Preparation of Lyocell Fibers from Solutions of Miscanthus Cellulose
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Dopes
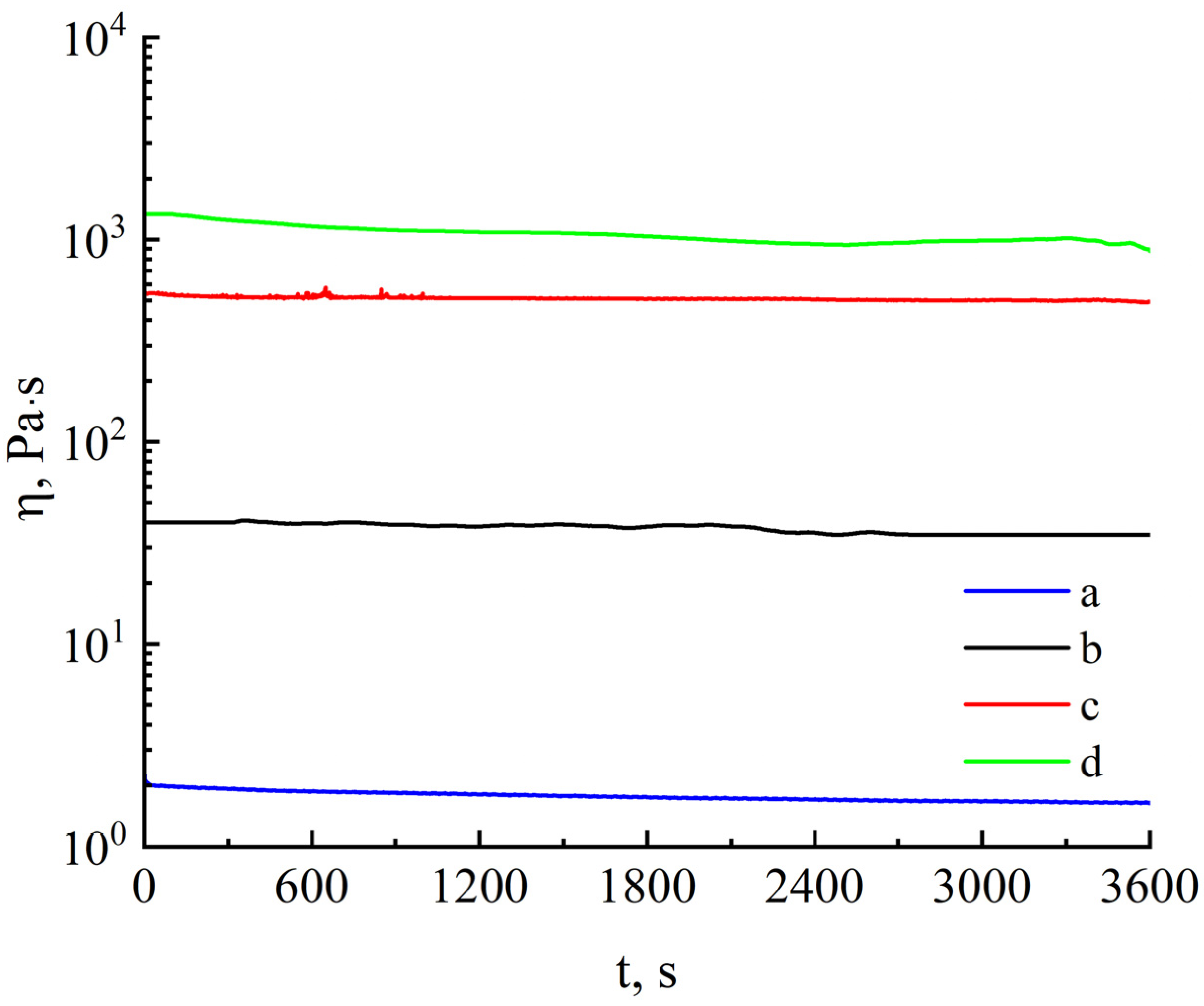
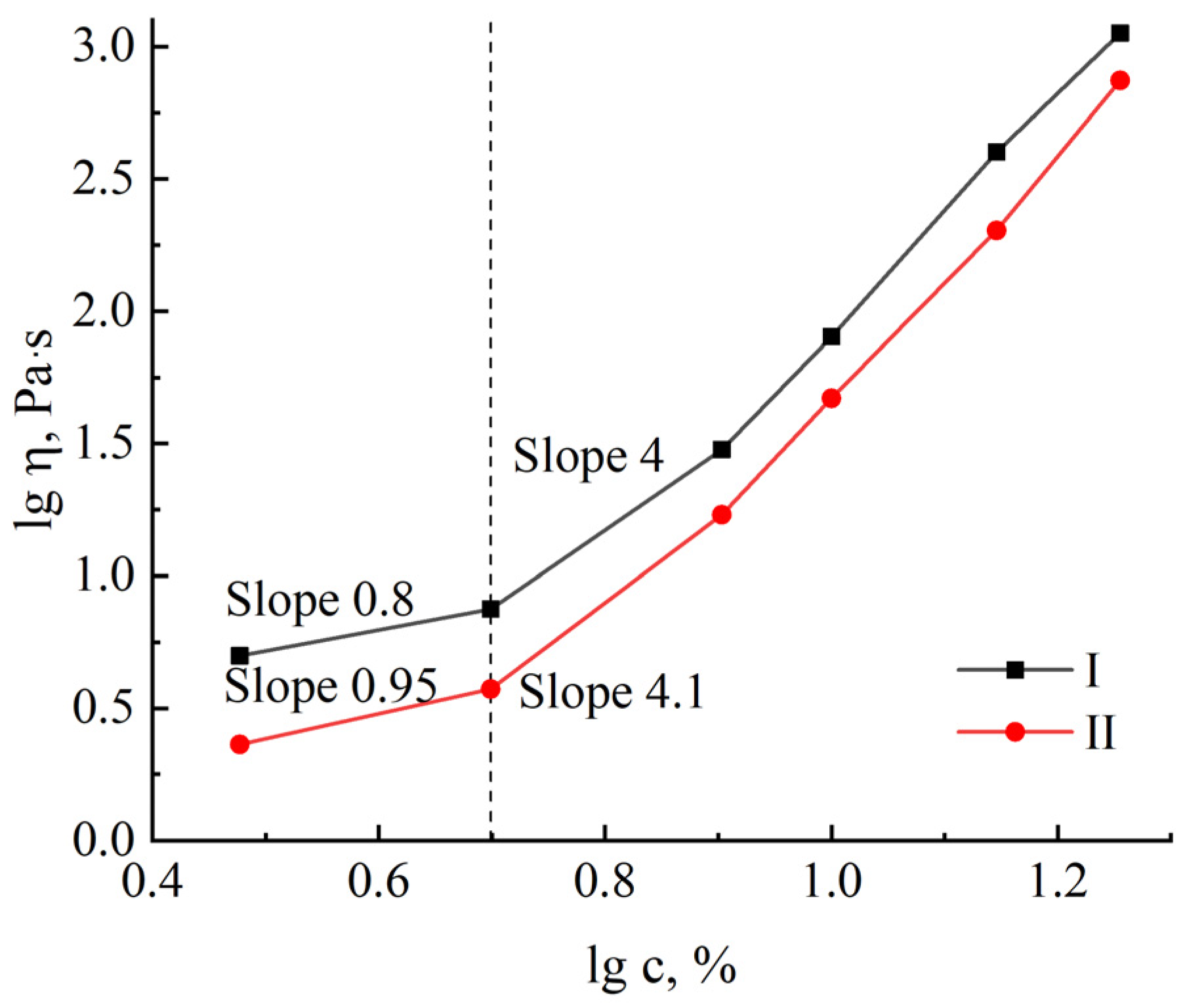
2.2.2. Rheology
2.2.3. Fibers Spinning
2.2.4. Structure and Morphology
2.2.5. Mechanical Testing
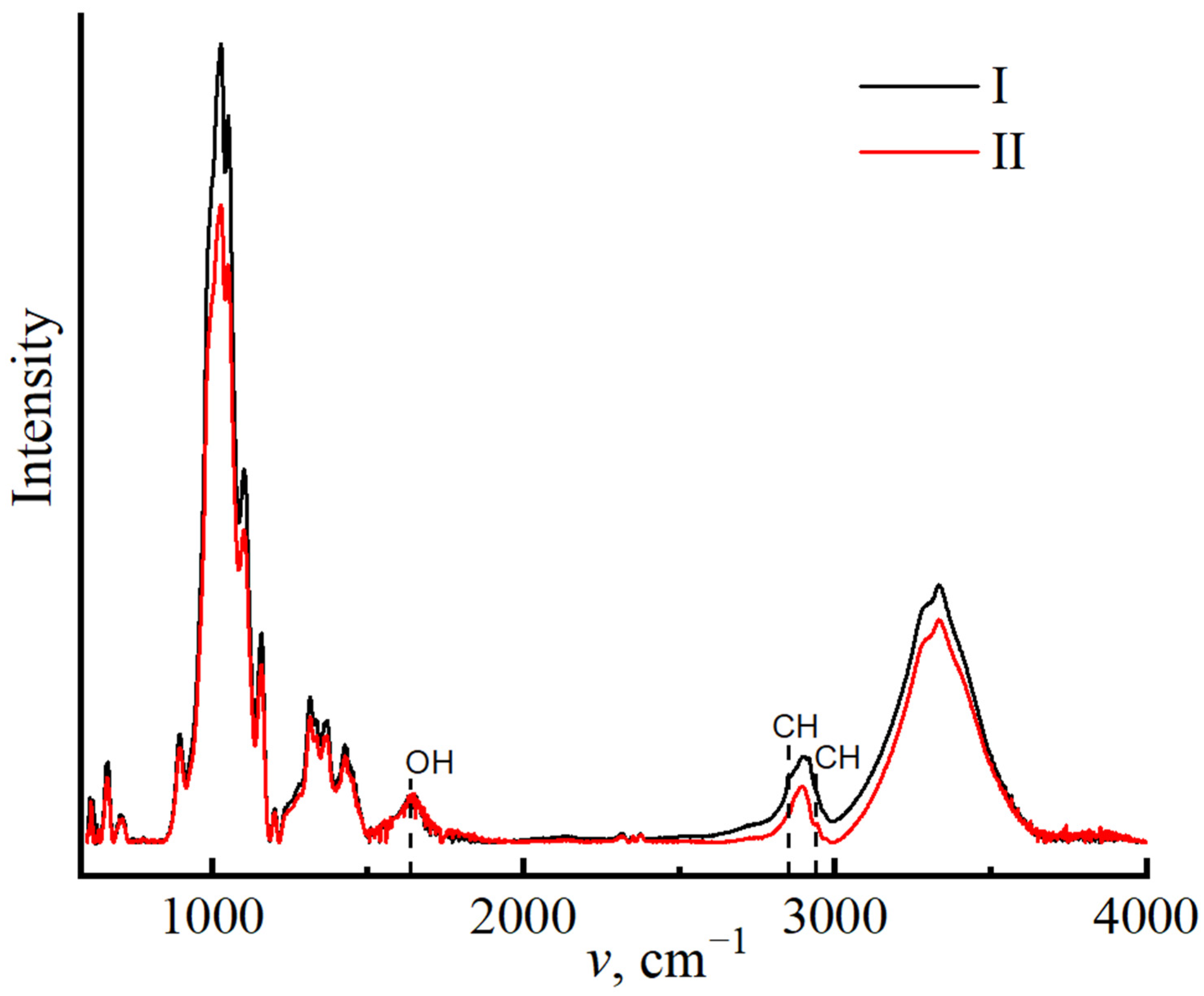
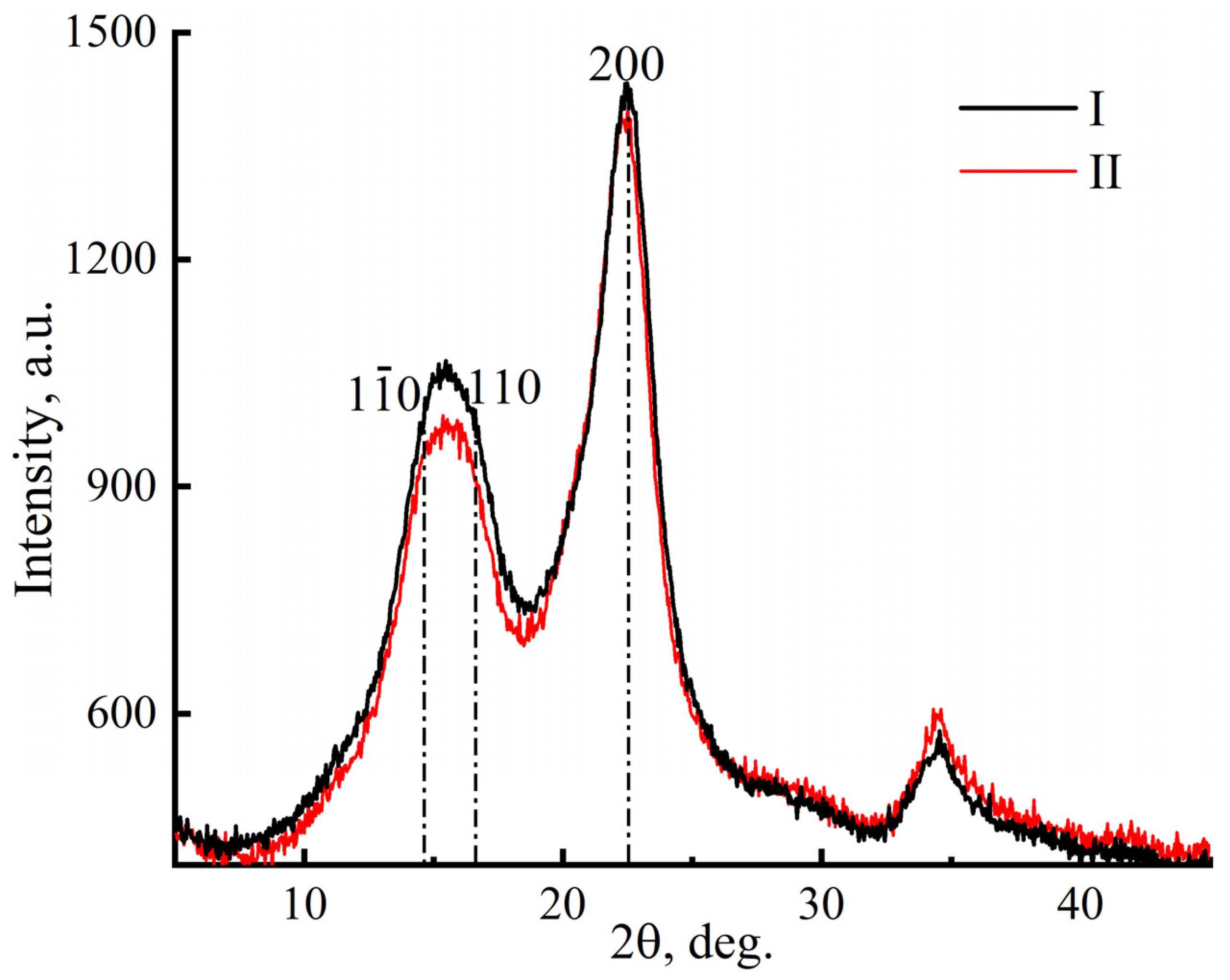
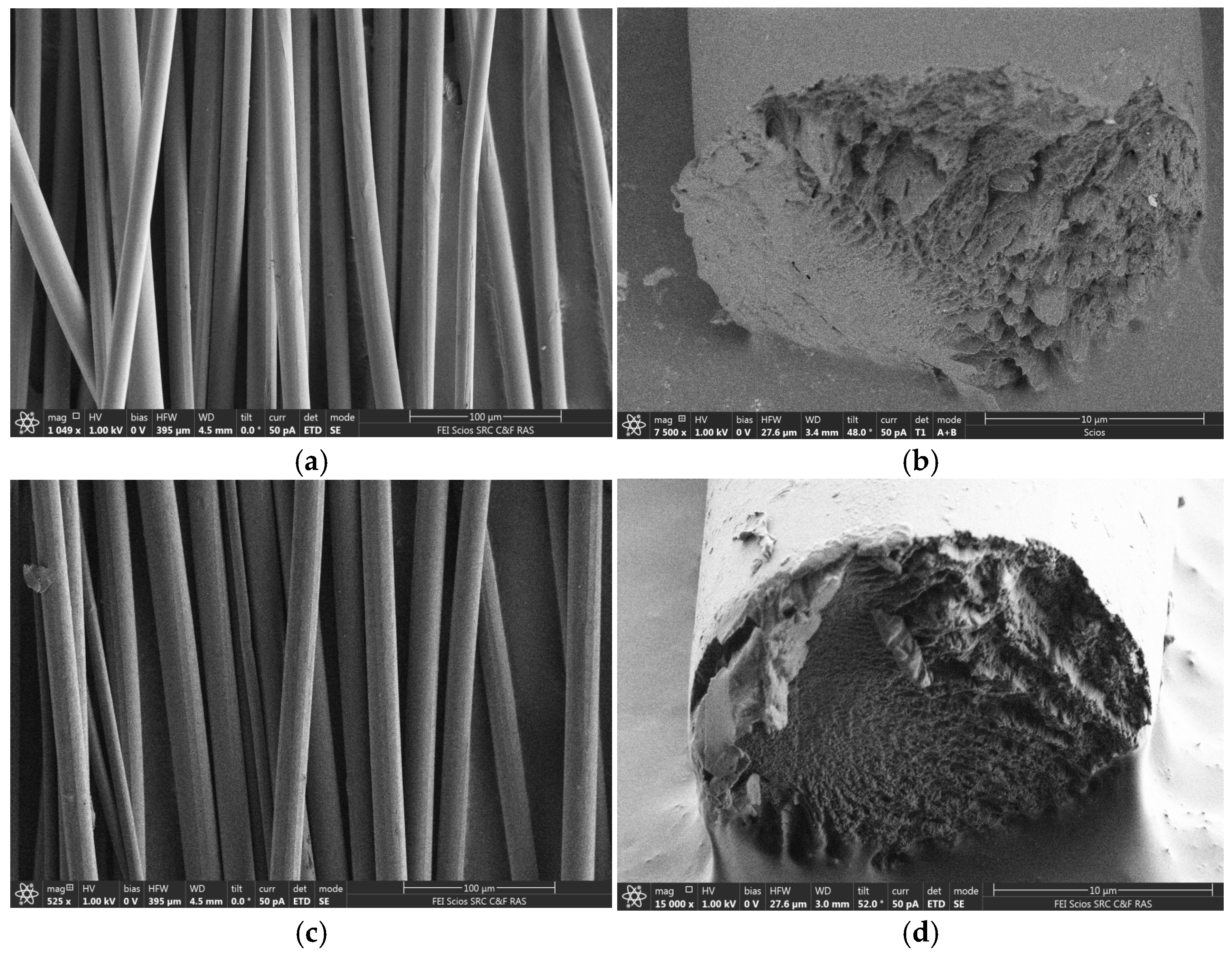
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kopeć, P.; Płażek, A. An attempt to restore the fertility of Miscanthus × giganteus. Agronomy 2023, 13, 323. [Google Scholar] [CrossRef]
- Touchell, D.H.; Lynch, N.; Shekasteband, R.; Dickey, A.N.; Chinn, M.C.; Whitfield, M.; Ranney, T.G. Biomass yields, reproductive fertility, compositional analysis, and genetic diversity of newly developed triploid giant miscanthus hybrids. GCB Bioenergy 2024, 16, e13174. [Google Scholar] [CrossRef]
- Yu, Y.; Li, M.; Song, T.; Zhang, S.; Wang, T. Genome-wide identification of Miscanthus ASR gene family reveals that MsASR4 is linked to NaCl tolerance. Ind. Crops Prod. 2024, 219, 119113. [Google Scholar] [CrossRef]
- Dorogina, O.V.; Nuzhdina, N.S.; Kozlova, M.V.; Zueva, G.A.; Vasilyeva, O.Y. Identification of populations by ISSR markers and a histochemical determination of transient starch in species of the genus Miscanthus anderss. Contemp. Probl. Ecol. 2023, 16, 67–75. [Google Scholar] [CrossRef]
- Magenau, E.; Clifton–Brown, J.; Awty–Carroll, D.; Ashman, C.; Ferrarini, A.; Kontek, M.; Martani, E.; Roderick, K.; Amaducci, S.; Davey, C.; et al. Site impacts nutrient translocation efficiency in intraspecies and interspecies miscanthus hybrids on marginal lands. GCB Bioenergy 2022, 14, 1035–1054. [Google Scholar] [CrossRef]
- Banerjee, S.; Dien, B.S.; Eilts, K.K.; Sacks, E.J.; Singh, V. Pilot-scale processing of Miscanthus × giganteus for recovery of anthocyanins integrated with production of microbial lipids and lignin-rich residue. Chem. Eng. J. 2024, 485, 150117. [Google Scholar] [CrossRef]
- Iqbal, Y.; Dai, Y.; Xue, S.; Yi, Z.; Chen, Z.; Li, M.; von Cossel, M. Organic Acid-Based Hemicellulose Fractionation and Cellulosic Ethanol Potential of Five Miscanthus Genotypes. Agronomy 2024, 14, 1389. [Google Scholar] [CrossRef]
- Sebastian, J.; Rouissi, T.; Brar, S.K. Miscanthus sp.—Perennial lignocellulosic biomass as feedstock for greener fumaric acid bioproduction. Ind. Crops Prod. 2022, 175, 114248. [Google Scholar] [CrossRef]
- Liu, W.; You, L.; Wang, S.; Li, J.; Chen, Z.; Si, B.; Iqbal, Y.; Xue, S.; Fu, T.; Yi, Z.; et al. Screening of Miscanthus Genotypes for Sustainable Production of Microcrystalline Cellulose and Cellulose Nanocrystals. Agronomy 2024, 14, 1255. [Google Scholar] [CrossRef]
- Lewandowski, I.; Scurlock, J.M.O.; Lindvall, E.; Christou, M. The development and current status of perennial rhizomatous grasses as energy crops in the US and Europe. Biomass Bioenergy 2003, 25, 335–361. [Google Scholar] [CrossRef]
- Lask, J.; Kam, J.; Weik, J.; Kiesel, A.; Wagner, M.; Lewandowski, I. A parsimonious model for calculating the greenhouse gas emissions of miscanthus cultivation using current commercial practice in the United Kingdom. GCB Bioenergy 2021, 13, 1087–1098. [Google Scholar] [CrossRef]
- Kapustyanchik, S.Y.; Yakimenko, V.N.; Gismatulina, Y.A.; Budaeva, V.V. Miscanthus—A promising energy crop for industrial processing. Ecol. Ind. Russ. 2021, 25, 66–71. [Google Scholar] [CrossRef]
- Semeshkina, P.S.; Mazurov, V.N.; Voinsky, S.M. Miscanthus KAMIS (Miscanthus Anderss.), RU Patent for Selection Achievements. 9365, 30 November 2017. Available online: https://miscanthus.eco/upload/iblock/718/7187dc5c4bb538c581c6377b0274cdb4.pdf (accessed on 19 August 2024). (In Russian).
- Gismatulina, Y.A.; Budaeva, V.V.; Kortusov, A.N.; Kashcheyeva, E.I.; Gladysheva, E.K.; Mironova, G.F.; Skiba, E.A.; Shavyrkina, N.A.; Korchagina, A.A.; Zolotukhin, V.N.; et al. Evaluation of chemical composition of Miscanthus × giganteus raised in different climate regions in Russia. Plants 2022, 11, 2791. [Google Scholar] [CrossRef]
- Xu, P.; Cheng, S.; Han, Y.; Zhao, D.; Li, H.; Wang, Y.; Zhang, G.; Chen, C. Natural Variation of Lignocellulosic Components in Miscanthus Biomass in China. Front. Chem. 2020, 8, 595143. [Google Scholar] [CrossRef]
- Waliszewska, B.; Mleczek, M.; Zborowska, M.; Golinski, P.; Rutkowski, P.; Szentner, K. Changes in the chemical composition and the structure of cellulose and lignin in elm wood exposed to various forms of arsenic. Cellulose 2019, 26, 6303–6315. [Google Scholar] [CrossRef]
- Wiener, J.; Kovačič, V.; Dejlová, P. Differences between flax and hemp. AUTEX Res. J. 2003, 3, 58–63. [Google Scholar] [CrossRef]
- Liu, M.; Thygesen, A.; Summerscales, J.; Meyer, A.S. Targeted pre-treatment of hemp bast fibres for optimal performance in biocomposite materials: A review. Ind. Crops Prod. 2017, 108, 660–683. [Google Scholar] [CrossRef]
- Makarov, I.S.; Golova, L.K.; Smyslov, A.G.; Vinogradov, M.I.; Palchikova, E.E.; Legkov, S.A. Flax Noils as a Source of Cellulose for the Production of Lyocell Fibers. Fibers 2022, 10, 45. [Google Scholar] [CrossRef]
- Jankauskienė, Z.; Butkutė, B.; Gruzdevienė, E.; Cesevičienė, J.; Fernando, A.L. Chemical composition and physical properties of dew- and water-retted hemp fibers. Ind. Crops Prod. 2015, 75, 206–211. [Google Scholar] [CrossRef]
- Day, A.; Ruel, K.; Neutelings, G.; Cronier, D.; David, H.; Hawkins, S.; Chabbert, B. Lignification in the flax stem: Evidence for an unusual lignin in bast fibers. Planta 2005, 222, 234–245. [Google Scholar] [CrossRef]
- Tarasov, D.; Leitch, M.; Fatehi, P. Lignin–carbohydrate complexes: Properties, applications, analyses, and methods of extraction: A review. Biotechnol. Biofuels 2018, 11, 269. [Google Scholar] [CrossRef]
- Deng, W.; Feng, Y.; Fu, J.; Guo, H.; Guo, Y.; Han, B.; Zhou, H. Catalytic conversion of lignocellulosic biomass into chemicals and fuels. Green Energy Environ. 2023, 8, 10–114. [Google Scholar] [CrossRef]
- Bochek, A.M. Effect of hydrogen bonding on cellulose solubility in aqueous and nonaqueous solvents. Russ. J. Appl. Chem. 2003, 76, 1711–1719. [Google Scholar] [CrossRef]
- Golova, L.K.; Kulichikhin, V.G.; Papkov, S.P. Mecanisme de dissolution de la cellulose dans les systemes solvants non aqueux. Vysokomolekularnye soedinenia. Ser. A 1986, 28, 1795–1809. [Google Scholar]
- Grinshpan, D.D.; Gonchar, A.N.; Tsygankova, N.G.; Makarevich, S.E.; Savitskaya, T.A.; Sheimo, E.V. Rheological properties of concentrated solutions of cellulose and its mixtures with other polymers in orthophosphoric acid. J. Eng. Phys. Thermophys. 2011, 84, 594–598. [Google Scholar] [CrossRef]
- Liebert, T. Cellulose solvents—Remarkable history, bright future. In Cellulose Solvents: For Analysis, Shaping and Chemical Modification; American Chemical Society: Washington, DC, USA, 2010; pp. 3–54. [Google Scholar] [CrossRef]
- Davidson, G.F. 12—The Dissolution of Chemically Modified Cotton Cellulose in Alkaline Solutions. Part I—In Solutions of Sodium Hydroxide, Particularly at Temperatures Below the Normal. J. Text. Inst. Trans. 1934, 25, T174–T196. [Google Scholar] [CrossRef]
- Yudianti, R.; Syampurwadi, A.; Onggo, H.; Karina, M.; Uyama, H.; Azuma, J. Properties of bacterial cellulose transparent film regenerated from dimethylacetamide–LiCl solution. Polym. Adv. Technol. 2016, 27, 1102–1107. [Google Scholar] [CrossRef]
- Lu, X.; Shen, X. Solubility of bacteria cellulose in zinc chloride aqueous solutions. Carbohydr. Polym. 2011, 86, 239–244. [Google Scholar] [CrossRef]
- Pavlyuchenko, M.M.; Kaputsky, F.N.; Grinshpan, D.D. Effect of organic solvent nature on the interaction of cellulose with nitrogen tetroxide. J. Appl. Chem. 1975, 48, 1822–1825. [Google Scholar]
- Hammer, R.B.; Turbak, A.F. Production of Rayon from Solutions of Cellulose in N2O4-DMF. In Solvent Spun Rayon, Modified Cellulose Fibers and Derivatives; American Chemical Society: Washington, DC, USA, 1977; Chapter 4. [Google Scholar]
- Seddon, K.R. Ionic Liquids for Clean Technology. J. Chem. Technol. Biotechnol. 1997, 68, 351–356. [Google Scholar] [CrossRef]
- Ye, Y.; Elabd, Y.A. Anion exchanged polymerized ionic liquids: High free volume single ion conductors. Polymer 2011, 52, 1309–1317. [Google Scholar] [CrossRef]
- Wilkes, A.G. The viscose process. In Regenerated Cellulose Fibers; John and Wiley and Sons: Hoboken, NJ, USA, 2001; pp. 37–61. [Google Scholar]
- Okano, T.; Sarko, A. Mercerization of cellulose. II. Alkali–cellulose intermediates and a possible mercerization mechanism. J. Appl. Polym. Sci. 1985, 30, 325–332. [Google Scholar] [CrossRef]
- Papkov, S.P. Ecological problems in the preparation of hydrocellulose fibres. Fibre Chem. 1991, 23, 93–95. [Google Scholar] [CrossRef]
- Golova, L.K. New Cellulose Fiber Lyocell. Russ. Chem. J. 2002, 46, 49–57. [Google Scholar]
- Higashi, T.; Toyama, T.; Sakurai, H.; Nakaza, M.; Omae, K.; Nakadate, T.; Yamaguchi, N. Cross-sectional Study of Respiratory Symptoms and Pulmonary Functions in Rayon Textile Workers with Special Reference to H2S Exposure. Ind. Health 1983, 21, 281–292. [Google Scholar] [CrossRef]
- GOST R 5982-84; Sulfite Viscose Cellulose, Technical Specifications. Russian State Standard: Moscow, Russia, 1998. Available online: https://files.stroyinf.ru/Data2/1/4294823/4294823279.pdf (accessed on 19 August 2024). (In Russian)
- Sevastyanova, Y.V.; Molodtsova, M.A.; Ivanov, K.A.; Tatarsky, K.O. Preparation of Na-Bisulfite Dissolving Wood Pulp (DWP) from Coniferous Wood Species. In Proceedings of the Scientific and Practical Conference on the Latest Advances in the Innovative Development of Pulp and Paper Industry: Technology, Equipment, Chemistry, Minsk, Belarus, 4–6 April 2017; Belarus State University of Technology: Minsk, Belarus, 2017; pp. 69–72. Available online: https://elib.belstu.by/bitstream/123456789/20711/1/12.Sevastyanova.pdf (accessed on 19 August 2024). (In Russian).
- Makarov, I.S.; Golova, L.K.; Vinogradov, M.I.; Egorov, Y.E.; Kulichikhin, V.G.; Mikhailov, Y.M. New Hydrated Cellulose Fiber Based on Flax Cellulose. Russ. J. Gen. Chem. 2021, 91, 1807–1815. [Google Scholar] [CrossRef]
- Johnson, D.L. Compounds Dissolved in Cyclic Amine Oxides. U.S. Patent 3447939A, 3 June 1969. Available online: https://patents.google.com/patent/US3447939A/en (accessed on 19 August 2024).
- Golova, L.K.; Romanov, V.V.; Lunina, O.B.; Platonov, V.A.; Papkov, S.P.; Khorozova, O.D.; Yakshin, V.V.; Belasheva, T.P.; Sokira, A.N. A Process for Preparing a Solution for Fiber Spinning. USSR Patent 1645308, 30 April 1991. Available online: https://patents.su/3-1645308-sposob-polucheniya-rastvora-dlya-formovaniya-volokon.html (accessed on 19 August 2024). (In Russian).
- Chanzy, H.; Paillet, M.; Hagege, R. Spinning of cellulose from N-methylmorpholine-N-oxide in the presence of additives. Polymer 1990, 31, 400–405. [Google Scholar] [CrossRef]
- Chavan, R.; Patra, A. Development and processing of lyocell. Indian J. Fibre Text. Res. 2004, 29, 483. [Google Scholar]
- Golova, L.K.; Makarov, I.S.; Plotnikova, E.P.; Shambilova, G.S.; Tereshin, A.K.; Kulichikhin, V.G. Solutions of mixtures of cellulose and synthetic polymers in N-methylmorpholine-N-oxide. Polym. Sci. Ser. A 2009, 51, 283–294. [Google Scholar] [CrossRef]
- Korchagina, A.A. Synthesis of cellulose nitrates from Miscanthus × giganteus var. KAMIS cellulose obtained under pilot production conditions. Izvestiya Vuzov. Prikladnaya Khimiya i Biotekhnologiya = Proceedings of Universities. Appl. Chem. Biotechnol. 2023, 13, 393–402. [Google Scholar] [CrossRef]
- Skiba, E.A.; Ovchinnikova, E.V.; Budaeva, V.V.; Banzaraktsaeva, S.P.; Surmina, M.A.; Chumachenko, V.A.; Mironova, G.F.; Kortusov, A.N.; Parmon, V.N.; Sakovich, G.V. Miscanthus bioprocessing using HNO3-pretreatment to improve productivity and quality of bioethanol and downstream ethylene. Ind. Crops Prod. 2022, 177, 114448. [Google Scholar] [CrossRef]
- Kriger, O.V.; Babich, O.O.; Dolganyuk, V.F.; Kozlova, O.V.; Sukhikh, S.A.; Larichev, T.A. Bioethanol Production from Miscanthus sinensis Cellulose by Bioconversion. Food Process. Tech. Technol. 2021, 51, 387–394. [Google Scholar] [CrossRef]
- Kashcheyeva, E.I.; Mironova, G.F.; Budaeva, V.V.; Khan, H. Bioconversion of oat hull and miscanthus cellulose to glucose solutions. Izvestiya Vuzov. Prikladnaya Khimiya i Biotekhnologiya = Proceedings of Universities. Appl. Chem. Biotechnol. 2019, 9, 654–664. [Google Scholar] [CrossRef]
- Aleshina, L.A.; Gladysheva, E.K.; Budaeva, V.V.; Mironova, G.F.; Skiba, E.A.; Sakovich, G.V. X-Ray Diffraction Data on the Bacterial Nanocellulose Synthesized by Komagataeibacter xylinus B-12429 and B-12431 Microbial Producers in Miscanthus- and Oat Hull-Derived Enzymatic Hydrolyzates. Crystallogr. Rep. 2022, 67, 391–397. [Google Scholar] [CrossRef]
- Skiba, E.A.; Shavyrkina, N.A.; Skiba, M.A.; Mironova, G.F.; Budaeva, V.V. Biosynthesis of Bacterial Nanocellulose from Low-Cost Cellulosic Feedstocks: Effect of Microbial Producer. Int. J. Mol. Sci. 2023, 24, 14401. [Google Scholar] [CrossRef]
- Sukhikh, S.; Babich, O.; Ivanova, S.; Kriger, O.; Prosekov, A.; Noskova, S.; Ulrikh, E.; Budenkova, E.; Kalashnikova, O. Production of nanocellulose from miscanthus biomass. Curr. Res. Green Sustain. Chem. 2024, 8, 100412. [Google Scholar] [CrossRef]
- Shulzhenko, D.V.; Bessonova, I.Y.; Azanov, M.V.; Dyachenko, L.R.; Fadeev, B.A.; Tyurin, E.T.; Zuikov, A.A. A process for Obtaining Cellulose from Cellulosic Raw Materials for Chemical Processing. RU Patent 2763878C1, 11 January 2022. Available online: https://patents.google.com/patent/RU2763878C1/ru (accessed on 19 August 2024). (In Russian).
- Kashcheyeva, E.I.; Korchagina, A.A.; Gismatulina, Y.A.; Gladysheva, E.K.; Budaeva, V.V.; Sakovich, G.V. Simultaneous Production of Cellulose Nitrates and Bacterial Cellulose from Lignocellulose of Energy Crop. Polymers 2024, 16, 42. [Google Scholar] [CrossRef]
- Bogolitsyn, K.; Parshina, A.; Mayorova, K.; Aksenov, A.; Polomarchuk, D.; Sinitsyna, O.; Sinitsyn, A. Enzymatic hydrolysis of cellulose-rich fraction of Arctic seaweeds using Penicillium- and Myceliophtora-based glycoside hydrolases. In Biomass Conversion and Biorefinery; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–12. [Google Scholar] [CrossRef]
- Salem, K.S.; Kasera, N.K.; Rahman, M.A.; Jameel, H.; Habibi, Y.; Eichhorn, S.J.; Lucia, L.A. Comparison and assessment of methods for cellulose crystallinity determination. Chem. Soc. Rev. 2023, 52, 6417. [Google Scholar] [CrossRef]
- Makarov, I.S.; Smyslov, A.G.; Palchikova, E.E.; Vinogradov, M.I.; Shandryuk, G.A.; Levin, I.S.; Kulichikhin, V.G. Nonwoven materials based on natural and artificial fibers. Cellulose 2024, 31, 1927–1940. [Google Scholar] [CrossRef]
- French, A.D. Increment in evolution of cellulose crystallinity analysis. Cellulose 2020, 27, 5445–5448. [Google Scholar] [CrossRef]
- French, A.D.; Santiago Cintrón, M. Cellulose polymorphy, crystallite size, and the Segal crystallinity index. Cellulose 2013, 20, 583–588. [Google Scholar] [CrossRef]
- Makarov, I.S.; Golova, L.K.; Vinogradov, M.I.; Levin, I.S.; Shandryuk, G.A.; Arkharova, N.A.; Golubev, Y.V.; Berkovich, A.K.; Eremin, T.V.; Obraztsova, E.D. The effect of alcohol precipitants on structural and morphological features and thermal properties of lyocell fibers. Fibers 2020, 8, 43. [Google Scholar] [CrossRef]
- Hennequin, L.M.; Tan, S.Y.; Jensen, E.; Fennell, P.; Hallett, J.P. Combining phytoremediation and biorefinery: Metal extraction from lead contaminated Miscanthus during pretreatment using the ionoSolv process. Ind. Crops Prod. 2022, 176, 114259. [Google Scholar] [CrossRef]
- Zhou, S.; Xue, Y.; Sharma, A.; Bai, X. Lignin valorization through thermochemical conversion: Comparison of hardwood, softwood and herbaceous lignin. ACS Sustain. Chem. Eng. 2016, 4, 6608–6617. [Google Scholar] [CrossRef]
- Baskakov, S.A.; Baskakova, Y.V.; Kabachkov, E.N.; Kichigina, G.A.; Kushch, P.P.; Kiryukhin, D.P.; Krasnikova, S.S.; Badamshina, E.R.; Vasil’ev, S.G.; Soldatenkov, T.A. Cellulose from Annual Plants and Its Use for the Production of the Films Hydrophobized with Tetrafluoroethylene Telomers. Molecules 2022, 27, 6002. [Google Scholar] [CrossRef]
- Vinogradov, G.V.; Malkin, A.J. Rheology of Polymers; Khimiya Publisher: Moscow, Russia, 1977; 440p, Available online: https://bigenc.ru/b/reologiia-polimerov-93e4c9 (accessed on 19 August 2024). (In Russian)
- Nelson, M.L.; O’Connor, R.T. Relation of certain infrared bands to cellulose crystallinity and crystal lattice type. Part I. Spectra of lattice types I, II, III and amorphous cellulose. J. Appl. Polym. Sci. 1964, 8, 1311–1324. [Google Scholar] [CrossRef]
- Carrillo, F.; Colom, X.; Suñol, J.J.; Saurina, J. Structural FTIR analysis and thermal characterization of lyocell and viscose-type fibres. Eur. Polym. J. 2004, 40, 2229–2234. [Google Scholar] [CrossRef]
- Su, H.; Wang, T.; Zhang, Y.; Zhang, Y.; Wang, H. Flame-retardant anti-fibrillation Lyocell fibers prepared by online-treatment of coagulated filament. Cellulose 2024, 31, 1279–1293. [Google Scholar] [CrossRef]
- Chakraborty, I.; Rongpipi, S.; Govindaraju, I.; Mal, S.S.; Gomez, E.W.; Gomez, E.D.; Mazumder, N. An insight into microscopy and analytical techniques for morphological, structural, chemical, and thermal characterization of cellulose. Microsc. Res. Tech. 2022, 85, 1990–2015. [Google Scholar] [CrossRef]
- Kaplan, D.L. Biopolymers from Renewable Resources; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; p. 420. [Google Scholar]
- Yan, H.; Li, W.; Liu, X.; Zhu, M.; Wang, M. Morphological Structure and Basic Characteristics of Miscanthus floridulus Fibers. ACS Omega 2022, 7, 19412–19419. [Google Scholar] [CrossRef]



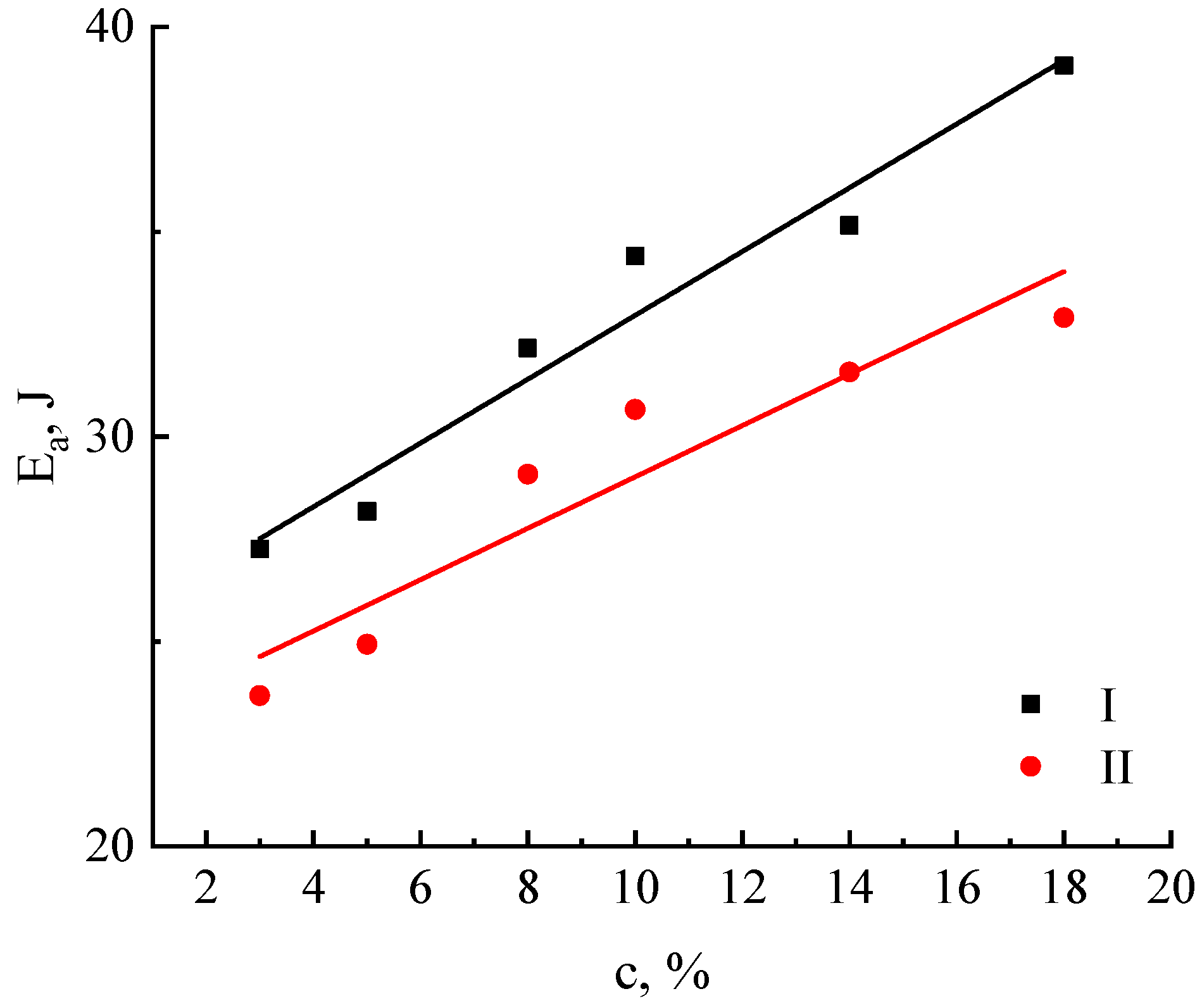
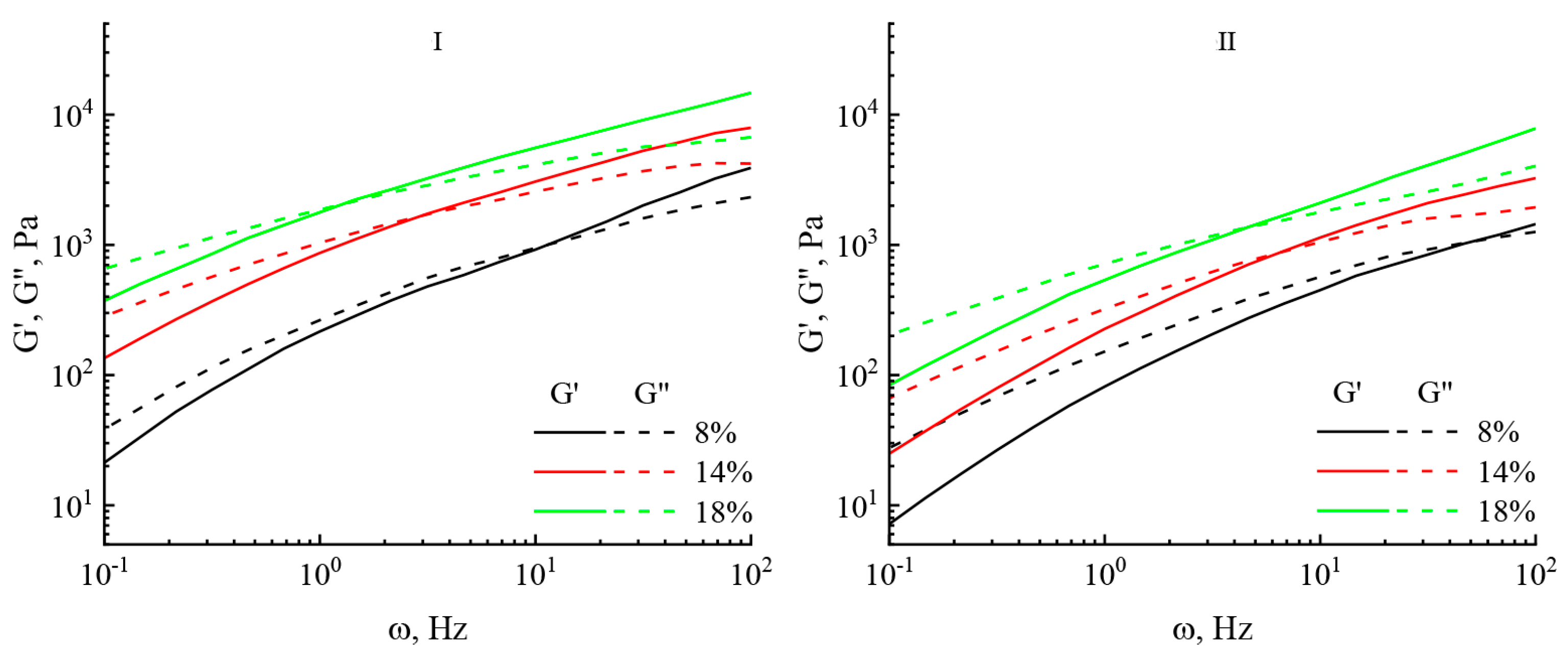

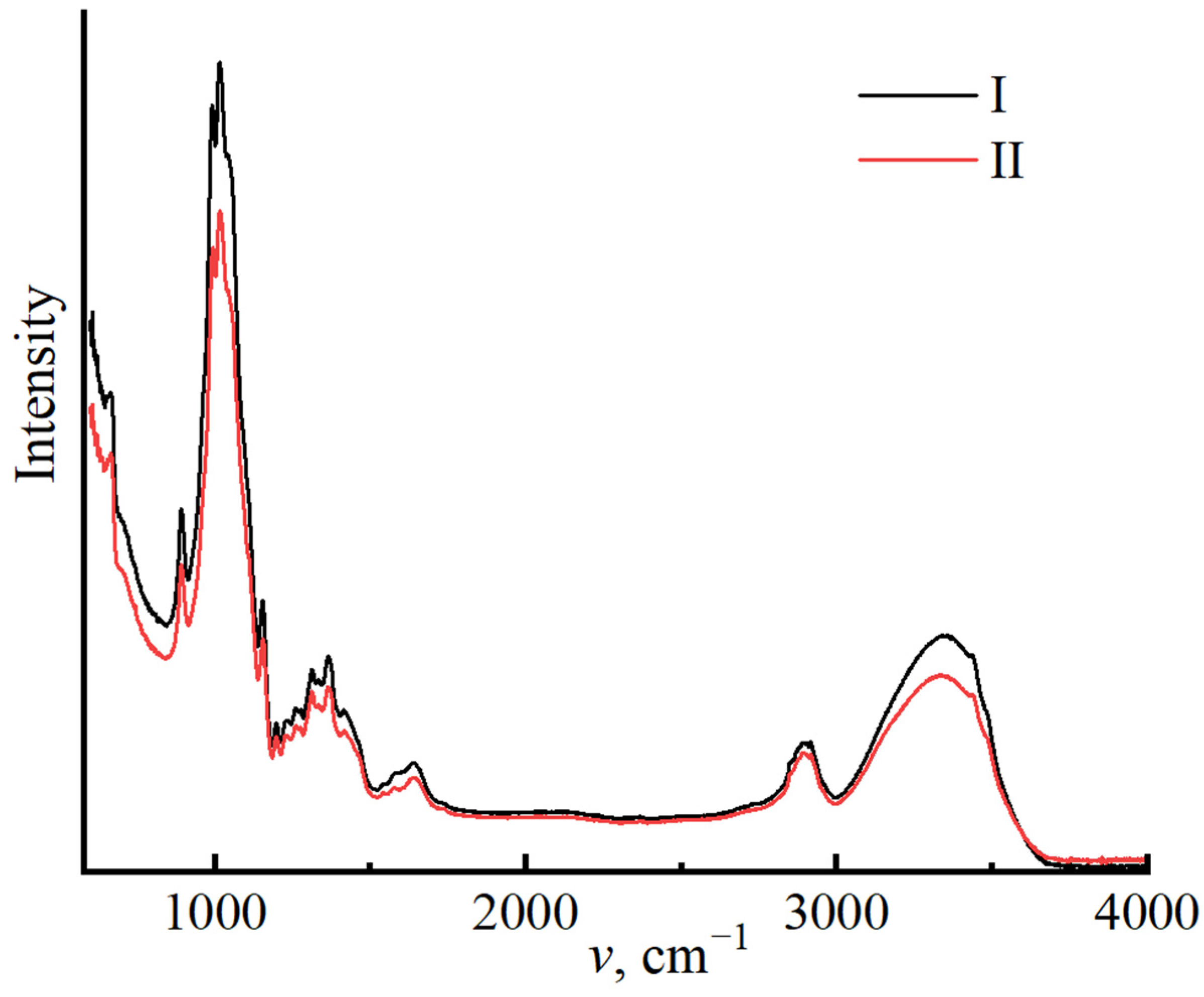
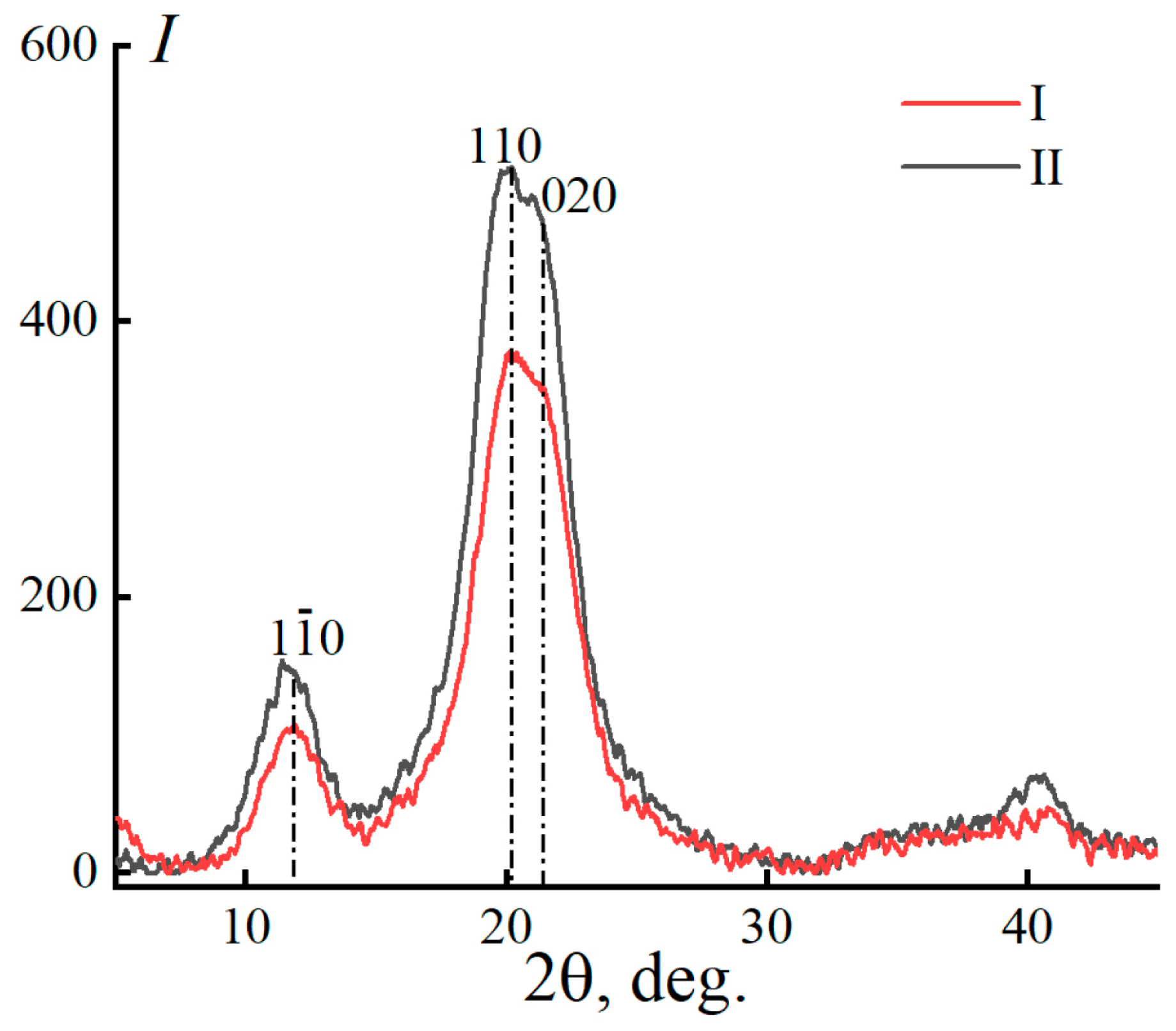
| Characteristics | Cellulose No. I | Cellulose No. II |
|---|---|---|
| α-cellulose, wt% | 88.2 ± 0.5 | 79.7 ± 0.5 |
| Pentosans, wt% | 1.6 ± 0.1 | 1.9 ± 0.1 |
| Acid-insoluble lignin, wt% | 0.80 ± 0.05 | 0.30 ± 0.05 |
| Ash, % | 0.40 ± 0.01 | 0.1 ± 0.01 |
| Degree of polymerization | 1200 ± 100 | 700 ± 50 |
| Moisture, % | 6.7 | 5.0 |
| Metal Contents, ppm | ||
|---|---|---|
| Cellulose No. I | Cellulose No. II | |
| Al | 47.66 | 25.98 |
| Ba | 2.54 | 0.72 |
| Ca | 1050.92 | 871.78 |
| Cr | 25.34 | 3.73 |
| Cu | 6.86 | 16.53 |
| Fe | 520.45 | 111.81 |
| Li | 7.34 | ≤0.02 |
| Mg | 87.47 | 103.22 |
| Mn | 4.22 | 0.08 |
| Na | 1666.38 | 1837.70 |
| Ni | 23.04 | 6.06 |
| Sn | 28.80 | 30.88 |
| Zn | 20.23 | 3.39 |
| Fiber | Diameter, µm | Tensile Strength, MPa | Elastic Modulus, GPa | Elongation at Break, % |
|---|---|---|---|---|
| no. I | 23–27 | 420–580 | 14.0–19.0 | 6.3–11.2 |
| no. II | 12–29 | 360–490 | 11.0–16.5 | 8.2–14.5 |
| Commercial Lyocell | 18 | 610 | 14 | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makarov, I.S.; Budaeva, V.V.; Gismatulina, Y.A.; Kashcheyeva, E.I.; Zolotukhin, V.N.; Gorbatova, P.A.; Sakovich, G.V.; Vinogradov, M.I.; Palchikova, E.E.; Levin, I.S.; et al. Preparation of Lyocell Fibers from Solutions of Miscanthus Cellulose. Polymers 2024, 16, 2915. https://doi.org/10.3390/polym16202915
Makarov IS, Budaeva VV, Gismatulina YA, Kashcheyeva EI, Zolotukhin VN, Gorbatova PA, Sakovich GV, Vinogradov MI, Palchikova EE, Levin IS, et al. Preparation of Lyocell Fibers from Solutions of Miscanthus Cellulose. Polymers. 2024; 16(20):2915. https://doi.org/10.3390/polym16202915
Chicago/Turabian StyleMakarov, Igor S., Vera V. Budaeva, Yulia A. Gismatulina, Ekaterina I. Kashcheyeva, Vladimir N. Zolotukhin, Polina A. Gorbatova, Gennady V. Sakovich, Markel I. Vinogradov, Ekaterina E. Palchikova, Ivan S. Levin, and et al. 2024. "Preparation of Lyocell Fibers from Solutions of Miscanthus Cellulose" Polymers 16, no. 20: 2915. https://doi.org/10.3390/polym16202915
APA StyleMakarov, I. S., Budaeva, V. V., Gismatulina, Y. A., Kashcheyeva, E. I., Zolotukhin, V. N., Gorbatova, P. A., Sakovich, G. V., Vinogradov, M. I., Palchikova, E. E., Levin, I. S., & Azanov, M. V. (2024). Preparation of Lyocell Fibers from Solutions of Miscanthus Cellulose. Polymers, 16(20), 2915. https://doi.org/10.3390/polym16202915










