Abstract
Essential for human development, water is increasingly polluted by diverse anthropogenic activities, containing contaminants like organic dyes, acids, antibiotics, inorganic salts, and heavy metals. Conventional methods fall short, prompting the exploration of advanced, cost-effective remediation. Recent research focuses on sustainable adsorption, with nano-modifications enhancing adsorbent efficacy against persistent waterborne pollutants. This review delves into recent advancements (2020–2023) in sustainable biopolymeric nanocomposites, spotlighting the applications of biopolymers like chitosan in wastewater remediation, particularly as adsorbents and filtration membranes along with their mechanism. The advantages and drawbacks of various biopolymers have also been discussed along with their modification in synthesizing biopolymeric nanocomposites by combining the benefits of biodegradable polymers and nanomaterials for enhanced physiochemical and mechanical properties for their application in wastewater treatment. The important functions of biopolymeric nanocomposites by adsorbing, removing, and selectively targeting contaminants, contributing to the purification and sustainable management of water resources, have also been elaborated on. Furthermore, it outlines the reusability and current challenges for the further exploration of biopolymers in this burgeoning field for environmental applications.
1. Introduction
Globally, human beings are facing two basic challenges, namely, the dearth of clean water and its contamination. Water is a fundamental need to sustain life. Natural and anthropogenic activities produce large quantities of micropollutants in water [1]. Industrial development and advancements in agricultural techniques accelerate the accumulation of non-degradable pollutants in aquatic life [2]. Organic and inorganic impurities end up in lakes, rivers, or oceans, and the oxygen content in these is then affected [3]. The organic content immediately starts consuming oxygen in the water, resulting in oxygen deficiency, which in turn leads to the death of fish and other aquatic animals. This is due to the unnaturally high consumption of oxygen by pollutants [4]. If inorganic nutrients such as nitrogen and phosphorus are discharged into the water, they provide a food source for algae and plankton. This new biomass is organic matter, and when it decomposes, it consumes additional amounts of oxygen [4]. Small quantities of nutrients can create a large amount of biomass and result in substantial oxygen depletion and extensive damage to the aquatic system. The impact of effluents from the specified wastewater is contingent upon the characteristics of the receiving water [5]. Discharges containing organic matter may pose harm in certain contexts. Conversely, in other areas, the release of phosphorus and nitrogen could lead to significant environmental harm by fostering biological growth [6]. Thus, it is imperative that the issuance of discharge permits and the selection of purification methods aligns with the ecological requirements of the region [2]. The rapid surge in global population and widespread industrialization poses significant challenges in ensuring access to safe drinking water [7]. This pressing issue underscores the urgency for the exploration of effective and cost-efficient water treatment methods. Addressing this need is crucial for sustaining the well-being of communities and ecosystems in the face of escalating demands on freshwater resources [7,8].
Different techniques and methods are used to treat wastewater purposely to maintain the quality and quantity of water contaminated by natural or anthropogenic activities [9]. Non-futuristic approaches like unplanned industrialization and urbanization and the use of pesticides, synthetic fertilizers, and antibiotics or medical waste play a significant role in polluting water; thus, the availability of freshwater is still a challenge [10]. Antifouling is one of the critical problems for treating wastewater [11]. Conventionally, different modifications have been observed in polysulfone membranes by adding poly(2-acrylamido-2-methyl-1-propanesulfonic acid) and Cu2O for the ultrafiltration of proteins, BSA, and humic acid from the water by increasing the antifouling properties [12,13]. A water treatment process typically involves many important steps, which may vary in order and complexity, depending upon the kind of contamination present in water. For the elimination of contaminants, different physical, chemical, and biological methods are recommended. The conventional methods of water treatment involve the use of strong chemicals and organic media. The main steps of water treatment are coagulation and flocculation [14,15]. Coagulation implies the addition of coagulants like alum or ferric chloride to the water, which neutralizes the electrical charges of particles present in wastewater, and flocculation implies soft stirring to promote the formation of substantial particles that settle down easily [14,16]. The process is followed by sedimentation, filtration, disinfection, and pH adjustment. The introduction of membranes in water treatment upgraded the process by reducing its cost and making it an eco-friendly approach [17]. To fulfil the demands of fresh water, recycling and reusing contaminated water were adopted. The tertiary step in the water treatment process concentrates on the removal of floating organic contaminants with phosphorus and nitrogen [18]. Advance treatment includes advanced oxidation processes (AOPs), which use strong oxidation reactions to break down complex and tenacious pollutants, membrane bioreactors; use membrane filtration and constructed wetlands; and use wetlands to treat wastewater [19,20,21]. Out of the throng of wastewater treatment processes, adsorption is the most recommended process because of its comprehensibility, efficiency, regeneration capacity, and cost-effectiveness [22]. The degree of adsorption is calculated using suitable adsorbents for components; it is essential to elucidate the physical and chemical aspects of the adsorbent and the related mechanism [23]. Since the effectiveness of each technique depends on the specific characteristics of the wastewater and the targeted contaminants, the choice of method often involves a combination of techniques to achieve optimal results. Table 1 depicts the advantages and disadvantages of different techniques utilized for treating varying types of wastewaters.
Nanomaterials are practical and efficient solutions to get through major roadblocks in creating effective remedial technologies for wastewater treatment [24]. The large surface-to-volume ratio and numerous reactive sites of nanomaterials make them highly reactive toward quick and efficient removal of water pollutants [25]. Nanostructured adsorbents can be specifically designed to target pollutants and possess a substantial capability for addressing contaminated water [26,27]. There is also growing research focusing on the synthesis of biodegradable polymers for wastewater remediation. Natural polymers called biopolymers are either produced from sustainable natural resources or biosynthesized by living organisms [28]. Biopolymers are mostly composed of polysaccharides, and polypeptides. Biopolymers can be divided into three categories: nature-derived, chemically produced, and microbial biopolymers [28,29]. Renewability, biocompatibility, environmental compatibility, biodegradability, and antimicrobial activity are only a few of the impressive interrelated biological, physical, and chemical characteristics of biopolymers [29,30]. Reactive functional groups such as carbonyl, amide, carboxyl, and hydroxyl are present in the skeleton of biopolymers, which make them suitable for wastewater treatment [31]. However, the cost inefficiency of the synthesis and purification procedures is a significant problem that has been noticed throughout the scaling up of biopolymers [32]. Recently, there has been a surge in the scientific significance of biopolymer nanocomposites owing to their versatile applications in addressing environmental issues and remediation challenges [33]. Biopolymeric nanocomposites have been used to remove heavy metals, natural organic matter, dyes, antibiotics, and other water pollutants such as coagulants, adsorbents, flocculants, membranes, and photocatalytic agents [11,34]. Biopolymeric nanocomposites have enhanced physiochemical, thermophysical, and mechanical properties compared to nanomaterials and polymers [35]. Inorganic nanofillers such as metal and metal oxide nanoparticles, nanoclays, and carbon nanomaterials can be included in a biopolymer matrix to produce biopolymeric nanocomposites [36,37,38].
The primary objective of this comprehensive review is to conduct a thorough analysis of the current status and progress in the field of biopolymeric nanocomposites, with a specific focus on their utilization for water remediation. This review explores production methods, properties, and applications of various biopolymeric nanocomposites, emphasizing particularly their role as filtration membranes and adsorbents in the context of wastewater treatment. Beginning with a brief introduction and discussion of the advantages associated with biopolymeric nanocomposites, this review then covers diverse synthesis methods, their properties, and recent advancements in applications and modifications in the composition of a biopolymeric nanocomposite. Furthermore, this paper openly discusses the functions, mechanism, reusability, limitations, and challenges of these materials, emphasizing their significant potential for further exploration and refinement in the field of water remediation. Moreover, this review article supports and advances the UN’s sustainable development goals, in particular, SDG 7 (Affordable and clean energy) and SDG 13 (Climate Action). Figure 1 shows a comprehensive overview of global water scarcity, the diverse array of pollutants impacting water quality, and stages of wastewater treatment—primary, secondary, and tertiary—aimed at removing inorganic, organic, and biological contaminants for effective water purification.
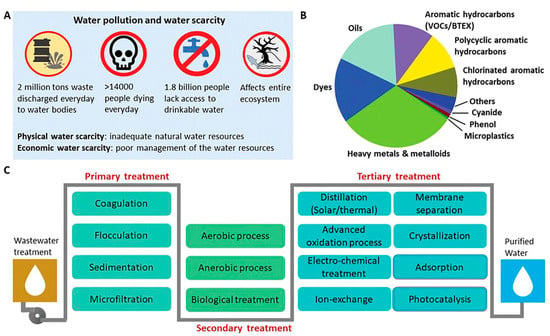
Figure 1.
General illustration of polluted water and technologies available. (A) Global scarcity of water and pollution caused by (B) different pollutants, and (C) different stages of technologies for wastewater treatment [39].

Table 1.
Advantages and disadvantages of different wastewater remediation techniques.
Table 1.
Advantages and disadvantages of different wastewater remediation techniques.
| Technique | Type of Wastewater | Advantages | Disadvantages | Refs. |
|---|---|---|---|---|
| Filtration | Pharmaceutical industry Fish processing | Simple and widely applicable Effective removal of suspended solids | Limited removal of contaminants Filter media can get clogged, require frequent maintenance | [40,41] |
| Coagulation | Domestic sewage Oil Surface water Algae-laden | Efficient removal of colloidal particles Enhances subsequent filtration processes | Formation of sludge imposes on proper disposal Requires careful control of coagulant dosage | [42] |
| Precipitation | Acidic decontamination of radioactive concrete Digested swine | Can reduce water hardness Effective for the removal of dissolved heavy metals | pH control is crucial for precipitation reactions Sludge production and disposal challenges | [43,44] |
| Adsorption | Urban Pharmaceutical Organic | High efficiency in removing organic pollutants Versatile with various adsorbent materials | Saturation of adsorption sites over time Regeneration of adsorbents can be complex | [45,46,47] |
| Flocculation | Pb (II)-polluted groundwater | Aggregation of particles for easier removal Enhanced sedimentation and filtration | Requires careful control of flocculant dosage Potential carryover of fine particles | [48] |
| Electrodialysis | N and P High-salt organic Carbocysteine | Selective removal of ions Continuous operation with minimal chemical usage | High energy consumption Scaling on membranes may occur | [49,50,51] |
| Membranes | Textile Microelectronic | Effective removal of particles, microorganisms, and ions Applicable for various contaminants | High operational and maintenance costs Membrane fouling can reduce efficiency | [52,53] |
| Ion exchange | Cu (II), Ni (II) Cu (II), Pb (II) Municipal Mining | Selective removal of specific ions Regeneration allows for extended use | Limited to ion-specific removal High regeneration chemical usage | [54,55,56,57] |
2. Why Biopolymeric Nanocomposites?
In water remediation processes, biopolymeric nanocomposites serve several important functions and advantages. Firstly, biopolymeric nanocomposites are often derived from natural sources, making them environmentally friendly. Their use in water remediation aligns with sustainable practices, contributing to eco-friendly and green approaches for water treatment [58]. The adsorption capacity of biopolymeric nanocomposites enables them to effectively remove pollutants from water. Biopolymeric nanocomposites, due to their high surface area and functional groups, can adsorb or attract contaminants present in water. Contaminants adhere to the surface or interact with the nanocomposite’s structure, facilitating their separation from the water. This includes pollutants such as heavy metals, dyes, organic compounds, antibiotics, and other impurities [59]. Additionally, biopolymeric nanocomposites can act as filtration agents. They can be designed with specific properties to trap or filter out particulate matter, microorganisms, microplastics, or other undesirable components from water, contributing to improved water quality [60]. Some biopolymeric nanocomposites possess ion exchange capabilities. This means they can exchange ions with contaminants in water, effectively reducing the concentration of harmful substances [61,62]. Furthermore, a notable feature of certain biopolymeric nanocomposites is their ability to be regenerated and reused. After adsorbing contaminants, these nanocomposites can undergo a regeneration process, allowing them to maintain their adsorption capacity for multiple treatment cycles [63]. Interestingly, some biopolymeric nanocomposites can be tailored for selective binding to specific contaminants, such as antibiotics or other chemical compounds. This selectivity enhances their efficiency in targeting particular pollutants without affecting the overall composition of water [64,65]. In summary, biopolymeric nanocomposites play a pivotal role in water remediation by adsorbing, removing, and selectively targeting contaminants, contributing to the purification and sustainable management of water resources.
3. Biopolymeric Nanocomposites
Biopolymers are defined as degradable polymers derived from natural sources such as chitosan, alginate, pectin, lignin, starch, cellulose, etc., along with some biodegradable synthetic polymers such as polylactic acid, polyhydroxybutyrate, polyhydroxyalkanoates, etc., and play a vital role in the formation of biopolymeric nanocomposites [28,66,67,68,69,70]. Those derived from synthetic sources, however, are not renewable and do not entirely adhere to the notions of renewability and degradability. Biopolymer-based nanocomposites are also known as bionanocomposites by some researchers [71]. The unique structure, physiochemical characteristics, chemical stability, and high reactivity of biopolymers make them attractive candidates. The presence of functional groups on biopolymers facilitates the absorption of water pollutants, and hence, biopolymers are suitable for wastewater treatment. Polysaccharides are one of the biopolymers that are frequently used because of their eco-friendliness, biodegradability, nontoxicity, etc. Through physical and chemical interactions, they can also bind to various substances [28]. They are the perfect choice for water treatment because of their adsorption capabilities [72]. Due to the growing societal concern for the environment, environmentally friendly and sustainable concerns have a wide spectrum of appeal. As a result, materials are created according to their life cycle between extraction and disposal. In the cycle, it is also necessary to assess their negative effects on the environment. The utilization of renewable sources rather than synthetic ones to make environmentally friendly polymeric nanocomposites, known as biopolymeric nanocomposites, avoids the challenges associated with plastic waste. These materials are entirely renewable in terms of energy and biodegradable, in addition to being environmentally benign. As a result, these materials can be disposed of at the end of their useful lives without endangering the environment. Biopolymeric nanocomposites use inorganic nanoparticles as nanofillers distributed in an organic biopolymer matrix to combine the advantages of both [33]. Nanofillers are classified as zero-dimensional (0D), one-dimensional (1D), and two-dimensional (2D) nanofillers depending on their dimensions in the nanoscale region. Fillers with all dimensions less than 100 nm are referred to as 0D nanofillers. Similarly, fillers with one or two dimensions less than 100 nm are called 2D and 1D nanofillers, respectively [73,74]. The morphology of the produced biopolymeric nanocomposites, the size of the nanomaterials, and the class of the polymers are some parameters to categorize biopolymeric nanocomposites [75,76].
Among the array of biopolymers, chitosan stands out due to its notable antimicrobial attributes, biodegradability, and impressive gelation properties [77]. These qualities position it as an exemplary material with versatile applications, extending beyond wastewater treatment to various scientific and technological fields. Non-toxicity, biodegradability, antimicrobial, antioxidant, and biocompatibility properties are significant beneficial properties of chitosan, which make it a universal biopolymeric nanocomposite candidate [66]. The positively charged amino groups present on the surface of chitosan enable its interaction with negatively charged contaminants or ions. Due to this reason, the application of chitosan is not only confined to environmental applications but also emerges in different fields of science and technology. Biopolymers such as chitosan with its distinctive gel-forming capacity, particularly when coupled with nanoparticles like Ag ZnO, TiO2, etc., [73,74,78] in bionanocomposites or nanocomposites, hold great promise in food packaging [79], biomedical [78,80,81], and textile applications [82,83], in addition to environmental applications [84,85]. Accompanied by interesting characteristics, chitosan has some drawbacks as well including limited selectivity for certain contaminants.
Alginate, derived from seaweed, has gel-forming ability in the presence of divalent cations and therefore it has good affinity toward metal ions and organic pollutants. It is biocompatible and suitable for encapsulation. However, it lacks in maintaining mechanical strength, stability, and preventing disintegration in aggressive chemical environments, which can affect its performance. Similarly, all the biopolymers have some pros and cons for their utilization in wastewater treatment. Table 2 describes the advantageous characteristics and limitations of biopolymers in wastewater treatment.

Table 2.
Advantages and limitations of biopolymers in wastewater remediation.
4. Synthesis of Biopolymeric Nanocomposites
Biopolymeric or polymeric nanocomposites, in general, have been synthesized using various methods, such as the template synthesis method, melt intercalation, polymer intercalation from a solution, and the in situ polymerization method as mentioned in Figure 2.
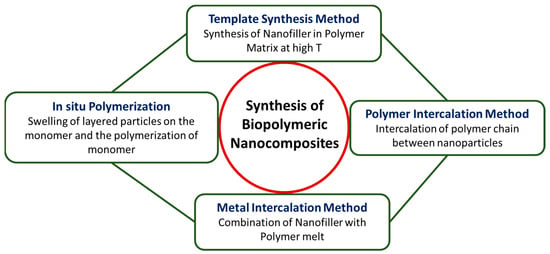
Figure 2.
Representation of various synthesis methods of polymer nanocomposites.
In the template method, filler material is synthesized in the presence of a polymer matrix at high temperatures. Consequently, the polymer facilitates the initiation and expansion of the inorganic host crystals while ensnaring them within its layers. Although it has the potential to produce exfoliated nanocomposites, filler aggregation cannot be neglected [123].
In the melt intercalation process, the high-molecular-weight polymer is heated to a high melting point and combined with the filler as the polymer melts. As a result, neither a solvent nor a chemical synthesis is required in this procedure. However, this procedure can be difficult for high-molecular-weight polymer chains in the filler interlayers owing to the thermodynamic and kinetic impacts on intercalation. Therefore, filler modification is necessary for exfoliating the polymer matrix under shear action [123].
In polymer intercalation using the solution method, the polymer is soluble in a solvent while the nanoparticles are dispersed in a solvent. In the aftermath, the polymer adheres to the delaminated sheets, with subsequent solvent evaporation. As the sheets reassemble during solvent evaporation, they entrap the polymer chains within their layers. This process results in the formation of a multilayered structure [124].
In the in situ intercalation method, the layered particle undergoes swelling in the monomer, initiating monomer polymerization thereafter. The resulting structure is considerably intercalated or exfoliated because of the monomer being present both inside and outside of the filler interlayers. This procedure results in the formation of stable nanocomposites [125,126]. To produce polymer nanocomposite materials, the selection of precursors, design, and synthetic techniques is crucial. Producing polymeric nanocomposites with specified properties involves a meticulous selection of monomers, fillers, and other composite materials, along with the application of distinct synthesis techniques. This highlights how important the design and synthesis processes are in the production of polymeric nanocomposites [127].
Chemical and mechanical methods are mostly efficient techniques for improving the dispersion of nanoparticles in polymeric nanocomposites. Enhancing the interaction or surface area between polymer matrices and nanoparticles is crucial in the design of polymeric nanocomposites [128]. The utilization of a surfactant is thought to be a useful method for enhancing the interaction between the organic phase of the polymer matrix and the inorganic phase of the nanoparticles. Several studies demonstrated the use of silane as a surfactant for inorganic phase surface modification and increasing their dispersion in the polymer matrix. The esterification process of hydrolyzed vinyltrimethoxysilane in an alcoholic solution was used to successfully silanize nanodiamonds [129]. Nanoparticles are compelled to disperse across the polymer matrix through agitation, one of the mechanical procedures. Ultrasonic or high-frequency sonication dispersion is also beneficial in this area since it offers more uniform dispersion as opposed to agitation approaches [130]. Nanoparticle aggregation problems can also be resolved via atomic layer deposition and plasma-assisted mechanochemistry [130]. There are various surfactants used for inorganic phase surface modification in polymer matrices. However, depending on the specific materials and applications, different surfactants may be employed. For the wastewater remediation process, the surfactants can either be cationic, for example, cetyltrimethylammonium bromide (CTAB) [131], used for the surface modification of negatively charged inorganic particles, or anionic (sodium dodecyl sulfate, sodium lauryl ether sulphate) [132,133] with vice versa surface modification, or non-ionic surfactants (Triton X-100, polyoxyethylene glycerol ester) [134], which do not possess a charged head, and are generally used for the dispersion of hydrophobic pollutants and oil droplets in wastewater. Additionally, triblock copolymers such as pluronic surfactants are used in wastewater treatment for the stabilization and dispersion of nanoparticles or colloids [135,136]. Furthermore, fluorinated surfactants, for example, perfluorooctanoic acid (PFOA), are also employed for the treatment of fluorinated compounds of industrial wastewater [137].
5. Properties of Polymeric and Biopolymeric Nanocomposites
The advancement of suitable polymer nanocomposites has significantly augmented the advantageous attributes of polymers, potentially introducing a novel set of features for the resultant materials, as depicted in Figure 3. The extent of improvement, however, hinges on factors such as the nanomaterial’s shape, size, aspect ratio, dispersion state, and interfacial interactions with the polymer matrix. The enhancement of mechanical properties, including the tensile strength, modulus, or stiffness, is often a primary motive for incorporating nanoparticles into polymer matrices. Nonetheless, achieving even dispersion is crucial, as poor compatibility between polymer matrices and inorganic particles can lead to flaws that adversely affect the mechanical properties of polymer nanocomposites. Utilizing nanoparticles has proven effective in addressing the dimensional stability of neat polymers at elevated temperatures, attributed to their high thermal expansion coefficient, contributing to the overall improvement in thermal stability [71]. Polymer materials often have low electrical conductivity. Conductive polymeric nanocomposites are made possible by combining polymer matrices with conductive nanoparticles and are useful in electronic circuits. These goods exhibit not just electrical conductivity but also particular polymeric component characteristics, including flexibility and cheap production costs [126,138].
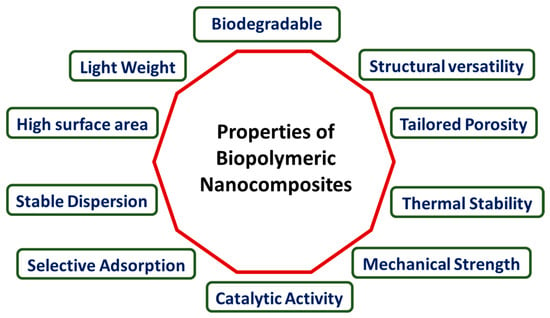
Figure 3.
Properties of biopolymeric nanocomposites.
In another aspect, biopolymeric nanocomposites exhibit a range of properties that make them advantageous for various applications, particularly in wastewater treatment. Some key properties include the following: Enhanced mechanical strength—biopolymeric nanocomposites often display improved mechanical properties compared to their individual components. The addition of nanomaterials reinforces the structural integrity of the biopolymer, enhancing its overall strength. High surface area—the nanoscale features of these composites contribute to a large surface-to-volume ratio. This property increases the available surface area for interactions, making them effective in adsorption processes. Biodegradability—biopolymeric components, such as chitosan, are inherently biodegradable. When combined with nanomaterials, the resulting nanocomposites often maintain biodegradability, making them environmentally friendly. Tailored porosity—nanocomposites can be engineered to have specific porosity levels. This tunable porosity enhances their adsorption capacity, making them suitable for capturing pollutants in wastewater. Chemical stability—the combination of biopolymers and nanomaterials can lead to enhanced chemical stability, ensuring the composite remains robust in various environmental conditions. Selective adsorption—the presence of nanomaterials provides selective adsorption capabilities, allowing the nanocomposites to target specific pollutants or contaminants in wastewater. Thermal stability—the incorporation of nanomaterials often improves the thermal stability of biopolymeric nanocomposites, making them suitable for applications involving varying temperature conditions. Versatility—biopolymeric nanocomposites can be versatile in terms of composition and structure, allowing for customization based on specific wastewater treatment requirements. Understanding and leveraging these properties contribute to the effectiveness of biopolymeric nanocomposites in addressing challenges related to water pollution and wastewater treatment.
6. Applications of Biopolymeric Nanocomposites in Wastewater Remediation
One of the significant hurdles to achieving sustainability and an eco-friendly world is protecting the existing water resources. Less than 1% of the world’s water supply is considered clean, while the remaining water is polluted as per international standards [139]. Municipal wastewater, industrial waste, and agricultural practices are the main causes of water contamination. Among the diverse categories of pollutants, including organic acids, heavy metals, pesticides, fertilizers, dyes, phenolic compounds, halogenated chemicals, and microorganisms, it is noteworthy that certain examples within each category exhibit dual characteristics of toxicity and non-biodegradability [34,84,140,141,142,143,144]. The intake of contaminated water also contributes to several ailments, such as cancer, fever, diarrhea, nasal septum rupture, skin irritation, chills, ulcers, organ damage, headache, abdominal pain, appetite loss, and a lot more. To ensure that all living species have access to clean water, these pollutants must be removed. In this respect, multiple cutting-edge technologies for water purification have since been created.
Conventional wastewater treatment plants mitigate water pollution by eliminating organic and suspended solids. However, with evolving standards and treatment approaches, there is a growing emphasis on the removal of both hazardous substances and organic matter. The methods used to remove these pollutants from sewage can be divided into three groups: physical, biological, and mechanical. There are several methods for treating contaminated water, including filtration, coagulation, precipitation, adsorption, flocculation, electrodialysis, membrane technologies, and ion exchange. Each of these procedures has both advantages and disadvantages. For instance, the precipitation process produces waste, which must be treated before disposal. The ion exchangers are quickly contaminated, lowering their capability for exchanging ions. However, considerable amounts of non-recyclable waste are produced during the flocculation and coagulation processes. Electrodialysis has limited application owing to its high operating costs and energy needs, while photocatalytic techniques require a lengthy reaction time to be effective. Adsorption and membrane technologies have gained a lot of attention in the past few years for water treatment. These techniques are also lacking in several areas that need to be addressed to make them an inexpensive and suitable solution for industrial use [145]. Recently, biopolymeric nanocomposites have become popular as filtration membranes and adsorbents for wastewater treatment.
6.1. Biopolymeric Nanocomposites as Filtration Membranes
Biopolymer-nanocomposite-based filtration membranes leverage the unique properties of nanomaterials integrated into biopolymer matrices, offering enhanced filtration performance, improved mechanical strength, and heightened resistance to fouling. These membranes hold great promise for diverse applications in water purification, separation processes, and environmental remediation. Both academia and industry have paid significant attention to water filtration membranes for desalination, microbial treatment, and ion permeation. Membrane-based separation technologies represent a pinnacle in advanced separation methodologies, lauded for their simplicity, adaptability, and cost-effectiveness. Operating as selective barriers, membranes facilitate the passage of desired materials while detaining undesired substances on their surface. Offering a diverse array of separation techniques, including ultrafiltration, reverse osmosis, and nanofiltration, these membranes stand out for their energy efficiency by eliminating the need for phase change and exhibit exceptional selectivity in removing trace pollutants from water [146].
Various membrane technologies cater to specific separation requirements, such as ultrafiltration, microfiltration, nanofiltration, forward and reverse osmosis, gas separation, membrane distillation, pervaporation, membrane bioreactors, and separation using liquid membranes, as represented in Figure 4. Reverse osmosis (RO) and nanofiltration membranes are especially extensively employed due to their high-water permeability, low-pressure requirements, and cost-effectiveness.
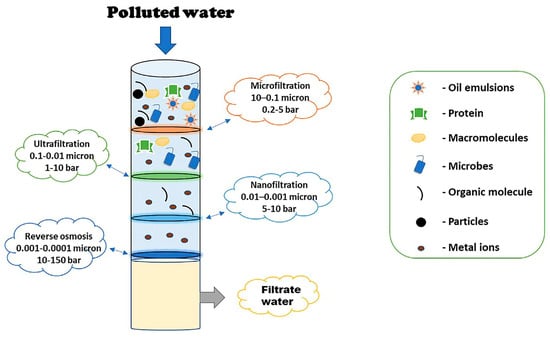
Figure 4.
A schematic representing the capability of different membranes for treating wastewater [145].
In the realm of wastewater treatment using adsorptive membranes, two fundamental approaches come into play: adsorption and rejection. When water-borne solutes encounter the membrane’s active layer, molecular sieving and filtration work collaboratively to reject solutes larger than the pore size. Simultaneously, smaller solutes penetrate the support layer, which acts as an adsorption microsphere. As these smaller solutes pass through the active layer, they form complexes, ultimately resulting in the production of filtered water through the absorptive membrane. This multifaceted process showcases the versatility and efficacy of membrane-based separation technologies in addressing the challenges of water purification and pollutant removal [34,84].
Inorganic membranes offer strong mechanical, structural, and thermal resistance. Despite their great selectivity, they are not suited for a wide range of applications due to their limited permeability. On the other hand, polymeric membranes have a low cost, easy manufacturing, excellent flexibility, chemical stability, and mechanical strength. Polyvinyl alcohol (PVA), polyether sulfone (PES), polyamide (PA), polyethylene (PE), polyvinylidene fluoride (PVDF), polyvinyl chloride (PVC), polypropylene (PP), polyacrylonitrile (PAN), polyimide (PI), chitosan, and alginate [147] are among the materials used to make polymeric membranes. Poly(ethylene glycol) (PEG), a non-biodegradable yet non-toxic polymer, undergoes modification with poly(vinylidene fluoride-co-hexafluoropropylene), incorporating methoxy PEG. This modification results in a material achieving a 99% rejection of humic acid and displaying robust antifouling properties, albeit with environmental considerations due to its non-biodegradable nature [28]. There are various reports on the use of polymeric membranes as filtration membranes for removing pollutants. However, there are several issues with the thermal and mechanical characteristics of current polymeric membranes used in water treatment techniques [148]. Filtration can be improved by using nanocomposite membranes, mixtures of nanofillers, and polymeric membranes. Nanofillers, which comprise metal/metal oxide nanoparticles and carbon-based nanoparticles, have received a lot of interest. It has been demonstrated that biopolymeric nanocomposites may successfully remove a variety of contaminants from wastewater to acceptable levels. Polymeric adsorptive membranes are powerful water pollution remediation solutions. Various kinds of persistent and developing chemical contaminants that are resistant to current approaches can be removed from wastewater using cellulosic and other polymeric membranes. Biopolymeric membranes captivate attention through their compelling integration of adsorption and filtration mechanisms. These membranes not only enhance membrane permeability but also elevate selectivity, rejection rates, and adsorption capacity. In addition to these performance improvements, the utilization of adsorptive membranes effectively tackles fouling issues, contributes to reduced operational costs, and enhances the reusability of the adsorbent. This harmonious synergy of adsorption and filtration mechanisms positions biopolymeric membranes as a promising avenue for advanced separation technologies. Several studies have been conducted to investigate the efficacy of biopolymeric nanocomposites in the removal of antibiotics from water sources. These investigations reveal the pivotal role played by these nanocomposites in the removal process.
In a study, Moradi et al. developed a high-performance thin-film composite nanofiltration membrane for antibiotic removal in pharmaceutical wastewater treatment. Utilizing furosemide-modified chitosan (CS@FS) composite-assisted pectin (PC) functionalization, the polyethersulfone (PES) nanofiltration membrane is enhanced in physicochemical characteristics, such as a smoother membrane surface and a reduced water contact angle. The optimized TFC membrane, TFC-0.5, achieves a 47.8 L/m2 h pure water flux, 94.2% flux recovery ratio, and 5.8% irreversible fouling ratio. Additionally, the CS@FS-co-PC nanofiltration membranes excel in pharmaceutical wastewater treatment, with a 92.0% ± 1.1 COD removal efficiency, 56.1 ± 1.0% TDS removal, and whole turbidity removal. The membrane’s high antibiotic rejection and antifouling abilities make it promising for pharmaceutical wastewater treatment applications [149]. Similarly, Gopal et al. have developed a nanocomposite for the removal of antibiotics from water, employing clay-nanosheets supported with an Fe-Cu nanocomposite. This innovative approach involves immobilizing the composite in a biodegradable chitosan-coated alginate–carboxymethyl chitosan matrix, forming nanocomposite beads suitable for use in column reactors. The study demonstrates effective ciprofloxacin (CIPRO) removal, achieving approximately 90% under optimal conditions in the batch mode, with a maximum removal capacity of 485.58 mg/g according to the Langmuir isotherm. Additionally, the nanocomposite’s performance is assessed against various environmental factors, including salts (NaCl and CaCl2) and micro-contaminants (humic acid and polyethylene), providing valuable insights into its robustness. The research explores reaction parameters in column reactors, such as the flow rate, initial CIPRO concentration, and bed height. The study also evaluates the residual toxicity of the composite beads, confirming a substantial reduction in toxic effects on environmentally relevant algae (Chlorella sp. and Scenedesmus obliquus) [150]. Palacio et al. tackled the global challenge of antibiotic contaminants in water, focusing on nalidixic acid removal using two cationic polymers: poly[(4-vinylbenzyl) trimethylammonium chloride] and N-alkylated chitosan. The removal processes are governed by electrostatic interactions, π–π interactions, and hydrogen bonding, as revealed by their effectiveness under varying conditions, with distinct removal rates—75.0% at pH 9 for poly[(4-vinylbenzyl) trimethylammonium chloride] and 65.0% at pH 7 for alkylated N-chitosan [151]. Valizadeh et al. innovatively tackled tetracycline (TC) antibiotic pollution by introducing a zinc ferrite/chitosan–curdlan magnetic composite. This environmentally friendly adsorbent proved to be highly efficient in TC removal, with optimal conditions at pH 6 and a composite dosage of 0.65 g/L. The adsorption process adhered to pseudo-first-order kinetics and Langmuir isotherm models, revealing a maximum adsorption capacity of 371.42 mg/g at 328 K. A thermodynamic analysis suggested a spontaneous endothermic result and adsorption. The magnetic composite demonstrated easy separation, regeneration capability, and consistent stability over successive cycles, and was a cost-effective solution for removing pharmaceutical pollutants from water [152].
Rawat et al. have recently developed chitosan-based beads by using an iron oxyhydroxide metal nanocomposite for the ultrafiltration of contaminated water and removing arsenic. Their study revealed that a dose of 2 g/L of IICBs can remove arsenic to <10 µg/L permissible limits (Figure 5) [153]. Similarly, an alginate-based nanocomposite has been developed by Ehsan et al. in association with a graphene oxide (GO) carbon network for the separation of oil from water, as depicted in Figure 6 [154]. The utilization of biopolymeric materials, combined with nanotechnology, presents a promising avenue for addressing the environmental concern of antibiotic contamination in water.
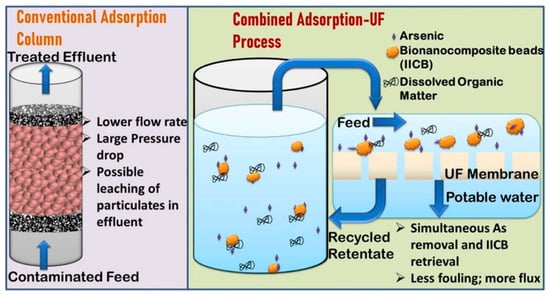
Figure 5.
Schematic representation of conventional treatment of contaminated water with adsorption (left) and with ultrafiltration membranes (right) by using iron oxyhydroxide chitosan beads (IICBs) as the biopolymeric-chitosan-based bionanocomposite [153].
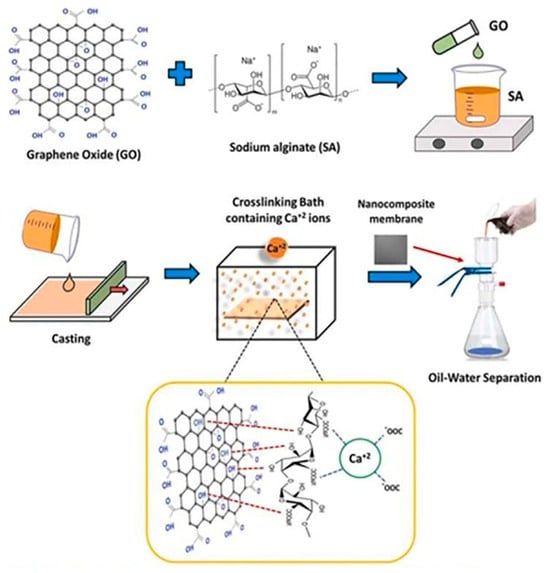
Figure 6.
Schematic representation of oil–water separation by using alginate-GO-based nanocomposite membranes [154].
Mechanism
The size exclusion mechanism operates by permitting molecules with dimensions smaller than the pore size to traverse the membrane, while larger species are impeded. Membranes featuring an accumulation of surface electric charge repel species carrying the same surface charges, facilitating the passage of neutral species as shown in Figure 7. The performance of membrane separation processes is significantly influenced by the physical and chemical interactions between chemicals and the membrane [58].
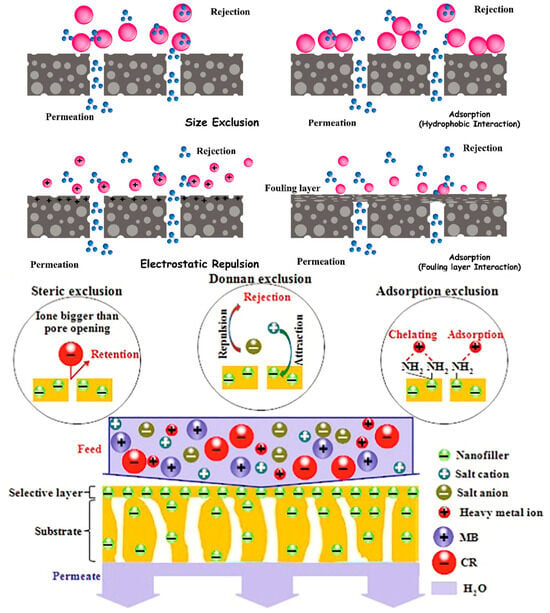
Figure 7.
Mechanistic approach of membrane separation and rejection [58] of inorganic salt, heavy metal ions, and organic dyes [155].
For instance, hydrophobic pollutants can engage in interactions with hydrophobic membrane surfaces through hydrophobic interactions, leading to the adsorption and retention of these species on the solid membrane. Conversely, the formation of biofilm on the membrane enhances the hydrophilicity of the surface, resulting in the rejection of hydrophobic pollutant species [58]. The mechanism of the separation of the rejection of pollutants such as inorganic salts, organic dyes, and heavy metal ions by using the biopolymeric nanocomposite membranes for nanofiltration is depicted in Figure 7 [155].
The exceptional nanofiltration performance and stability of the thin-film composite nanofiltration membrane composed of a chitosan hydrogel covalent organic framework interlayered with tannic acid-Fe3+ involve the establishment of stable chemical bonding interaction between the substrate and polyamide layer and an increase in the degree of cross-linking within the polyamide layer, along with reduced thickness. The water permeability reached 16.17 L m−2 h−1 bar−1, marking a substantial increase to 185% compared to the TFC-control membrane’s 8.74 L m−2 h−1 bar−1. Furthermore, the membrane exhibited high rejections for norfloxacin (94.89%), ciprofloxacin (99.07%), and ofloxacin (99.10%). Notably, the flux recovery rate was impressive at 98.32% (alginate) and 97.99% (BSA), indicating remarkable antifouling performance (Figure 8) [156].
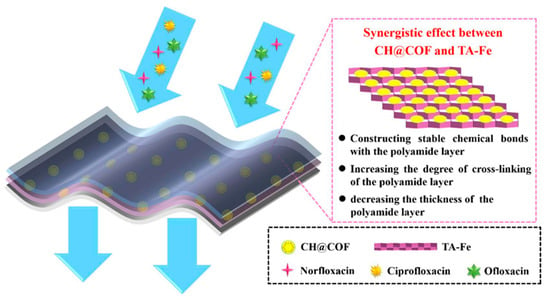
Figure 8.
Pictorial representation of a thin-film composite nanofiltration membrane composed of chitosan hydrogel covalent organic framework interlayered with tannic acid-Fe3+ to remove norfloxacin, ciprofloxacin, and ofloxacin antibiotics from water [156].
In recent years, the escalating global need for lithium resources has been fueled by the rapid expansion of the new energy sector. Regarding this, Zhang et al. have designed nanofiltration membranes, specifically with a positive charge, by utilizing modified chitosan as hydroxypropyltrimethyl ammonium chloride chitosan (HACC). The study revealed decreased thickness and increased hydrophilicity due to the interfacial polymerization process with HACC. Moreover, pore size remained unchanged, while the incorporation of the quaternary ammonium group in HACC significantly enhanced the antibacterial efficacy of the nanofiltration membranes. The optimized nanofiltration membrane, NF-HACC-0.3, significantly improved the separation factor and doubled the flux compared to the original membrane. This innovative approach of modified biopolymeric membranes shows high-performance capabilities in effectively separating magnesium ions (Mg2+) and lithium ions (Li+), and therefore serves as a valuable solution for the extraction as well as recovery of lithium resources from brine, addressing the growing demand in a sustainable manner [157]. Table 3 represents several biopolymer nanocomposite membranes that have been employed to filter out various types of water contaminants, along with treatment technology and their advantages.

Table 3.
Recent studies on wastewater remediation by several biopolymeric nanocomposites as filtration membranes.
6.2. Biopolymeric Nanocomposites as Adsorbents
For many years, biopolymers by themselves were utilized in the water purification process. Heavy metals, oil spills, and other particulates are successfully removed from wastewater using biopolymers. Furthermore, biopolymers and their derivatives can adsorb or capture heavy metals and have stronger adsorbing and chelating effects. The main contributors to water contamination are dyes, which are used in a variety of sectors, including printing, textiles, and painting. Most dyes that are released into water are poisonous and may have an adverse effect on photosynthetic activity by lowering sunlight penetration, which would therefore have an adverse effect on aquatic as well as human life. Therefore, it is crucial to get rid of these harmful dyes and save the environment. A variety of methods, including physical, chemical, and biological techniques, are employed for this purpose. The most popular physiochemical technique for achieving this goal is adsorption. Recently, MXene has been incorporated with chitosan/lignosulfonate nanospheres for removing heavy metals, viz., Cu(II), Co(II), Ni(II), Pb(II), and Cr(VI), from wastewater [170].
Biopolymers have been used extensively to eliminate harmful dyes and heavy toxic metal ions from aqueous solutions owing to their biodegradability, biocompatibility, and presence of multiple functional groups. However, their low thermal stability, poor mechanical properties, and small surface area limit their applications.
GO/polyamidoamine nanocomposites have been investigated for the adsorption of Pb, Cu, Mn, and Cd heavy metal ions [171]. Magnetite nanoparticles were used to modify GO nanosheets before covalently attaching a dithiocarbamate-terminated highly branched polyamidoamine dendrimer to their surface. This study utilizes ultrasound-assisted magnetic solid-phase extraction for concentrating Ni(II), Cr(III), Cu(II), Pb(II), and Cd(II) to demonstrate their sorbent efficacy [172]. Hayati et al. demonstrated that the PAMAM/CNT nanocomposite is a super-adsorbent capable of absorbing unusually large amounts of heavy metals from single- and binary-component liquid phases [173]. ZnO nanoparticles were immobilized on the chitosan/silica hybrid to form an effective chitosan/silica/ZnO nanocomposite, which was used to remove methylene blue (MB) from wastewater using an adsorption process with a 293.3 mg/g adsorption capacity [85]. Similarly, a stable chitosan-TiO2 nanocomposite (CTNC) was synthesized for the quantitative and selective elimination of Rose Bengal dye from industrial wastewater with a 79.365 mg/g adsorption capacity [174]. A polyamidoamine dendrimer was successfully mounted on titania nanoparticles to create a novel nanohybrid with encapsulation potential for phenol removal from industrial wastewater [175]. Paleos and coworkers produced and characterized a variety of poly(propylene imine) dendrimers functionalized with extended aliphatic chains. These dendrimers have been shown to encapsulate polycyclic aromatic hydrocarbons from water down to the few-ppb level [176]. Huang et al. recently introduced carbon microspheres as an outstanding adsorbent by utilizing a chitosan biopolymer as depicted in Figure 9. A series of Cu/Al-doped nitrogen-containing carbon microspheres (Cu/Al@NC-x, x = 1, 2, 3) were then synthesized via a facile one-pot hydrothermal strategy. In experimental batch adsorption studies, these microspheres exhibited exceptional efficacy in removing oxytetracycline contaminants, contributing to water quality improvement. The subsequent thermodynamic analysis revealed a spontaneous endothermic process for Cu/Al@NC-2 adsorbing oxytetracycline (ΔH° > 0, ΔG° < 0). Notably, even after five adsorption cycles, Cu/Al@NC-2 maintained an excellent 92.25% removal efficiency for oxytetracycline [177]. Similarly, chitosan- and alginate-modified carbonized fibers have been recently developed by Li et al. to remove Zn(II), Pb(II), and Cd(II) heavy metal ions at pH = 6 and an optimized 30 °C temperature with a 0.1 mol/L ionic strength for maximum adsorption (Figure 10) [178]. Shan et al. delineated the mechanism for As(III) removal, emphasizing chemisorption as the predominant process. An Fe/Mn-doped chitosan-GO granular adsorbent facilitates the adsorption of most As(III) through inner-sphere complexation, specifically with Fe-O groups associated with ferrihydrite and goethite. This process coincides with the oxidation of As(III) to As(V), catalyzed by O2 and MnO2, followed by complexation with Fe-O groups. Additionally, a minor fraction of As is adsorbed through complexation with oxygen-containing functional groups, such as -OH and single -COOH, present in the chitosan-GO-based nanocomposite (Figure 11) [179]. Similarly, Zheng et al. has developed the composite nanofiber membrane based on the modified chitosan as carboxymethyl chitosan with a synthetic biodegradable polymer, polyvinyl alcohol, PVA, and GO by using the electrospinning method for the adsorption of heavy metal ions (Ni2+, Cu2+, Ag+, and Pb2+). The study shows the reduction in the nanofiber diameter and increased crystallinity through the addition of GO with improved intermolecular hydrogen bonding with the polymeric matrix. The improved adsorption capacity of the biopolymeric membrane for Ni2+, Cu2+, Ag+, and Pb2+ was observed at 262.1, 237.9, 319.3, and 413.6 mg/g, respectively [180]. Similar results have been observed by Thakur et al. with more than a 90% removal efficiency [181]. Chitosan and dialdehyde cellulose have also been explored for the removal of heavy metal ions using a Schiff base reaction and followed by the graft copolymerization of acrylic acid [182].
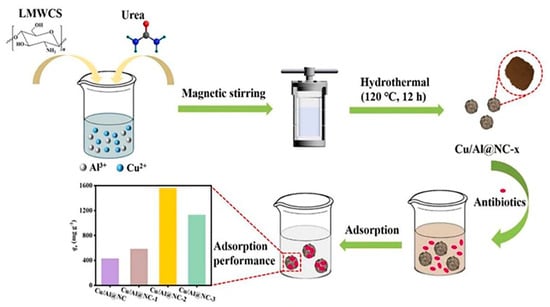
Figure 9.
Schematic illustration of formation of low-molecular-weight chitosan (LMWCS)-Cu/Al with nitrogen-doped carbon microspheres, with hydrothermal method, as an excellent high-performance adsorbent for oxytetracycline antibiotic removal [177].
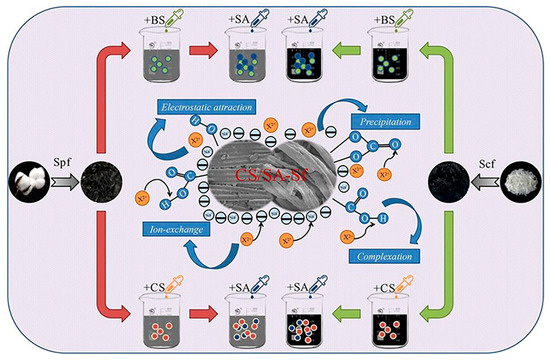
Figure 10.
Schematic illustration of the preparation of semi-carbonized plant fiber (Spf) and chemical fiber (Scf) using dodecyl dimethyl betaine (BS) and chitosan (CS) as modifiers to enhance Sfs. Sodium alginate (SA) served as the composite modifier to further modify BS-Sf and CS-Sf (dodecyl dimethyl betaine and chitosan-modified semi-carbonized fibers), resulting in the preparation of BS/SA-Sf and CS/SA-Sf (sodium-alginate-composite-modified BS-Sf and CS-Sf) to remove Zn(II), Pb(II), and Cd(II) heavy metal ions from polluted water [178].
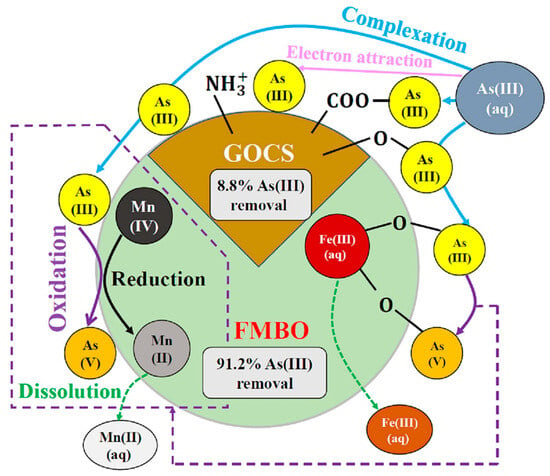
Figure 11.
Schematic illustration of mechanism of removal of As (III) by using binary-doped Fe-Mn with chitosan-GO granular adsorbent [179].
Li et al. formulated a bifunctional composite microsphere adsorbent, CS/DS@ZIF-8, resulting from the combination of a zeolite imidazolate framework (ZIF-8) with chitosan microspheres doped with silica (CS/DS), utilizing the electrospraying method. Characterization analyses indicated a superior crystallinity, increased specific surface area, diverse distribution of pore size, heightened thermal stability. Adsorption studies revealed that CS/DS@ZIF-8 adhered to the Langmuir model as well as the pseudo-second-order kinetics model, displaying maximal capacities of 340.94 mg/g for Pb2+ and 308.27 mg/g for Cu2+. These results demonstrated sustained adsorption rates of 81.3% for Pb2+ and 72.9% for Cu2+ over five cycles. This innovative microsphere effectively addresses both chemical and biological pollutants for water remediation (Figure 12) [183]. Wang et al. utilized advanced techniques, specifically freeze–drying and 3D printing, to fabricate a chitosan/hydroxyapatite, CS/HAP, composite adsorbent and a series of monolithic polylactic acid (PLA), PLA@CS/HAP, filters. This innovative method imparted distinctive macroscopic microchannel structures to the monolithic PLA@CS/HAP filters, significantly enhancing their Cu2+ removal capacity. The study revealed that the adsorption process aligned with Freundlich and pseudo-second-order models, suggesting a multi-layer adsorption with chemisorption characteristics for the CS/HAP composite adsorbent. Cu2+ reusability experiments demonstrated the resilience of PLA@CS/HAP filters, maintaining consistent Cu2+ removal capacity over five consecutive adsorption–desorption cycles, with a significant removal efficiency of 97.17% [184].
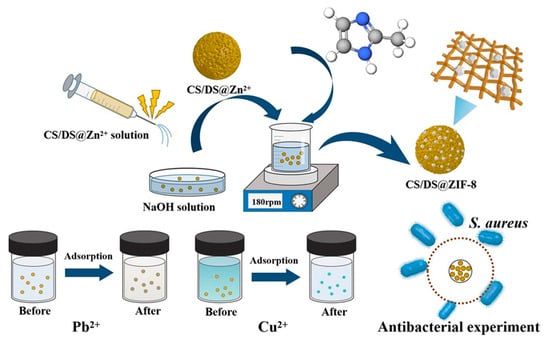
Figure 12.
Schematic illustration of silica-doped chitosan with zeolite imidazolate framework (ZIF-8) composite microsphere for Pb2+ and Cu2+ heavy metal ion removal with significant antibacterial activity [183].
In another study, Ghiorghita et al. utilized a chitosan biopolymer, revealing the potential of ultra-lightweight thiourea–chitosan (CSTU) aerogels. These aerogels, with low densities (0.0021–0.0103 g/cm3) and high specific surface areas (416.64–447.26 m2/g), excelled in swiftly removing heavy metal ions. CSTU aerogels demonstrated impressive recycling stability (up to 80% removal efficiency after five cycles) and potent antimicrobial properties against bacterial strains. These findings underscore the CSTU aerogels’ potential in wastewater treatment and circular economy practices through biological decontamination [185]. Aspartame is a low-calorie artificial sweetener that has faced controversy and concerns due to potential health risks associated with its consumption. Khan et al. developed a green hydrogel nanocomposite, GTBCH, via free-radical polymerization for efficient removal of aspartame from wastewater. The robust adsorption capacity (392.04 mg g−1), as determined using the Langmuir model, can be ascribed to the enhanced interactions between AS and GTBCH. Their diffusion studies revealed aspartame uptake occurring through surface adsorption, liquid film, and intraparticle diffusion mechanisms, respectively [186]. In another study, Kebria et al. investigated the efficacy of a chitosan/polyethyleneimine composite xerogel for removing perfluorobutanesulfonic acid (PFBS) from aqueous solutions via static adsorption. The study covered a wide concentration range (ppb to ppm), revealing a maximum PFBS adsorption capacity of 305 mg/g within 24 h. Chemical characterization indicated electrostatic interactions and hydrogen bond formation between the xerogels’ amine groups and PFBS molecules; findings were confirmed using molecular dynamics simulations. This research offers a viable solution for PFBS removal, highlighting the composite xerogel’s potential in water treatment [187]. Basirun et al. synthesized a polymeric hydrogel, [HIMP][TS], through the functionalization of thiosalicylate-based ionic liquids, and integrated it into polyvinyl alcohol (PVA)–alginate beads for solid biomaterial support. The study focused on an effective treatment method for the removal of toxic manganese (Mn) heavy metal from industrial wastewater, employing an adsorption-based approach with an alginate adsorbent, incorporating the functionalized thiosalicylate-based ionic liquid [188]. Several innovative composite materials have been explored to tailor their mechanical characteristics and surface area, crucial for augmenting adsorption capacity, as outlined in Table 4.

Table 4.
Recent studies on wastewater remediation using several biopolymeric nanocomposites as adsorbents.
7. Reusability of Biopolymeric Nanocomposites
Biopolymeric nanocomposites, a blend of natural polymers and nano-dimensional particles, are composed of eco-friendly components, proven to be remarkably useful in treating wastewater. Biopolymeric nanocomposites have crucial reusability. Their reusable nature means that after being used once to remove contaminants from water, they can undergo regeneration or be reintroduced into the treatment process multiple times [63]. This eco-friendly approach showcases the potential of biopolymeric nanocomposites as valuable tools in addressing water pollution challenges.
Reusability acts as a gauge for determining the stability of photocatalysts within environmental remediation systems. In another study, Salehi et al. demonstrated the elimination of organophosphorus pesticides, viz., chlorpyrifos and diazinon, from an aquatic region by using a MOF-based biopolymeric nanocomposite as a nanoadsorbent hydrogel. The adsorbent hydrogel was composed of xanthan gum, acrylamide, HKUST-1 as MOF material, and Fe3O4 magnetic nanoparticles. The reusability and cost-effective stability or sustainability of the fabricated hydrogel were best after four repeated cycles [202]. Similarly, Sudarmono et al. have evaluated the reusability of a chitosan-Fe3O4 nanoparticle (4:1)-based biopolymeric nanocomposite for the photodegradation of methylene blue. This study revealed high stability and reusability, in a respective 4:1 ratio, up to five cycles with an initial increase in photocatalytic degradation ability (13%) with a simultaneous decrease in the mass of the nanocomposite, which subsequently acts as a photocatalyst by 40%, as shown in Figure 13a,b [203].
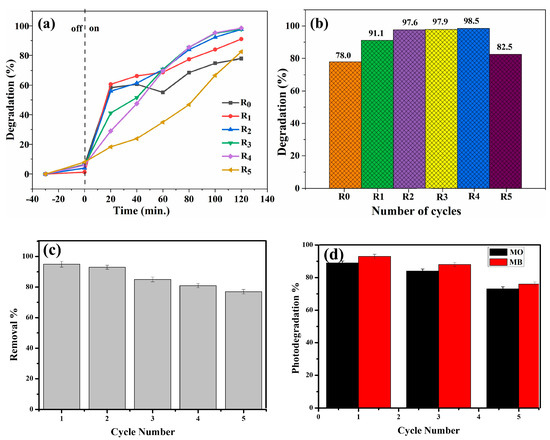
Figure 13.
Reusability of biopolymeric nanocomposites: (a) chitosan-Fe3O4 nanocomposite for photocatalytic ability and (b) photodegradation of methylene blue dye for 120 min UV irradiation [203], and chitosan-based ternary nanocomposite with TiO2 and Ag nanoparticles on cellulose fabric (c) for removal of Cu (II) ions and (d) for photodegradation of methyl orange and methylene blue dye [204].
Recently, Rehan et al. have developed a chitosan-based ternary biopolymeric nanocomposite with TiO2 and Ag nanoparticles, which were further deposited on cellulose fabric to evaluate the wastewater treatment efficacy in terms of removing methyl orange and methyl blue dye contaminants and Cu (II) ions from polluted water. Since the effectiveness of biopolymeric nanocomposites lies in their capacity to capture and eliminate pollutants from wastewater, their reusable feature enhances the sustainability of the treatment process, contributing to both environmental and economic advantages. Therefore, to demonstrate the stability and practical application of the cellulose-fabric-deposited chitosan nanocomposite, it was evaluated for reusability. In this regard, disodium ethylenediamine tetraacetate (Na2EDTA) was used as an eluent for the desorption test with the implication of five repeated cycles, and the test revealed a very reduced amount of loss (19%) in the adsorption ability of the nanocomposite fabric with the removal percentage of Cu (II) ions decreasing from 95% to 77% as depicted in Figure 13c. The reason for this decrement is due to the decrease in the number of actives responsible for the metal ion removal. Similarly, the stability and reusability of the nanocomposite cellulose fabric have been evaluated for the degradation of methyl orange and methylene blue organic dyes. The results revealed a 73% and 76% photocatalytic degradation of methyl orange and methylene blue dye, respectively, after five repeated cycles, as depicted in Figure 13d [204]. The main reasons for the decrease in the degradation activity are (i) a reduced mass after washing and drying, (ii) blockage of pores and active sites due to the accumulation of intermediate particles, and (iii) adherence of dye molecules to the surface of the photocatalyst after the fifth cycle [203].
8. Limitations and Challenges
Biopolymeric nanocomposites, while holding great promise for wastewater remediation, do have certain limitations that need to be considered. One of the primary challenges is the adaptation of biopolymeric nanocomposites for large-scale industrial applications. Ensuring scalability of synthesis methods and their integration into existing wastewater treatment systems is a complex task. Some biopolymeric nanocomposites may exhibit specificity in adsorption, limiting their effectiveness to certain types of contaminants. Ensuring broad-spectrum applicability requires addressing the specificity of adsorption. While some biopolymeric nanocomposites can be regenerated and reused, the efficiency of regeneration processes is either low or may vary. Enhancing the regeneration efficiency is crucial for maximizing the lifespan of these materials. Moreover, the cost of the production and implementation of biopolymeric nanocomposites can be a limiting factor. Ensuring cost-effectiveness compared to alternative treatment methods is essential for widespread adoption. The durability and stability of biopolymeric nanocomposites under different environmental conditions need thorough consideration. Long-term stability and resistance to degradation are crucial for sustained performance. The synthesis of certain biopolymeric nanocomposites may involve intricate processes. Simplifying and optimizing synthesis methods are essential for reducing complexity and enhancing efficiency. While selectivity can be an advantage, it may also limit the applicability of biopolymeric nanocomposites to specific types of contaminants. Achieving a balance between selectivity and versatility is a key challenge. The lack of standardized protocols for the synthesis and application of biopolymeric nanocomposites can hinder widespread adoption. Establishing standardized procedures is important for ensuring consistency and reliability. The availability of modified or functionalized biopolymeric nanocomposites tailored for specific contaminants may be limited. Expanding the range of available materials is crucial for addressing diverse water quality challenges. The environmental impact of synthesis processes for biopolymeric nanocomposites needs consideration. Ensuring that these processes align with sustainable and eco-friendly practices is essential.
9. Conclusions and Future Perspective
In conclusion, the synthesis and application of biopolymeric nanocomposites represent a promising avenue for advancing wastewater remediation strategies. Despite the significant advantages offered by these materials, including eco-friendliness, high adsorption capacity, filtration capabilities, ion exchange properties, and selective binding, several challenges and limitations must be addressed to fully realize their potential. The primary hurdle lies in adapting biopolymeric nanocomposites for large-scale industrial applications, requiring scalable synthesis methods and integration into existing wastewater treatment systems. Specificity in adsorption, regeneration efficiency, production costs, and the lack of standardized protocols further underscore the need for comprehensive research and development in this field.
To overcome challenges, future research efforts should prioritize enhancing the scalability of synthesis methods to facilitate seamless integration into industrial processes. Developing efficient regeneration protocols is imperative to prolong the lifespan of these materials and maximize their reusability. Additionally, optimizing production processes and exploring cost-effective alternatives are essential to ensure the economic viability of biopolymeric nanocomposites in comparison to conventional treatment methods. The balance between selectivity and versatility is crucial, and future studies should aim to strike this equilibrium, broadening the applicability of biopolymeric nanocomposites to diverse contaminants without sacrificing specificity. Standardized protocols for synthesis and application are paramount to ensure consistency and reliability across different studies, fostering widespread adoption and comparability of results. Looking ahead, the exploration of modified or functionalized biopolymeric nanocomposites tailored for specific contaminants should be a priority. Expanding the range of available materials will contribute to addressing diverse water quality challenges, catering to the unique characteristics of different pollutants. Moreover, a concerted effort toward sustainable and eco-friendly synthesis processes is necessary to align with global environmental goals and ensure the long-term viability of biopolymeric nanocomposites in water remediation.
In summary, this review provides an in-depth analysis of the current state of biopolymeric nanocomposites in wastewater remediation, offering insights into their advantages, functions, limitations, and challenges. Future perspectives outlined herein aim to guide and inspire further research endeavors, ultimately contributing to the evolution of and improvement in biopolymeric nanocomposites for sustainable and effective water treatment solutions.
Author Contributions
Conceptualization, A.; methodology, A. and M.M.; formal analysis, A. and M.M.; investigation, A. and M.M.; resources, A.; data curation, A.; writing—original draft preparation, A., M.M. and S.T.; writing—review and editing, A., M.M. and S.T.; visualization, A.; supervision, A. and D.K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
This work is supported by the Technology Innovation Program (#20010170) funded by the Ministry of Trade, Industry & Energy (MOTIE), Republic of Korea.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Oladoye, P.O. Natural, Low-Cost Adsorbents for Toxic Pb(II) Ion Sequestration from (Waste)Water: A State-of-the-Art Review. Chemosphere 2022, 287, 132130. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.; Zare, E.N.; Makvandi, P.; Zheng, X.; Iftekhar, S.; Wu, A.; Padil, V.V.T.; Mokhtari, B.; Varma, R.S.; Tay, F.R.; et al. Cytotoxic Aquatic Pollutants and Their Removal by Nanocomposite-Based Sorbents. Chemosphere 2020, 258, 127324. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, M.; Samsudin, M.F.R.; Sufian, S. Modelling and Optimization of Photocatalytic Degradation of Phenol via TiO2 Nanoparticles: An Insight into Response Surface Methodology and Artificial Neural Network. J. Photochem. Photobiol. A Chem. 2019, 384, 112039. [Google Scholar] [CrossRef]
- Devlin, M.; Brodie, J. Nutrients and Eutrophication. In Marine Pollution—Monitoring, Management and Mitigation; Reichelt-Brushett, A., Ed.; Springer Nature: Cham, Switzerland, 2023; pp. 75–100. ISBN 978-3-031-10127-4. [Google Scholar]
- Khademian, E.; Salehi, E.; Sanaeepur, H.; Galiano, F.; Figoli, A. A Systematic Review on Carbohydrate Biopolymers for Adsorptive Remediation of Copper Ions from Aqueous Environments-Part A: Classification and Modification Strategies. Sci. Total Environ. 2020, 738, 139829. [Google Scholar] [CrossRef]
- Shah, A.I.; Din Dar, M.U.; Bhat, R.A.; Singh, J.P.; Singh, K.; Bhat, S.A. Prospectives and Challenges of Wastewater Treatment Technologies to Combat Contaminants of Emerging Concerns. Ecol. Eng. 2020, 152, 105882. [Google Scholar] [CrossRef]
- Mansoori, S.; Davarnejad, R.; Matsuura, T.; Ismail, A.F. Membranes Based on Non-Synthetic (Natural) Polymers for Wastewater Treatment. Polym. Test. 2020, 84, 106381. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Ahmadi, S.; Ghosh, S.; Othmani, A.; Osagie, C.; Meskini, M.; AlKafaas, S.S.; Malloum, A.; Khanday, W.A.; Jacob, A.O.; et al. Recent Advances on Sustainable Adsorbents for the Remediation of Noxious Pollutants from Water and Wastewater: A Critical Review. Arab. J. Chem. 2023, 16, 105303. [Google Scholar] [CrossRef]
- Russo, T.; Fucile, P.; Giacometti, R.; Sannino, F. Sustainable Removal of Contaminants by Biopolymers: A Novel Approach for Wastewater Treatment. Current State and Future Perspectives. Processes 2021, 9, 719. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Abdullah, A.Z.; Ibrahim, M.H.; Tan, K.B.; Gholami, Z.; Amouzgar, P. Application of Chitosan and Its Derivatives as Adsorbents for Dye Removal from Water and Wastewater: A Review. Carbohydr. Polym. 2014, 113, 115–130. [Google Scholar] [CrossRef]
- Mangla, D.; Annu; Sharma, A.; Ikram, S. Critical Review on Adsorptive Removal of Antibiotics: Present Situation, Challenges and Future Perspective. J. Hazard. Mater. 2022, 425, 127946. [Google Scholar] [CrossRef]
- Singh, R.; Purkait, M.K. Role of Poly(2-Acrylamido-2-Methyl-1-Propanesulfonic Acid) in the Modification of Polysulfone Membranes for Ultrafiltration. J. Appl. Polym. Sci. 2017, 134, 45290. [Google Scholar] [CrossRef]
- Singh, R.; Yadav, V.S.K.; Purkait, M.K. Cu2O Photocatalyst Modified Antifouling Polysulfone Mixed Matrix Membrane for Ultrafiltration of Protein and Visible Light Driven Photocatalytic Pharmaceutical Removal. Sep. Purif. Technol. 2019, 212, 191–204. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, J.; Yan, Y.; Yang, L.; Xing, G.; Li, H.; Wu, P.; Wang, M.; Zheng, H. Application of Coagulation/Flocculation in Oily Wastewater Treatment: A Review. Sci. Total Environ. 2021, 765, 142795. [Google Scholar] [CrossRef] [PubMed]
- Rajala, K.; Grönfors, O.; Hesampour, M.; Mikola, A. Removal of Microplastics from Secondary Wastewater Treatment Plant Effluent by Coagulation/Flocculation with Iron, Aluminum and Polyamine-Based Chemicals. Water Res. 2020, 183, 116045. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, K.Q.; Barzinjy, A.A.; Hamad, S.M. Iron Oxide Nanoparticles: Preparation Methods, Functions, Adsorption and Coagulation/Flocculation in Wastewater Treatment. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100661. [Google Scholar] [CrossRef]
- Samer, M. Biological and Chemical Wastewater Treatment Processes. In Wastewater Treatment Engineering; IntechOpen: London, UK, 2015. [Google Scholar] [CrossRef]
- Sharma, S.; Ahammed, M.M. Application of Modified Water Treatment Residuals in Water and Wastewater Treatment: A Review. Heliyon 2023, 9, e15796. [Google Scholar] [CrossRef] [PubMed]
- Dereli, R.K.; van der Zee, F.P.; Ozturk, I.; van Lier, J.B. Treatment of Cheese Whey by a Cross-Flow Anaerobic Membrane Bioreactor: Biological and Filtration Performance. Environ. Res. 2019, 168, 109–117. [Google Scholar] [CrossRef]
- Li, S.; Wu, S.; Cheng, X.; Dong, H.; Qiang, Z.; Xu, D. Adsorption, Boiling or Membrane Filtration for Disinfection by-Product Removal: How to Make Our Drinking Water Safer? Sci. Total Environ. 2024, 912, 169468. [Google Scholar] [CrossRef]
- Iqbal, A.; Cevik, E.; Bozkurt, A.; Mustafa, A.; Asiri, S.; Alagha, O.; Qahtan, T.F. Tailored Multifunctional Molybdenum-Iron Nanosheets for Enhanced Membrane Filtration and Excellent Electrocatalytic Performance for Hydrogen Evolution Reaction. J. Clean. Prod. 2023, 421, 138486. [Google Scholar] [CrossRef]
- Mandal, S.; Calderon, J.; Marpu, S.B.; Omary, M.A.; Shi, S.Q. Mesoporous Activated Carbon as a Green Adsorbent for the Removal of Heavy Metals and Congo Red: Characterization, Adsorption Kinetics, and Isotherm Studies. J. Contam. Hydrol. 2021, 243, 103869. [Google Scholar] [CrossRef]
- Jun, B.M.; Lee, H.K.; Park, S.; Kim, T.J. Purification of Uranium-Contaminated Radioactive Water by Adsorption: A Review on Adsorbent Materials. Sep. Purif. Technol. 2021, 278, 119675. [Google Scholar] [CrossRef]
- Dhariwal, S.; Mittal, M. Wastewater Treatment with Perovskite-Based Photocatalysts: Environmental Sustainability from a Green Perspective. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Sharma, T.; Yadav, S.; Mittal, M. Recent Advancement in the Sustainable Synthesis of BiOX (X = I, Br, and Cl) Nanomaterials and Their Applications in the Photocatalysis. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Singh, S.; Garg, R.; Jana, A.; Bathula, C.; Naik, S.; Mittal, M. Current Developments in Nanostructurally Engineered Metal Oxide for Removal of Contaminants in Water. Ceram. Int. 2023, 49, 7308–7321. [Google Scholar] [CrossRef]
- Mittal, M.; Jana, A. Role of Nanophotocatalysts in Water Remediation. In Nanotechnology for Sustainable Agriculture, Food and Environment; CRC Press: Boca Raton, FL, USA, 2023; pp. 171–182. [Google Scholar]
- Shekh, M.I.; Annu; Ahmed, S. Chapter 1—Biopolymers: An Overview. In Advanced Applications of Biobased Materials; Ahmed, S., Annu, Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 3–21. ISBN 978-0-323-91677-6. [Google Scholar]
- Van de Velde, K.; Kiekens, P. Biopolymers: Overview of Several Properties and Consequences on Their Applications. Polym. Test. 2002, 21, 433–442. [Google Scholar] [CrossRef]
- Fredi, G.; Dorigato, A. Compatibilization of Biopolymer Blends: A Review. Adv. Ind. Eng. Polym. Res. 2023. [Google Scholar] [CrossRef]
- Hoque, M.; Alam, M.; Wang, S.; Zaman, J.U.; Rahman, M.S.; Johir, M.A.H.; Tian, L.; Choi, J.-G.; Ahmed, M.B.; Yoon, M.-H. Interaction Chemistry of Functional Groups for Natural Biopolymer-Based Hydrogel Design. Mater. Sci. Eng. R Rep. 2023, 156, 100758. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Eloffy, M.G.; Guibal, E.; Alghamdi, H.M.; Elwakeel, K.Z. Use of Biopolymers in Wastewater Treatment: A Brief Review of Current Trends and Prospects. Chin. J. Chem. Eng. 2023, 64, 292–320. [Google Scholar] [CrossRef]
- Kolya, H.; Kang, C.-W. Next-Generation Water Treatment: Exploring the Potential of Biopolymer-Based Nanocomposites in Adsorption and Membrane Filtration. Polymers 2023, 15, 3421. [Google Scholar] [CrossRef]
- Rajendra, S.; Sadasivuni, K.K.; Deshmukh, K.; Mehta, A.; Basu, S.; Meshram, J.S.; Al-Maadeed, M.A.A.; Karim, A. Natural Polymer Based Composite Membranes for Water Purification: A Review. Polym. Technol. Mater. 2019, 58, 1295–1310. [Google Scholar] [CrossRef]
- Rahmatpour, A.; Hesarsorkh, A.H.A. Chitosan and Silica Nanoparticles-Modified Xanthan Gum-Derived Bio-Nanocomposite Hydrogel Film for Efficient Uptake of Methyl Orange Acidic Dye. Carbohydr. Polym. 2024, 328, 121721. [Google Scholar] [CrossRef] [PubMed]
- Emam, H.E.; Badawi, A.K.; Adeola, A.O.; Nomngongo, P.N. Advanced Polymeric Nanocomposites for Water Treatment Applications: A Holistic Perspective. Polymers 2022, 14, 2462. [Google Scholar] [CrossRef]
- Tharwat, R.M.; Mahmoud, M.E.; Abdelfattah, A.M.; Hassan, S.S.M. Decorated Xanthan Gum/Alginate Mingled Hydrogel Beads@La(III)-MOFs@reduced Graphene Oxide@graphene Quantum Dots Nanohybrid for Adsorptive Capture and Recovery of U(VI). J. Mol. Liq. 2023, 390, 122960. [Google Scholar] [CrossRef]
- Mudgal, D.; Yadav, N.; Singh, J.; Srivastava, G.K.; Mishra, V. Xanthan Gum-Based Copper Nano-Magnetite Doped Carbon Aerogel: A Promising Candidate for Environmentally Friendly Catalytic Dye Degradation. Int. J. Biol. Macromol. 2023, 253, 127491. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Gusain, R.; Pandey, S.; Ray, S.S. Hydrogel Nanocomposite Adsorbents and Photocatalysts for Sustainable Water Purification. Adv. Mater. Interfaces 2023, 10, 2201375. [Google Scholar] [CrossRef]
- Virpiranta, H.; Okyere Abayie, S.; Mäkikangas, J.; Puirava, M.; Koivula, K.; Leiviskä, T. Treatment of Fish Processing Plant Wastewater Using Dissolved Air Flotation and Pilot-Scale Biochar Column Filtration. J. Environ. Chem. Eng. 2023, 11, 110853. [Google Scholar] [CrossRef]
- Hong Tran, G.; Khanh Tran, T.; Leu, H.-J.; Richards, D.; Lo, S.-S. An Integrated System Combining Electrochemical Oxidation and Filtration Processes to Remove Chlorine from Pharmaceutical Industry Wastewater. Arab. J. Chem. 2024, 17, 105611. [Google Scholar] [CrossRef]
- Geng, Y.; Nie, Y.; Du, H.; Ma, T.; Li, L.; Can, Z.; Xue, N.; Shen, Q. Coagulation Performance and Floc Characteristics of Fe–Ti–V Ternary Inorganic Coagulant for Organic Wastewater Treatment. J. Water Process Eng. 2023, 56, 104344. [Google Scholar] [CrossRef]
- Oh, M.; Lee, K.; Jeon, M.K.; Foster, R.I.; Lee, C.-H. Chemical Precipitation–Based Treatment of Acidic Wastewater Generated by Chemical Decontamination of Radioactive Concrete. J. Environ. Chem. Eng. 2023, 11, 110306. [Google Scholar] [CrossRef]
- Bai, W.; Tang, R.; Wu, G.; Wang, W.; Yuan, S.; Xiao, L.; Zhan, X.; Hu, Z.-H. Co-Precipitation of Heavy Metals with Struvite from Digested Swine Wastewater: Role of Suspended Solids. J. Hazard. Mater. 2023, 455, 131633. [Google Scholar] [CrossRef]
- Santos, A.F.; Lopes, D.V.; Alvarenga, P.; Gando-Ferreira, L.M.; Quina, M.J. Phosphorus Removal from Urban Wastewater through Adsorption Using Biogenic Calcium Carbonate. J. Environ. Manag. 2024, 351, 119875. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Yang, S.; Liu, X.; Zhu, C.; Qi, F.; Wang, K.; Wang, J.; Wang, Q.; Wang, T.; Ma, P. Adsorption of Antibiotics from Wastewater by Cabbage-Based N, P Co-Doped Mesoporous Carbon Materials. J. Clean. Prod. 2023, 391, 136174. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Chen, M.; Feng, Q.; Zhang, X.; Wang, S.; Zhao, R.; Jiang, T. Adsorption and Photocatalytic Degradation of Quinolone Antibiotics from Wastewater Using Functionalized Biochar. Environ. Pollut. 2023, 336, 122409. [Google Scholar] [CrossRef] [PubMed]
- Ban, Y.; Liu, L.; Du, J.; Ma, C. Investigation of the Treatment Efficiency and Mechanism of Microporous Flocculation Magnetic Fluidized Bed (MFMFB) Reactor for Pb(II)-Containing Wastewater. Sep. Purif. Technol. 2024, 334, 125963. [Google Scholar] [CrossRef]
- Cao, Y.; Li, X.; Zhang, L. Construction of Bipolar Membrane Electrodialysis Reactor for Removal and Recovery of Nitrogen and Phosphorus from Wastewater. Int. J. Electrochem. Sci. 2023, 18, 100051. [Google Scholar] [CrossRef]
- Jia, Z.; Li, F.; Zhang, X.; Zhao, X. Effects of Cation Exchange Membrane Properties on the Separation of Salt from High-Salt Organic Wastewater by Electrodialysis. Chem. Eng. J. 2023, 475, 146287. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, H.; Cheng, Y.; Ma, X.; Chu, Y.; Hu, X. Treatment of Carbocysteine Wastewater by Bipolar Membrane Electrodialysis: From Lab-to Pilot-Scale. J. Memb. Sci. 2023, 687, 122056. [Google Scholar] [CrossRef]
- Luo, X.; Jiang, L.; Zhao, R.; Wang, Y.; Xiao, X.; Ghazouani, S.; Yu, L.; Mai, Z.; Matsuyama, H.; Jin, P. Energy-Efficient Trehalose-Based Polyester Nanofiltration Membranes for Zero-Discharge Textile Wastewater Treatment. J. Hazard. Mater. 2024, 465, 133059. [Google Scholar] [CrossRef]
- Li, X.; Lin, L.; Zhang, X.; Dai, R.; Wu, Z.; Wang, Z. Performance and Fouling Characteristics in a Pilot-Scale Reverse Osmosis Membrane System for Microelectronic Wastewater Treatment. Sep. Purif. Technol. 2024, 337, 126333. [Google Scholar] [CrossRef]
- Yang, X.; Ma, N.; Jia, Y.; Huang, J.; Zhang, X. Separation and Recovery Process of Copper (II) and Nickel (II) from Wastewater Using Ion Exchange Fiber. ChemistrySelect 2021, 6, 12985–12997. [Google Scholar] [CrossRef]
- Lin, Z.; Li, F.; Liu, X.; Su, J. Preparation of Corn Starch/Acrylic Acid/Itaconic Acid Ion Exchange Hydrogel and Its Adsorption Properties for Copper and Lead Ions in Wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2023, 671, 131668. [Google Scholar] [CrossRef]
- Medri, V.; Papa, E.; Landi, E.; Maggetti, C.; Pinelli, D.; Frascari, D. Ammonium Removal and Recovery from Municipal Wastewater by Ion Exchange Using a Metakaolin K-Based Geopolymer. Water Res. 2022, 225, 119203. [Google Scholar] [CrossRef] [PubMed]
- Lebron, Y.A.R.; Silva, A.F.R.; Moreira, V.R.; de Souza Santos, L.V.; Amaral, M.C.S. Hybrid Membrane Distillation and Ion Exchange Process for Resources Recovery from Mining Wastewater. Desalination 2024, 573, 117224. [Google Scholar] [CrossRef]
- Al-Hazmi, H.E.; Łuczak, J.; Habibzadeh, S.; Hasanin, M.S.; Mohammadi, A.; Esmaeili, A.; Kim, S.-J.; Khodadadi Yazdi, M.; Rabiee, N.; Badawi, M.; et al. Polysaccharide Nanocomposites in Wastewater Treatment: A Review. Chemosphere 2024, 347, 140578. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.N.; Christopher, L.P. Natural Cellulose-Chitosan Cross-Linked Superabsorbent Hydrogels with Superior Swelling Properties. ACS Sustain. Chem. Eng. 2018, 6, 8736–8742. [Google Scholar] [CrossRef]
- Risch, P.; Adlhart, C. A Chitosan Nanofiber Sponge for Oyster-Inspired Filtration of Microplastics. ACS Appl. Polym. Mater. 2021, 3, 4685–4694. [Google Scholar] [CrossRef]
- Jørgensen, S.E. Examination of the Applicability of Cellulose Ion Exchangers for Water and Waste Water Treatment. Water Res. 1979, 13, 1239–1247. [Google Scholar] [CrossRef]
- Zhang, Z.; Ahmed, A.I.S.; Malik, M.Z.; Ali, N.; khan, A.; Ali, F.; Hassan, M.O.; Mohamed, B.A.; Zdarta, J.; Bilal, M. Cellulose/Inorganic Nanoparticles-Based Nano-Biocomposite for Abatement of Water and Wastewater Pollutants. Chemosphere 2023, 313, 137483. [Google Scholar] [CrossRef]
- He, S.; Li, J.; Cao, X.; Xie, F.; Yang, H.; Wang, C.; Bittencourt, C.; Li, W. Regenerated Cellulose/Chitosan Composite Aerogel with Highly Efficient Adsorption for Anionic Dyes. Int. J. Biol. Macromol. 2023, 244, 125067. [Google Scholar] [CrossRef]
- Maity, S.K.; Tyagi, U.; Sirohi, S.; Pani, B.; Kumar, K.; Nikita; Kumar, G. Fabrication of Biocompatible Chitosan/Graphene Based Nanocomposite for the Competitive Adsorption of Heavy Metal Ions from Wastewater in Binary and Ternary Systems: Scale-up and Upgradation Studies. J. Water Process Eng. 2023, 56, 104555. [Google Scholar] [CrossRef]
- Li, L.; Iqbal, J.; Zhu, Y.; Wang, F.; Zhang, F.; Chen, W.; Wu, T.; Du, Y. Chitosan/Al2O3-HA Nanocomposite Beads for Efficient Removal of Estradiol and Chrysoidin from Aqueous Solution. Int. J. Biol. Macromol. 2020, 145, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Annu; Ahmed, S.; Ahmed, S.; Ikram, S. Chitin and Chitosan: History, Composition and Properties. In Chitosa; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 1–24. ISBN 9781119364849. [Google Scholar]
- Annu; Ahmed, S.; Ikram, S. Perspectives of Chitosan and Alginate Membranes for Biomedical Applications. In Natural Polymers: Derivatives, Blends and Composites; Ahmed, S., Ikram, S., Eds.; Nova Science Publishers, Incorporated: Hauppauge, NY, USA, 2017; Volume 2, pp. 157–182. ISBN 9781536104400. [Google Scholar]
- isverya, S.; Annu; Ali, A.; Sudha, P.N. 28—Pullulan-Based Bionanocomposites in Tissue Engineering and Regenerative Medicine. In Bionanocomposites in Tissue Engineering and Regenerative Medicine; Ahmed, S., Annu, Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2021; pp. 533–547. ISBN 978-0-12-821280-6. [Google Scholar]
- Khatoon, F.; Shabbir, M.; Annu. An Introduction to Regenerated Cellulose: Morphologies and Applications. In Regenerated Cellulose and Composites: Morphology-Property Relationship; Shabbir, M., Ed.; Springer Nature: Singapore, 2023; pp. 1–7. ISBN 978-981-99-1655-9. [Google Scholar]
- Oyewo, O.A.; Elemike, E.E.; Onwudiwe, D.C.; Onyango, M.S. Metal Oxide-Cellulose Nanocomposites for the Removal of Toxic Metals and Dyes from Wastewater. Int. J. Biol. Macromol. 2020, 164, 2477–2496. [Google Scholar] [CrossRef]
- Annu; Ahmed, S. 1—Bionanocomposites: An Overview. In Bionanocomposites in Tissue Engineering and Regenerative Medicine; Ahmed, S., Annu, Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2021; pp. 1–6. ISBN 978-0-12-821280-6. [Google Scholar]
- Gomez-Maldonado, D.; Erramuspe, I.B.V.; Peresin, M.S. Natural Polymers as Alternative Adsorbents and Treatment Agents for Water Remediation. BioResources 2019, 14, 10093–10160. [Google Scholar] [CrossRef]
- Annu; Ahmed, S.; Kaur, G.; Sharma, P.; Singh, S.; Ikram, S. Evaluation of the Antioxidant, Antibacterial and Anticancer (Lung Cancer Cell Line A549) Activity of: Punica Granatum Mediated Silver Nanoparticles. Toxicol. Res. 2018, 7, 923–930. [Google Scholar] [CrossRef]
- Annu; Ahmed, S.; Kaur, G.; Sharma, P.; Singh, S.; Ikram, S. Fruit Waste (Peel) as Bio-Reductant to Synthesize Silver Nanoparticles with Antimicrobial, Antioxidant and Cytotoxic Activities. J. Appl. Biomed. 2018, 16, 221–231. [Google Scholar] [CrossRef]
- Amass, W.; Amass, A.; Tighe, B. A Review of Biodegradable Polymers: Uses, Current Developments in the Synthesis and Characterization of Biodegradable Polyesters, Blends of Biodegradable Polymers and Recent Advances in Biodegradation Studies. Polym. Int. 1998, 47, 89–144. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M.A.; Hinrichsen, G.I. Biofibres, Biodegradable Polymers and Biocomposites: An Overview. Macromol. Mater. Eng. 2000, 276–277, 1–24. [Google Scholar] [CrossRef]
- Annu; Manzoor, K.; Ahmad, S.; Soundarajan, A.; Ikram, S.; Ahmed, S. Chapter 30—Chitosan Based Nanomaterials for Biomedical Applications. In Handbook of Nanomaterials for Industrial Applications; Mustansar Hussain, C., Ed.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2018; pp. 543–562. ISBN 978-0-12-813351-4. [Google Scholar]
- Annu; Ali, A.; Ahmed, S. Green Synthesis of Metal, Metal Oxide Nanoparticles, and Their Various Applications. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–45. ISBN 978-3-319-48281-1. [Google Scholar]
- Annu; Ali, A.; Ahmed, S. Eco-Friendly Natural Extract Loaded Antioxidative Chitosan/Polyvinyl Alcohol Based Active Films for Food Packaging. Heliyon 2021, 7, e06550. [Google Scholar] [CrossRef]
- Annu; Ahmed, S.; Nirala, R.K.; Kumar, R.; Ikram, S. Green Synthesis of Chitosan/Nanosilver Hybrid Bionanocomposites with Promising Antimicrobial, Antioxidant and Anticervical Cancer Activity. Polym. Polym. Compos. 2021, 29, S199–S210. [Google Scholar] [CrossRef]
- Annu; Bhat, Z.I.; Imtiyaz, K.; Rizvi, M.M.A.; Ikram, S.; Shin, D.K. Comparative Study of ZnO-and-TiO2-Nanoparticles-Functionalized Polyvinyl Alcohol/Chitosan Bionanocomposites for Multifunctional Biomedical Applications. Polymers 2023, 15, 3477. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Sheikh, J.; Annu; Singh, A. An Eco-Friendly Route to Develop Cellulose-Based Multifunctional Finished Linen Fabric Using ZnO NPs and CS Network. J. Ind. Eng. Chem. 2021, 97, 383–389. [Google Scholar] [CrossRef]
- Annu; Pandit, P.; Maity, S.; Bhattacharya, T.; Shekh, M.I.; Ahmed, S. Chapter 30—Chitosan Biobased Materials in Textile Industry. In Advanced Applications of Biobased Materials; Ahmed, S., Annu, Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 717–735. ISBN 978-0-323-91677-6. [Google Scholar]
- Althomali, R.H.; Alamry, K.A.; Hussein, M.A.; Tay, G.S. Versatile Applications Of Biopolymer Nanocomposites: A Review. ChemistrySelect 2022, 7, e202200843. [Google Scholar] [CrossRef]
- Hassan, H.; Salama, A.; El-ziaty, A.K.; El-Sakhawy, M. New Chitosan/Silica/Zinc Oxide Nanocomposite as Adsorbent for Dye Removal. Int. J. Biol. Macromol. 2019, 131, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Arputharaj, E.; Dahms, H.-U.; Patel, A.K.; Huang, Y.-L. Chitosan-Based Nanocomposites for Removal of Cr(VI) and Synthetic Food Colorants from Wastewater. Bioresour. Technol. 2022, 351, 127018. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.A.; Ahmed, M.A.; Mohamed, A.A. Synthesis, Characterization and Application of Chitosan/Graphene Oxide/Copper Ferrite Nanocomposite for the Adsorptive Removal of Anionic and Cationic Dyes from Wastewater. RSC Adv. 2023, 13, 5337–5352. [Google Scholar] [CrossRef] [PubMed]
- Billah, R.E.K.; Azoubi, Z.; López-Maldonado, E.A.; Majdoubi, H.; Lgaz, H.; Lima, E.C.; Shekhawat, A.; Tamraoui, Y.; Agunaou, M.; Soufiane, A.; et al. Multifunctional Cross-Linked Shrimp Waste-Derived Chitosan/MgAl-LDH Composite for Removal of As(V) from Wastewater and Antibacterial Activity. ACS Omega 2023, 8, 10051–10061. [Google Scholar] [CrossRef]
- El Shahawy, A.; Mubarak, M.F.; El Shafie, M.; Abdulla, H.M. Fe(Iii) and Cr(vi) Ions’ Removal Using AgNPs/GO/Chitosan Nanocomposite as an Adsorbent for Wastewater Treatment. RSC Adv. 2022, 12, 17065–17084. [Google Scholar] [CrossRef]
- Dou, Z.; Xie, X. Chitosan–Montmorillonite–Fe Nanocomposite Hydrogel for Phosphate Recovery and Reuse. ACS ES&T Eng. 2023, 3, 682–689. [Google Scholar] [CrossRef]
- Landge, V.K.; Hakke, V.S.; Kakunuri, M.; Babu, G.U.B.; Boczkaj, G.; Sonawane, S.H. Synthesis of Bimetallic Co–Pt/Cellulose Nanocomposites for Catalytic Reduction of p-Nitrophenol. React. Chem. Eng. 2022, 7, 641–652. [Google Scholar] [CrossRef]
- Almuslem, A.S.; Alnaim, N.; Ibrahim, S.S.; Ibrahim, M.A. Green Synthesis and Characteristics of Cellulose Nanocrystal/Poly Acrylic Acid Nanocomposite Thin Film for Organic Dye Adsorption during Water Treatment. Polymers 2023, 15, 2154. [Google Scholar] [CrossRef]
- Jing, J.; Feng, Y.; Wu, S.; Ye, Z.; Yang, L.; Li, J.; Chen, Y.; Yang, F. β-FeOOH/TiO2/Cellulose Nanocomposite Aerogel as a Novel Heterogeneous Photocatalyst for Highly Efficient Photo-Fenton Degradation. RSC Adv. 2023, 13, 14190–14197. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.M.; Abdelwahab, S.M.; Elsawy, N.M.; Ahmed, N.A.; Raafat, A.I. E-Beam Irradiation-Induced Synthesis of Hydroxyethyl Cellulose/(Cu2O-RGO)/BiVO4-Based Nanocomposite for Photocatalytic Remediation of Wastewater under Visible Light. Int. J. Biol. Macromol. 2024, 258, 128681. [Google Scholar] [CrossRef]
- Eyni Gavabari, S.; Goudarzi, A.; Shahrousvand, M.; Asfaram, A. Preparation of Novel Polyurethane/Activated Carbon/Cellulose Nano-Whisker Nanocomposite Film as an Efficient Adsorbent for the Removal of Methylene Blue and Basic Violet 16 Dyes from Wastewater. Sep. Purif. Technol. 2024, 330, 125285. [Google Scholar] [CrossRef]
- Ahmadi, A.; Foroutan, R.; Esmaeili, H.; Peighambardoust, S.J.; Hemmati, S.; Ramavandi, B. Montmorillonite Clay/Starch/CoFe2O4 Nanocomposite as a Superior Functional Material for Uptake of Cationic Dye Molecules from Water and Wastewater. Mater. Chem. Phys. 2022, 284, 126088. [Google Scholar] [CrossRef]
- Atta, M.M.; Abou-Laila, M.T.; Abdelwahed, M.H.; Dwidar, S.A.; Desouky, O. Structural, Mechanical, and Thermal Features of PVA/Starch/Graphene Oxide Nanocomposite Enriched with WO3 as Gamma–Ray Radiation Shielding Materials for Medical Applications. Polym. Eng. Sci. 2023, 63, 3843–3854. [Google Scholar] [CrossRef]
- Al-Qahtani, S.D.; Ibarhiam, S.; Sallam, S.; Almotairy, A.R.Z.; Al-bonayan, A.M.; Munshi, A.M.; El-Metwaly, N.M. Magnetic Sodium Alginate Grafted with Waste Carbonaceous Material for Diclofenac Sodium Removal: Optimization of Operational Parameters and Process Mechanism. RSC Adv. 2023, 13, 6466–6480. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, G.A.; Sayed, A.; Abdel-raouf, M.E.-S.; Danial, M.Y.F.; Amin, A. Efficient Removal of Cr(VI) from Aqueous Solutions Using Chitosan/Na-Alginate Bio-Based Nanocomposite Hydrogel. J. Appl. Polym. Sci. 2023, 140, e53886. [Google Scholar] [CrossRef]
- Li, J.; Lei, L.; Liu, Z.; Qiu, J.; Wang, H. Sodium Alginate/Polyethyleneimine/Polydopamine@cellulose Nanofiber Composite Aerogel as a Novel Adsorbent for Cr(VI) and Dyes Removal. Polym. Eng. Sci. 2023, 63, 3492–3506. [Google Scholar] [CrossRef]
- Davarnejad, R.; Hassanvand, Z.R.; Mansoori, S.; Kennedy, J.F. Metronidazole Elimination from Wastewater through Photo-Fenton Process Using Green-Synthesized Alginate-Based Hydrogel Coated Bimetallic Iron-copper Nanocomposite Beads as a Reusable Heterogeneous Catalyst. Bioresour. Technol. Rep. 2022, 18, 101068. [Google Scholar] [CrossRef]
- Hosseini, H.; Pirahmadi, P.; Shakeri, S.E.; Khoshbakhti, E.; Sharafkhani, S.; Fakhri, V.; Saeidi, A.; McClements, D.J.; Chen, W.-H.; Su, C.-H.; et al. A Novel Environmentally Friendly Nanocomposite Aerogel Based on the Semi-Interpenetrating Network of Polyacrylic Acid into Xanthan Gum Containing Hydroxyapatite for Efficient Removal of Methylene Blue from Wastewater. Int. J. Biol. Macromol. 2022, 201, 133–142. [Google Scholar] [CrossRef]
- Abu Elella, M.H.; Goda, E.S.; Abdallah, H.M.; Shalan, A.E.; Gamal, H.; Yoon, K.R. Innovative Bactericidal Adsorbents Containing Modified Xanthan Gum/Montmorillonite Nanocomposites for Wastewater Treatment. Int. J. Biol. Macromol. 2021, 167, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- El-Kholy, S.A.; Radwan, E.K.; El-Naggar, M.E.; El-Wakeel, S.T.; El-Tantawy El Sayed, I. Sponge-like Zinc Oxide Nanoparticles Loaded Xanthan Gum/Cationic Chitosan Cryogel: Synthesis, Characterization, Microbicidal and Adsorption of Synthetic Dye and Heavy Metal. J. Environ. Chem. Eng. 2023, 11, 110652. [Google Scholar] [CrossRef]
- Cong, S.-Q.; Wang, B.; Wang, H.; Zheng, Q.-C.; Yang, Q.-R.; Yang, R.-T.; Li, Q.-L.; Wang, W.-S.; Cui, X.-J.; Luo, F.-X. Fe3O4-Lignin@Pd-NPs: A Highly Active, Stable and Broad-Spectrum Nanocomposite for Water Treatment. Int. J. Biol. Macromol. 2024, 256, 128233. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.J.; Lau, C.Y.; Farid, M.U.; Islam, M.K.; An, A.K.; Yi, J.; Leu, S.-Y. Woody Biomass Derived Nano Lignin-Enabled Membrane with High Structural Stability for Efficient Wastewater Treatment. Process Saf. Environ. Prot. 2024, 182, 595–607. [Google Scholar] [CrossRef]
- Yao, G.; Wang, K.; Wang, M.; Shao, X.; Qiu, F.; Zhang, T. Magnetic FeS@Lignin-Derived Carbon Nanocomposites as an Efficient Adsorbent for Multistage Collaborative Selective Recovery of Tellurium (IV) from Wastewater. J. Environ. Chem. Eng. 2021, 9, 106135. [Google Scholar] [CrossRef]
- Hassan, A.F.; El-Naggar, G.A.; Esmail, G.; Shaltout, W.A. Efficient Adsorption of Methylene Blue on Novel Triple-Nanocomposites of Potassium Kappa-Carrageenan, Calcium Alginate and Nanohydroxyapatite Obtained from Sea Scallop Shells. Appl. Surf. Sci. Adv. 2023, 13, 100388. [Google Scholar] [CrossRef]
- Bateni, A.; Valizadeh, K.; Salahshour, Y.; Behroozi, A.H.; Maleki, A. Fabrication and Characterization of Pectin-Graphene Oxide-Magnesium Ferrite-Zinc Oxide Nanocomposite for Photocatalytic Degradation of Diclofenac in an Aqueous Solution under Visible Light Irradiation. J. Environ. Manag. 2022, 324, 116358. [Google Scholar] [CrossRef]
- Ibrar, I.; Alsaka, L.; Yadav, S.; Altaee, A.; Zhou, J.L.; Shon, H.K. Kappa Carrageenan-Vanillin Composite Hydrogel for Landfill Leachate Wastewater Treatment. Desalination 2023, 565, 116826. [Google Scholar] [CrossRef]
- Qi, X.; Zeng, Q.; Tong, X.; Su, T.; Xie, L.; Yuan, K.; Xu, J.; Shen, J. Polydopamine/Montmorillonite-Embedded Pullulan Hydrogels as Efficient Adsorbents for Removing Crystal Violet. J. Hazard. Mater. 2021, 402, 123359. [Google Scholar] [CrossRef]
- Pan, X.; Cheng, S.; Zhang, C.; Jiao, Y.; Lin, X.; Dong, W.; Qi, X. Mussel-Inspired Magnetic Pullulan Hydrogels for Enhancing Catalytic Degradation of Antibiotics from Biomedical Wastewater. Chem. Eng. J. 2021, 409, 128203. [Google Scholar] [CrossRef]
- Khan, S.A.; Abbasi, N.; Hussain, D.; Khan, T.A. Sustainable Mitigation of Paracetamol with a Novel Dual-Functionalized Pullulan/Kaolin Hydrogel Nanocomposite from Simulated Wastewater. Langmuir 2022, 38, 8280–8295. [Google Scholar] [CrossRef] [PubMed]
- Mpelane, S.; Mpupa, A.; Mlambo, M.; Bingwa, N.; Mketo, N.; Nomngongo, P.N. Magnetic Zeolite@β-Cyclodextrin-Gum Arabic Nanocomposite for Adsorptive Removal of Levofloxacin. J. Hazard. Mater. Adv. 2023, 11, 100354. [Google Scholar] [CrossRef]
- Goswami, R.; Gogoi, M.; Borah, A.; Sarmah, H.; Borah, A.R.; Feng, X.; Hazarika, S. Quantum Dot- β-Cyclodextrin Nanofiller Decorated Thin Film Nanocomposite Membrane for Removal of Cationic and Anionic Dyes from Aqueous Solution. Mater. Today Chem. 2024, 35, 101871. [Google Scholar] [CrossRef]
- El-Aassar, M.R.; Ibrahim, O.M.; Hashem, F.S.; Ali, A.S.M.; Elzain, A.A.; Mohamed, F.M. Fabrication of Polyaniline@β-Cyclodextrin Nanocomposite for Adsorption of Carcinogenic Phenol from Wastewater. ACS Appl. Bio Mater. 2022, 5, 4504–4515. [Google Scholar] [CrossRef] [PubMed]
- Zia, Q.; Tabassum, M.; Lu, Z.; Khawar, M.T.; Song, J.; Gong, H.; Meng, J.; Li, Z.; Li, J. Porous Poly(L–Lactic Acid)/Chitosan Nanofibres for Copper Ion Adsorption. Carbohydr. Polym. 2020, 227, 115343. [Google Scholar] [CrossRef] [PubMed]
- Kyshkarova, V.; Marcin Behunova, D.; Václavíková, M.; Melnyk, I. V Hybrid Composite Sorbents Based on SiO2/PLGA for Fe(III) Ions Removal. Appl. Nanosci. 2022, 12, 1201–1212. [Google Scholar] [CrossRef]
- Milovanovic, S.; Markovic, D.; Pantic, M.; Pavlovic, S.M.; Knapczyk-Korczak, J.; Stachewicz, U.; Novak, Z. Development of Advanced Floating Poly(Lactic Acid)-Based Materials for Colored Wastewater Treatment. J. Supercrit. Fluids 2021, 177, 105328. [Google Scholar] [CrossRef]
- Soltaninejad, V.; Ahghari, M.R.; Taheri-Ledari, R.; Maleki, A.; Shalan, A.E. A Versatile Nanocomposite Made of Cd/Cu, Chlorophyll and PVA Matrix Utilized for Photocatalytic Degradation of the Hazardous Chemicals and Pathogens for Wastewater Treatment. J. Mol. Struct. 2022, 1256, 132456. [Google Scholar] [CrossRef]
- Joshi, P.; Raturi, A.; Srivastava, M.; Khatri, O.P. Graphene Oxide, Kaolinite Clay and PVA-Derived Nanocomposite Aerogel as a Regenerative Adsorbent for Wastewater Treatment Applications. J. Environ. Chem. Eng. 2022, 10, 108597. [Google Scholar] [CrossRef]
- Saleem, A.; Iqbal, A.; Younas, U.; Ashraf, A.; Al-Mijalli, S.H.; Ali, F.; Pervaiz, M.; Saeed, Z.; Nazir, A.; Iqbal, M. Antimicrobial Attributes and Enhanced Catalytic Potential of PVA Stabilized Ag-NiO2 Nanocomposite for Wastewater Treatment. Arab. J. Chem. 2024, 17, 105545. [Google Scholar] [CrossRef]
- Fawaz, J.; Mittal, V. Synthesis of Polymer Nanocomposites: Review of Various Techniques. In Synthesis Techniques for Polymer Nanocomposites; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 1–30. ISBN 9783527670307. [Google Scholar]
- Amiri, M.J.; Raayatpisheh, M.; Radi, M.; Amiri, S. Preparation and Characterization of Biopolymer-Based Adsorbents and Their Application for Methylene Blue Removal from Wastewater. Sci. Rep. 2023, 13, 17263. [Google Scholar] [CrossRef] [PubMed]
- Mittal, V. In-Situ Synthesis of Polymer Nanocomposites; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; pp. 1–25. [Google Scholar] [CrossRef]
- Zaferani, S.H. Introduction of Polymer-Based Nanocomposites. In Polymer-Based Nanocomposites for Energy and Environmental Applications; Woodhead Publishing: Sawston, UK, 2018; pp. 1–25. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Romero-Fierro, D.; Arcentales-Vera, B.; Pardo, S.; Bucio, E. Interaction between Filler and Polymeric Matrix in Nanocomposites: Magnetic Approach and Applications. Polymers 2021, 13, 2998. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Mai, Y.W. Permeability Modelling of Polymer-Layered Silicate Nanocomposites. Compos. Sci. Technol. 2007, 67, 2895–2902. [Google Scholar] [CrossRef]
- Hajiali, F.; Shojaei, A. Silane Functionalization of Nanodiamond for Polymer Nanocomposites-Effect of Degree of Silanization. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 254–263. [Google Scholar] [CrossRef]
- Nguyen, Q.T.; Baird, D.G. An Improved Technique for Exfoliating and Dispersing Nanoclay Particles into Polymer Matrices Using Supercritical Carbon Dioxide. Polymer 2007, 48, 6923–6933. [Google Scholar] [CrossRef]
- Ulhaq, I.; Ahmad, W.; Ahmad, I.; Yaseen, M.; Ilyas, M. Engineering TiO2 Supported CTAB Modified Bentonite for Treatment of Refinery Wastewater through Simultaneous Photocatalytic Oxidation and Adsorption. J. Water Process Eng. 2021, 43, 102239. [Google Scholar] [CrossRef]
- Barra Caracciolo, A.; Ademollo, N.; Cardoni, M.; Di Giulio, A.; Grenni, P.; Pescatore, T.; Rauseo, J.; Patrolecco, L. Assessment of Biodegradation of the Anionic Surfactant Sodium Lauryl Ether Sulphate Used in Two Foaming Agents for Mechanized Tunnelling Excavation. J. Hazard. Mater. 2019, 365, 538–545. [Google Scholar] [CrossRef]
- Najim, A.A.; Ismail, Z.Z.; Hummadi, K.K. Biodegradation Potential of Sodium Dodecyl Sulphate (SDS) by Mixed Cells in Domestic and Non-Domestic Actual Wastewaters: Experimental and Kinetic Studies. Biochem. Eng. J. 2022, 180, 108374. [Google Scholar] [CrossRef]
- Ríos, F.; Caparrós-Salvador, F.; Lechuga, M.; Fernández-Serrano, M. Complete Biodegradability Assessment of Polyoxyethylene Glycerol Ester Non-Ionic Surfactant: Aerobic, Anaerobic, Combined Biodegradation and Inhibitory Effects. Water Res. 2024, 248, 120857. [Google Scholar] [CrossRef]
- Ghimici, L.; Brunchi, C.-E. Titanium Dioxide Separation from Water by PEG and Pluronic Type Polymers. Sep. Purif. Technol. 2013, 103, 306–312. [Google Scholar] [CrossRef]
- Khosroshahi, M.M.; Jafarzadeh, Y.; Nasiri, M.; Khayet, M. Novel Polyvinyl Chloride Ultrafiltration Membranes Blended with Amphiphilic Polyethylene Glycol-Block-Poly(1, 2-Dichloroethylene) Copolymer for Oily Wastewater Treatment. J. Water Process Eng. 2023, 56, 104433. [Google Scholar] [CrossRef]
- Licul-Kucera, V.; Frömel, T.; Kruså, M.; van Wezel, A.P.; Knepper, T.P. Finding a Way out? Comprehensive Biotransformation Study of Novel Fluorinated Surfactants. Chemosphere 2023, 339, 139563. [Google Scholar] [CrossRef]
- Jeon, I.Y.; Baek, J.B. Nanocomposites Derived from Polymers and Inorganic Nanoparticles. Materials 2010, 3, 3654–3674. [Google Scholar] [CrossRef]
- The Global Water Crisis At a Glance. Available online: https://concernusa.org/news/global-water-crisis-causes/ (accessed on 12 January 2023).
- Olajire, A.A.; Bamigbade, L.A. Green Synthesis of Chitosan-Based Iron@silver Nanocomposite as Adsorbent for Wastewater Treatment. Water Resour. Ind. 2021, 26, 100158. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Naggar, M.E.; Malhat, F.M.; El Sharkawi, H.M. Efficient Removal of Pesticides and Heavy Metals from Wastewater and the Antimicrobial Activity of F-MWCNTs/PVA Nanocomposite Film. J. Clean. Prod. 2019, 206, 315–325. [Google Scholar] [CrossRef]
- Shahadat, M.; Jha, A.; Shahid-ul-Islam; Adnan, R.; Ali, S.W.; Ismail, I.M.I.; Oves, M.; Ahammad, S.Z. Recent Advances in Chitosan-Polyaniline Based Nanocomposites for Environmental Applications: A Review. Polymer 2022, 254, 124975. [Google Scholar] [CrossRef]
- Abd El-Aziz, M.E.; Morsi, S.M.M.; Kamal, K.H.; Khattab, T.A. Preparation of Isopropyl Acrylamide Grafted Chitosan and Carbon Bionanocomposites for Adsorption of Lead Ion and Methylene Blue. Polymers 2022, 14, 4485. [Google Scholar] [CrossRef]
- Saya, L.; Gautam, D.; Malik, V.; Singh, W.R.; Hooda, S. Natural Polysaccharide Based Graphene Oxide Nanocomposites for Removal of Dyes from Wastewater: A Review. J. Chem. Eng. Data 2021, 66, 11–37. [Google Scholar] [CrossRef]
- Agrawal, A.; Sharma, A.; Awasthi, K.K.; Awasthi, A. Metal Oxides Nanocomposite Membrane for Biofouling Mitigation in Wastewater Treatment. Mater. Today Chem. 2021, 21, 100532. [Google Scholar] [CrossRef]
- Ray, P.; Singh, P.S.; Polisetti, V. Synthetic Polymeric Membranes for the Removal of Toxic Pollutants and Other Harmful Contaminants from Water. In Removal of Toxic Pollutants through Microbiological and Tertiary Treatment: New Perspectives; Elsevier: Amsterdam, The Netherlands, 2020; pp. 43–99. [Google Scholar] [CrossRef]
- Yáñez, O.; Alegría-Arcos, M.; Suardiaz, R.; Morales-Quintana, L.; Castro, R.I.; Palma-Olate, J.; Galarza, C.; Catagua-González, Á.; Rojas-Pérez, V.; Urra, G.; et al. Calcium-Alginate-Chitosan Nanoparticle as a Potential Solution for Pesticide Removal, a Computational Approach. Polymers 2023, 15, 3020. [Google Scholar] [CrossRef]
- Potara, M.; Focsan, M.; Craciun, A.M.; Botiz, I.; Astilean, S. Polymer-Coated Plasmonic Nanoparticles for Environmental Remediation: Synthesis, Functionalization, and Properties. In New Polymer Nanocomposites for Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 361–387. [Google Scholar] [CrossRef]
- Moradi, G.; Heydari, R.; Zinadini, S.; Rahimi, M. Chitosan-Furosemide/Pectin Surface Functionalized Thin Film Nanofiltration Membrane with Improved Antifouling Behavior for Pharmaceutical Wastewater Treatment. J. Ind. Eng. Chem. 2023, 124, 368–380. [Google Scholar] [CrossRef]
- Gopal, G.; Nirmala, M.J.; Mukherjee, A. A Novel Chitosan-Coated Fe–Cu CNS Loaded with CMC–Alginate Composite for Adsorptive Removal of Ciprofloxacin from Water. Surf. Interfaces 2023, 39, 102981. [Google Scholar] [CrossRef]
- Palacio, D.A.; Muñoz, C.; Meléndrez, M.; Rabanal-León, W.A.; Murillo-López, J.A.; Palencia, M.; Rivas, B.L. Comparative Study of the Removal Efficiency of Nalidixic Acid by Poly[(4-Vinylbenzyl)Trimethylammonium Chloride] and N-Alkylated Chitosan through the Ultrafiltration Technique and Its Approximation through Theoretical Calculations. Polymers 2023, 15, 3185. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, K.; Bateni, A.; Sojoodi, N.; Rafiei, R.; Behroozi, A.H.; Maleki, A. Preparation and Characterization of Chitosan-Curdlan Composite Magnetized by Zinc Ferrite for Efficient Adsorption of Tetracycline Antibiotics in Water. Int. J. Biol. Macromol. 2023, 235, 123826. [Google Scholar] [CrossRef] [PubMed]
- Rawat, S.; Chaudhary, M.; Maiti, A. Synergistic Arsenic Removal Using Chitosan-Based Nanocomposite Beads and Cross-Flow Ultrafiltration: A Significant Reduction of Membrane Fouling. J. Environ. Chem. Eng. 2023, 11, 109431. [Google Scholar] [CrossRef]
- Ehsan, M.; Razzaq, H.; Razzaque, S.; Kanwal, M.; Hussain, I. Engineering Nanocomposite Membranes of Sodium Alginate-Graphene Oxide for Efficient Separation of Oil-Water and Antifouling Performance. J. Environ. Chem. Eng. 2023, 11, 109185. [Google Scholar] [CrossRef]
- Kamari, S.; Shahbazi, A. High–Performance Nanofiltration Membrane Blended by Fe3O4@SiO2–CS Bionanocomposite for Efficient Simultaneous Rejection of Salts/Heavy Metals Ions/Dyes with High Permeability, Retention Increase and Fouling Decline. Chem. Eng. J. 2021, 417, 127930. [Google Scholar] [CrossRef]
- Tang, L.; Gong, J.; Li, J.; Fang, S.; Wang, Y.; Zhou, H. Synergistic Effect between Tannic Acid-Fe3+ and Chitosan Hydrogel-Coated Covalent Organic Framework: Endowing Better Nanofiltration Performance and Stability. Sep. Purif. Technol. 2023, 323, 124469. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, Y.; Yu, Q.; Sun, H.; Chen, K.; Ye, H.; Tang, S.; Zhang, H.; Li, P.; Jason Niu, Q. Advanced Mg2+/Li+ Separation Nanofiltration Membranes by Introducing Hydroxypropyltrimethyl Ammonium Chloride Chitosan as a Co-Monomer. Appl. Surf. Sci. 2023, 616, 156434. [Google Scholar] [CrossRef]
- Batool, M.; Abbas, M.A.; Khan, I.A.; Khan, M.Z.; Saleem, M.; Khan, A.U.; Deen, K.M.; Batool, M.; Khan, A.L.; Zhu, S.; et al. Response Surface Methodology Modeling Correlation of Polymer Composite Carbon Nanotubes/Chitosan Nanofiltration Membranes for Water Desalination. ACS ES&T Water 2023, 3, 1406–1421. [Google Scholar] [CrossRef]
- Gadkari, R.R.; Ali, S.W.; Das, A.; Alagirusamy, R. Silver Nanowires Embedded Chitosan/Poly-Lactic Acid Electrospun Nanocomposite Web Based Nanofibrous Multifunctional Membrane for Safe Water Purification. Adv. Sustain. Syst. 2022, 6, 2100360. [Google Scholar] [CrossRef]
- Zhou, W.; Li, Y.; Zhao, X.; Wang, J.; Zhu, X.; Lai, C.; Wu, D.; Cheng, X.; Xu, J.; Liang, H. PH-Responsive Chitosan Sacrificial Layer for Simultaneous Enhancement of Ultrafiltration Performance and Sustainable Membrane Fouling Control. ACS Appl. Polym. Mater. 2023, 5, 6875–6885. [Google Scholar] [CrossRef]
- Nalatambi, S.; Oh, K.S.; Yoon, L.W.; Tee, L.H. Evaluation of Antibacterial Property and Greywater Treatment Performance Using Composite Chitosan/Graphene Oxide Membrane. Mater. Chem. Phys. 2023, 295, 127160. [Google Scholar] [CrossRef]
- Khosravi, M.J.; Hosseini, S.M.; Vatanpour, V. Performance Improvement of PES Membrane Decorated by Mil-125(Ti)/Chitosan Nanocomposite for Removal of Organic Pollutants and Heavy Metal. Chemosphere 2022, 290, 133335. [Google Scholar] [CrossRef] [PubMed]
- Al Momani, D.E.; Arshad, F.; Zou, L. Chitosan/MoS2/GO Membrane for Catalytic Degradation of Organic Contaminants. Environ. Technol. Innov. 2023, 32, 103410. [Google Scholar] [CrossRef]
- Amiri, S.; Asghari, A.; Vatanpour, V.; Rajabi, M. Fabrication of Chitosan-Aminopropylsilane Graphene Oxide Nanocomposite Hydrogel Embedded PES Membrane for Improved Filtration Performance and Lead Separation. J. Environ. Manag. 2021, 294, 112918. [Google Scholar] [CrossRef]
- Khoerunnisa, F.; Nurhayati, M.; Annisa, N.A.A.; Fatimah, S.; Nashrah, N.; Hendrawan, H.; Ko, Y.-G.; Ng, E.-P.; Opaprakasit, P. Effects of Benzalkonium Chloride Contents on Structures, Properties, and Ultrafiltration Performances of Chitosan-Based Nanocomposite Membranes. Membranes 2022, 12, 268. [Google Scholar] [CrossRef]
- Amiri, S.; Asghari, A.; Vatanpour, V.; Rajabi, M. Fabrication and Characterization of a Novel Polyvinyl Alcohol-Graphene Oxide-Sodium Alginate Nanocomposite Hydrogel Blended PES Nanofiltration Membrane for Improved Water Purification. Sep. Purif. Technol. 2020, 250, 117216. [Google Scholar] [CrossRef]
- Bai, L.; Wu, H.; Ding, J.; Ding, A.; Zhang, X.; Ren, N.; Li, G.; Liang, H. Cellulose Nanocrystal-Blended Polyethersulfone Membranes for Enhanced Removal of Natural Organic Matter and Alleviation of Membrane Fouling. Chem. Eng. J. 2020, 382, 122919. [Google Scholar] [CrossRef]
- Xing, W.; Wu, Y.; Lu, J.; Lin, X.; Yu, C.; Dong, Z.; Yan, Y.; Li, C. Biomass-Based Synthesis of Green and Biodegradable Molecularly Imprinted Membranes for Selective Recognition and Separation of Tetracycline. Nano 2020, 15, 2050004. [Google Scholar] [CrossRef]
- Yogarathinam, L.T.; Goh, P.S.; Ismail, A.F.; Gangasalam, A.; Ahmad, N.A.; Samavati, A.; Mamah, S.C.; Zainol Abidin, M.N.; Ng, B.C.; Gopal, B. Nanocrystalline Cellulose Incorporated Biopolymer Tailored Polyethersulfone Mixed Matrix Membranes for Efficient Treatment of Produced Water. Chemosphere 2022, 293, 133561. [Google Scholar] [CrossRef] [PubMed]
- Othman, Z.; Mackey, H.R.; Mahmoud, K.A. MXene/Chitosan/Lignosulfonate (MCL) Nanocomposite for Simultaneous Removal of Co(II), Cr(VI), Cu(II), Ni(II) and Pb(II) Heavy Metals from Wastewater. 2D Mater. 2023, 10, 24004. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, B.; He, S.; Man, R. Preparation of Graphene-Oxide/Polyamidoamine Dendrimers and Their Adsorption Properties toward Some Heavy Metal Ions. J. Chem. Eng. Data 2014, 59, 1719–1726. [Google Scholar] [CrossRef]
- Lotfi, Z.; Mousavi, H.Z.; Sajjadi, S.M. Covalently Bonded Dithiocarbamate-Terminated Hyperbranched Polyamidoamine Polymer on Magnetic Graphene Oxide Nanosheets as an Efficient Sorbent for Preconcentration and Separation of Trace Levels of Some Heavy Metal Ions in Food Samples. J. Food Meas. Charact. 2020, 14, 293–302. [Google Scholar] [CrossRef]
- Hayati, B.; Maleki, A.; Najafi, F.; Daraei, H.; Gharibi, F.; McKay, G. Super High Removal Capacities of Heavy Metals (Pb2+ and Cu2+) Using CNT Dendrimer. J. Hazard. Mater. 2017, 336, 146–157. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Abdelbar, N.M.; Mohamed, A.A. Molecular Imprinted Chitosan-TiO2 Nanocomposite for the Selective Removal of Rose Bengal from Wastewater. Int. J. Biol. Macromol. 2018, 107, 1046–1053. [Google Scholar] [CrossRef]
- Hayati, B.; Arami, M.; Maleki, A.; Pajootan, E. Application of Dendrimer/Titania Nanohybrid for the Removal of Phenol from Contaminated Wastewater. Desalin. Water Treat. 2016, 57, 6809–6819. [Google Scholar] [CrossRef]
- Arkas, M.; Tsiourvas, D.; Paleos, C.M. Functional Dendrimeric “Nanosponges” for the Removal of Polycyclic Aromatic Hydrocarbons from Water. Chem. Mater. 2003, 15, 2844–2847. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, L.; Niu, G.; Hua, M.; Zhu, L.; Cui, P.; Li, X.; Chao, Y.; Zhu, W.; Liu, Z. Adsorptive Removal of Oxytetracycline in Wastewater by Cu/Al Doped Carbon Microspheres Prepared from Low-Molecular-Weight Chitosan. J. Environ. Chem. Eng. 2023, 11, 109496. [Google Scholar] [CrossRef]
- Li, W.; Guo, M.; Wang, Y.; Deng, H.; Lei, H.; Yu, C.; Liu, Z. Selective Adsorption of Heavy Metal Ions by Different Composite-Modified Semi-Carbonized Fibers. Sep. Purif. Technol. 2024, 328, 125022. [Google Scholar] [CrossRef]
- Shan, H.; Mo, H.; Liu, Y.; Zeng, C.; Peng, S.; Zhan, H. As(III) Removal by a Recyclable Granular Adsorbent through Dopping Fe-Mn Binary Oxides into Graphene Oxide Chitosan. Int. J. Biol. Macromol. 2023, 237, 124184. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ma, H.; Wang, J.; Fan, C. Preparation of Graphene Oxide/Carboxymethyl Chitosan/Polyvinyl Alcohol Composite Nanofiber Membranes by Electrospinning for Heavy Metal Adsorption. J. Appl. Polym. Sci. 2023, e54840. [Google Scholar] [CrossRef]
- Thakur, M.; Rajput, J.K.; Kumar, R. Study of Morphological Aspects in the Efficient Adsorptive Removal of Heavy Metal Ions Using Graphene Oxide-Chitosan Based Magnetic Nanocomposite (0.4Fe0x:6x@GCS). J. Hazard. Mater. Adv. 2023, 11, 100362. [Google Scholar] [CrossRef]
- El-Sayed, E.S.A.; Dacrory, S.; Essawy, H.A.; Ibrahim, H.S.; Ammar, N.S.; Kamel, S. Sustainable Grafted Chitosan-Dialdehyde Cellulose with High Adsorption Capacity of Heavy Metal. BMC Chem. 2023, 17, 117. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jiang, Q.; Sun, L.; Zhang, J.; Han, Z.; Xu, S.; Cheng, Z. Adsorption of Heavy Metals and Antibacterial Activity of Silicon-Doped Chitosan Composite Microspheres Loaded with ZIF-8. Sep. Purif. Technol. 2024, 328, 124969. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Qiu, S.; Wang, C.; Zhang, H.; Guo, J.; Wang, S.; Ma, H. 3D-Printed Filters for Efficient Heavy Metal Removal from Water Using PLA@CS/HAP Composites. Polymers 2023, 15, 4144. [Google Scholar] [CrossRef]
- Ghiorghita, C.-A.; Lazar, M.M.; Platon, I.-V.; Humelnicu, D.; Doroftei, F.; Dinu, M.V. Feather-Weight Cryostructured Thiourea-Chitosan Aerogels for Highly Efficient Removal of Heavy Metal Ions and Bacterial Pathogens. Int. J. Biol. Macromol. 2023, 235, 123910. [Google Scholar] [CrossRef]
- Khan, S.A.; Hussain, D.; Abbasi, N.; Khan, T.A. Deciphering the Adsorption Potential of a Functionalized Green Hydrogel Nanocomposite for Aspartame from Aqueous Phase. Chemosphere 2022, 289, 133232. [Google Scholar] [CrossRef]
- Shirzad Kebria, M.R.; Bono, L.; Khoshhal Salestan, S.; Armirotti, A.; Carzino, R.; Athanassiou, A.; Fragouli, D. Efficient Removal of Perfluorobutanesulfonic Acid from Water through a Chitosan/Polyethyleneimine Xerogel. Chem. Eng. J. 2023, 466, 143236. [Google Scholar] [CrossRef]
- Basirun, A.A.; Karim, W.A.W.A.; Wei, N.C.; Wu, J.; Wilfred, C.D. Manganese Removal Using Functionalised Thiosalicylate-Based Ionic Liquid: Water Filtration System Application. Molecules 2023, 28, 5777. [Google Scholar] [CrossRef]
- Dinh, V.P.; Nguyen, M.D.; Nguyen, Q.H.; Do, T.T.T.; Luu, T.T.; Luu, A.T.; Tap, T.D.; Ho, T.H.; Phan, T.P.; Nguyen, T.D.; et al. Chitosan-MnO2 Nanocomposite for Effective Removal of Cr (VI) from Aqueous Solution. Chemosphere 2020, 257, 127147. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Nabil, G.M.; Elweshahy, S.M.T. Novel NTiO2-Chitosan@NZrO2-Chitosan Nanocomposite for Effective Adsorptive Uptake of Trivalent Gadolinium and Samarium Ions from Water. Powder Technol. 2021, 378, 246–254. [Google Scholar] [CrossRef]
- Wang, J.; Ma, R.; Li, L.; Gu, P.; Wang, X. Chitosan Modified Molybdenum Disulfide Composites as Adsorbents for the Simultaneous Removal of U(VI), Eu(III), and Cr(VI) from Aqueous Solutions. Cellulose 2020, 27, 1635–1648. [Google Scholar] [CrossRef]
- Reghioua, A.; Barkat, D.; Jawad, A.H.; Abdulhameed, A.S.; Al-Kahtani, A.A.; Alothman, Z.A. Parametric Optimization by Box–Behnken Design for Synthesis of Magnetic Chitosan-Benzil/ZnO/Fe3O4 Nanocomposite and Textile Dye Removal. J. Environ. Chem. Eng. 2021, 9, 105166. [Google Scholar] [CrossRef]
- Lakkaboyana, S.K.; Soontarapa, K.; Vinaykumar; Marella, R.K.; Kannan, K. Preparation of Novel Chitosan Polymeric Nanocomposite as an Efficient Material for the Removal of Acid Blue 25 from Aqueous Environment. Int. J. Biol. Macromol. 2021, 168, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Abootorabi, Z.; Sohrabi, M.R.; Mortazavinik, S. Removing Diazo Direct Red 81 Using Chitosan/Zero-Valent Iron Nanocomposite from Aqueous Solutions and Process Optimization. Int. J. Environ. Anal. Chem. 2023, 103, 1168–1185. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Nguyen, N.T.; Nguyen, V.A. In Situ Synthesis and Characterization of ZnO/Chitosan Nanocomposite as an Adsorbent for Removal of Congo Red from Aqueous Solution. Adv. Polym. Technol. 2020, 2020, 3892694. [Google Scholar] [CrossRef]
- Muinde, V.M.; Onyari, J.M.; Wamalwa, B.; Wabomba, J.N. Adsorption of Malachite Green Dye from Aqueous Solutions Using Mesoporous Chitosan–Zinc Oxide Composite Material. Environ. Chem. Ecotoxicol. 2020, 2, 115–125. [Google Scholar] [CrossRef]
- Mohammed, M.I.; Ismael, M.K.; Gönen, M. Synthesis of Chitosan-Silica Nanocomposite for Removal of Methyl Orange from Water: Composite Characterization and Adsorption Performance. IOP Conf. Ser. Mater. Sci. Eng. 2020, 745, 012084. [Google Scholar] [CrossRef]
- Abbasi, M. Synthesis and Characterization of Magnetic Nanocomposite of Chitosan/SiO2/Carbon Nanotubes and Its Application for Dyes Removal. J. Clean. Prod. 2017, 145, 105–113. [Google Scholar] [CrossRef]
- Almasian, A.; Olya, M.E.; Mahmoodi, N.M. Synthesis of Polyacrylonitrile/Polyamidoamine Composite Nanofibers Using Electrospinning Technique and Their Dye Removal Capacity. J. Taiwan Inst. Chem. Eng. 2015, 49, 119–128. [Google Scholar] [CrossRef]
- Ghasempour, A.; Pajootan, E.; Bahrami, H.; Arami, M. Introduction of Amine Terminated Dendritic Structure to Graphene Oxide Using Poly(Propylene Imine) Dendrimer to Evaluate Its Organic Contaminant Removal. J. Taiwan Inst. Chem. Eng. 2017, 71, 285–297. [Google Scholar] [CrossRef]
- Samani, F.N.; Darvishi, R.; Moshkriz, A.; Darvish, M. Oxidized Pectin-Cross-Linked O-Carboxymethyl Chitosan/EDTriAA Intercalated LDH: An Antibiotic Adsorbent Hydrogel. J. Polym. Environ. 2023, 31, 3131–3148. [Google Scholar] [CrossRef]
- Mehdi Salehi, M.; Hassanzadeh-Afruzi, F.; Esmailzadeh, F.; Choopani, L.; Rajabi, K.; Naeimy Kuzekanan, H.; Azizi, M.; Eshrati yeganeh, F.; Demchuk, O.M.; Maleki, A. Chlorpyrifos and Diazinon Elimination through PAAm-g-XG/HKUST-1@Fe3O4 Biopolymer Nanoadsorbent Hydrogel from Wastewater: Preparation, Characterization, Kinetics and Isotherm. Sep. Purif. Technol. 2024, 334, 126097. [Google Scholar] [CrossRef]
- Sudarmono; Istiqomah, N.I.; Budianti, S.I.; Cuana, R.; Puspitarum, D.L.; Mahardhika, L.J.; Chotimah; Suharyadi, E. Magnetically Separable and Reusable Fe3O4/Chitosan Nanocomposites Green Synthesized Utilizing Moringa Oleifera Extract for Rapid Photocatalytic Degradation of Methylene Blue. Results Chem. 2024, 7, 101245. [Google Scholar] [CrossRef]
- Rehan, M.; Elhaddad, E. An Efficient Multi-Functional Ternary Reusable Nanocomposite Based on Chitosan@TiO2@Ag NP Immobilized on Cellulosic Fiber as a Support Substrate for Wastewater Treatment. Environ. Pollut. 2024, 340, 122850. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).