Surface Wetting Behaviors of Hydroxyl-Terminated Polybutadiene: Molecular Mechanism and Modulation
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
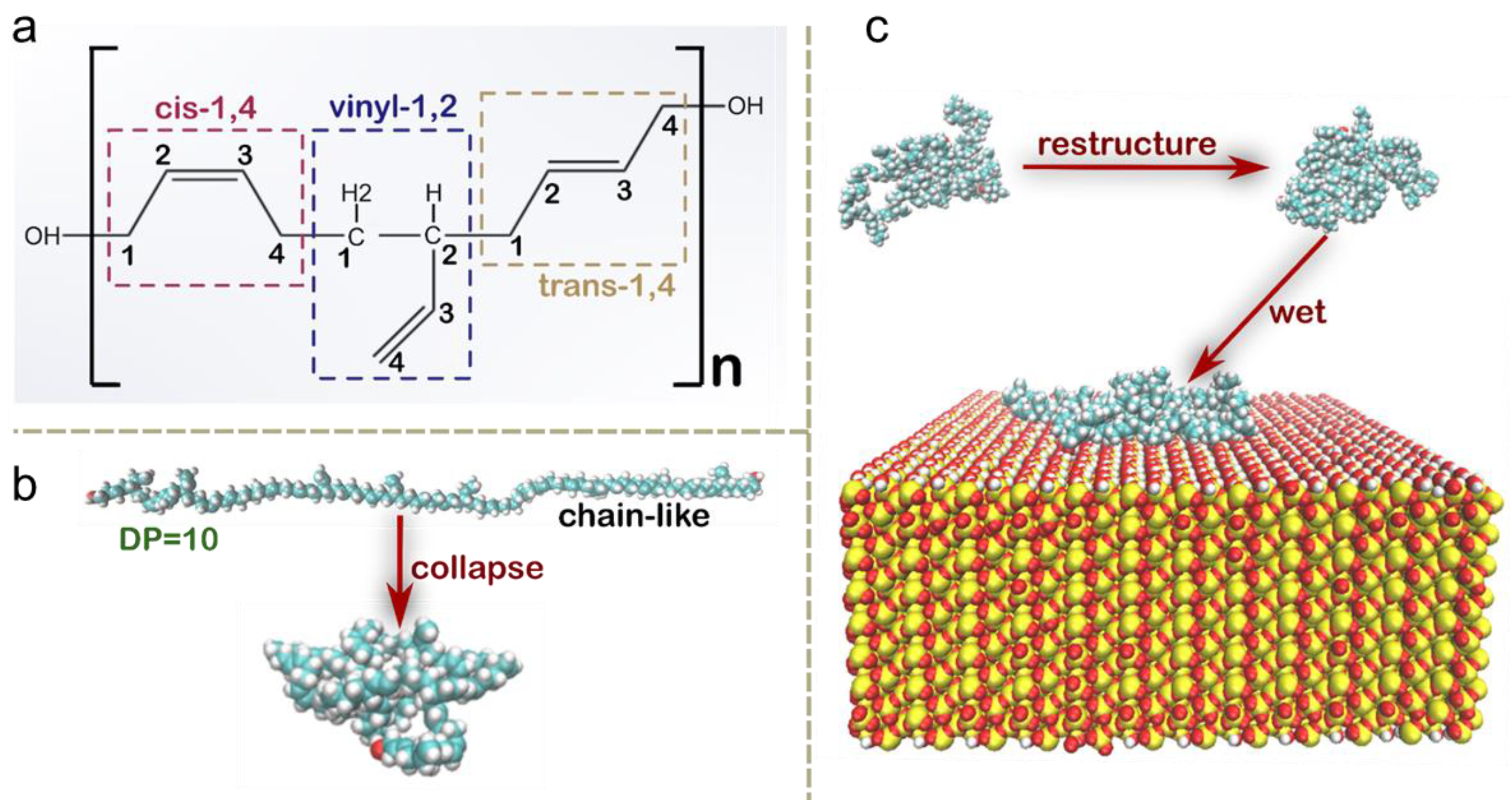
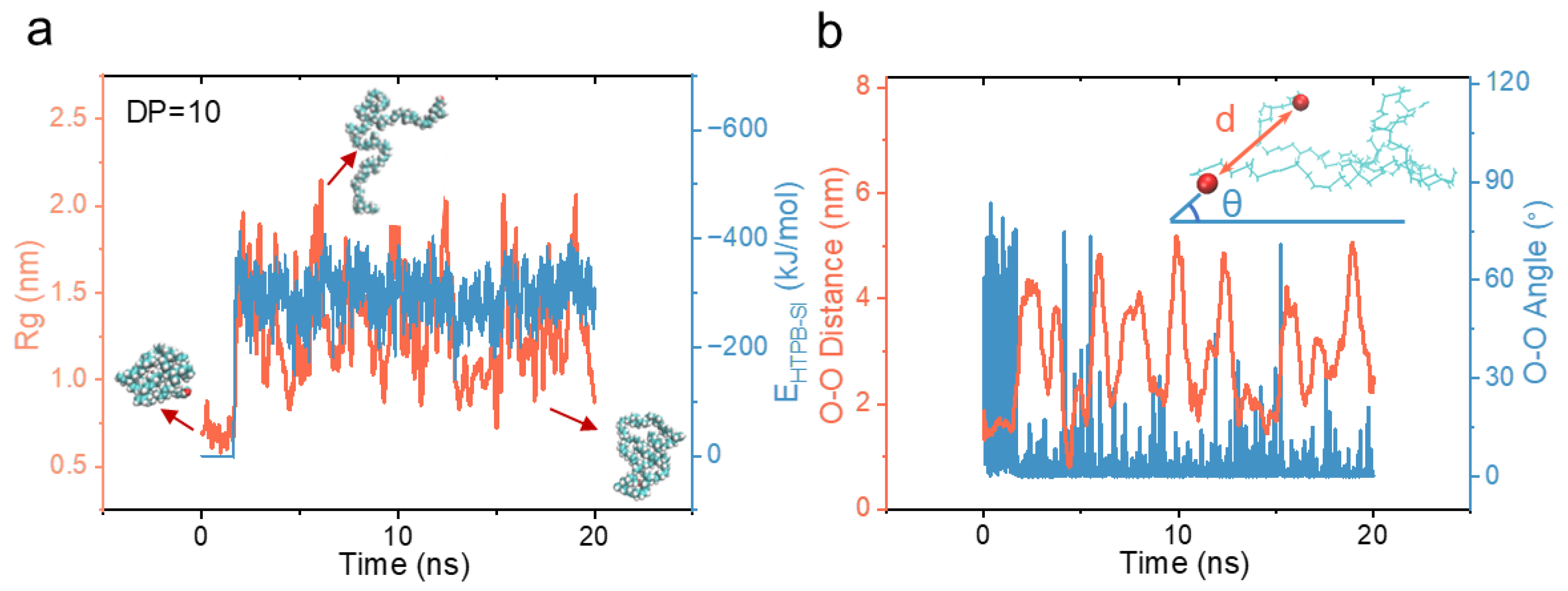
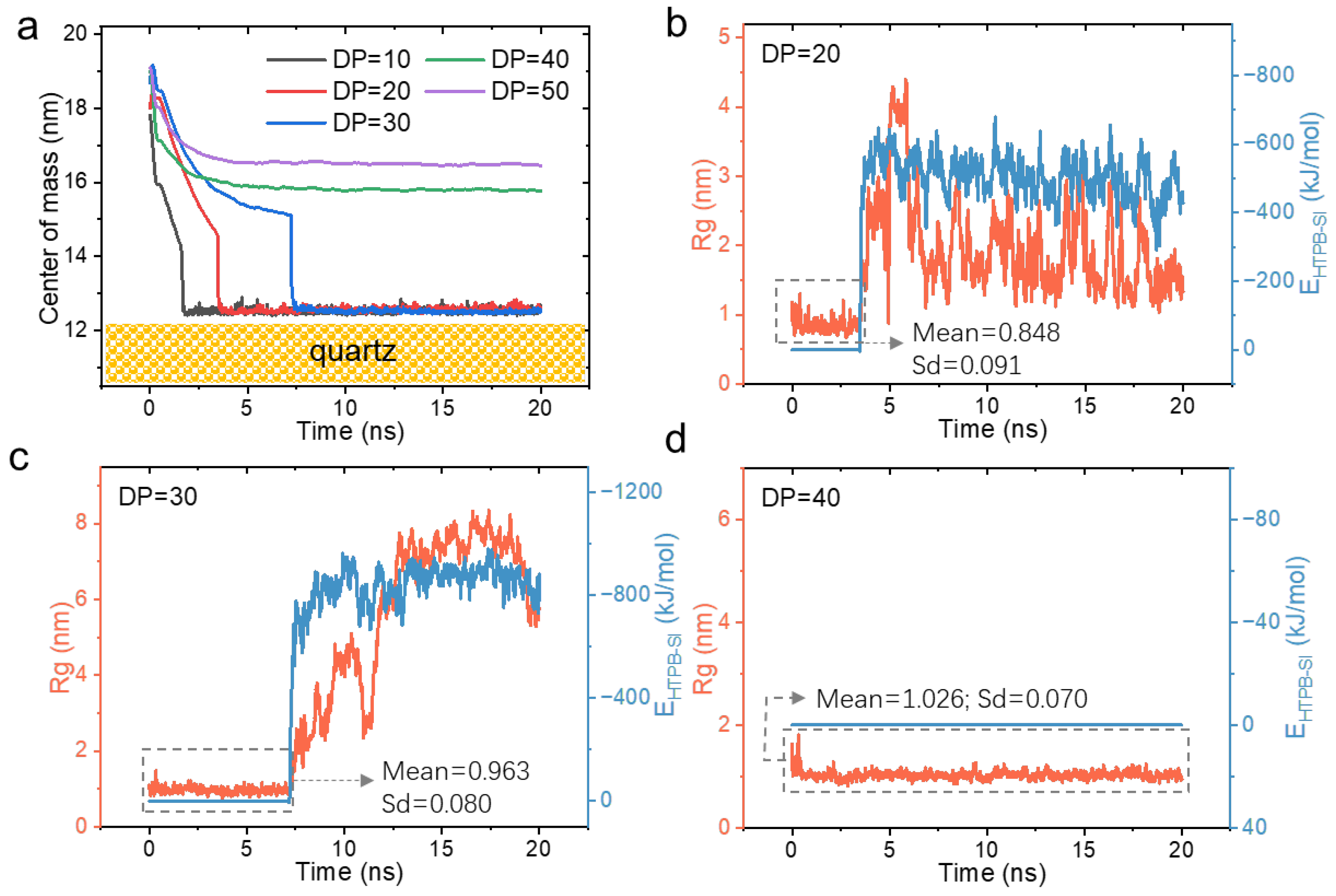
3.1. Molecular Conformational Transitions of HTPB During Its Surface Wetting Process
3.2. The Contributions of Enthalpy and Entropy in Determining the Surface Wetting of HTPB
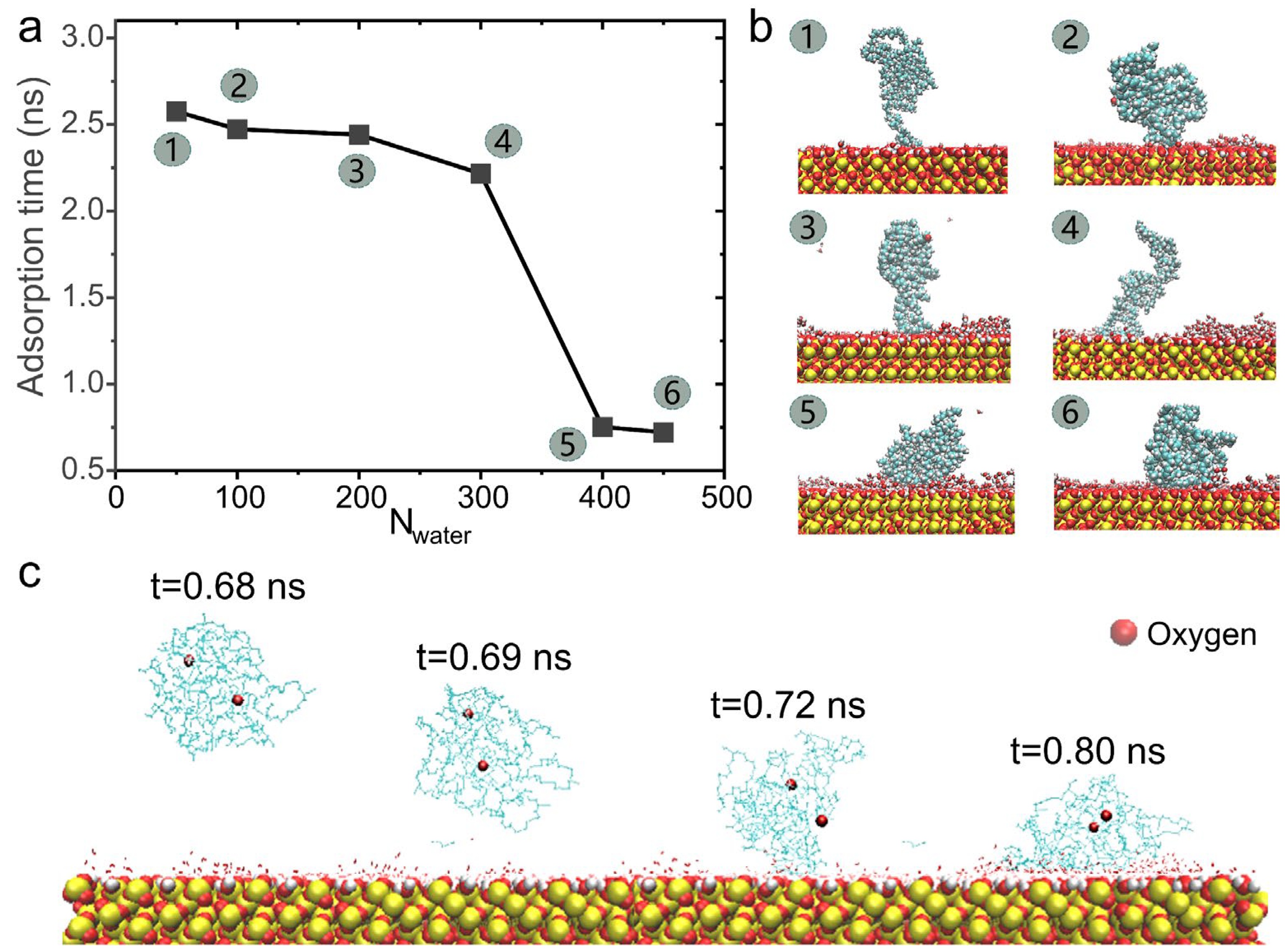
3.3. Regulation of the Surface Wetting Effect by Controlling Entropy–Enthalpy Competition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Du, C.; Wang, J.; Chen, Z.; Chen, D. Durable superhydrophobic and superoleophilic filter paper for oil–water separation prepared by a colloidal deposition method. Appl. Surf. Sci. 2014, 313, 304–310. [Google Scholar] [CrossRef]
- Dhakal, S.; Mondal, M.; Mirzazadeh, A.; Banerjee, S.; Ghosh, A.; Rangachari, V. α-Synuclein emulsifies TDP-43 prion-like domain-RNA liquid droplets to promote heterotypic amyloid fibrils. Commun. Biol. 2023, 6, 1227. [Google Scholar] [CrossRef] [PubMed]
- Karpitschka, S.; Das, S.; van Gorcum, M.; Perrin, H.; Andreotti, B.; Snoeijer, J.H. Droplets move over viscoelastic substrates by surfing a ridge. Nat. Commun. 2015, 6, 7891. [Google Scholar] [CrossRef] [PubMed]
- Ejenstam, L.; Ovaskainen, L.; Rodriguez-Meizoso, I.; Wagberg, L.; Pan, J.; Swerin, A.; Claesson, P.M. The effect of superhydrophobic wetting state on corrosion protection—The AKD example. J. Colloid Interface Sci. 2013, 412, 56–64. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Yang, J.H.; Li, L.L.; Cui, C.X.; Li, Y.; Liu, S.Q.; Zhou, X.M.; Qu, L.B. Facile Fabrication of superhydrophobic copper-foam and electrospinning polystyrene fiber for combinational oil-water separation. Polymers 2019, 11, 97. [Google Scholar] [CrossRef]
- Liu, K.; Yao, X.; Jiang, L. Recent developments in bio-inspired special wettability. Chem. Soc. Rev. 2010, 39, 3240–3255. [Google Scholar] [CrossRef]
- Chen, K.; Ren, Q.; Li, J.; Chen, D.; Li, C. A highly stretchable and self-healing hydroxy-terminated polybutadiene elastomer. J. Saudi. Chem. Soc. 2020, 24, 1034–1041. [Google Scholar] [CrossRef]
- Chang, K.; Jia, H.; Gu, S.Y. A transparent, highly stretchable, self-healing polyurethane based on disulfide bonds. Eur. Polym. J. 2019, 112, 822–831. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, J.; Hu, C.; Yang, B.; Ning, Z. Sustainable polyurethane networks with high self-healing and mechanical properties based on dual dynamic covalent bonds. Macromol. Chem. Phy. 2023, 224, 2200322. [Google Scholar] [CrossRef]
- Gopala Krishnan, P.S.; Ayyaswamy, K.; Nayak, S.K. Hydroxy terminated polybutadiene: Chemical modifications and applications. J. Macromol. Sci. A 2012, 50, 128–138. [Google Scholar] [CrossRef]
- Zhou, Q.; Jie, S.; Li, B.G. Preparation of hydroxyl-terminated polybutadiene with High Cis-1,4 Content. Ind. Eng. Chem. Res. 2014, 53, 17884–17893. [Google Scholar] [CrossRef]
- Chen, S.; Tang, Y.; Yu, H.; Guan, X.; DeLuca, L.T.; Zhang, W.; Shen, R.; Ye, Y. Combustion enhancement of hydroxyl-terminated polybutadiene by doping multiwall carbon nanotubes. Carbon 2019, 144, 472–480. [Google Scholar] [CrossRef]
- Malkappa, K.; Rao, B.N.; Suresh, G.; Ramana, C.V.; Jana, T. Organic/inorganic hybrid nanocolloids of water dispersible polyurethanes with antibacterial activity. Colloid Polym. Sci. 2017, 296, 95–106. [Google Scholar] [CrossRef]
- Sekkar, V.; Bhagawan, S.S.; Prabhakaran, N.; Rao, M.R.; Ninan, K.N. Polyurethanes based on hydroxyl terminated polybutadiene: Modelling of network parameters and correlation with mechanical properties. Polymer 2000, 41, 6773–6786. [Google Scholar] [CrossRef]
- Liu, Y.; He, J.; Xian, W.; Li, Y. Impact velocity and temperature effects on the shock wave propagation and spallation of hydroxyl-terminated polybutadiene: A molecular dynamics study. ACS Appl. Polym. Mater. 2023, 5, 8937–8948. [Google Scholar] [CrossRef]
- Yang, L.; Xie, K.; Pei, J.; Sui, X.; Wang, N. Compressive mechanical properties of HTPB propellant at low, intermediate, and high strain rates. J. Appl. Polym. Sci. 2016, 133, 43512. [Google Scholar] [CrossRef]
- Chen, X.; Lai, J.; Chang, X.L.; Zhang, Y.; Zhang, L.; Wang, C. Compressive mechanical properties of HTPB propellant at low temperatures and high strain rates. Results Phys. 2017, 7, 4079–4084. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, M.; Miao, Y.; Wang, H.; Chen, T.; Fan, X.; Chang, H. Dynamic mechanical response and damage mechanism of HTPB propellant under impact loading. Materials 2020, 13, 3031. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.M.; Lu, Z.J.; Pan, G.Q.; Qi, Y.X.; Yi, J.J.; Bai, H.J. Synthesis of hydroxyl-terminated polybutadiene possessing high content of 1,4-units via anionic polymerization. Chinese. J. Polym. Sci. 2010, 28, 715–720. [Google Scholar] [CrossRef]
- Lee, I.; Pathani, T.R.; Bates, F.S. Sustainable poly(lactide-b-butadiene) multiblock copolymers with enhanced mechanical properties. Macromolecules 2013, 46, 7387–7398. [Google Scholar] [CrossRef]
- Burelo, M.; Martinez, A.; Hernandez-Varela, J.D.; Stringer, T.; Ramirez-Melgarejo, M.; Yau, A.Y.; Luna-Barcenas, G.; Trevino-Quintanilla, C.D. Recent developments in synthesis, properties, applications and recycling of bio-based elastomers. Molecules 2024, 29, 387. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Yumei, D.; Yulong, H.; Le, Y.; Moldenaers, P.; Weimin, Y.; Czigany, T.; Thomas, S. Miscibility, morphology, thermal, and mechanical properties of a DGEBA based epoxy resin toughened with a liquid rubber. Polymer 2008, 49, 278–294. [Google Scholar] [CrossRef]
- Furusho, Y.; Endo, T. Supramolecular polymer gels formed from carboxy-terminated telechelic polybutadiene and polyamidine through amidinium-carboxylate salt bridge. J. Polym. Sci. Pol. Chem. 2014, 52, 1815–1824. [Google Scholar] [CrossRef]
- Yuan, B.; Wang, G.; Tian, W.; Zhou, L.; Li, C. Fabrication of hydroxy-terminated polybutadiene with piezoelectric property by functionalized branch chain modification. Molecules 2023, 28, 1810. [Google Scholar] [CrossRef]
- Ma, J.; Song, X.; Peng, B.; Zhao, T.; Luo, J.; Shi, R.; Zhao, S.; Liu, H. Multiscale molecular dynamics simulation study of polyoxyethylated alcohols self-assembly in emulsion systems. Chem. Eng. Sci. 2021, 231, 116252. [Google Scholar] [CrossRef]
- Taguta, J.; O’Connor, C.T.; McFadzean, B. The relationship between enthalpy of immersion and flotation response. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 263–270. [Google Scholar] [CrossRef]
- Wu, P.; Tan, B.T.; Jeong, J.I.; Yang, J.H.; Wu, S.N.; Anariba, F. Entropy of resilience: Formulism and validation using strained-induced surface wettability study on thin film alloys. J. Alloys Compd. 2020, 846, 156357. [Google Scholar] [CrossRef]
- Lowe, A.R.; Wong, W.S.Y.; Tsyrin, N.; Chorazewski, M.A.; Zaki, A.; Geppert-Rybczynska, M.; Stoudenets, V.; Tricoli, A.; Faik, A.; Grosu, Y. The Effect of surface entropy on the heat of non-wetting liquid intrusion into nanopores. Langmuir 2021, 37, 4827–4835. [Google Scholar] [CrossRef]
- Chien, C.C.; Shekar, S.; Niedzwiecki, D.J.; Shepard, K.L.; Drndic, M. Single-stranded DNA translocation recordings through solid-state nanopores on glass chips at 10 MHz measurement bandwidth. ACS Nano 2019, 13, 10545–10554. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, G.; Gao, L.; Xu, D.; Wan, H.; Dai, X.; Zhang, X.; Tao, L.; Yan, L.T. Configurational entropy-enabled thermostability of cell membranes in extremophiles: From molecular mechanism to bioinspired design. Nano Lett. 2023, 23, 1109–1118. [Google Scholar] [CrossRef]
- Wan, H.X.; Xu, D.; Dong, X.W.; Yang, K.; Yan, L.T. Insight into Biophysicochemical Principles of Biopolymers through Simulation and Theory. Chin. J. Polym. Sci. 2023, 41, 1342–1354. [Google Scholar] [CrossRef]
- Jiang, B.Y.; Zhou, Y.; Ji, B.; Zheng, Y.N.; Huang, J.S.; Wang, X.H. Investigation on the effect of functional groups on the wettability of coal dust: Experiments and theoretical validation. Fuel 2023, 351, 128987. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, R.; Gao, P.; Dai, Y.; Xia, F. Revealing the role of surface wettability in ionic detection signals of nanofluidic-based chemical sensors. Anal. Chem. 2022, 94, 16411–16417. [Google Scholar] [CrossRef]
- Dai, L.; Zhang, W.Q.; Ding, D.F.; Luo, C.H.; Jiang, L.; Huang, Y.; Xia, F. Outer-surface functionalized solid-state nanochannels for enhanced sensing properties: Progress and perspective. ACS Nano 2024, 18, 7677–7687. [Google Scholar] [CrossRef] [PubMed]
- Dhar, M.; Sarkar, D.; Das, A.; Rahaman, S.K.A.; Ghosh, D.; Manna, U. ‘Rewritable’ and ‘liquid-specific’ recognizable wettability pattern. Nat. Commun. 2024, 15, 5838. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Wang, S.K.; Zhou, L.; Li, X.; Zhang, H.C. The disentanglement and shear properties of amorphous polyethylene during friction: Insights from molecular dynamics simulations. Appl. Surf. Sci. 2022, 580, 152301. [Google Scholar] [CrossRef]
- Zheng, W.X.; Sun, C.Z.; Wen, B.Y.; Bai, B.F.; Lichtfouse, E. Effects of molecular chain length on the contact line movement in water/n-alkane/solid systems. Polymers 2019, 11, 2081. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Song, X.; Luo, J.; Zhao, T.; Yu, H.; Peng, B.; Zhao, S. Molecular dynamics simulation insight into interfacial stability and fluidity properties of microemulsions. Langmuir 2019, 35, 13636–13645. [Google Scholar] [CrossRef]
- Yang, K.; Ma, Y.Q. Computer simulation of the translocation of nanoparticles with different shapes across a lipid bilayer. Nat. Nanotechnol. 2010, 5, 579–583. [Google Scholar] [CrossRef]
- Ou, L.L.; Chen, H.B.; Yuan, B.; Yang, K. Membrane-specific binding of 4 nm lipid nanoparticles mediated by an entropy-driven interaction mechanism. ACS Nano 2022, 16, 18090–18100. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. ‘VMD: Visual molecular dynamics’. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Essmann, U.; Perera, L.; Berkowitz, M.L.; Darden, T.; Lee, H.; Pedersen, L.G. A smooth particle mesh Ewald method. J. Chem. Phys. 1995, 103, 8577–8593. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Dai, X.B.; Gao, L.J.; Xu, D.; Wan, H.X.; Wang, Y.M.; Yan, L.T. The entropy-controlled strategy in self-assembling systems. Chem. Soc. Rev. 2023, 52, 6806. [Google Scholar] [CrossRef] [PubMed]
- Jurado, E.; Vicaria, J.M.; García-Martín, J.F.; García-Román, M. Wettability of aqueous solutions of eco-friendly surfactants (ethoxylated alcohols and polyoxyethylene glycerin esters). J. Surfactants Deterg. 2012, 15, 251–258. [Google Scholar] [CrossRef]
- Bernardini, C.; Stoyanov, S.D.; Cohen Stuart, M.A.; Arnaudov, L.N.; Leermakers, A.M. Polymers at the water/air interface, surface pressure isotherms, and molecularly detailed modeling. Langmuir 2010, 26, 11850–11861. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Liu, Z.; Yuan, B.; Yang, K. Surface Wetting Behaviors of Hydroxyl-Terminated Polybutadiene: Molecular Mechanism and Modulation. Polymers 2024, 16, 3085. https://doi.org/10.3390/polym16213085
Zhang X, Liu Z, Yuan B, Yang K. Surface Wetting Behaviors of Hydroxyl-Terminated Polybutadiene: Molecular Mechanism and Modulation. Polymers. 2024; 16(21):3085. https://doi.org/10.3390/polym16213085
Chicago/Turabian StyleZhang, Xinke, Zhikun Liu, Bing Yuan, and Kai Yang. 2024. "Surface Wetting Behaviors of Hydroxyl-Terminated Polybutadiene: Molecular Mechanism and Modulation" Polymers 16, no. 21: 3085. https://doi.org/10.3390/polym16213085
APA StyleZhang, X., Liu, Z., Yuan, B., & Yang, K. (2024). Surface Wetting Behaviors of Hydroxyl-Terminated Polybutadiene: Molecular Mechanism and Modulation. Polymers, 16(21), 3085. https://doi.org/10.3390/polym16213085








