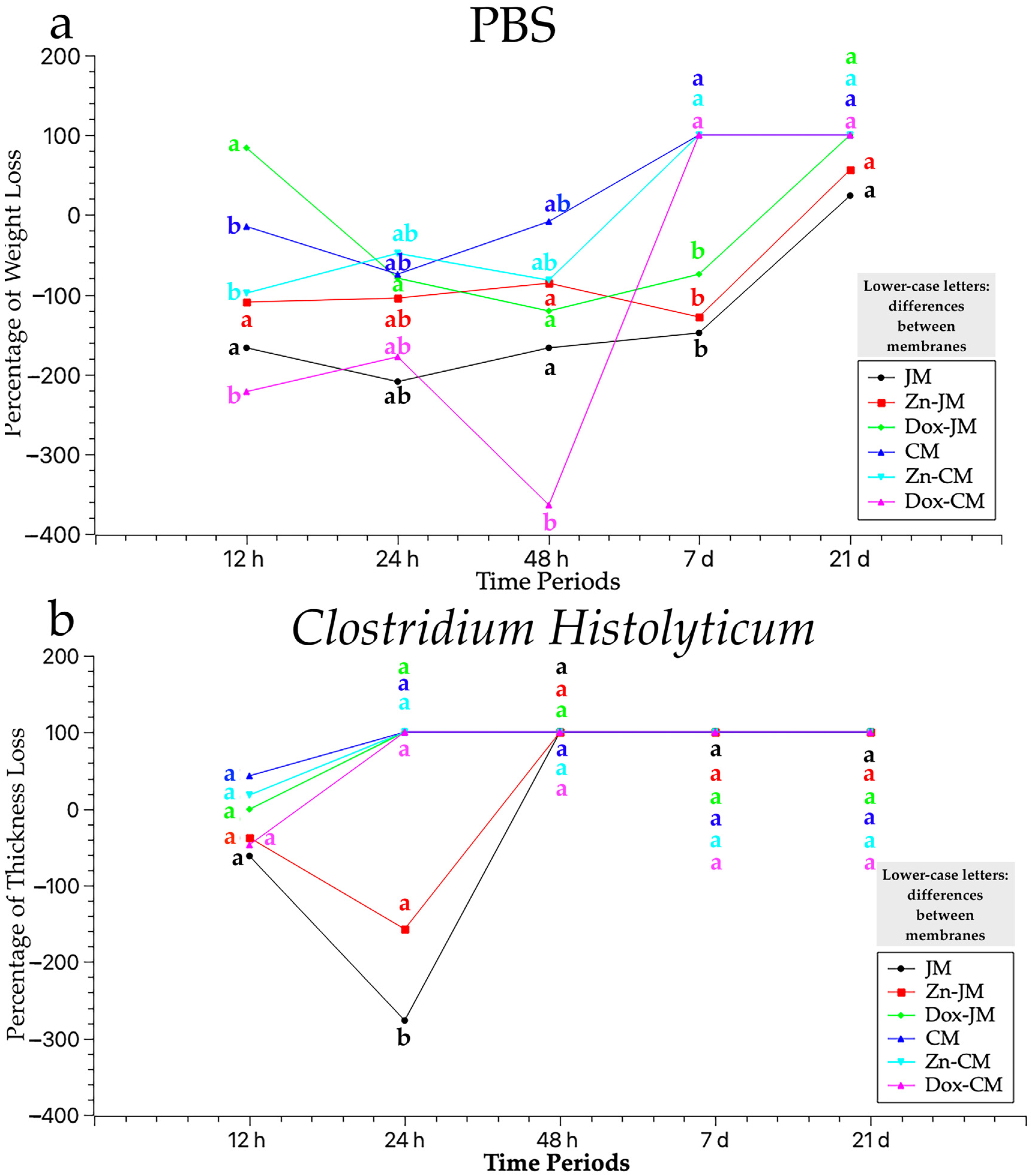
3.3. Weight Assessment After PBS Degradation Assay
3.4. Weight Evaluation After C. histolyticum Collagenase Degradation Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CH | C. histolyticum |
| CM | Unfunctionalized Collprotect® Membrane |
| Dox-CM | Collprotect® functionalized with doxycycline |
| Dox-JM | Jason® functionalized with doxycycline |
| GBR | Guided Bone Regeneration |
| GTR | Guided Tissue Regeneration |
| JM | Unfunctionalized Jason® membrane |
| MMP | Matrix Metalloproteinase |
| MNGCs | Multinucleated Giant Cells |
| PBS | Phosphate Buffer Saline |
| Th | Thickness |
| W | Weight |
| Zn-CM | Collprotect® functionalized with zinc-ions |
| Zn-JM | Jason® functionalized with zinc-ions |
References
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical Applications of Collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Ortolani, E.; Quadrini, F.; Bellisario, D.; Santo, L.; Polimeni, A.; Santarsiero, A. Mechanical Qualification of Collagen Membranes Used in Dentistry. Ann. Ist. Super. Sanita 2015, 51, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Gelin, A.; Masson-Meyers, D.; Amini, F.; Moharamzadeh, K.; Tayebi, L. Collagen: The Superior Material for Full-Thickness Oral Mucosa Tissue Engineering. J. Oral Biosci. 2024, 66, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Ottenbacher, N.; Alkildani, S.; Korzinskas, T.; Pissarek, J.; Ulm, C.; Jung, O.; Sundag, B.; Bellmann, O.; Stojanovic, S.; Najman, S.; et al. Novel Histomorphometrical Approach to Evaluate the Integration Pattern and Functionality of Barrier Membranes. Dent. J. 2021, 9, 127. [Google Scholar] [CrossRef]
- Barbeck, M.; Lorenz, J.; Kubesch, A.; Böhm, N.; Booms, P.; Choukroun, J.; Sader, R.; Kirkpatrick, C.J.; Ghanaati, S. Porcine Dermis-Derived Collagen Membranes Induce Implantation Bed Vascularization Via Multinucleated Giant Cells: A Physiological Reaction? J. Oral Implantol. 2015, 41, e238–e251. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, C.; Linde, A.; Gottlow, J.; Nyman, S. Healing of Bone Defects by Guided Tissue Regeneration. Plast. Reconstr. Surg. 1988, 81, 672–676. [Google Scholar] [CrossRef]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided Bone Regeneration: Materials and Biological Mechanisms Revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef]
- Bornert, F.; Herber, V.; Sandgren, R.; Witek, L.; Coelho, P.G.; Pippenger, B.E.; Shahdad, S. Comparative Barrier Membrane Degradation over Time: Pericardium Versus Dermal Membranes. Clin. Exp. Dent. Res. 2021, 7, 711–718. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V.; Janowski, G.M. A Novel Spatially Designed and Functionally Graded Electrospun Membrane for Periodontal Regeneration. Acta Biomater. 2011, 7, 216–224. [Google Scholar] [CrossRef]
- Bozkurt, A.; Apel, C.; Sellhaus, B.; van Neerven, S.; Wessing, B.; Hilgers, R.-D.; Pallua, N. Differences in Degradation Behavior of Two Non-Cross-Linked Collagen Barrier Membranes: An in Vitro and in Vivo Study. Clin. Oral Implant. Res. 2014, 25, 1403–1411. [Google Scholar] [CrossRef]
- Thoma, D.S.; Zeltner, M.; Hilbe, M.; Hämmerle, C.H.F.; Hüsler, J.; Jung, R.E. Randomized Controlled Clinical Study Evaluating Effectiveness and Safety of a Volume-Stable Collagen Matrix Compared to Autogenous Connective Tissue Grafts for Soft Tissue Augmentation at Implant Sites. J. Clin. Periodontol. 2016, 43, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Vallecillo-Rivas, M.; Toledano-Osorio, M.; Vallecillo, C.; Toledano, M.; Osorio, R. The Collagen Origin Influences the Degradation Kinetics of Guided Bone Regeneration Membranes. Polymers 2021, 13, 3007. [Google Scholar] [CrossRef] [PubMed]
- Soldatos, N.K.; Stylianou, P.; Koidou, V.P.; Angelov, N.; Yukna, R.; Romanos, G.E. Limitations and Options Using Resorbable versus Nonresorbable Membranes for Successful Guided Bone Regeneration. Quintessence Int. 2017, 48, 131–147. [Google Scholar] [CrossRef] [PubMed]
- Kapogianni, E.; Alkildani, S.; Radenkovic, M.; Xiong, X.; Krastev, R.; Stöwe, I.; Bielenstein, J.; Jung, O.; Najman, S.; Barbeck, M.; et al. The Early Fragmentation of a Bovine Dermis-Derived Collagen Barrier Membrane Contributes to Transmembraneous Vascularization—A Possible Paradigm Shift for Guided Bone Regeneration. Membranes 2021, 11, 185. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.; Asady, S.; Toledano-Osorio, M.; García-Godoy, F.; Serrera-Figallo, M.-A.; Benítez-García, J.A.; Osorio, R. Differential Biodegradation Kinetics of Collagen Membranes for Bone Regeneration. Polymers 2020, 12, 1290. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.-M.G.; Kowolik, M.J.; Janowski, G.M. Recent Advances in the Development of GTR/GBR Membranes for Periodontal Regeneration--a Materials Perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef]
- Dunn, R.M. Cross-Linking in Biomaterials: A Primer for Clinicians. Plast. Reconstr. Surg. 2012, 130, 18S–26S. [Google Scholar] [CrossRef]
- Vallecillo, C.; Toledano-Osorio, M.; Vallecillo-Rivas, M.; Toledano, M.; Osorio, R. In Vitro Biodegradation Pattern of Collagen Matrices for Soft Tissue Augmentation. Polymers 2021, 13, 2633. [Google Scholar] [CrossRef]
- Caballé-Serrano, J.; Munar-Frau, A.; Delgado, L.; Pérez, R.; Hernández-Alfaro, F. Physicochemical Characterization of Barrier Membranes for Bone Regeneration. J. Mech. Behav. Biomed. Mater. 2019, 97, 13–20. [Google Scholar] [CrossRef]
- Barrino, F.; Vassallo, V.; Cammarota, M.; Lepore, M.; Portaccio, M.; Schiraldi, C.; La Gatta, A. A Comprehensive in Vitro Characterization of Non-Crosslinked, Diverse Tissue-Derived Collagen-Based Membranes Intended for Assisting Bone Regeneration. PLoS ONE 2024, 19, e0298280. [Google Scholar] [CrossRef]
- Toledano, M.; Vallecillo, C.; Serrera-Figallo, M.-A.; Vallecillo-Rivas, M.; Gutierrez-Corrales, A.; Lynch, C.D.; Toledano-Osorio, M. Doped Electrospinned Material-Guides High Efficiency Regional Bone Regeneration. Polymers 2023, 15, 1726. [Google Scholar] [CrossRef] [PubMed]
- Toledano-Osorio, M.; Manzano-Moreno, F.J.; Ruiz, C.; Toledano, M.; Osorio, R. Testing Active Membranes for Bone Regeneration: A Review. J. Dent. 2021, 105, 103580. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Fan, L.; Alkildani, S.; Liu, L.; Emmert, S.; Najman, S.; Rimashevskiy, D.; Schnettler, R.; Jung, O.; Xiong, X.; et al. Barrier Membranes for Guided Bone Regeneration (GBR): A Focus on Recent Advances in Collagen Membranes. Int. J. Mol. Sci. 2022, 23, 14987. [Google Scholar] [CrossRef]
- Castillo-Dalí, G.; Castillo-Oyagüe, R.; Terriza, A.; Saffar, J.-L.; Batista-Cruzado, A.; Lynch, C.D.; Sloan, A.J.; Gutiérrez-Pérez, J.-L.; Torres-Lagares, D. Pre-Prosthetic Use of Poly(Lactic-Co-Glycolic Acid) Membranes Treated with Oxygen Plasma and TiO2 Nanocomposite Particles for Guided Bone Regeneration Processes. J. Dent. 2016, 47, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.; Vallecillo-Rivas, M.; Osorio, M.T.; Muñoz-Soto, E.; Toledano-Osorio, M.; Vallecillo, C.; Toledano, R.; Lynch, C.D.; Serrera-Figallo, M.-A.; Osorio, R. Zn-Containing Membranes for Guided Bone Regeneration in Dentistry. Polymers 2021, 13, 1797. [Google Scholar] [CrossRef] [PubMed]
- Yusa, K.; Yamamoto, O.; Fukuda, M.; Koyota, S.; Koizumi, Y.; Sugiyama, T. In Vitro Prominent Bone Regeneration by Release Zinc Ion from Zn-Modified Implant. Biochem. Biophys. Res. Commun. 2011, 412, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Osorio, R.; Yamauti, M.; Osorio, E.; Ruiz-Requena, M.E.; Pashley, D.H.; Tay, F.R.; Toledano, M. Zinc Reduces Collagen Degradation in Demineralized Human Dentin Explants. J. Dent. 2011, 39, 148–153. [Google Scholar] [CrossRef]
- Adamuz-Jiménez, A.; Manzano-Moreno, F.-J.; Vallecillo, C. Regeneration Membranes Loaded with Non-Antibiotic Anti-2 Microbials: A Review. Polymers 2023, 16, 95. [Google Scholar] [CrossRef]
- Toledano-Osorio, M.; Manzano-Moreno, F.J.; Toledano, M.; Medina-Castillo, A.L.; Costela-Ruiz, V.J.; Ruiz, C.; Osorio, R. Doxycycline-Doped Polymeric Membranes Induced Growth, Differentiation and Expression of Antigenic Phenotype Markers of Osteoblasts. Polymers 2021, 13, 1063. [Google Scholar] [CrossRef]
- Toledano-Osorio, M.; Manzano-Moreno, F.J.; Toledano, M.; Osorio, R.; Medina-Castillo, A.L.; Costela-Ruiz, V.J.; Ruiz, C. Doxycycline-Doped Membranes Induced Osteogenic Gene Expression on Osteoblastic Cells. J. Dent. 2021, 109, 103676. [Google Scholar] [CrossRef]
- Graziani, F.; Gennai, S.; Roldán, S.; Discepoli, N.; Buti, J.; Madianos, P.; Herrera, D. Efficacy of Periodontal Plastic Procedures in the Treatment of Multiple Gingival Recessions. J. Clin. Periodontol. 2014, 41 (Suppl. S15), S63–S76. [Google Scholar] [CrossRef] [PubMed]
- Bueno, J.; Sánchez, M.C.; Toledano-Osorio, M.; Figuero, E.; Toledano, M.; Medina-Castillo, A.L.; Osorio, R.; Herrera, D.; Sanz, M. Antimicrobial Effect of Nanostructured Membranes for Guided Tissue Regeneration: An in Vitro Study. Dent. Mater. 2020, 36, 1566–1577. [Google Scholar] [CrossRef]
- Yu, X.; Sun, H.; Yang, J.; Liu, Y.; Zhang, Z.; Wang, J.; Deng, F. Evaluation of Bone-Regeneration Effects and Ectopic Osteogenesis of Collagen Membrane Chemically Conjugated with Stromal Cell-Derived Factor-1 in Vivo. Biomed. Mater. 2019, 15, 015009. [Google Scholar] [CrossRef] [PubMed]
- Khorsand, B.; Elangovan, S.; Hong, L.; Kormann, M.S.D.; Salem, A.K. A Bioactive Collagen Membrane That Enhances Bone Regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.; Vallecillo, C.; Gutierrez-Corrales, A.; Torres-Lagares, D.; Toledano-Osorio, M.; Serrera-Figallo, M.-A. Histomorphometric Analysis of Differential Regional Bone Regeneration Induced by Distinct Doped Membranes. Polymers 2022, 14, 2078. [Google Scholar] [CrossRef] [PubMed]
- Kozlovsky, A.; Aboodi, G.; Moses, O.; Tal, H.; Artzi, Z.; Weinreb, M.; Nemcovsky, C.E. Bio-Degradation of a Resorbable Collagen Membrane (Bio-Gide) Applied in a Double-Layer Technique in Rats. Clin. Oral Implant. Res. 2009, 20, 1116–1123. [Google Scholar] [CrossRef]
- Sunandhakumari, V.J.; Vidhyadharan, A.K.; Alim, A.; Kumar, D.; Ravindran, J.; Krishna, A.; Prasad, M. Fabrication and In Vitro Characterization of Bioactive Glass/Nano Hydroxyapatite Reinforced Electrospun Poly(ε-Caprolactone) Composite Membranes for Guided Tissue Regeneration. Bioengineering 2018, 5, 54. [Google Scholar] [CrossRef]
- Sela, M.N.; Babitski, E.; Steinberg, D.; Kohavi, D.; Rosen, G. Degradation of Collagen-Guided Tissue Regeneration Membranes by Proteolytic Enzymes of Porphyromonas Gingivalis and Its Inhibition by Antibacterial Agents. Clin. Oral Implant. Res. 2009, 20, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.; Yoganarasimha, S.; Carrico, C.; Madurantakam, P. Incorporation of Fibrin Matrix into Electrospun Membranes for Periodontal Wound Healing. Bioengineering 2019, 6, 57. [Google Scholar] [CrossRef]
- Shi, X.; Li, X.; Tian, Y.; Qu, X.; Zhai, S.; Liu, Y.; Jia, W.; Cui, Y.; Chu, S. Physical, Mechanical, and Biological Properties of Collagen Membranes for Guided Bone Regeneration: A Comparative in Vitro Study. BMC Oral Health 2023, 23, 510. [Google Scholar] [CrossRef]
- Meyer, M. Processing of Collagen Based Biomaterials and the Resulting Materials Properties. Biomed. Eng. Online 2019, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Cavalu, S.; Roiu, G.; Pop, O.; Heredea, D.A.P.; Costea, T.O.; Costea, C.F. Nano-Scale Modifications of Amniotic Membrane Induced by UV and Antibiotic Treatment: Histological, AFM and FTIR Spectroscopy Evidence. Materials 2021, 14, 863. [Google Scholar] [CrossRef] [PubMed]
- Ratiu, C.; Brocks, M.; Costea, T.; Moldovan, L.; Cavalu, S. PRGF-Modified Collagen Membranes for Guided Bone Regeneration: Spectroscopic, Microscopic and Nano-Mechanical Investigations. Appl. Sci. 2019, 9, 1035. [Google Scholar] [CrossRef]
- Rothamel, D.; Schwarz, F.; Fienitz, T.; Smeets, R.; Dreiseidler, T.; Ritter, L.; Happe, A.; Zöller, J. Biocompatibility and Biodegradation of a Native Porcine Pericardium Membrane: Results of in Vitro and in Vivo Examinations. Int. J. Oral Maxillofac. Implant. 2012, 27, 146–154. [Google Scholar]
- Ramos, E.U.; Leandro, M.N.C.; Criales, J.O.C.; Buitron, M.R.O.; Verástegui, E.S.; Carbajal, W.M.; Adrianzén, R.C.S.; Grijalva, A.E.E.; Baylon, A.A.B.; Bassi, A.P.F. Evaluation of Porcine Collagen Membranes Used with Guided Bone Regeneration for Critical Defects: A Histological, Histomorphometric, Immunohistochemical, and Inflammatory Profile Analysis. Eur. J. Dent. 2024, 18, 898–906. [Google Scholar] [CrossRef]
- Sbricoli, L.; Guazzo, R.; Annunziata, M.; Gobbato, L.; Bressan, E.; Nastri, L. Selection of Collagen Membranes for Bone Regeneration: A Literature Review. Materials 2020, 13, 786. [Google Scholar] [CrossRef]
- Zhao, D.; Dong, H.; Niu, Y.; Fan, W.; Jiang, M.; Li, K.; Wei, Q.; Palin, W.M.; Zhang, Z. Electrophoretic Deposition of Novel Semi-Permeable Coatings on 3D-Printed Ti-Nb Alloy Meshes for Guided Alveolar Bone Regeneration. Dent. Mater. 2022, 38, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Gordon, J.; Hook, M. Collagen Binding Proteins of Gram-Positive Pathogens. Front. Microbiol. 2021, 12, 628798. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Wu, Z.; Leung, A.; Chen, X.; Landao-Bassonga, E.; Gao, J.; Chen, L.; Zheng, M.; Yao, F.; Yang, H.; et al. Fabrication of a Silver Nanoparticle-Coated Collagen Membrane with Anti-Bacterial and Anti-Inflammatory Activities for Guided Bone Regeneration. Biomed. Mater. 2018, 13, 065014. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, S.; Luo, P.; Deng, S.; Shan, Z.; Fang, J.; Liu, X.; Xie, J.; Liu, R.; Wu, S.; et al. Optimizing the Bio-Degradability and Biocompatibility of a Biogenic Collagen Membrane Through Cross-Linking and Zinc-Doped Hydroxyapatite. Acta Biomater. 2022, 143, 159–172. [Google Scholar] [CrossRef]
- Toledano-Osorio, M.; de Luna-Bertos, E.; Toledano, M.; Manzano-Moreno, F.J.; García-Recio, E.; Ruiz, C.; Osorio, R.; Sanz, M. Doxycycline-Doped Collagen Membranes Accelerate in Vitro Osteoblast Proliferation and Differentiation. J. Periodontal Res. 2023, 58, 296–307. [Google Scholar] [CrossRef]
- Mayrand, D.; Grenier, D. Detection of Collagenase Activity in Oral Bacteria. Can. J. Microbiol. 1985, 31, 134–138. [Google Scholar] [CrossRef] [PubMed]
| Attribute | Jason® Membrane | Collprotect® Membrane |
|---|---|---|
| Origin | Porcine pericardium | Porcine dermis |
| Composition | Native collagen type III | Native collagen type I and III |
| Crosslinking | No cross-linked | Naturally cross-linked |
| Structure | Natural multilayered collagen structure | Natural 3D collagen structure, rough and porous |
| Thickness | 0.05–0.35 mm | 0.4 mm |
| Degradation time | Slow degradation rate 12–24 weeks | Intermediate degradation rate 4–8 weeks |
| (a) | ||||||||||||
| JM | Zn-JM | Dox-JM | CM | Zn-CM | Dox-CM | |||||||
| PBS | C.H | PBS | C.H | PBS | C.H | PBS | C.H | PBS | C.H | PBS | C.H | |
| t0 | 0.021 (0.027) | 0.041 (0.004) | 0.021 (0.018) | 0.018 (0.025) | 0.024 (0.019) | 0.012 (0.016) | 0.122 (0.048) | 0.078 (0.089) | 0.056 (0.056) | 0.060 (0.076) | 0.041 (0.048) | 0.063 (0.048) |
| 12 h | 0.056 (0.021) | 0.034 (0.004) | 0.044 (0.010) | 0.029 (0.005) | 0.0038 (0.006) | 0.024 (0.005) | 0.140 (0.053) | 0.069 (0.058) | 0.111 (0.085) | 0.046 (0.037) | 0.132 (0.027) | 0.060 (0.048) |
| 24 h | 0.065 (0.011) | 0.079 (0.095) | 0.043 (0.010) | 0.054 (0.131) | 0.043 (0.036) | 0 (0) | 0.213 (0.100) | 0 (0) | 0.083 (0.090) | 0 (0) | 0.114 (0.069) | 0 (0) |
| 48 h | 0.056 (0.009) | 0 (0) | 0.039 (0.005) | 0 (0) | 0.053 (0.029) | 0 (0) | 0.132 (0.014) | 0 (0) | 0.102 (0.078) | 0 (0) | 0.190 (0.055) | 0 (0) |
| 7 d | 0.052 (0.005) | 0 (0) | 0.048 (0.014) | 0 (0) | 0.042 (0.011) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 21 d | 0.016 (0.023) | 0 (0) | 0.009 (0.013) | 0 (0) | 0.011 (0.017) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| (b) | ||||||||||||
| 0–12 h | 0.008 | 0.003 | 0.003 | 0.207 | <0.001 | 0.889 | 0.320 | 0.023 | 0.172 | 0.623 | <0.001 | <0.001 |
| 0–24 h | <0.001 | 0.251 | 0.005 | 0.434 | 0.020 | 0.001 | 0.026 | 0.018 | 0.505 | 0.032 | 0.020 | 0.001 |
| 0–48 h | 0.002 | <0.001 | 0.010 | 0.045 | <0.001 | 0.001 | 0.566 | 0.018 | 0.227 | 0.032 | <0.001 | 0.001 |
| 0–7 d | 0.004 | <0.001 | 0.002 | 0.045 | 0.021 | 0.001 | <0.001 | 0.018 | 0.040 | 0.032 | 0.021 | 0.001 |
| 0–21 d | 0.668 | <0.001 | 0.124 | 0.045 | 0.021 | 0.001 | <0.001 | 0.018 | 0.040 | 0.032 | 0.021 | 0.001 |
| (a) | ||||||||||||
| JM | Zn-JM | Dox-JM | CM | Zn-CM | Dox-CM | |||||||
| PBS | C.H | PBS | C.H | PBS | C.H | PBS | C.H | PBS | C.H | PBS | C.H | |
| t0 | 0.006 (0) | 0.005 (0.001) | 0.003 (0) | 0.003 (0) | 0.003 (0) | 0.003 (0) | 0.010 (0.001) | 0.009 (0.001) | 0.006 (0.005) | 0.038 (0.044) | 0.009 (0.001) | 0.009 (0.001) |
| 12 h | 0.006 (0) | 0.004 (0) | 0.003 (0) | 0.002 (0) | 0.003 (0) | 0.002 (0) | 0.009 (0.001) | 0.004 (0.003) | 0.006 (0.004) | 0.002 (0.001) | 0.008 (0.001) | 0.001 (0.002) |
| 24 h | 0.005 (0.001) | 0.002 (0.001) | 0.003 (0) | 0.001 (0.001) | 0.003 (0) | 0 (0) | 0.008 (0.001) | 0 (0) | 0.005 (0.004) | 0 (0) | 0.007 (0.001) | 0 (0) |
| 48 h | 0.005 (0) | 0 (0) | 0.003 (0) | 0 (0) | 0.003 (0) | 0 (0) | 0.007 (0) | 0 (0) | 0.003 (0.003) | 0 (0) | 0.007 (0.001) | 0 (0) |
| 7 d | 0.005 (0) | 0 (0) | 0.004 (0.001) | 0 (0) | 0.003 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 21 d | 0.002 (0.003) | 0 (0) | 0.002 (0.003) | 0 (0) | 0.001 (0.002) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| (b) | ||||||||||||
| 0–12 h | 0.304 | 0.002 | 0.084 | <0.001 | 0.035 | <0.001 | 0.070 | <0.001 | 0.116 | 0.025 | 0.035 | <0.001 |
| 0–24 h | 0.064 | <0.001 | 0.885 | <0.001 | <0.001 | <0.001 | 0.003 | <0.001 | 0.591 | 0.019 | <0.001 | <0.001 |
| 0–48 h | <0.001 | <0.001 | 1.000 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.118 | 0.019 | <0.001 | <0.001 |
| 0–7 d | 0.001 | <0.001 | 0.106 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | 0.019 | <0.001 | <0.001 |
| 0–21 d | <0.001 | <0.001 | 0.107 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | 0.019 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallecillo, C.; Osorio, M.T.; Infante, N.; Ávalos, M.J.; Vallecillo-Rivas, M.; Lynch, C.D.; Toledano, M. In Vitro Degradation of Collagen-Based Membranes for Guided Bone Regeneration After Zn-Ions or Doxycycline Functionalization. Polymers 2024, 16, 3109. https://doi.org/10.3390/polym16223109
Vallecillo C, Osorio MT, Infante N, Ávalos MJ, Vallecillo-Rivas M, Lynch CD, Toledano M. In Vitro Degradation of Collagen-Based Membranes for Guided Bone Regeneration After Zn-Ions or Doxycycline Functionalization. Polymers. 2024; 16(22):3109. https://doi.org/10.3390/polym16223109
Chicago/Turabian StyleVallecillo, Cristina, María T. Osorio, Nuria Infante, María Jesús Ávalos, Marta Vallecillo-Rivas, Christopher D. Lynch, and Manuel Toledano. 2024. "In Vitro Degradation of Collagen-Based Membranes for Guided Bone Regeneration After Zn-Ions or Doxycycline Functionalization" Polymers 16, no. 22: 3109. https://doi.org/10.3390/polym16223109
APA StyleVallecillo, C., Osorio, M. T., Infante, N., Ávalos, M. J., Vallecillo-Rivas, M., Lynch, C. D., & Toledano, M. (2024). In Vitro Degradation of Collagen-Based Membranes for Guided Bone Regeneration After Zn-Ions or Doxycycline Functionalization. Polymers, 16(22), 3109. https://doi.org/10.3390/polym16223109








