Innovative Polymeric Coatings with Dual Antifouling and Light-Activated Bactericidal Functions for Urinary Catheter Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis and Characterization of pSB and pSBCC
2.3. Preparation and Characterization of pSB and pSBCC Coatings
2.4. Quantitative Analysis of FITC-BSA Adsorption on pSB-Coated Substrates
2.5. Evaluation of L929 Cell Adhesion and Cytotoxicity of pSB-Coated Substrates
2.6. Assessment of Bacteria Adhesion to pSB or pSBCC Coated PDMS
2.7. Evaluation of Bactericidal Effects Under Light Illumination
2.8. Statistical Analysis
3. Results and Discussion
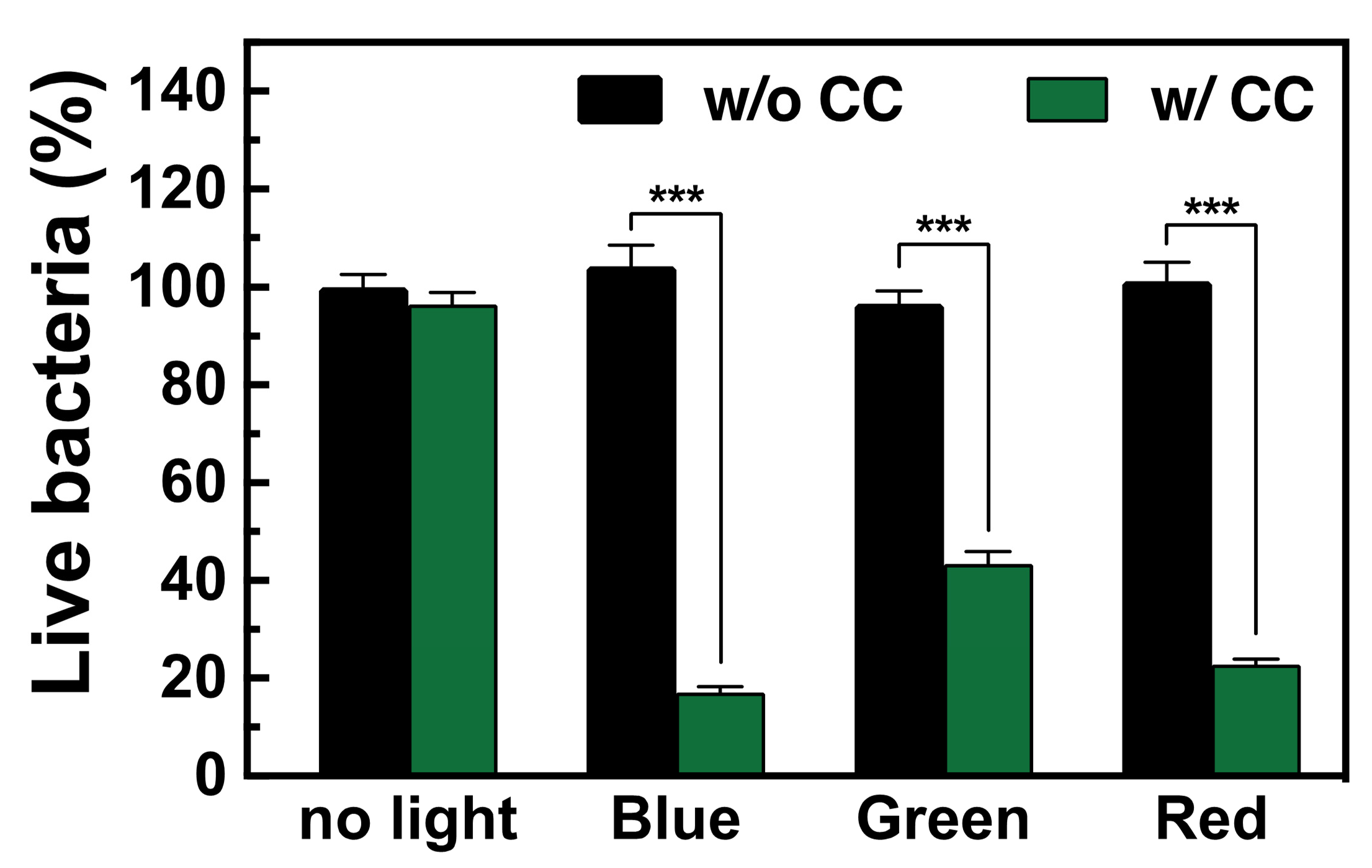
3.1. Light-Induced Bactericidal Properties of CC
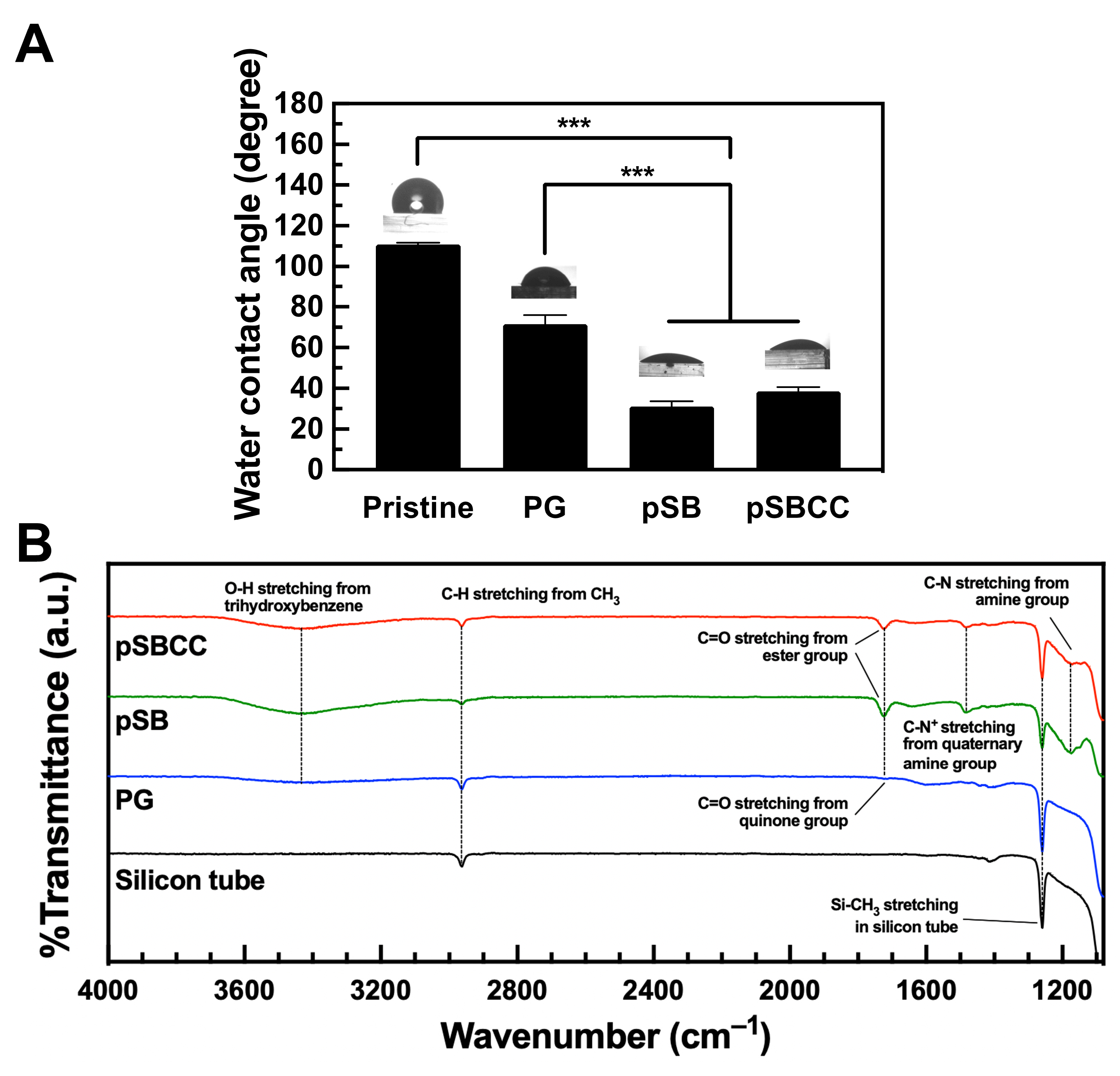
3.2. Characterization of pSB and pSBCC
3.3. Surface Characterization of pSB-Coated Substrates
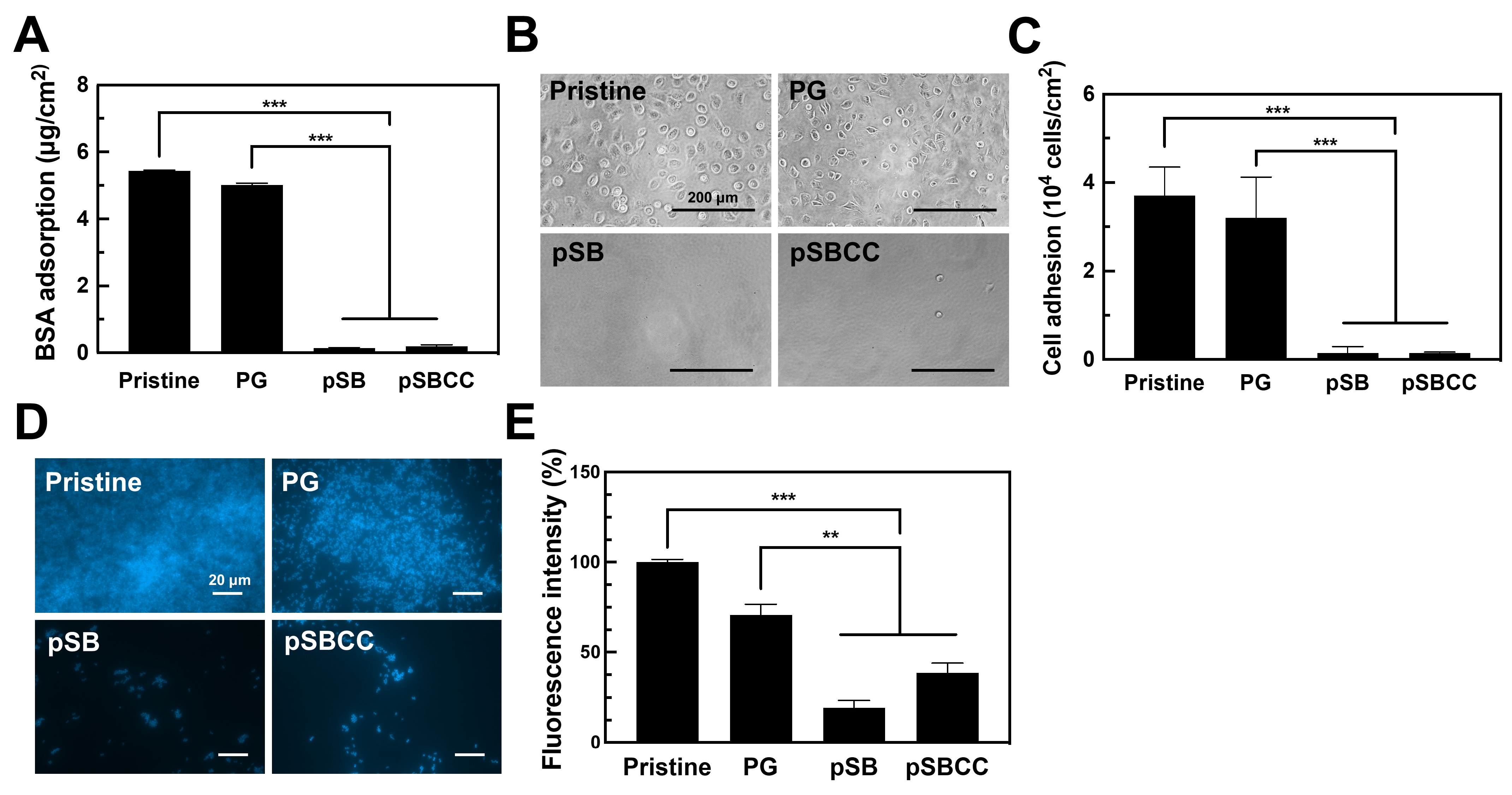
3.4. Antifouling Efficacy of pSB and pSBCC Coated Surfaces
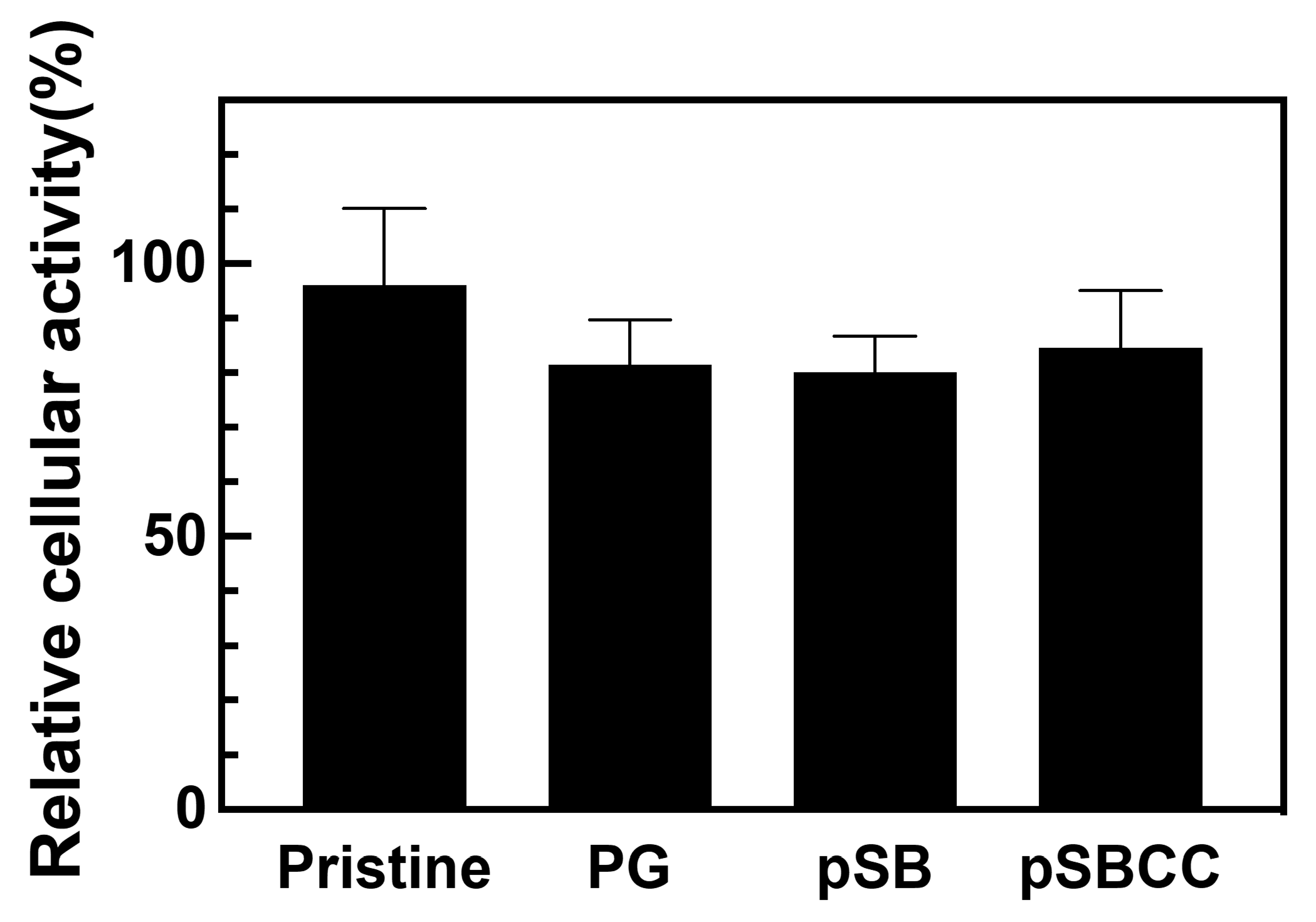
3.5. Cytotoxicity Assessment of pSB-Coated Silicone Tubes
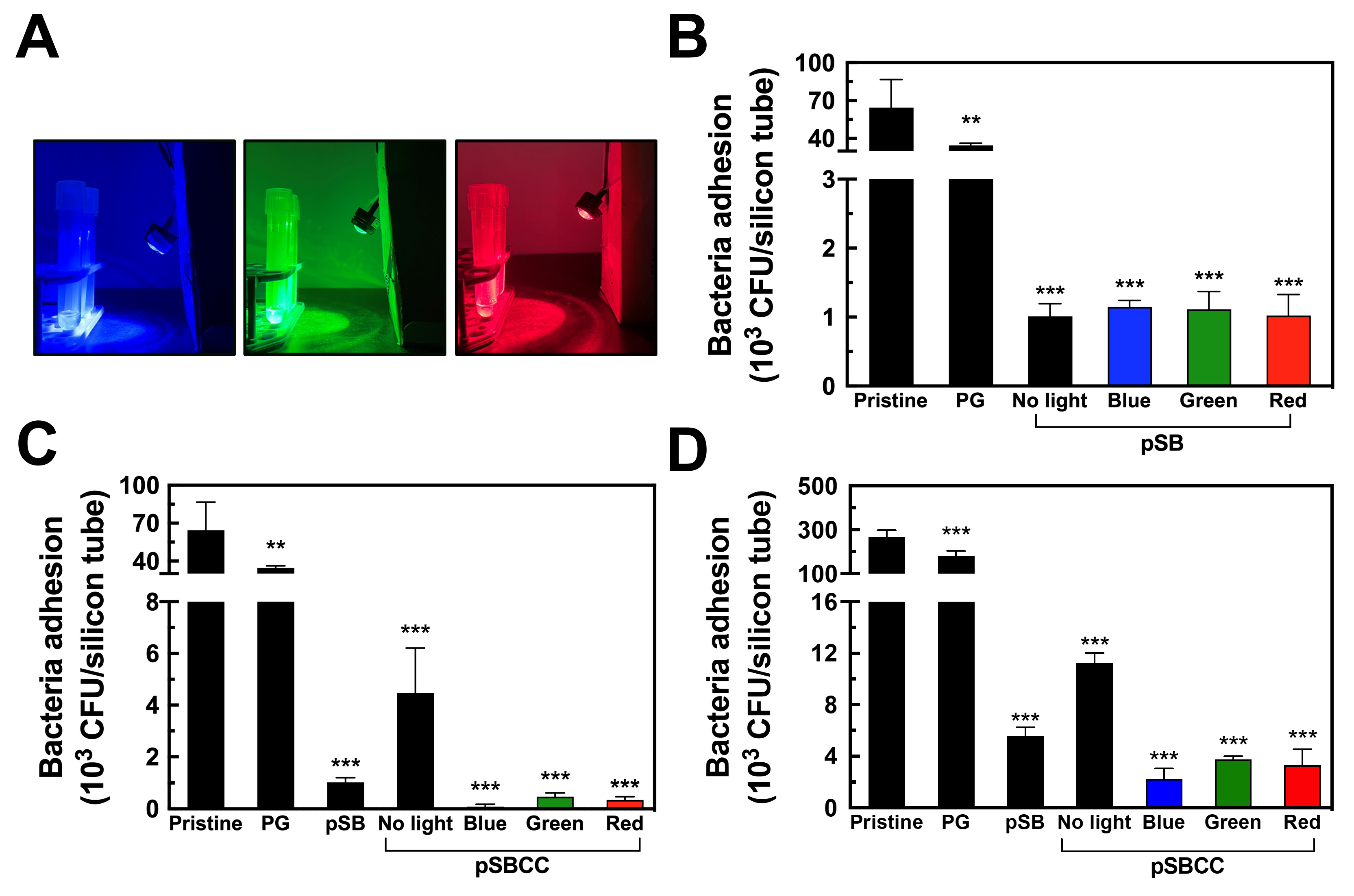
3.6. Bactericidal Efficacy of pSBCC Coating with Light Illumination
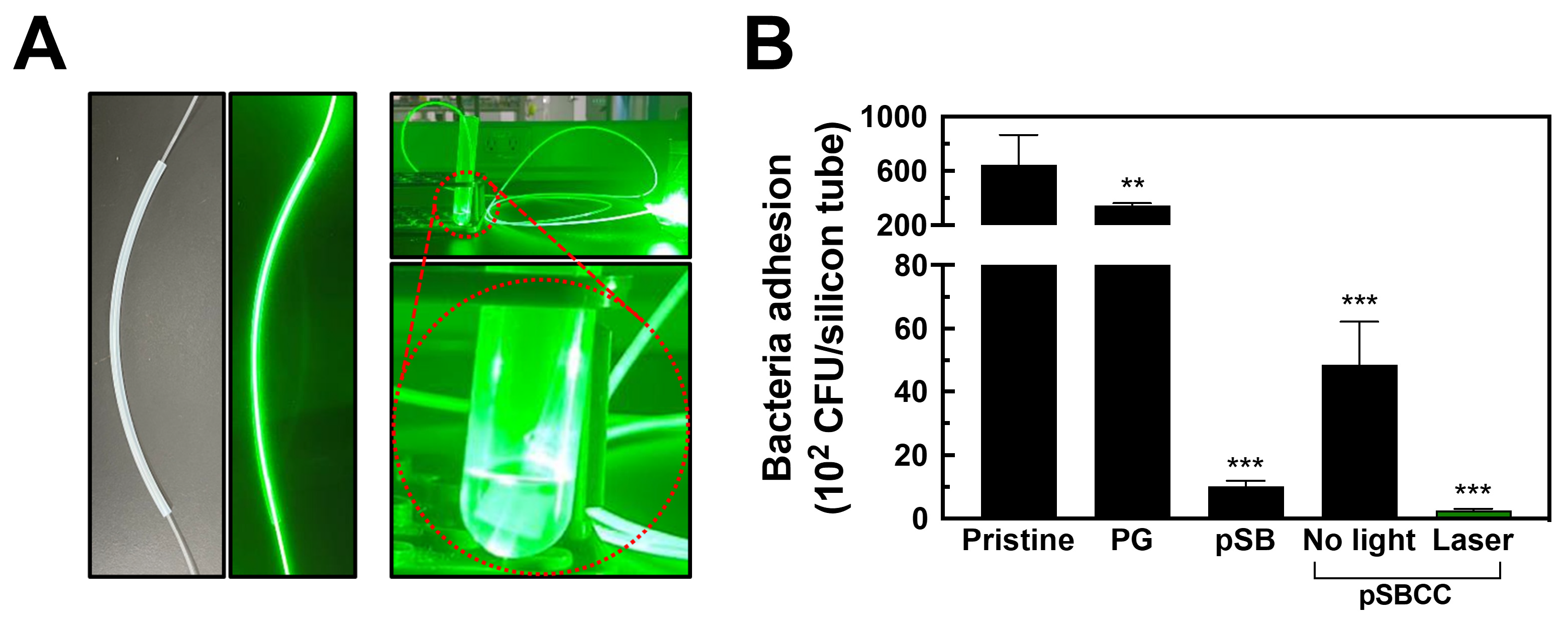
3.7. Irradiation of Bacteria Through Optical Fiber-Guided Green Laser Illumination Inside Silicone Tubes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warren, J.W. Catheter-associated urinary tract infections. Int. J. Antimicrob. Agents 2001, 17, 299–303. [Google Scholar] [CrossRef]
- Tambyah, P.A.; Oon, J. Catheter-associated urinary tract infection. Curr. Opin. Infect. Dis. 2012, 25, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Saint, S.; Greene, M.T.; Krein, S.L.; Rogers, M.A.; Ratz, D.; Fowler, K.E.; Edson, B.S.; Watson, S.R.; Meyer-Lucas, B.; Masuga, M.; et al. A Program to Prevent Catheter-Associated Urinary Tract Infection in Acute Care. N. Engl. J. Med. 2016, 374, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Yu, Q.; Chen, H. Responsive and Synergistic Antibacterial Coatings: Fighting Against Bacteria in a Smart and Effective Way. Adv. Healthc. Mater. 2019, 8, 1801381. [Google Scholar] [CrossRef] [PubMed]
- Singha, P.; Locklin, J.; Handa, H. A review of the recent advances in antimicrobial coatings for urinary catheters. Acta Biomater. 2017, 50, 20–40. [Google Scholar] [CrossRef]
- Yeh, S.L.; Deval, P.; Tsai, W.B. Fabrication of Transparent PEGylated Antifouling Coatings via One-Step Pyrogallol Deposition. Polymers 2023, 15, 2731. [Google Scholar] [CrossRef]
- Venault, A.; Wu, Y.L.; Maggay, I.V.; Chang, Y. Rapid attainment of full wettability through surface PEGylation of hydrophobic polyvinylidene fluoride membranes via spray-coating for enhanced anti-biofouling performances. Sep. Purif. Technol. 2024, 349, 127917. [Google Scholar] [CrossRef]
- Yeh, S.L.; Wang, T.C.; Yusa, S.; Thissen, H.; Tsai, W.B. Conjugation of Polysulfobetaine via Poly(pyrogallol) Coatings for Improving the Antifouling Efficacy of Biomaterials. Acs Omega 2021, 6, 3517–3524. [Google Scholar] [CrossRef]
- Chiao, Y.H.; Lin, H.T.; Ang, M.B.M.Y.; Teow, Y.H.; Wickramasinghe, S.R.; Chang, Y. Surface Zwitterionization via Grafting of Epoxylated Sulfobetaine Copolymers onto PVDF Membranes for Improved Permeability and Biofouling Mitigation. Ind. Eng. Chem. Res. 2023, 62, 2913–2923. [Google Scholar] [CrossRef]
- Song, B.Y.; Zhang, E.S.; Shi, Y.J.; Wang, W.; Zhu, H.; Gallagher, S.J.; Fischer, S.; Rigney, J.; Kim, E.; Cao, Z.Q. Zwitterionic Hydrogel Coating with Antisediment Properties for Marine Antifouling Applications. Acs Appl. Mater. Inter. 2024, 16, 27908–27916. [Google Scholar] [CrossRef]
- Yoshikawa, C.; Takagi, R.; Nakaji-Hirabayashi, T.; Ochi, T.; Kawamura, Y.; Thissen, H. Marine Antifouling Coatings Based on Durable Bottlebrush Polymers. Acs Appl. Mater. Inter. 2022, 14, 32497–32509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chao, T.; Chen, S.F.; Jiang, S.Y. Superlow fouling sulfobetaine and carboxybetaine polymers on glass slides. Langmuir 2006, 22, 10072–10077. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Ziats, N.P.; Tierney, B.P.; Nakabayashi, N.; Anderson, J.M. Protein adsorption from human plasma is reduced on phospholipid polymers. J. Biomed. Mater. Res. 1991, 25, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.Y.; Easton, C.D.; Thissen, H.; Tsai, W.B. Aminomalononitrile-Assisted Multifunctional Antibacterial Coatings. Acs Biomater. Sci. Eng. 2020, 6, 3349–3360. [Google Scholar] [CrossRef]
- Sakala, G.P.; Reches, M. Peptide-Based Approaches to Fight Biofouling. Adv. Mater. Interfaces 2018, 5, 1800073. [Google Scholar] [CrossRef]
- Li, F.Y.; Huang, T.; Pasic, P.; Easton, C.D.; Voelcker, N.H.; Heath, D.E.; O’Brien-Simpson, N.M.; O’Connor, A.J.; Thissen, H. One step antimicrobial coatings for medical device applications based on low fouling polymers containing selenium nanoparticles. Chem. Eng. J. 2023, 467, 143546. [Google Scholar] [CrossRef]
- Riool, M.; de Breij, A.; de Boer, L.; Kwakman, P.H.S.; Cordfunke, R.A.; Cohen, O.; Malanovic, N.; Emanuel, N.; Lohner, K.; Drijfhout, J.W.; et al. Controlled Release of LL-37-Derived Synthetic Antimicrobial and Anti-Biofilm Peptides SAAP-145 and SAAP-276 Prevents Experimental Biomaterial-Associated Infection. Adv. Funct. Mater. 2017, 27, 1606623. [Google Scholar] [CrossRef]
- Mitra, D.; Kang, E.T.; Neoh, K.G. Polymer-Based Coatings with Integrated Antifouling and Bactericidal Properties for Targeted Biomedical Applications. Acs Appl. Polym. Mater. 2021, 3, 2233–2263. [Google Scholar] [CrossRef]
- Su, C.C.; Ye, Y.M.; Qiu, H.F.; Zhu, Y.B. Solvent-Free Fabrication of Self-Regenerating Antibacterial Surfaces Resisting Biofilm Formation. Acs Appl. Mater. Inter. 2021, 13, 10553–10563. [Google Scholar] [CrossRef]
- Wang, J.L.; Wang, S.; Zhong, P.; Chen, Y.Y.; Zhao, Z.Z.; Liu, W.Q. Multimechanism Long-Term Antibacterial Coating with Simultaneous Antifouling, Contact-Active, and Release-Kill Properties. Acs Appl. Polym. Mater. 2024, 6, 5726–5737. [Google Scholar] [CrossRef]
- Li, W.L.; Hua, G.P.; Cai, J.F.; Zhou, Y.M.; Zhou, X.; Wang, M.; Wang, X.M.; Fu, B.Q.; Ren, L. Multi-Stimulus Responsive Multilayer Coating for Treatment of Device-Associated Infections. J. Funct. Biomater. 2022, 13, 24. [Google Scholar] [CrossRef] [PubMed]
- Li, K.J.; Tang, H.B.; Peng, J.Y.; Gao, S.; Du, Z.L.; Chen, G.; Wu, D.M.; Liu, G.Y. Smart Lubricant Coating with Urease-Responsive Antibacterial Functions for Ureteral Stents to Inhibit Infectious Encrustation. Adv. Funct. Mater. 2023, 34, 2307760. [Google Scholar] [CrossRef]
- Ricardo, S.I.C.; Anjos, I.I.L.; Monge, N.; Faustino, C.M.C.; Ribeiro, I.A.C. A glance at antimicrobial strategies to prevent catheter-associated medical infections. ACS Infect. Dis. 2020, 6, 3109–3130. [Google Scholar] [CrossRef] [PubMed]
- Misba, L.; Khan, A.U. Domestic LED bulb induced photodynamic effect of Toluidine Blue O-embedded silicone catheters against urinary tract infection. Photodiagn. Photodyn. Ther. 2023, 42, 103590. [Google Scholar] [CrossRef]
- Jalvo, B.; Faraldos, M.; Bahamonde, A.; Rosal, R. Antimicrobial and antibiofilm efficacy of self-cleaning surfaces functionalized by TiO2 photocatalytic nanoparticles against Staphylococcus aureus and Pseudomonas putida. J. Hazard. Mater. 2017, 340, 160–170. [Google Scholar] [CrossRef]
- Jiang, C.; Scholle, F.; Jin, F.; Wei, Q.; Wang, Q.; Ghiladi, R.A. Chlorophyllin as a photosensitizer in photodynamic antimicrobial materials. Cellulose 2024, 31, 2475–2491. [Google Scholar] [CrossRef]
- Clerici, D.J.; da Silveira, C.H.; Iglesias, B.A.; Santos, R.C.V. The first evidence of antibiofilm action of Proteus mirabilis with tetra-cationic porphyrins containing cisplatin by antimicrobial photodynamic therapy. Microb. Pathog. 2023, 174, 105859. [Google Scholar] [CrossRef] [PubMed]
- Caires, C.S.; Silva, C.M.; Lima, A.R.; Alves, L.M.; Lima, T.H.; Rodrigues, A.C.; Chang, M.R.; Oliveira, S.L.; Whitby, C.; Nascimento, V.A. Photodynamic inactivation of methicillin-resistant Staphylococcus aureus by a natural food colorant (E-141ii). Molecules 2020, 25, 4464. [Google Scholar] [CrossRef]
- Zhang, Z.; Qin, J.; Wang, Z.; Chen, F.; Liao, X.; Hu, X.; Dong, L. Sodium copper chlorophyll mediated photodynamic treatment inactivates Escherichia coli via oxidative damage. Food Res. Int. 2022, 157, 111472. [Google Scholar] [CrossRef]
- Phasupan, P.; Le, T.D.; Nguyen, L.T. Assessing the photodynamic efficacy of different photosensitizer-light treatments against foodborne bacteria based on the number of absorbed photons. J. Photochem. Photobiol. B Biol. 2021, 221, 112249. [Google Scholar] [CrossRef]
- Liu, X.X.; Yu, L.F.; Wei, J.F.; Huang, Y.Y.; Yang, L.; Ning, J.H.; Su, Q.P.; Li, H.L.; Xin, J.L.; Jia, K.L. Mussel-Inspired Antimicrobial and Antifouling Coating Constructed by the Combination of Zwitterionic Copolymers and Silver Nanoparticles. Langmuir 2024, 40, 8654–8664. [Google Scholar] [CrossRef]
- Song, B.Y.; Zhang, E.S.; Han, X.F.; Zhu, H.; Shi, Y.J.; Cao, Z.Q. Engineering and Application Perspectives on Designing an Antimicrobial Surface. Acs Appl. Mater. Inter. 2020, 12, 21330–21341. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.W.; Tsai, W.B. Fabrication of tunable micropatterned substrates for cell patterning via microcontact printing of polydopamine with poly(ethylene imine)-grafted copolymers. Acta Biomater. 2012, 8, 3678–3686. [Google Scholar] [CrossRef]
- Meng, L.W.; Huang, C.X.; Liu, X.; Qu, H.Y.; Wang, Q.L. Zwitterionic coating assisted by dopamine with metal-phenolic networks loaded on titanium with improved biocompatibility and antibacterial property for artificial heart. Front. Bioeng. Biotech. 2023, 11, 1167340. [Google Scholar] [CrossRef] [PubMed]
- Sileika, T.S.; Kim, H.-D.; Maniak, P.; Messersmith, P.B. Antibacterial Performance of Polydopamine-Modified Polymer Surfaces Containing Passive and Active Components. Acs Appl. Mater. Inter. 2011, 3, 4602–4610. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D.G.; Sileika, T.S.; Messersmith, P.B. Molecular diversity in phenolic and polyphenolic precursors of tannin-inspired nanocoatings. Chem. Commun. 2014, 50, 7265–7268. [Google Scholar] [CrossRef]
- Dhand, C.; Harini, S.; Venkatesh, M.; Dwivedi, N.; Ng, A.; Liu, S.P.; Verma, N.K.; Ramakrishna, S.; Beuerman, R.W.; Loh, X.J.; et al. Multifunctional Polyphenols- and Catecholamines-Based Self-Defensive Films for Health Care Applications. Acs Appl. Mater. Inter. 2016, 8, 1220–1232. [Google Scholar] [CrossRef]
- Drynan, J.W.; Clifford, M.N.; Obuchowicz, J.; Kuhnert, N. The chemistry of low molecular weight black tea polyphenols. Nat. Prod. Rep. 2010, 27, 417–462. [Google Scholar] [CrossRef]
- Sileika, T.S.; Barrett, D.G.; Zhang, R.; Lau, K.H.A.; Messersmith, P.B. Colorless Multifunctional Coatings Inspired by Polyphenols Found in Tea, Chocolate, and Wine. Angew. Chem. Int. Edit. 2013, 52, 10766–10770. [Google Scholar] [CrossRef]
- Deval, P.; Lin, C.H.; Tsai, W.B. Fabrication of Polysulfobetaine Gradient Coating via Oxidation Polymerization of Pyrogallol to Modulate Biointerfaces. Acs Omega 2022, 7, 7125–7133. [Google Scholar] [CrossRef]
- Yeh, S.L.; Deval, P.; Wu, J.G.; Luo, S.C.; Tsai, W.B. One-step electrochemical deposition of antifouling polymers with pyrogallol for biosensing applications. J. Mater. Chem. B 2022, 10, 2504–2511. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Liao, T.-Y.; Thissen, H.; Tsai, W.-B. One-step aminomalononitrile-based coatings containing zwitterionic copolymers for the reduction of biofouling and the foreign body response. Acs Biomater. Sci. Eng. 2019, 5, 6454–6462. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.; Neal, S.L. Efficiency comparison of the imidazole plus RNO method for singlet oxygen detection in biorelevant solvents. Anal. Bioanal. Chem. 2019, 411, 5287–5296. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Garcez, A.; Barros, L.; Fernandes, M.; Fujii, D.; Suzuki, S.; Nepomuceno, R. Fluorescence image and microbiological analysis of biofilm retained around healthy and inflamed orthodontic miniscrews. Photodiagn. Photodyn. Ther. 2020, 30, 101707. [Google Scholar] [CrossRef]
- Chang, R.; Hsu, C.-F.; Tsai, W.-B. Fabrication of chlorophyll-incorporated nanogels for potential applications in photothermal cancer therapy. Acs Omega 2018, 3, 16057–16062. [Google Scholar] [CrossRef]
- Chang, R.; Tsai, W.B. Fabrication of Photothermo-Responsive Drug-Loaded Nanogel for Synergetic Cancer Therapy. Polymers 2018, 10, 1098. [Google Scholar] [CrossRef]
- An, T.; Lee, N.; Cho, H.-J.; Kim, S.; Shin, D.-S.; Lee, S.-M. Ultra-selective detection of Fe2+ ion by redox mechanism based on fluorescent polymerized dopamine derivatives. RSC Adv. 2017, 7, 30582–30587. [Google Scholar] [CrossRef]
- Huang, L.; Xuan, Y.; Koide, Y.; Zhiyentayev, T.; Tanaka, M.; Hamblin, M.R. Type I and Type II mechanisms of antimicrobial photodynamic therapy: An in vitro study on gram-negative and gram-positive bacteria. Lasers Surg. Med. 2012, 44, 490–499. [Google Scholar] [CrossRef]
- Heo, S.-Y.; Lee, Y.; Kim, T.-H.; Heo, S.-J.; Shin, H.; Lee, J.; Yi, M.; Kang, H.W.; Jung, W.-K. Anti-Cancer Effect of Chlorophyllin-Assisted Photodynamic Therapy to Induce Apoptosis through Oxidative Stress on Human Cervical Cancer. Int. J. Mol. Sci. 2023, 24, 11565. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Y.; Gao, J.; Jin, X.; Wang, Z.; Wang, B.; Li, K.; Li, Y. Detection and comparison of reactive oxygen species (ROS) generated by chlorophyllin metal (Fe, Mg and Cu) complexes under ultrasonic and visible-light irradiation. Ultrason. Sonochem. 2011, 18, 1028–1034. [Google Scholar] [CrossRef]
- Buchovec, I.; Lukseviciūtė, V.; Kokstaite, R.; Labeikyte, D.; Kaziukonyte, L.; Luksiene, Z. Inactivation of Gram (−) bacteria Salmonella enterica by chlorophyllin-based photosensitization: Mechanism of action and new strategies to enhance the inactivation efficiency. J. Photochem. Photobiol. B Biol. 2017, 172, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Pablos, C.; Marugán, J.; van Grieken, R.; Hamilton, J.W.; Ternan, N.G.; Dunlop, P.S. Assessment of Photoactivated Chlorophyllin Production of Singlet Oxygen and Inactivation of Foodborne Pathogens. Catalysts 2024, 14, 507. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.-H.; Chen, G.-H.; Tsai, W.-B. Innovative Polymeric Coatings with Dual Antifouling and Light-Activated Bactericidal Functions for Urinary Catheter Applications. Polymers 2024, 16, 2974. https://doi.org/10.3390/polym16212974
Chen P-H, Chen G-H, Tsai W-B. Innovative Polymeric Coatings with Dual Antifouling and Light-Activated Bactericidal Functions for Urinary Catheter Applications. Polymers. 2024; 16(21):2974. https://doi.org/10.3390/polym16212974
Chicago/Turabian StyleChen, Po-Hsun, Guan-Hua Chen, and Wei-Bor Tsai. 2024. "Innovative Polymeric Coatings with Dual Antifouling and Light-Activated Bactericidal Functions for Urinary Catheter Applications" Polymers 16, no. 21: 2974. https://doi.org/10.3390/polym16212974
APA StyleChen, P.-H., Chen, G.-H., & Tsai, W.-B. (2024). Innovative Polymeric Coatings with Dual Antifouling and Light-Activated Bactericidal Functions for Urinary Catheter Applications. Polymers, 16(21), 2974. https://doi.org/10.3390/polym16212974






