Polyacrylonitrile Ultrafiltration Membrane for Separation of Used Engine Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymer Characterization
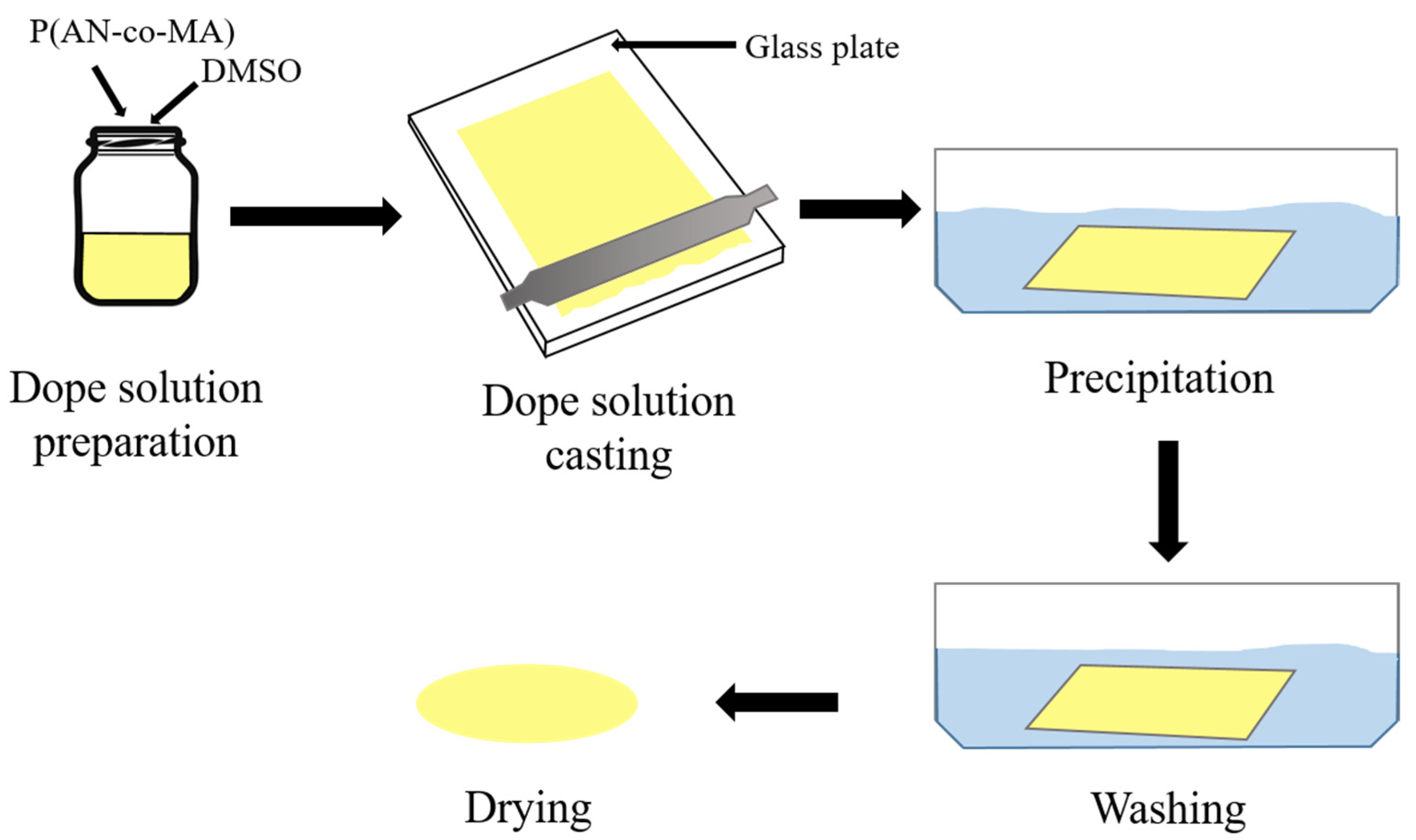
2.3. Preparation of P(AN-co-MA) Solution
2.4. Membrane Fabrication by Phase Inversion Method
2.5. Characterization of P(AN-co-MA) Membranes
2.6. Analysis of Oil Composition
2.7. Dynamic Viscosity Measurements
3. Results
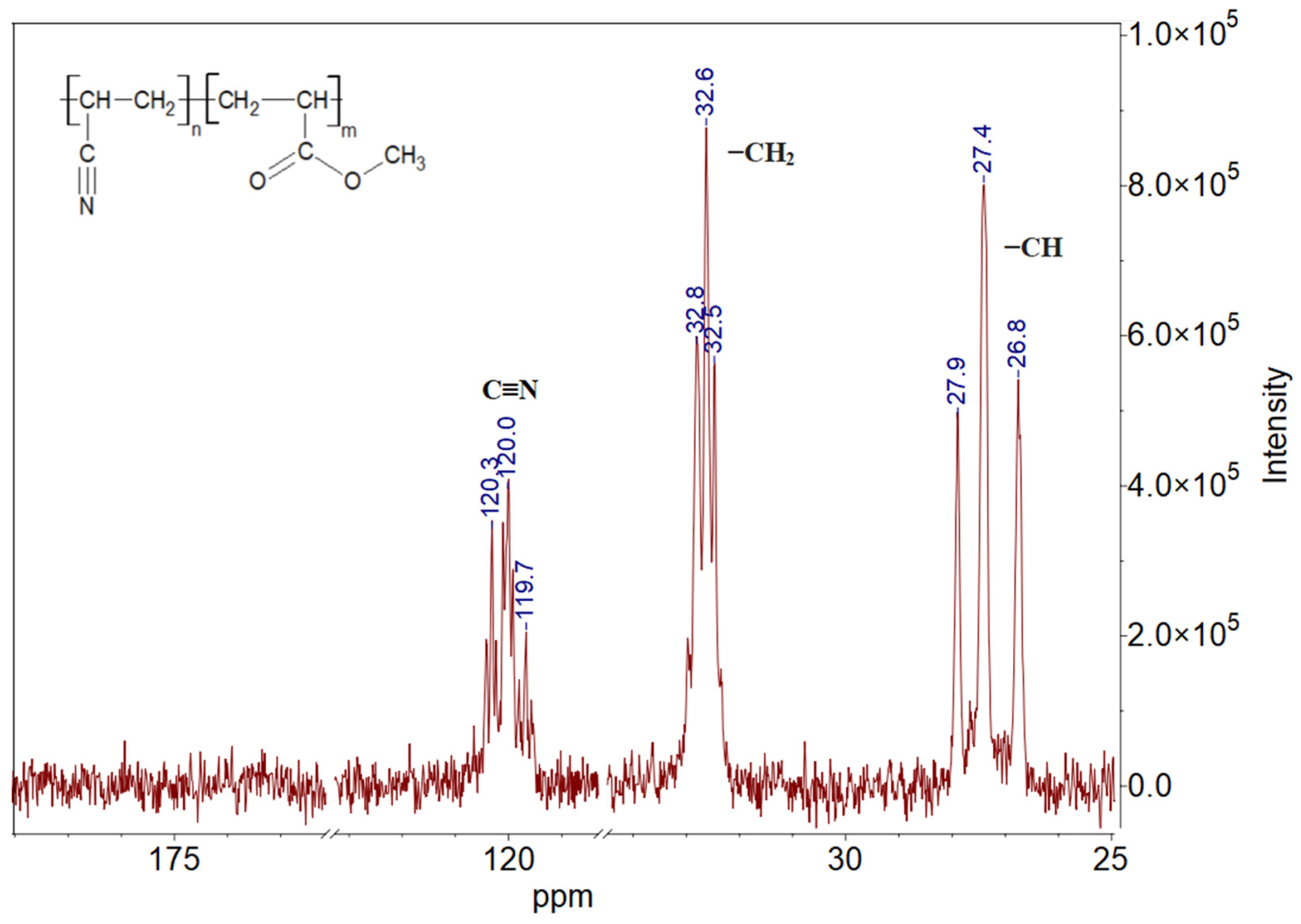
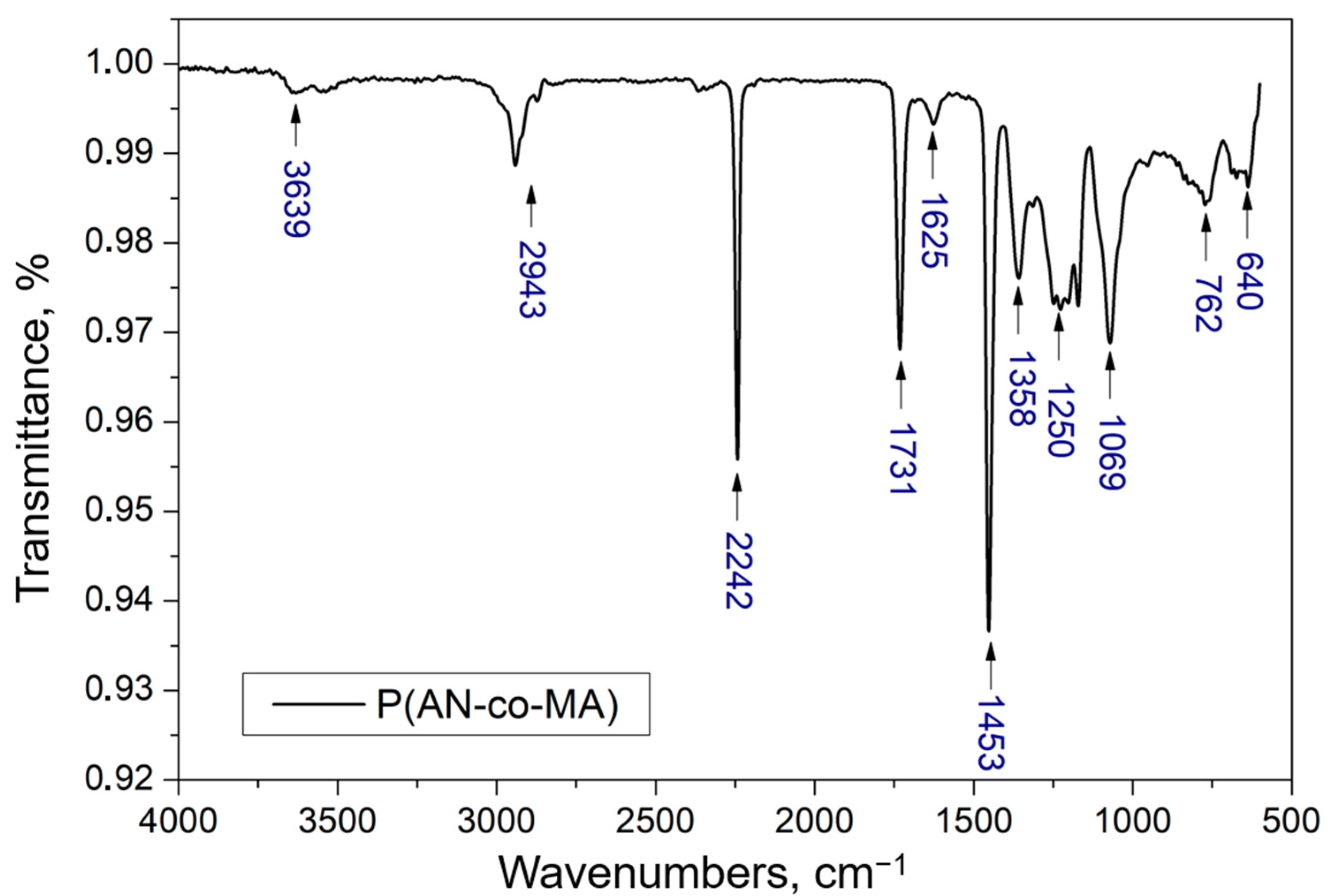
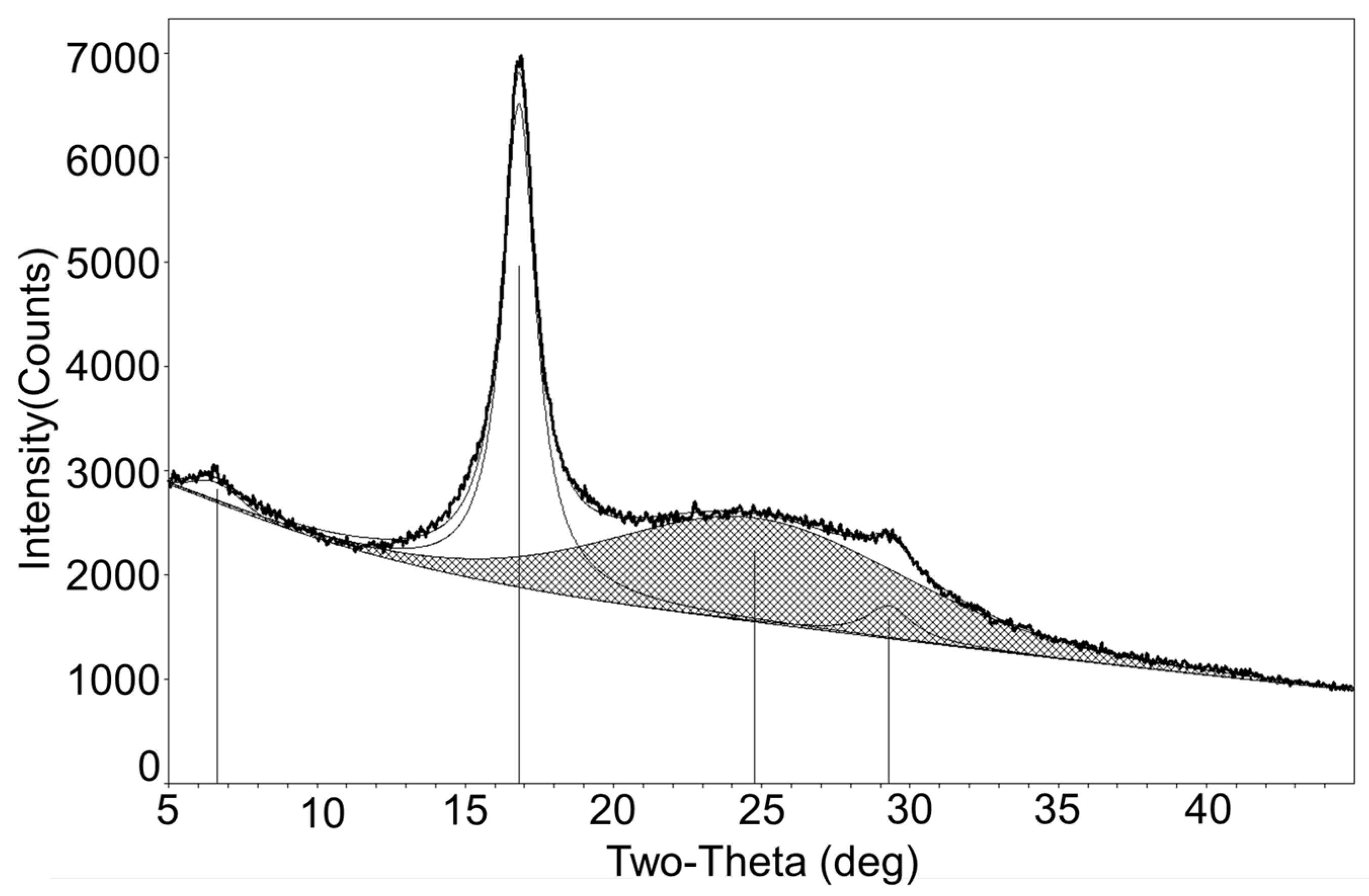
3.1. Polymer Characterization Results
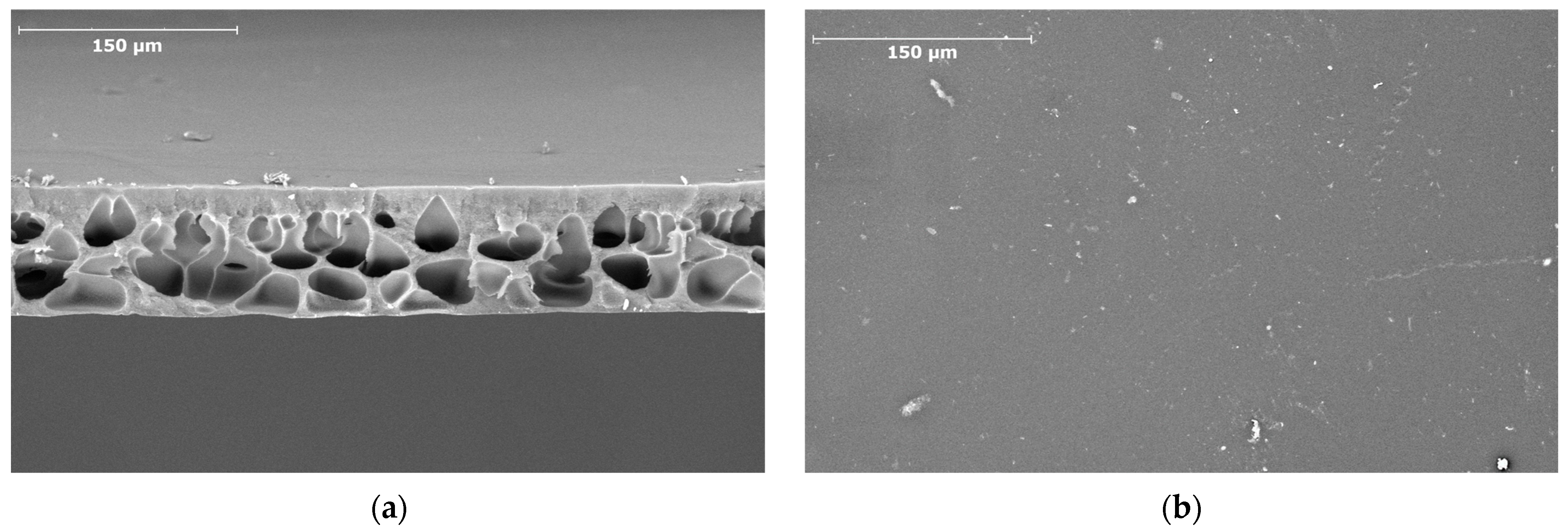
3.2. Preparation and Characterization of Membranes
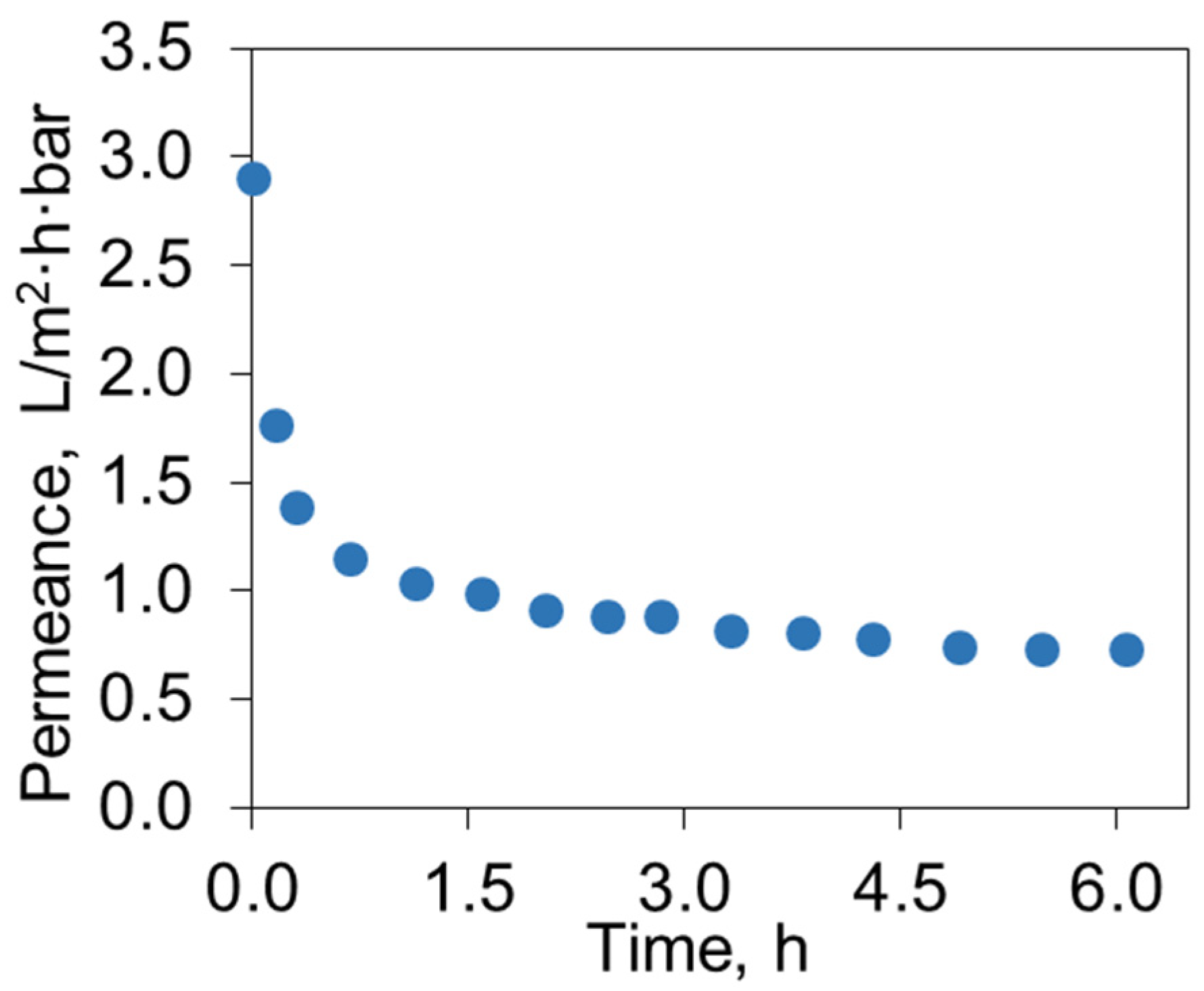
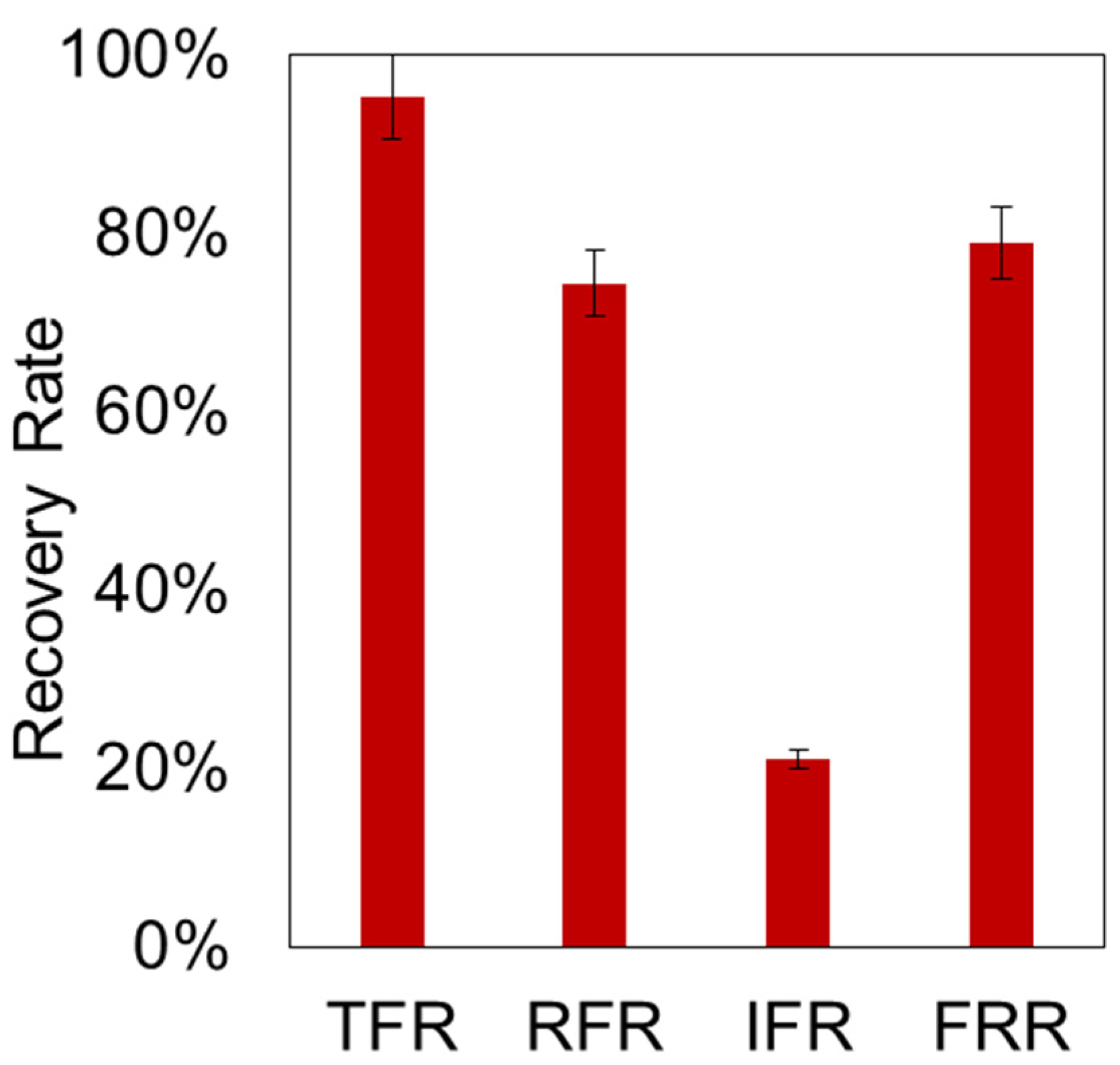
3.3. Ultrafiltration of Used Engine Oil
3.4. FTIR Spectroscopy
3.5. Some Physicochemical Properties of Used Engine Oil, Permeate, and Retentate
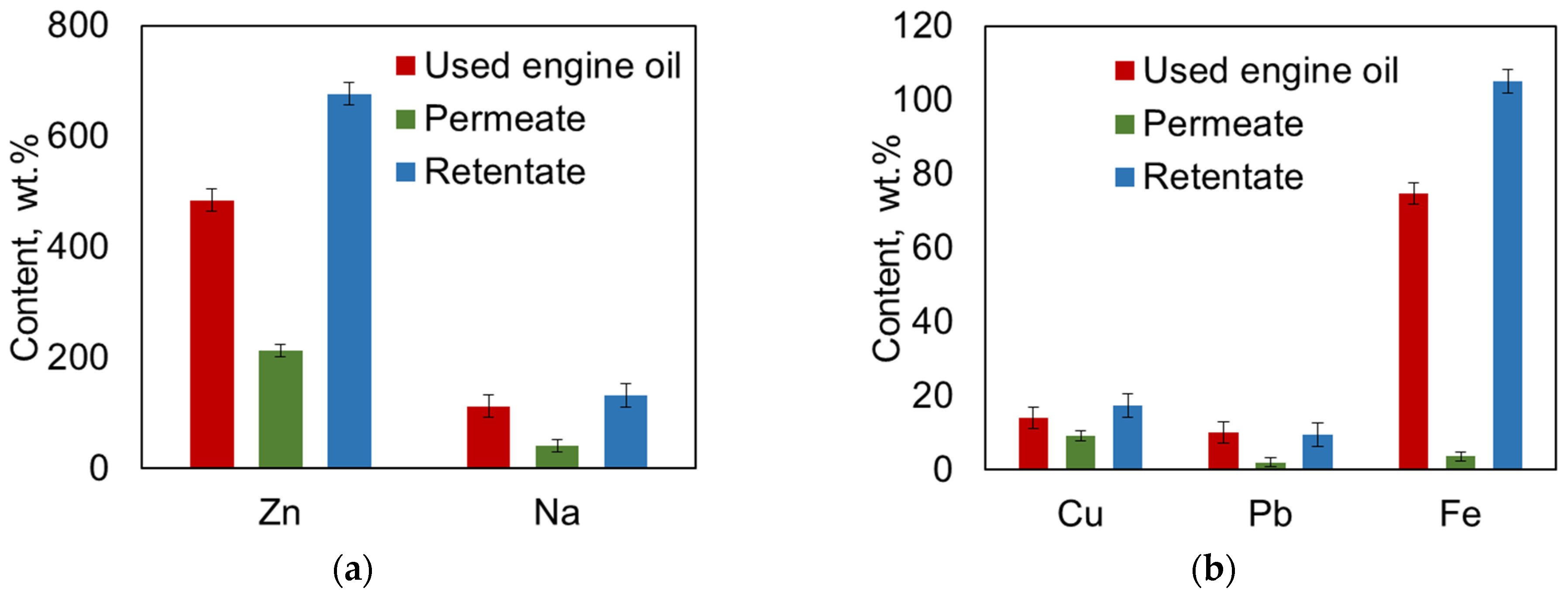
3.6. Elemental Analysis of Used Oil, Permeate, and Retentate
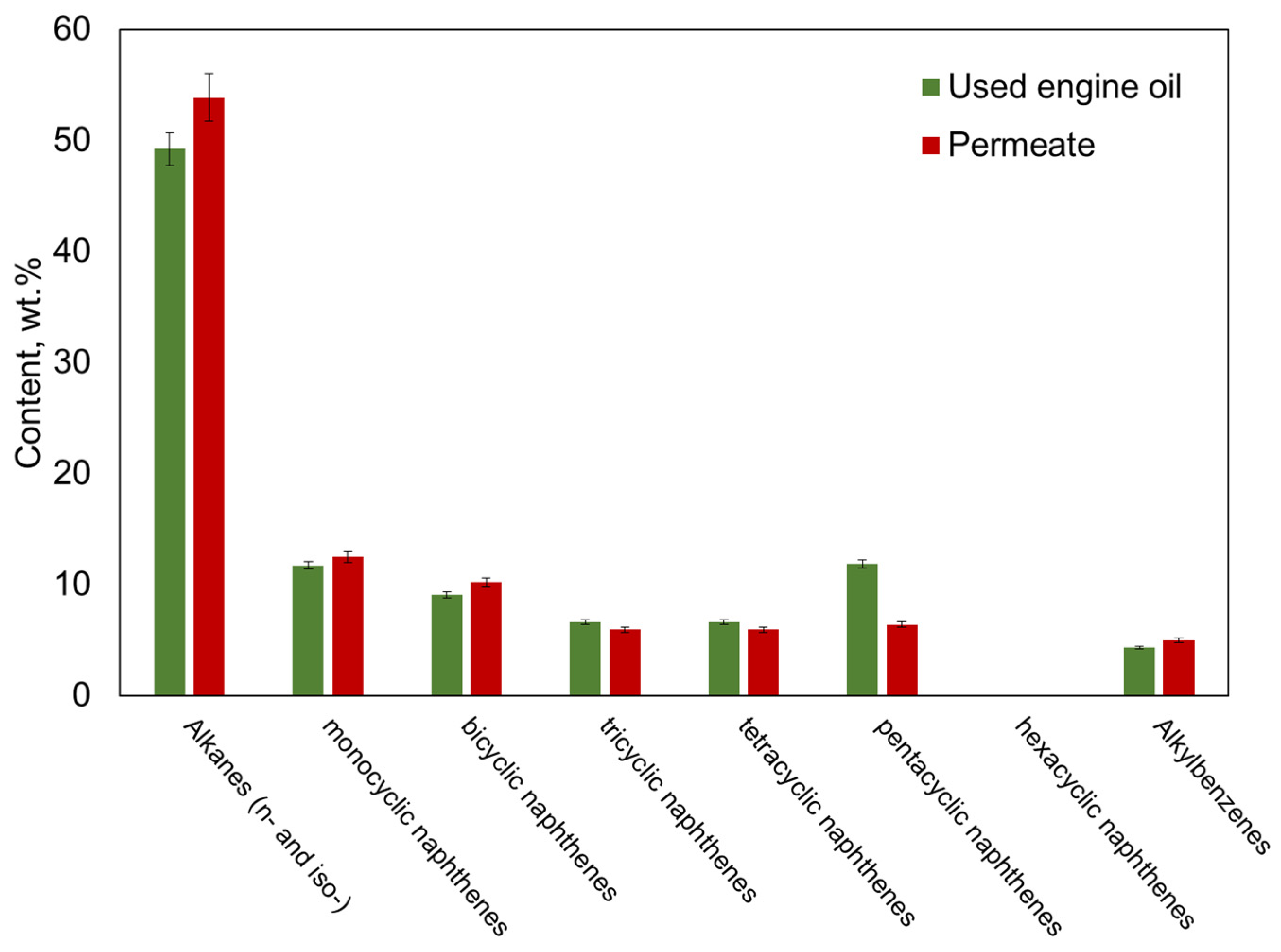
3.7. Structural Group Analysis by Mass Spectrometry
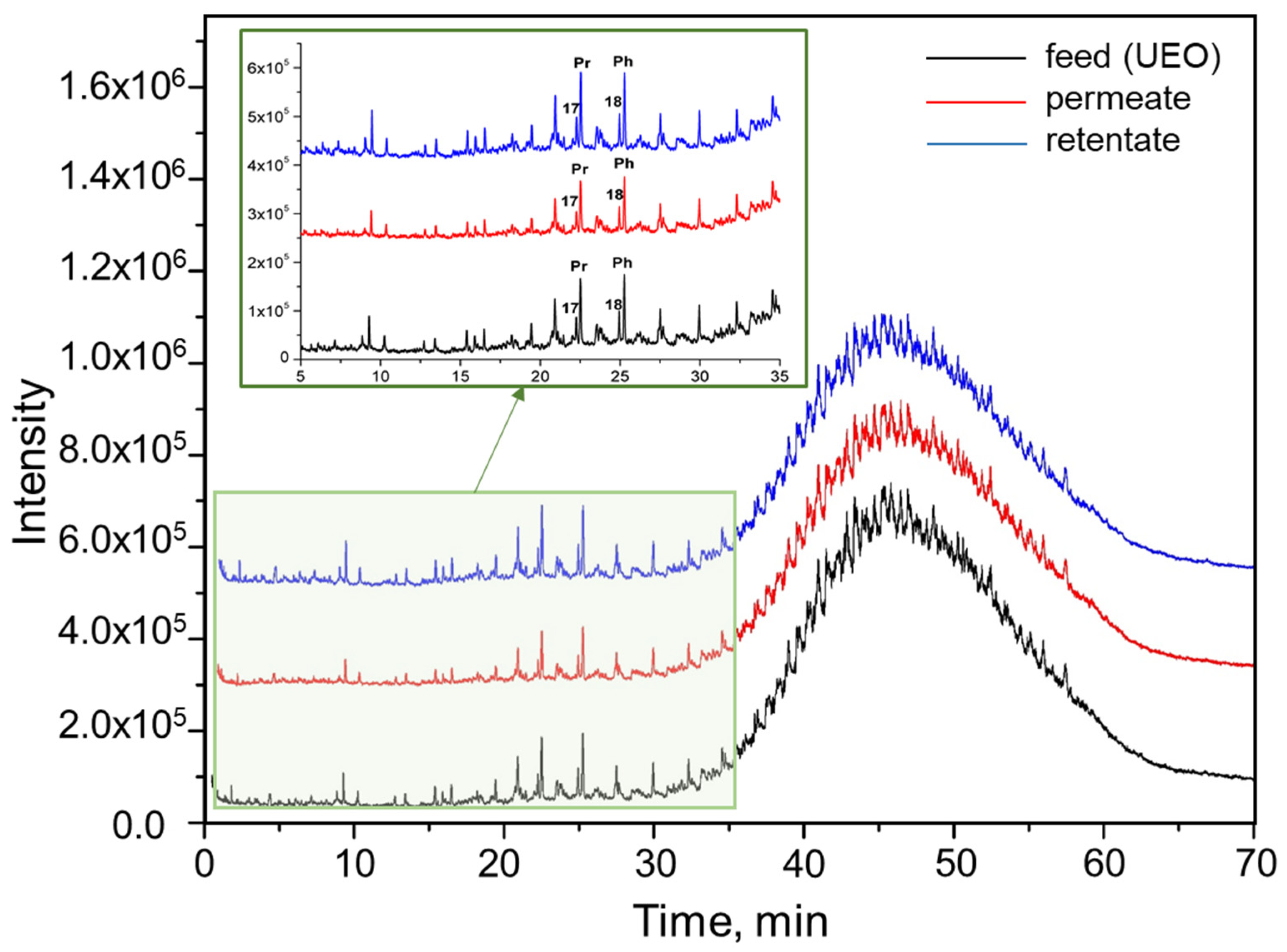
3.8. GS-MS Analysis of UEO, Permeate, and Retentate
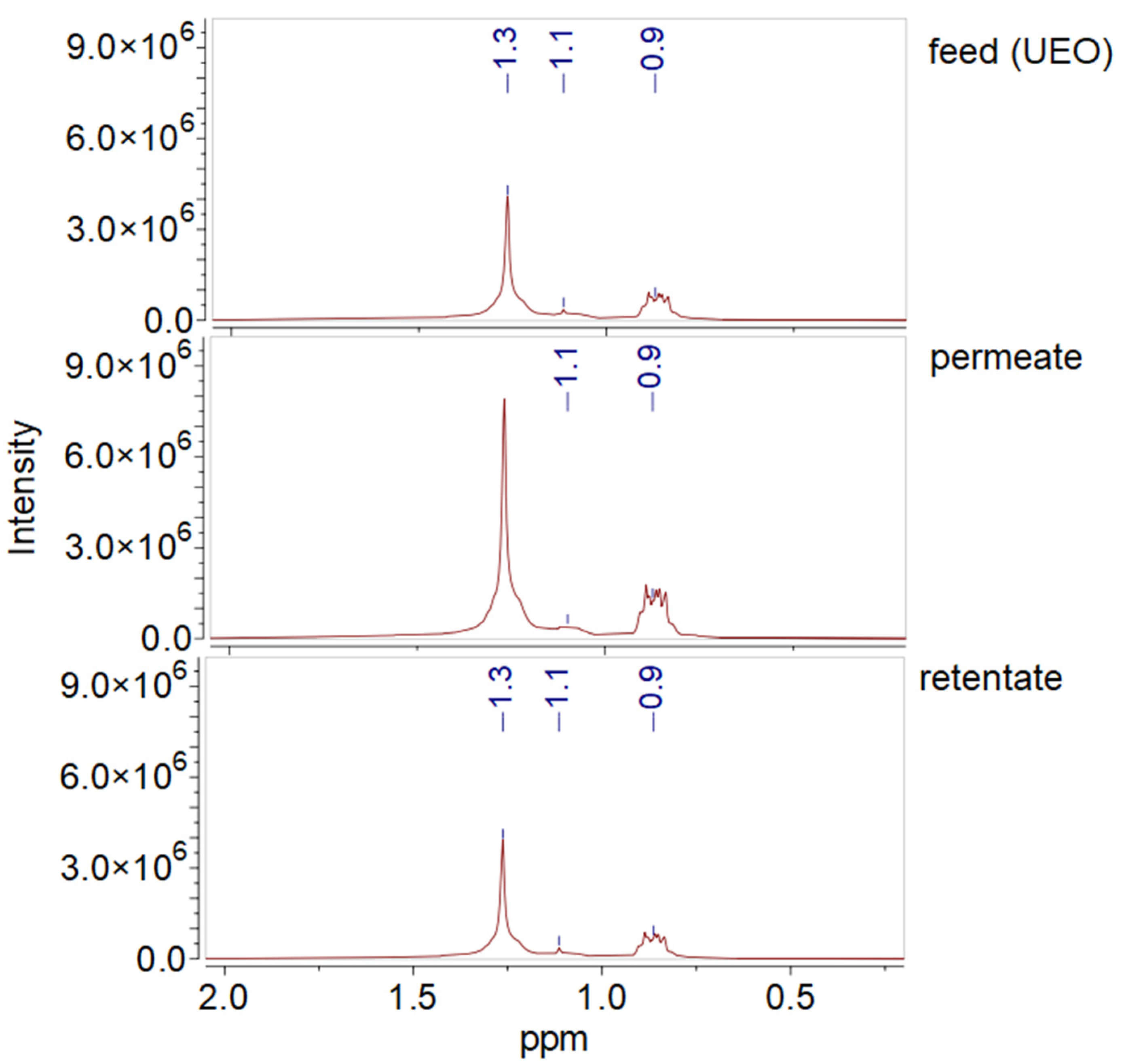
3.9. NMR Analysis of UEO and Filtration Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AES | atomic emission spectrometry; |
| AN | acrylonitrile; |
| Cf | metal content in the cell; |
| Cp | metal content in the permeate; |
| DMSO | dimethyl sulfoxide; |
| FRR | flux recovery ratio; |
| FTIR | Fourier-transform infrared; |
| GC-MS | gas chromatography–mass spectrometry; |
| GE | grapheme; |
| GmbH | Gesellschaft mit beschränkter Haftung; |
| HFCMs | hollow fiber composite membranes; |
| I | intensity of the molecular ion peak; |
| IFR | irreversible fouling ratio; |
| J | liquid flux; |
| K | coefficient; |
| KAUST | King Abdullah University of Science and Technology; |
| m | mass; |
| MA | methyl acrylate; |
| MD | mass spectrometric detector; |
| Mn | number average molecular weight; |
| Mw | weight average molecular weight; |
| MFP | mean flow pore size; |
| MWCO | molecular weight cut-off; |
| NIPS | non-solvent induced phase separation; |
| NMR | nuclear magnetic resonance; |
| P | permeability; |
| ∆p | trans-membrane pressure; |
| PAN | polyacrylonitrile; |
| P(AN-co-MA) | poly(acrylonitrile-co-methyl acrylate); |
| PES | polyethersulphone; |
| PGF | particle glass fibers; |
| PI | polyimide; |
| PP | polypropylene; |
| PTFE | polytetrafluoroethylene; |
| PVDF | polyvinylidenefluoride; |
| R | rejection; |
| RFR | reversible fouling ratio; |
| SEM | scanning electron microscopy; |
| t | filtration time; |
| TAN | total acid number; |
| TFR | total fouling ratio; |
| density; | |
| UEO | used engine oil; |
| V-SEP | Vibratory Shear Enhanced Process; |
| XRD | X-ray diffraction. |
References
- Widodo, S.; Ariono, D.; Khoiruddin, K.; Hakim, A.N.; Wenten, I.G. Recent advances in waste lube oils processing technologies. Environ. Prog. Sustain. Energy 2018, 37, 1867–1881. [Google Scholar] [CrossRef]
- Boadu, K.O.; Joel, O.F.; Essumang, D.K.; Evbuomwan, B.O. A review of methods for removal of contaminants in used lubricating oil. Chem. Sci. Int. J. 2019, 26, 1–11. [Google Scholar] [CrossRef]
- Rațiu, S.A.; Mihon, N.L.; Armioni, M.D. Study on the recycling methods of used engine oil. Romanian J. Automot. Eng. 2020, 26, 63–68. [Google Scholar]
- Pinheiro, C.T.; Quina, M.J.; Gando-Ferreira, L.M. Management of waste lubricant oil in Europe: A circular economy approach. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2015–2050. [Google Scholar] [CrossRef]
- Anisuzzaman, S.M.; Jumaidi, M.H.; Nasir, N.N.M. Used lubricating oil recovery process and treatment methods: A review. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1195, 012031. [Google Scholar] [CrossRef]
- Islam, M.S.; Sanzida, N.; Rahman, M.M.; Alam, M.D. From the value chain to environmental management of used lube oil: A baseline study in Bangladesh. Case Stud. Chem. Environ. Eng. 2021, 4, 100159. [Google Scholar] [CrossRef]
- Sánchez-Alvarracín, C.; Criollo-Bravo, J.; Albuja-Arias, D.; García-Ávila, F.; Pelaez-Samaniego, M.R. Characterization of used lubricant oil in a Latin-American medium-size city and analysis of options for its regeneration. Recycling 2021, 6, 10. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Q.; Ke, L.; Peng, Y.; Liu, Y.; Wu, Q.; Tian, X.; Dai, L.; Ruan, R.; Jiang, L. Review on the catalytic pyrolysis of waste oil for the production of renewable hydrocarbon fuels. Fuel 2021, 283, 119170. [Google Scholar] [CrossRef]
- Ratiu, S.A.; Tirian, G.O.; Mihon, N.L.; Armioni, M.D. Overview on globally applied used engine oil recycling technologies. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1220, 012034. [Google Scholar] [CrossRef]
- Mandloi, H.; Thakur, L.S. A Review on Recycle of Waste Lubricant Oil and its Properties Enhancement. Int. J. Res. Appl. Sci. Eng. Technol. 2023, 11, 4368–4371. [Google Scholar] [CrossRef]
- Nissar, A.; Hanief, M.; Mir, F.Q. Critical retrospection and evaluation of waste engine oil recycling methods. Int. J. Energy Water Resour. 2023, 7, 453–464. [Google Scholar] [CrossRef]
- Sarkar, S.; Datta, D.; Deepak, K.S.; Mondal, B.K.; Das, B. Comprehensive investigation of various re-refining technologies of used lubricating oil: A review. J. Mater. Cycles Waste Manag. 2023, 25, 1935–1965. [Google Scholar] [CrossRef]
- Kupareva, A.; Mäki-Arvela, P.; Murzin, D.Y. Technology for rerefining used lube oils applied in Europe: A review. J. Chem. Technol. Biotechnol. 2013, 88, 1780–1793. [Google Scholar] [CrossRef]
- Duong, A.; Chattopadhyaya, G.; Kwok, W.Y.; Smith, K.J. An experimental study of heavy oil ultrafiltration using ceramic membranes. Fuel 1997, 76, 821–828. [Google Scholar] [CrossRef]
- Kutowy, O.; Guerin, P.; Tweddle, T.A.; Woods, J. Use of membranes for oil upgrading. In Proceedings of the 35th Canadian Chemical Engineering Conference, Calgary, AB, Canada, 7 October 1985; Volume 1, p. 241. [Google Scholar]
- Mynin, V.N.; Smirnova, E.B.; Katsereva, O.V.; Komyagin, E.A.; Terpugov, G.V.; Smirnov, V.N. Treatment and regeneration of used lube oils with inorganic membranes. Chem. Technol. Fuels Oils 2004, 40, 345–350. [Google Scholar] [CrossRef]
- Psoch, C.; Wendler, B.; Goers, B.; Wozny, G.; Ruschel, B. Waste oil conditioning via microfiltration with ceramic membranes in cross flow. J. Membr. Sci. 2004, 245, 113–121. [Google Scholar] [CrossRef]
- Gourgouillon, D.; Schrive, L.; Sarrade, S.; Rios, G.M. An environmentally friendly process for the regeneration of used oils. Environ. Sci. Technol. 2000, 34, 3469–3473. [Google Scholar] [CrossRef]
- Gourgouillon, D.; Schrive, L.; Sarrade, S.; Rios, G.M. Improvement of the ultrafiltration of highly viscous liquids by injection of pressurized carbon dioxide. Sep. Sci. Technol. 2000, 35, 2045–2061. [Google Scholar] [CrossRef]
- Sarrade, S.; Schrive, L.; Gourgouillon, D.; Rios, G.M. Enhanced filtration of organic viscous liquids by supercritical CO2 addition and fluidification. Application to used oil regeneration. Sep. Purif. Technol. 2001, 25, 315–321. [Google Scholar] [CrossRef]
- Rodriguez, C.; Sarrade, S.; Schrive, L.; Dresch-Bazile, M.; Paolucci, D.; Rios, G.M. Membrane fouling in cross-flow ultrafiltration of mineral oil assisted by pressurised CO2. Desalination 2002, 144, 173–178. [Google Scholar] [CrossRef]
- Fedosov, S.V.; Markelov, A.V.; Sokolov, A.V.; Osadchy, Y.P. Coagulation and Ultrafiltration: A Hybrid Process for Purification of Used Engine Oils. Membr. Membr. Technol. 2022, 4, 297–305. [Google Scholar] [CrossRef]
- Shiohara, A.; Prieto-Simon, B.; Voelcker, N.H. Porous polymeric membranes: Fabrication techniques and biomedical applications. J. Mater. Chem. B 2021, 9, 2129–2154. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Karki, S.; Ingole, P.G. Current advances and opportunities in the development of nanofiltration (NF) membranes in the area of wastewater treatment, water desalination, biotechnological and pharmaceutical applications. J. Environ. Chem. Eng. 2022, 10, 108109. [Google Scholar] [CrossRef]
- Chikkatti, B.S.; Sajjan, A.M.; Banapurmath, N.R. The state of understanding of the electrochemical behaviours of a valve-regulated lead–acid battery comprising manganese dioxide-impregnated gel polymer electrolyte. Mater. Adv. 2023, 4, 6192–6198. [Google Scholar] [CrossRef]
- Chikkatti, B.S.; Kanaki, L.S.; Sajjan, A.M.; Banapurmath, N.R.; Umarfarooq, M.A.; Hosmath, R.S.; Badruddin, I.A.; Arabi, A.I.A.; Kamangar, S. Exploring the Electrochemical Performance of Molybdenum Disulfide Nanoparticles Entrenched in Miscible Poly (methyl methacrylate)-Poly (lactic acid) Blends as Freestanding Electrodes for Supercapacitors. Polymers 2024, 16, 2184. [Google Scholar] [CrossRef]
- Rajur, S.H.; Chikkatti, B.S.; Barnawi, A.B.; Bhutto, J.K.; Khan, T.Y.; Sajjan, A.M.; Banapurmath, N.R.; Raju, A.B. Unleashing the electrochemical performance of zirconia nanoparticles on valve-regulated lead acid battery. Heliyon 2024, 10, e29724. [Google Scholar] [CrossRef]
- Agboola, O.; Fayomi, O.S.I.; Ayodeji, A.; Ayeni, A.O.; Alagbe, E.E.; Sanni, S.E.; Okoro, E.E.; Moropeng, L.; Sadiku, R.; Kupolati, K.W.; et al. A review on polymer nanocomposites and their effective applications in membranes and adsorbents for water treatment and gas separation. Membranes 2021, 11, 139. [Google Scholar] [CrossRef]
- Yong, W.F.; Zhang, H. Recent advances in polymer blend membranes for gas separation and pervaporation. Prog. Mater. Sci. 2021, 116, 100713. [Google Scholar] [CrossRef]
- Grushevenko, E.A.; Borisov, I.L.; Volkov, A.V. High-Selectivity Polysiloxane Membranes for Gases and Liquids Separation (A Review). Pet. Chem. 2021, 61, 959–976. [Google Scholar] [CrossRef]
- Golubev, G.S.; Volkov, V.V.; Borisov, I.L.; Volkov, A.V. High free volume polymers for pervaporation. Curr. Opin. Chem. Eng. 2022, 36, 100788. [Google Scholar] [CrossRef]
- Olasupo, A.; Suah, F.B.M. Recent advances in the removal of pharmaceuticals and endocrine-disrupting compounds in the aquatic system: A case of polymer inclusion membranes. J. Hazard. Mater. 2021, 406, 124317. [Google Scholar] [CrossRef] [PubMed]
- Yushkin, A.A.; Balynin, A.V.; Nebesskaya, A.P.; Efimov, M.N.; Muratov, D.G.; Karpacheva, G.P. Oil Deasphalting Using Ultrafiltration PAN Membranes. Membr. Membr. Technol. 2023, 5, 454–466. [Google Scholar] [CrossRef]
- Chisca, S.; Musteata, V.E.; Zhang, W.; Vasylevskyi, S.; Falca, G.; Abou-Hamad, E.; Emwas, A.-H.; Altunkaya, M.; Nunes, S.P. Polytriazole membranes with ultrathin tunable selective layer for crude oil fractionation. Science 2022, 376, 1105. [Google Scholar] [CrossRef] [PubMed]
- Yushkin, A.; Basko, A.; Balynin, A.; Efimov, M.; Lebedeva, T.; Ilyasova, A.; Pochivalov, K.; Volkov, A. Effect of Acetone as Co-Solvent on Fabrication of Polyacrylonitrile Ultrafiltration Membranes by Non-Solvent Induced Phase Separation. Polymers 2022, 14, 4603. [Google Scholar] [CrossRef]
- Yushkin, A.A.; Balynin, A.V.; Nebesskaya, A.P.; Efimov, M.N.; Bakhtin, D.S.; Baskakov, S.A.; Kanatieva, A.Y. Fabrication of Ultrafiltration Membranes from PAN Composites with Hydrophilic Particles for Separation of Heavy Oil Components. Membr. Membr. Technol. 2023, 13, 331–344. [Google Scholar] [CrossRef]
- Yushkin, A.A.; Balynin, A.V.; Nebesskaya, A.P.; Chernikova, E.V.; Muratov, D.G.; Efimov, M.N.; Karpacheva, G.P. Acrylonitrile–Acrylic Acid Copolymer Ultrafiltration Membranes for Selective Asphaltene Removal from Crude Oil. Membranes 2023, 13, 775. [Google Scholar] [CrossRef]
- Shi, T.P.; Hu, Y.X.; Xu, Z.M.; Su, T.; Wang, R.A. Characterizing petroleum vacuum residue by supercritical fluid extraction and fractionation. Ind. Eng. Chem. Res. 1997, 36, 3988–3992. [Google Scholar] [CrossRef]
- Cao, Y.; Yan, F.; Li, J.; Liang, X.; He, B. Used lubricating oil recycling using a membrane filtration: Analysis of efficiency. Desalin. Water Treat. 2009, 11, 73–80. [Google Scholar] [CrossRef]
- Rouzegari, F.; Sargolzaei, J.; Ramezanian, N. A composite ultrafiltration membrane for regeneration of used engine oil. Energy Sour. Part A. 2020, 42, 1–16. [Google Scholar] [CrossRef]
- White, L.S.; Nitsch, A.R. Solvent recovery from lube oil filtrates with a polyimide membrane. J. Membr. Sci. 2000, 179, 267–274. [Google Scholar] [CrossRef]
- Ariono, D.; Widodo, S.; Khoiruddin, K.; Wardani, A.K.; Wenten, I.G. The Influence of Operating Parameters on Membrane Performance in Used Lube Oil Processing. IOP Conf. Ser. Mater. Sci. Eng. 2018, 395, 012018. [Google Scholar] [CrossRef]
- Widodo, S.; Khoiruddin, K.; Ariono, D.; Subagjo, S.; Wenten, I.G. Re-refining of waste engine oil using ultrafiltration membrane. J. Environ. Chem. Eng. 2020, 8, 103789. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, K.; Sun, G.; Zhao, W.; Jiang, Q.; Xiao, C. Robust design of durable PTFE/graphene hollow fiber composite membrane for high-temperature lubricant recycling. J. Water Process Eng. 2023, 55, 104163. [Google Scholar] [CrossRef]
- New Logic Research. V SEP Membrane Filtration of Waste Oil: A Cost-Effective and Environmentally Sound Processing Solution; New Logic Research Inc.: Emeryville, CA, USA; Available online: www.vsep.com (accessed on 12 May 2016).
- Matveev, D.; Vasilevsky, V.; Volkov, V.; Plisko, T.; Shustikov, A.; Volkov, A.; Bildyukevich, A. Fabrication of ultrafiltration membranes from non-toxic solvent dimethylsulfoxide: Benchmarking of commercially available acrylonitrile co-polymers. J. Environ. Chem. Eng. 2022, 10, 107061. [Google Scholar] [CrossRef]
- Ahmad, T.; Guria, C.; Mandal, A. A review of oily wastewater treatment using ultrafiltration membrane: A parametric study to enhance the membrane performance. J. Water Process Eng. 2020, 36, 101289. [Google Scholar] [CrossRef]
- Yushkin, A.A.; Balynin, A.V.; Efimov, M.N.; Muratov, D.G.; Karpacheva, G.P.; Volkov, A.V. Formation of Multilayer Membranes from One Polymer Using IR Treatment. Membr. Membr. Technol. 2022, 4, 251–257. [Google Scholar] [CrossRef]
- Mulder, M. Basic Principles of Membrane Technology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Polyakova, A.A. Mass-spectral analysis in oil processing and petrochemistry. In Non-Standard Methods; VNIINP: Moscow, Russia, 1988. [Google Scholar]
- ASTM D1500-21; Standard Test Method for ASTM Color of Petroleum Products (ASTM Color Scale). ASTM International: West Conshohocken, PA, USA, 2021. [CrossRef]
- Hlaváč, P.; Božiková, M.; Presová, R. Temperature relations of selected engine oils dynamic viscosity. Acta Technol. Agric. 2014, 17, 105–108. [Google Scholar] [CrossRef]
- ASTM D664-18e2; Standard Test Method for Acid Number of Petroleum Products by Potentiometric Titration. ASTM International: West Conshohocken, PA, USA, 2018. [CrossRef]
- ASTM D974-14; Standard Test Method for Acid and Base Number by Color-Indicator Titration. ASTM International: West Conshohocken, PA, USA, 2014. [CrossRef]
- Bajaj, P.; Paliwal, D.K.; Gupta, A.K. Acrylonitrile–acrylic acids copolymers. I. Synthesis and characterization. J. Appl. Polym. Sci. 1993, 49, 823–833. [Google Scholar] [CrossRef]
- Minagawa, M.; Miyano, K.; Takahashi, M.; Yoshii, F. Infrared characteristic absorption bands of highly isotactic poly (acrylonitrile). Macromolecules 1988, 21, 2387–2391. [Google Scholar] [CrossRef]
- Eldin, M.M.; Elaassar, M.R.; Elzatahry, A.A.; Al-Sabah, M.M.B. Poly (acrylonitrile-co-methyl methacrylate) nanoparticles: I. Preparation and characterization. Arab. J. Chem. 2017, 10, 1153–1166. [Google Scholar] [CrossRef]
- Zhou, D.Y.; Wang, G.Z.; Li, W.S.; Li, G.L.; Tan, C.L.; Rao, M.M.; Liao, Y.H. Preparation and performances of porous polyacrylonitrile–methyl methacrylate membrane for lithium-ion batteries. J. Power Sources 2008, 184, 477–480. [Google Scholar] [CrossRef]
- Toms, R.V.; Balashov, M.S.; Gervald, A.Y.; Prokopov, N.I.; Plutalova, A.V.; Berkovich, A.K.; Chernikova, E.V. Influence of synthesis method on the properties of carbon fiber precursors based on acrylonitrile and acrylic acid copolymers. Polym. Sci. Ser. B 2020, 62, 660–670. [Google Scholar] [CrossRef]
- Zhang, H.; Quan, L.; Gao, A.; Tong, Y.; Shi, F.; Xu, L. Thermal analysis and crystal structure of poly (acrylonitrile-co-itaconic acid) copolymers synthesized in water. Polymers 2020, 12, 221. [Google Scholar] [CrossRef]
- Parlayıcı, Ş.; Yar, A.; Pehlivan, E.; Avcı, A. ZnO-TiO2 doped polyacrylonitrile nano fiber-Mat for elimination of Cr (VI) from polluted water. J. Anal. Sci. Technol. 2019, 10, 24. [Google Scholar] [CrossRef]
- Chennam, P.K.; Kachlík, M.; Říhová, M.; Čičmancová, V.; Maca, K.; Macak, J.M. Synthesis of centrifugally spun polyacrylonitrile-carbon fibers. J. Mater. Res. Technol. 2004, 28, 2199–2205. [Google Scholar] [CrossRef]
- Apel, P.Y.; Velizarov, S.; Volkov, A.V.; Eliseeva, T.V.; Nikonenko, V.V.; Parshina, A.V.; Pismenskaya, N.D.; Popov, K.I.; Yaroslavtsev, A.B. Fouling and Membrane Degradation in Electromembrane and Baromembrane Processes. Membr. Membr. Technol. 2022, 4, 69–92. [Google Scholar] [CrossRef]
- Bellamy, L. The Infra-Red Spectra of Complex Molecules; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Sarkar, S.; Datta, D.; Das, B. Advance recovery approach for efficient recovery of waste lubricating oil by different material formulations. Mater. Today: Proc. 2022, 49, 1891–1898. [Google Scholar] [CrossRef]
- Hamidullayevna, A.Z.; Ismailovich, I.K. Causes of changes in the properties of motor oils in the high temperature zone of the engine. Am. J. Appl. Sci. Technol. 2023, 3, 1–5. [Google Scholar] [CrossRef]
- Kupareva, A.; Mäki-Arvela, P.; Grénman, H.; Eränen, K.; Sjöholm, R.; Reunanen, M.; Murzin, D.Y. Chemical characterization of lube oils. Energy Fuels 2013, 27, 27–34. [Google Scholar] [CrossRef]
- An, D.; Guo, C.; Chen, Y. Analysis of polycyclic aromatic hydrocarbon (PAH) mixtures using diffusion-ordered nmr spectroscopy and adsorption by powdered activated carbon and biochar. Materials 2018, 11, 460. [Google Scholar] [CrossRef]
- Urlaub, J.; Norwig, J.; Schollmayer, C.; Holzgrabe, U. 1H NMR analytical characterization of mineral oil hydrocarbons (PARAFFINS) for pharmaceutical use. J. Pharm. Biomed. Anal. 2019, 169, 41–48. [Google Scholar] [CrossRef] [PubMed]




| Mechanical Properties | Values |
|---|---|
| Tensile strength, MPa | 7.2 ± 0.6 |
| Young’s modulus, MPa | 152 ± 24 |
| Elongation at break, % | 8 ± 2 |
| Filtration Characteristics | Values |
|---|---|
| Water permeance, L/m2·h·bar | 54.15 ± 0.34 |
| Toluene permeance, L/m2·h·bar | 16.48 ± 0.15 |
| UEO solution permeance (100 g/L in touene), L/m2·h·bar | 0.75 ± 0.03 |
| Samples | Optical Density | Density, g/sm3 | Dynamic Viscosity, mPa·s | The Acid Number, mg KOH/g |
|---|---|---|---|---|
| Used oil | 1.024 ± 0.008 | 0.856 ± 0.001 | 59 ± 2 | 0.43 ± 0.03 |
| Permeate | 0.690 ± 0.050 | 0.854 ± 0.001 | 49 ± 1 | 0.33 ± 0.02 |
| Retentate | 1.199 ± 0.003 | 0.858 ± 0.002 | 61 ± 2 | 0.45 ± 0.02 |
| Samples | S(Pr)/S(C17H36) | S(Ph)/S(C18H38) | S(Pr)/S(Ph) |
|---|---|---|---|
| Used oil | 2.80 ± 0.10 | 2.7 ± 0.1 | 0.94 ± 0.01 |
| Permeate | 2.80 ± 0.20 | 2.8 ± 0.3 | 0.89 ± 0.04 |
| Retentate | 2.79 ± 0.03 | 2.7 ± 0.3 | 0.90 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nebesskaya, A.; Kanateva, A.; Borisov, R.; Yushkin, A.; Volkov, V.; Volkov, A. Polyacrylonitrile Ultrafiltration Membrane for Separation of Used Engine Oil. Polymers 2024, 16, 2910. https://doi.org/10.3390/polym16202910
Nebesskaya A, Kanateva A, Borisov R, Yushkin A, Volkov V, Volkov A. Polyacrylonitrile Ultrafiltration Membrane for Separation of Used Engine Oil. Polymers. 2024; 16(20):2910. https://doi.org/10.3390/polym16202910
Chicago/Turabian StyleNebesskaya, Alexandra, Anastasia Kanateva, Roman Borisov, Alexey Yushkin, Vladimir Volkov, and Alexey Volkov. 2024. "Polyacrylonitrile Ultrafiltration Membrane for Separation of Used Engine Oil" Polymers 16, no. 20: 2910. https://doi.org/10.3390/polym16202910
APA StyleNebesskaya, A., Kanateva, A., Borisov, R., Yushkin, A., Volkov, V., & Volkov, A. (2024). Polyacrylonitrile Ultrafiltration Membrane for Separation of Used Engine Oil. Polymers, 16(20), 2910. https://doi.org/10.3390/polym16202910










