Influence of Partially Carboxylated Powdered Lignocellulose from Oat Straw on Technological and Strength Properties of Water-Swelling Rubber
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Carboxylation of Cellulose
2.3. Preparation of Elastomer Composites
2.4. Measurements
2.4.1. Fourier Transform Infrared Spectroscopy
2.4.2. Thermogravimetric Analysis (TGA)
2.4.3. Hildebrand–Scatchard Solubility Parameter Calculation
2.4.4. Scanning Electron Microscope (SEM)
2.4.5. Determination of Curing Optimum, Vulcanization and Physical-Mechanical Tests
2.4.6. Determination of Viscosity by Mooney
2.4.7. Determination of Sorption Properties
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Circular Economy. Available online: https://environment.ec.europa.eu/topics/circular-economy_en (accessed on 1 April 2021).
- Borrega, M.; Hinkka, V.; Hörhammer, H.; Kataja, K.; Kenttä, E.; Ketoja, J.A.; Palmgren, R.; Salo, M.; Sundqvist-Andberg, H.; Tanaka, A. Utilizing and Valorizing Oat and Barley Straw as an Alternative Source of Lignocellulosic Fibers. Materials 2022, 15, 7826. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, L.; Rodríguez, A.; Pérez, A.; Moral, A.; Serrano, L. Alternative Raw Materials and Pulping Process Using Clean Technologies. Ind. Crops Prod. 2008, 28, 11–16. [Google Scholar] [CrossRef]
- Yusupova, N.F.; Tayirova, D.B.; Allanazarova, M.B. Use Annual Plants as an Additional Raw Materials for Obtaining Technical Cellulose. Cent. Asian J. Med. Nat. Sci. 2022, 3, 620–623. [Google Scholar]
- Lou, Y.; Chester, S. Kinetics of Swellable Packers under Downhole Conditions. Int. J. Appl. Mech. 2014, 6, 1450073. [Google Scholar] [CrossRef]
- Sadana, A.; Kovalchuk, A.; Cook, C. Delayed Oil Swell Packer for Openhole Zonal Isolation of Long Laterals Wells. In Proceedings of the International Petroleum Technology Conference 2020, IPTC 2020, Dhahran, Saudi Arabia, 13–15 January 2020. [Google Scholar]
- Seyger, R.; Resink, S.; Harms, H.; Hibberd, R. The Future of Swelling Elastomers: An Elastomer Manufacturer’s View of Swelling Elastomer Developments and Market Trends. J. Eng. Res. [TJER] 2013, 10, 50–64. [Google Scholar] [CrossRef]
- Mujtaba, M.; Fernandes Fraceto, L.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; Araujo de Medeiros, G.; do Espírito Santo Pereira, A.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic Biomass from Agricultural Waste to the Circular Economy: A Review with Focus on Biofuels, Biocomposites and Bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Borrero-López, A.M.; Valencia, C.; Franco, J.M. Lignocellulosic Materials for the Production of Biofuels, Biochemicals and Biomaterials and Applications of Lignocellulose-Based Polyurethanes: A Review. Polymers 2022, 14, 881. [Google Scholar] [CrossRef]
- Wijaya, C.J.; Ismadji, S.; Gunawan, S. A Review of Lignocellulosic-Derived Nanoparticles for Drug Delivery Applications: Lignin Nanoparticles, Xylan Nanoparticles, and Cellulose Nanocrystals. Molecules 2021, 26, 676. [Google Scholar] [CrossRef]
- Shen, F.; Xiong, X.; Fu, J.; Yang, J.; Qiu, M.; Qi, X.; Tsang, D.C.W. Recent Advances in Mechanochemical Production of Chemicals and Carbon Materials from Sustainable Biomass Resources. Renew. Sustain. Energy Rev. 2020, 130, 109944. [Google Scholar] [CrossRef]
- Sverguzova, S.V.; Shaikhiev, I.G.; Grechina, A.S.; Shaikhieva, K.I. Use of Waste from Processing Biomass of Oat as Sorption Materials for Removing Pollutants from Water Media (Literature Review). Econ. Constr. Environ. Manag. 2018, 2, 51–60. (In Russian) [Google Scholar]
- Kuvshinova, L.A.; Udoratina, E.V.; Karaseva, Y.S.; Cherezova, E.N. Physical-mechanical Properties and Morphology of Lignocellulose Powder Modifiers for Vulcanized Rubbers. Russ. J. Appl. Chem. 2023, 96, 281–291. [Google Scholar] [CrossRef]
- Kulasinski, K. Effects of Water Adsorption in Hydrophilic Polymers; Méndez-Vilas, A., Solano-Martín, A., Eds.; Formatex Research Center: Badajoz, Spain, 2016. [Google Scholar]
- Necolau, M.I.; Bălănucă, B.; Frone, A.N.; Damian, C.M. Tailoring an Effective Interface between Nanocellulose and the Epoxidized Linseed Oil Network through Functionalization. ACS Omega 2023, 8, 15896–15908. [Google Scholar] [CrossRef]
- Ivanova, A.V.; Ushmarin, N.F.; Egorov, E.N.; Sandalov, S.I.; Kol’tsov, N.I. An Investigation of the Effect of Methyl Cellulose and Sodium Polyacrylate on the Hydrosorption Properties of a Vulcanisate Based on Chloroprene Rubber. Int. Polym. Sci. Technol. 2018, 45, 311–314. [Google Scholar] [CrossRef]
- Huang, X.; Wang, A.; Xu, X.; Liu, H.; Shang, S. Enhancement of Hydrophobic Properties of Cellulose Fibers via Grafting with Polymeric Epoxidized Soybean Oil. ACS Sustain. Chem. Eng. 2017, 5, 1619–1627. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, W.-C.; Li, F.-X.; Yu, J.-Y. Swelling and Dissolution of Cellulose in NaOH Aqueous Solvent Systems. Cellul. Chem. Technol. 2013, 47, 671–679. [Google Scholar]
- Galikhanov, M.F.; Akhmedzyanova, D.M.; Nikitin, N.R. The Development and the Study of the Properties of Hydrosorption Material Based on a Blended Thermoplastic Vulcanisate. Int. Polym. Sci. Technol. 2017, 44, 9–14. [Google Scholar] [CrossRef]
- Vaidya, N.Y. Method and Apparatus for Controlling Elastomer Swelling in Downhole Applications. U.S. Patent No. 7,938,191, 10 May 2011. [Google Scholar]
- Mensah, B.; Oduro, E. Preparation and Characterization of Hydrophilic and Water-Swellable Elastomeric Nanocomposites. Polym. Eng. Sci. 2023, 63, 738–754. [Google Scholar] [CrossRef]
- Duan, P.; Sadana, A.; Furlan, W. Packers Having Controlled Swelling and Methods of Manufacturing Thereof. U.S. Patent No. 10,738,560, 11 August 2020. [Google Scholar]
- Qamar, S.Z.; Pervez, T.; Akhtar, M.; Al-Kharusi, M.S.M. Design and Manufacture of Swell Packers: Influence of Material Behavior. Mater. Manuf. Process. 2012, 27, 721–726. [Google Scholar] [CrossRef]
- Evers, R.; Koloy, T.R.; Abrahamsen, T. Design Methodology for Swellable Elastomer Packers for Well Construction Operations. In SPE Annual Technical Conference and Exhibition; Society of Petroleum Engineers: New Orleans, LA, USA, 2013; Volume SPE-166499, pp. 2986–2994. [Google Scholar]
- Liu, C.; Ding, J.; Zhou, L.; Chen, S. Mechanical Properties, Water-Swelling Behavior, and Morphology of Water-Swellable Rubber Prepared Using Crosslinked Sodium Polyacrylate. J. Appl. Polym. Sci. 2006, 102, 1489–1496. [Google Scholar] [CrossRef]
- Mohan, Y.M.; Murthy, P.S.K.; Sreeramulu, J.; Raju, K.M. Swelling Behavior of Semi-Interpenetrating Polymer Network Hydrogels Composed of Poly(Vinyl Alcohol) and Poly(Acrylamide-Co-Sodium Methacrylate). J. Appl. Polym. Sci. 2005, 98, 302–314. [Google Scholar] [CrossRef]
- Jiang, X.L.; Hu, K.; Yang, P.; Ren, J. Study on Preparation and Properties of Water Swellable Rubber Modified by Interpenetrating Polymer Networks. Plast. Rubber Compos. 2013, 42, 327–333. [Google Scholar] [CrossRef]
- Zhang, S.T. Preparation, Water Absorbent and Mechanical Properties of Water Swellable Rubber. Plast. Rubber Compos. 2012, 41, 326–331. [Google Scholar] [CrossRef]
- Cherezova, E.N.; Karaseva, Y.S.; Galikhanov, M.F. Influence of Sodium Carboxymethyl Cellulose on the Properties of Highly Filled Vulcanizates Based on Nitrile-Butadiene Rubber. Prom. Proizv. Ispol’z. Elastom. 2021, 3, 33–37. (In Russian) [Google Scholar] [CrossRef]
- Lopatina, S.S.; Vaniev, M.A.; Sychev, N.V.; Nilidin, D.A.; Savchenko, Y.Y.; Bruk, A.D. Efficiency of Application of Hydrolyzed Polyacrylamide and Copolymer Acrylamide with Potassium Acrylate as a Water-Swelling Agent in Rubbers. Key Eng. Mater. 2019, 816, 208–213. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, D.; Zhang, J. Novel Polyvinyl Alcohol/Styrene Butadiene Rubber Latex/Carboxymethyl Cellulose Nanocomposites Reinforced with Modified Halloysite Nanotubes. J. Nanomater. 2013, 2013, 542421. [Google Scholar] [CrossRef]
- Cherezova, E.N.; Karaseva, Y.S. Water-Swelling Rubbers Filled With Modified Cotton Powder. ChemChemTech 2022, 65, 71–78. [Google Scholar] [CrossRef]
- Polgar, L.M.; Fallani, F.; Cuijpers, J.; Raffa, P.; Broekhuis, A.A.; van Duin, M.; Picchioni, F. Water-Swellable Elastomers: Synthesis, Properties and Applications. Rev. Chem. Eng. 2018, 35, 45–72. [Google Scholar] [CrossRef]
- Cherezova, E.; Nakyp, A.; Karaseva, Y.; Zhapparbergenov, R.; Akylbekov, N. Application of Epoxidized Soybean Oil in Highly Filled Water-Swelling Rubbers. Eng. Sci. 2023, 25, 936. [Google Scholar] [CrossRef]
- Cherezova, E.N.; Nakyp, A.M.; Karaseva, Y.S. Use of Powdered Cellulose, Obtained from Cotton Fiber Waste, in the Composition of Hydro-Swelling Rubber. Butlerov. Commun. 2021, 68, 47–53. (In Russian) [Google Scholar]
- Cherezova, E.N.; Karaseva, Y.S.; Nakyp, A.M. Evaluation of the Durability of Limited Swelling Rubber Filled with Modified Powdered Cellulose from Cotton Waste. Polym. Sci. Ser. D 2023, 16, 681–686. [Google Scholar] [CrossRef]
- Abramas, L.A.; Sukmansky, O.B.; Vakhromeev, A.G.; Bogdanov, V.S.; Bragana, O.A.; Yakovleva, N.T. RU2227146C2 Method for Preparing Carboxymethylcellolose Potassium Salt. 2004. Available online: https://patents.google.com/patent/RU2227146C2/ru?oq=+Abrams%2c+L.A.;+Sukmansky%2c+O.B.;+Vakhromeev%2c+A.G.;+Bogdanov%2c+V.S.;+Braga%2c+O.A.;+Yakovleva%2c+N.T.+RU2227146C2+Method+for+Preparing+Carboxymethylcellolose+Potassium+Salt+2004 (accessed on 1 April 2021).
- Altunina, L.K.; Tikhonova, L.D.; Yarmukhametova, E.G. Method for Deriving Carboxymethyl Cellulose. Eurasian Chem. J. 2016, 3, 49. [Google Scholar] [CrossRef]
- Nüchter, M.; Ondruschka, B.; Bonrath, W.; Gum, A. Microwave Assisted Synthesis—A Critical Technology Overview. Green Chem. 2004, 6, 128–141. [Google Scholar] [CrossRef]
- Cheprasova, M.Y.; Markin, V.I.; Bazarnova, N.G.; Kotalevskii, I.V. Carboxymethylation of Wood in Different Solvents by the Action of Microwave Radiation. Russ. J. Bioorg. Chem. 2012, 38, 726–729. [Google Scholar] [CrossRef]
- Momzyakova, K.S.; Deberdeev, T.R.; Valishina, Z.T.; Deberdeev, R.Y.; Ibragimov, A.V.; Alexandrov, A.V. Research of Physical and Chemical Properties of Powder Cellulose from Various Type of Raw Materials. Mater. Sci. Forum 2020, 992, 791–795. [Google Scholar] [CrossRef]
- Korte, J.R.; Thurston, J.J.; Goodson, J.E. Water Swelling Rubber Compound for Use In Reactive Packers and Other Downhole Tools. US20090084550A1, 22 May 2012. [Google Scholar]
- Cherezova, E.N.; Karaseva, Y.S.; Momzyakova, K.S. Hydrophilic Rubber Based on Butadiene–Nitrile Rubber and Phytogenic Powdered Cellulose. Polym. Sci. Ser. D 2022, 15, 118–121. [Google Scholar] [CrossRef]
- Vaniev, M.A.; Lopatina, S.S.; Sychev, N.V.; Savchenko, Y.Y.; Bruk, A.D.; Novakov, I.A. Properties of Water-Swellable Compounds Based on Nitrile Rubber With Varied Acrylonitrile Content. Rubber Chem. Technol. 2021, 94, 591–599. [Google Scholar] [CrossRef]
- Wei, D.; He, N.; Zhao, J.; Wang, Z. Mechanical, Water-Swelling, and Morphological Properties of Water-Swellable Thermoplastic Vulcanizates Based on High Density Polyethylene/Chlorinated Polyethylene/Nitrile Butadiene Rubber/Cross-Linked Sodium Polyacrylate Blends. Polym.-Plast. Technol. Eng. 2015, 54, 616–624. [Google Scholar] [CrossRef]
- Cherezova, E.N.; Balabanova, F.B.; Shalyminova, D.P.; Saigitbatalova, S.S.; Liakumovich, A.G. 2,6-Bis(3,5-Di-Tert-Butyl-4-Hydroxybenzyl)Cycloalkanone: Synthesis and Prospects for Use as Stabilizer for Ethylene-Propylene Rubber. Russ. J. Appl. Chem. 2014, 87, 42–47. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, D. Preparation and Characterization of Water-Swellable Natural Rubbers. J. Appl. Polym. Sci. 2001, 80, 115–121. [Google Scholar] [CrossRef]
- Koenig, J.L. Fourier Transform Infrared Spectroscopy of Polymers. In Spectroscopy: NMR, Fluorescence, FT-IR; Springer: Berlin/Heidelberg, Germany, 1984; pp. 87–154. [Google Scholar]
- Liu, S.S.; Li, X.P.; Qi, P.J.; Song, Z.J.; Zhang, Z.; Wang, K.; Qiu, G.X.; Liu, G.Y. Determination of Three-Dimensional Solubility Parameters of Styrene Butadiene Rubber and the Potential Application in Tire Tread Formula Design. Polym. Test. 2020, 81, 106170. [Google Scholar] [CrossRef]
- Askadskii, A.A.; Matveev, Y.I. Chemical Structure and Physical Properties of Polymers; Khimiya: Moscow, Russia, 1983. [Google Scholar]
- Mohd Hanif, H.; Yong, K.C.; Lee, S.Y. Evaluating the Efficacy of a Newly Developed Palm-Based Process Aid on Nitrile Rubber Composites. J. Rubber Res. 2021, 24, 51–59. [Google Scholar] [CrossRef]
- Zhdanov, Y.A.; Alekseev, Y.E.; Alekseeva, V.G. Chemical Modification of Cellulose in a Superbasic Medium. Polym. Sci. 1993, 35, 1193–1197. [Google Scholar]
- Hon, D.N.S.; Shiraishi, N. Wood and Cellulosic Chemistry, Revised, and Expanded; CRC Press: Boca Raton, FL, USA, 2000; ISBN 9780429175336. [Google Scholar]
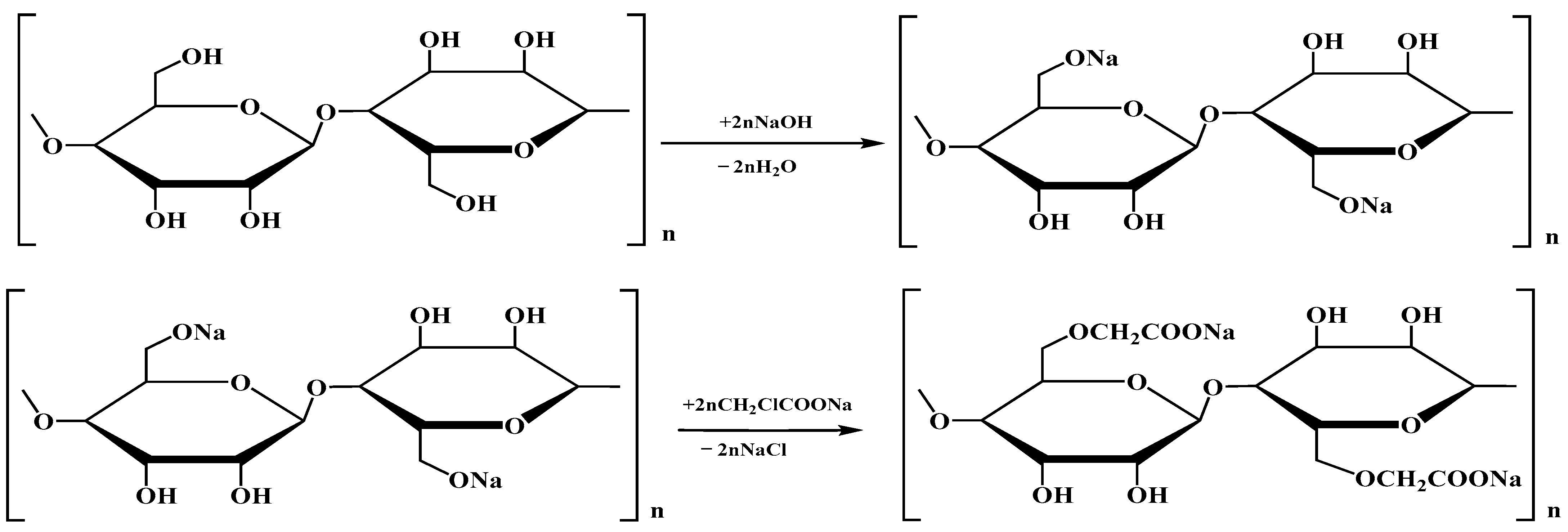
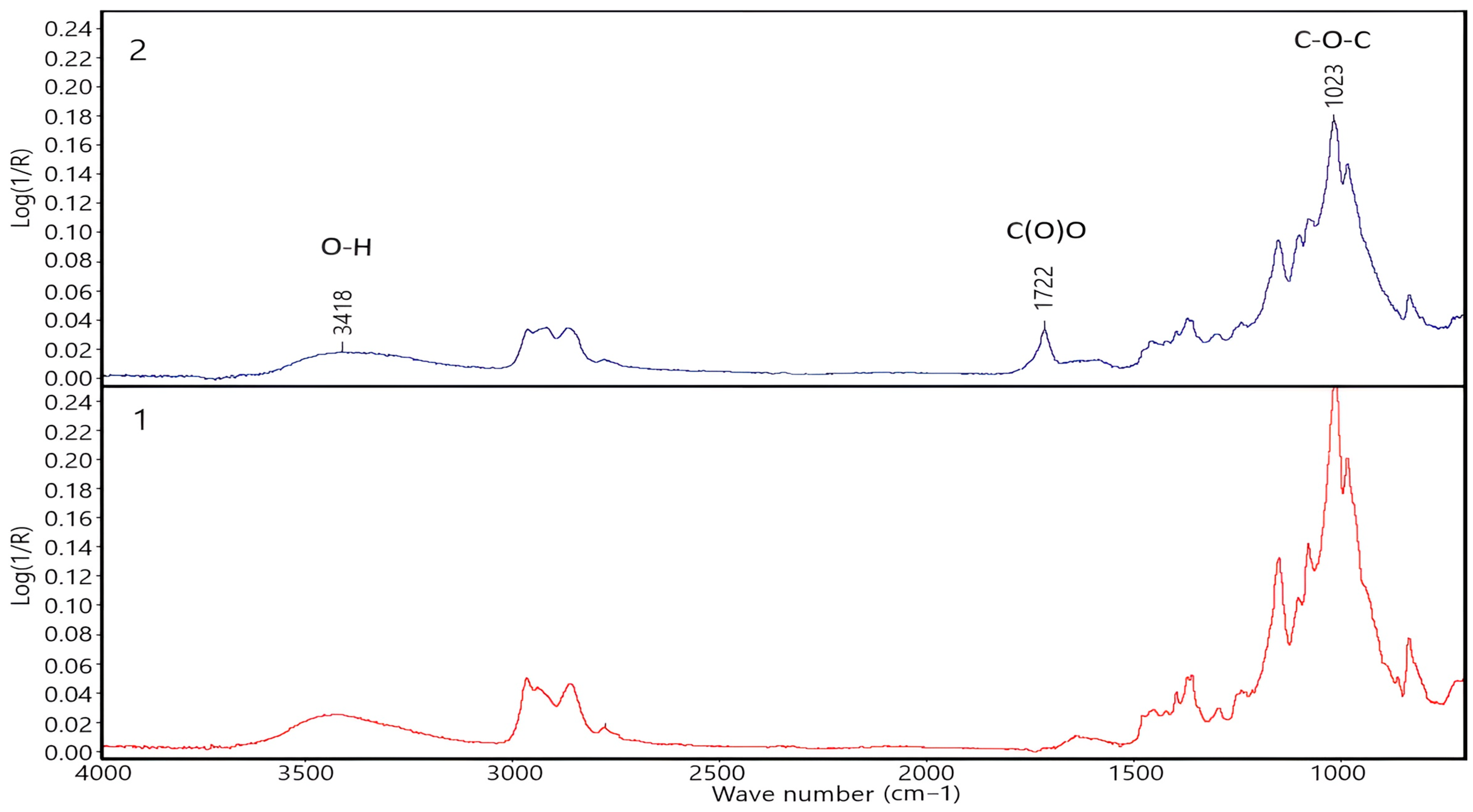

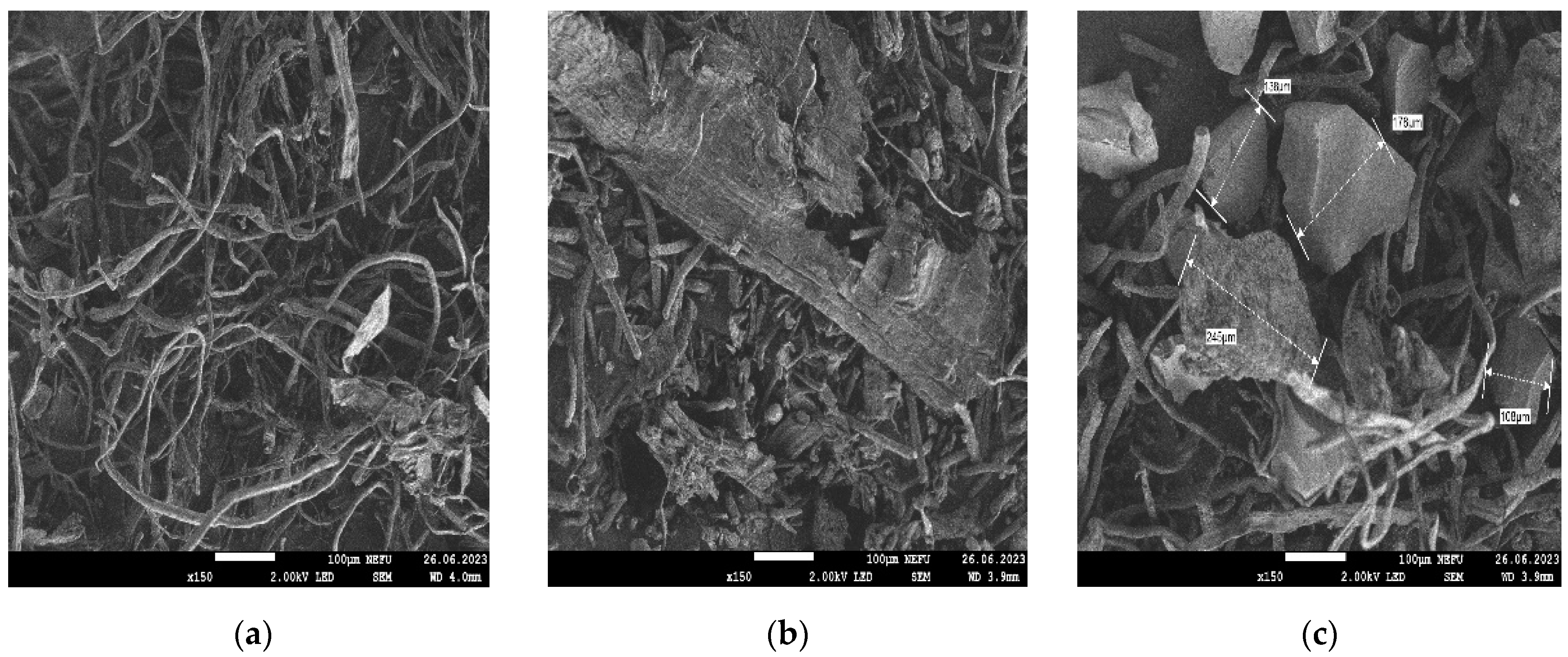

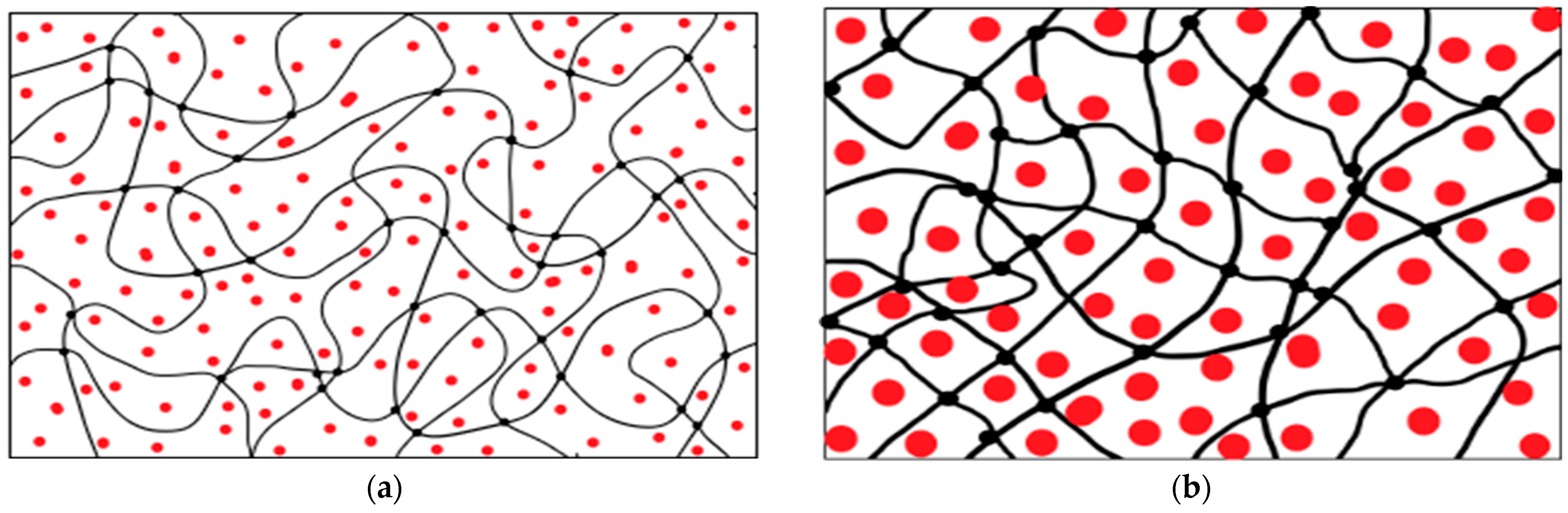
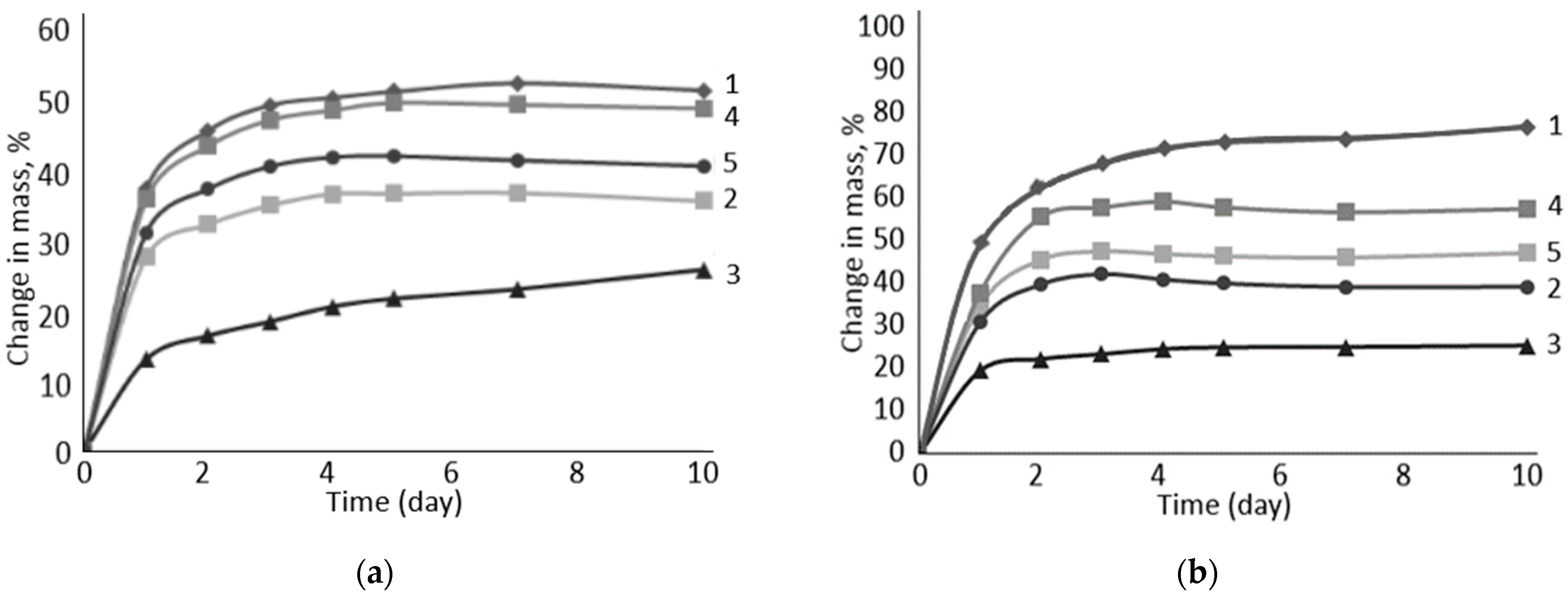

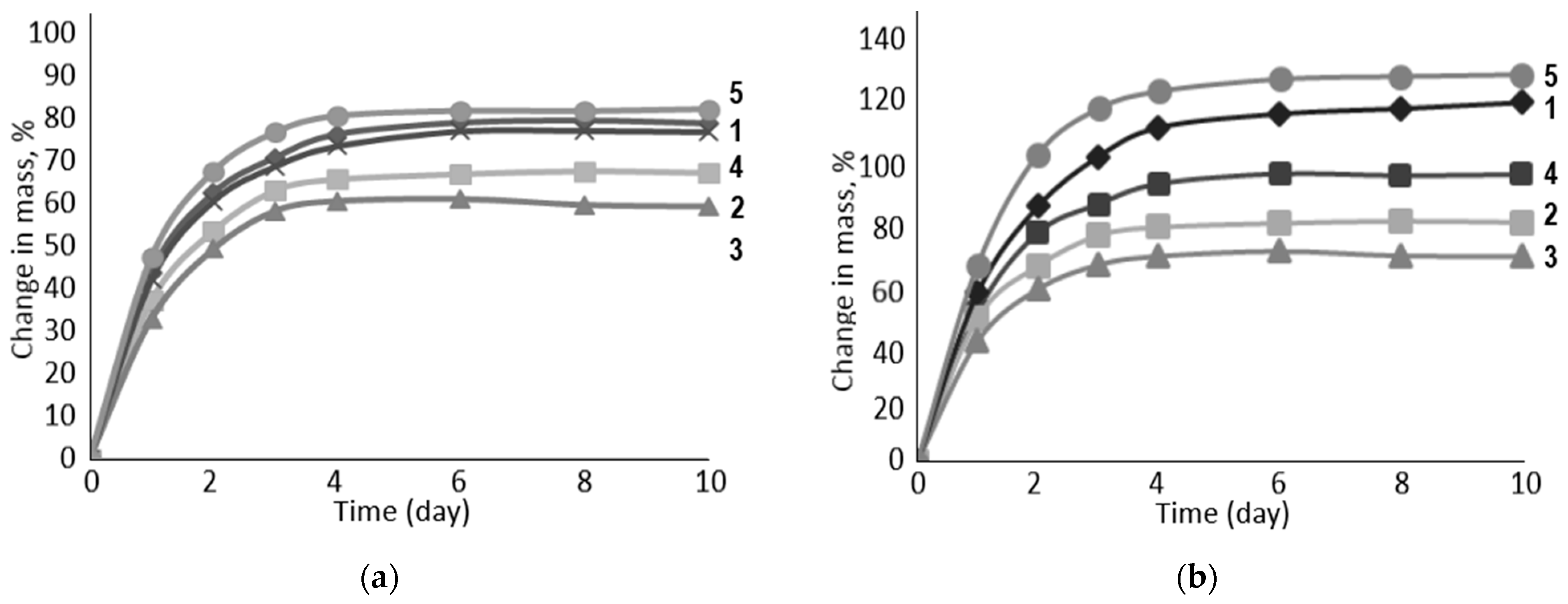

| Name of Aqueous Solution | Ion Content, g/L | Density, kg/m3 | |||||
|---|---|---|---|---|---|---|---|
| Na+ | Ca2+ | Mg2+ | Cl− | Total | |||
| I | Model sodium chloride solution | 59 | 11 | - | 110 | 180 | 1100 |
| II | Model sodium chloride solution | 26 | 12 | - | 62 | 100 | 1060 |
| III | Formation water (sodium chloride) | 70 | 11 | 3 | 139 | 223 | 1157 |
| IV | Formation water (sodium chloride) | 34 | 5 | 2 | 59 | 100 | 1070 |
| V | NaOH 10% | 57.5 | - | - | - | 57.5 | 1108 |
| VI | NaOH 5% | 28.8 | - | - | - | 28.8 | 1054 |
| Component | Cohesive Energy | Van der Waals Volume | Solubility Parameter | Compatibility of the Components with BNKS-28 AMN |
|---|---|---|---|---|
| ΔΕi, J/mol | NAƩVi × 106, m3/mol | δ, (MJ/m3)1/2 | β, MJ/m3 | |
| BNKS-28 AMN | 12,123 | 61.4 | 18.0 | - |
| Cellulose | 16,543 | 85.3 | 17.6 | 0.16 |
| Na-CMC | 21,639 | 117.4 | 17.8 | 0.04 |
| Oxal T-92 | 20,404 | 96.1 | 18.7 | 0.49 |
| Sample No | Quantity, Mass Fraction, % | |||
|---|---|---|---|---|
| BRC | Na-CMC | PC-Oat | CMC-Oat | |
| Without plasticizer | ||||
| 1 | 100 | - | - | - |
| 2 | 50 | 50 | - | - |
| 3 | 50 | 25 | 25 | - |
| 4 | 50 | - | 50 | - |
| 5 | 50 | 25 | - | 25 |
| 6 | 50 | - | - | 50 |
| Plasticizer T-92 (30 phr) | ||||
| 7 | 100 | - | - | - |
| 8 | 50 | 50 | - | - |
| 9 | 50 | 25 | 25 | - |
| 10 | 50 | - | 50 | - |
| 11 | 50 | 25 | - | 25 |
| 12 | 50 | - | - | 50 |
| Composition of Rubber Compounds (Ratio, Mass Fraction, %) | ts, min | Mmin, dN·m | Mmax, dN·m | t90, min | |
|---|---|---|---|---|---|
| Without plasticizer | |||||
| 1 | Control sample (BRC) | 1.2 | 24 | 54 | 19.8 |
| 2 | BRC/Na-CMC (50: 50) | 1.5 | 26 | 60 | 18.8 |
| 3 | BRC/PC-Oat (50:50) | 1.7 | 35 | 67 | 17.6 |
| 4 | BRC/CMC-Oat (50:50) | 1.8 | 24 | 53 | 17.4 |
| 5 | BRC/Na-CMC/PC-Oat (50:25:25) | 1.6 | 28 | 62 | 18.1 |
| 6 | BRC/Na-CMC/CMC-Oat (50:25:25) | 1.6 | 26 | 56 | 18.2 |
| Plasticizer T-92 (30 phr) | |||||
| 7 | Control sample (BRC) | 1.8 | 18 | 42 | 18.3 |
| 8 | BRC/Na-CMC (50:50) | 2.1 | 17 | 40 | 18.6 |
| 9 | BRC/PC-Oat (50:50) | 2.5 | 20 | 49 | 17.3 |
| 10 | BRC/CMC-Oat (50:50) | 2.4 | 14 | 36 | 17.6 |
| 11 | BRC/Na-CMC/PC-Oat (50:25:25) | 2.3 | 18 | 44 | 17.8 |
| 12 | BRC/Na-CMC/CMC-Oat (50:25:25) | 2.2 | 15 | 37 | 18.8 |
| Indicator | Swelling Filler | |||||
|---|---|---|---|---|---|---|
| –(Control without SF) | Na-CMC | PC-Oat | CMC-Oat | Na-CMC + PC-Oat | Na-CMC + CMC-Oat | |
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Mooney Viscosity ML(1 + 4) 100 °C | 48.7 | 74.6 | 180.6 | 62.2 | 104.9 | 75.3 |
| Plasticizer T-92 (30 phr) | ||||||
| 7 | 8 | 9 | 10 | 11 | 12 | |
| Mooney Viscosity ML(1 + 4) 100 °C | 43.5 | 64.3 | 160.1 | 55.4 | 96.3 | 67.8 |
| Properties | Swelling Filler | |||||
|---|---|---|---|---|---|---|
| –(Control without SF) | Na-CMC | PC-Oat | CMC-Oat | Na-CMC + PC-Oat | Na-CMC + CMC-Oat | |
| Without plasticizer | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | |
| TS, MPa | 13.8 | 3.6 | 8.6 | 4.1 | 5.3 | 4.6 |
| ε, % | 400 | 310 | 30 | 320 | 70 | 280 |
| Plasticizer T-92 | ||||||
| 7 | 8 | 9 | 10 | 11 | 12 | |
| TS, MPa | 14.8 | 3.4 | 7.2 | 3.9 | 4.8 | 4.3 |
| ε, % | 480 | 340 | 50 | 350 | 90 | 320 |
| N | Swelling Filler | Aqueous Media (Table 1) | |||||
|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | VI | ||
| Volume Change in the Samples, % | |||||||
| 1 | Na-CMC | 59 | 94 | 61 | 86 | 84 | 125 |
| 2 | Na-CMC + PC-Oat | 39 | 45 | 43 | 47 | 73 | 88 |
| 3 | PC-Oat | 26 | 28 | 24 | 29 | 64 | 77 |
| 4 | Na-CMC + CMC-Oat | 54 | 63 | 57 | 76 | 87 | 107 |
| 5 | CMC-Oat | 45 | 51 | 52 | 66 | 91 | 134 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherezova, E.; Karaseva, Y.; Nakyp, A.; Nuriev, A.; Islambekuly, B.; Akylbekov, N. Influence of Partially Carboxylated Powdered Lignocellulose from Oat Straw on Technological and Strength Properties of Water-Swelling Rubber. Polymers 2024, 16, 282. https://doi.org/10.3390/polym16020282
Cherezova E, Karaseva Y, Nakyp A, Nuriev A, Islambekuly B, Akylbekov N. Influence of Partially Carboxylated Powdered Lignocellulose from Oat Straw on Technological and Strength Properties of Water-Swelling Rubber. Polymers. 2024; 16(2):282. https://doi.org/10.3390/polym16020282
Chicago/Turabian StyleCherezova, Elena, Yulia Karaseva, Abdirakym Nakyp, Airat Nuriev, Bakytbek Islambekuly, and Nurgali Akylbekov. 2024. "Influence of Partially Carboxylated Powdered Lignocellulose from Oat Straw on Technological and Strength Properties of Water-Swelling Rubber" Polymers 16, no. 2: 282. https://doi.org/10.3390/polym16020282
APA StyleCherezova, E., Karaseva, Y., Nakyp, A., Nuriev, A., Islambekuly, B., & Akylbekov, N. (2024). Influence of Partially Carboxylated Powdered Lignocellulose from Oat Straw on Technological and Strength Properties of Water-Swelling Rubber. Polymers, 16(2), 282. https://doi.org/10.3390/polym16020282








