Preparation of Polyethylene Clay Composites via Melt Intercalation Using Hydrophobic and Superhydrophobic Organoclays and Comparison of Their Textural, Mechanical and Thermal Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
Preparation of Hydrophobic and Superhydrophobic Organoclay
2.3. Preparation of Polyethylene/Organoclay Composites
Melt Intercalation
2.4. Characterization of Polyethylene Clay Composites
3. Results and Discussion
3.1. HRTEM Analysis
3.2. FTIR Analysis
3.3. XRD Analysis
3.4. DSC Analysis
3.5. Mechanical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Utracki, L.A. Compatibilization of polymer blends. Can. J. Chem. Eng. 2002, 80, 1008–1016. [Google Scholar] [CrossRef]
- Ilcikova, M.; Galeziewska, M.; Mrlik, M.; Osicka, J.; Masar, M.; Slouf, M.; Maslowski, M.; Kracalik, M.; Pietrasik, R.; Mosnacek, J.; et al. The effect of short polystyrene brushes grafted from graphene oxide on the behavior of miscible PMMA/SAN blends. Polymer 2020, 211, 123088. [Google Scholar] [CrossRef]
- Din, S.H.; Shah, M.A.; Sheikh, N.A.; Butt, M.M. Nano-composites and their applications: A review. Charact. Appl. Nanomater. 2020, 3, 40–48. [Google Scholar] [CrossRef]
- Oladele, I.O.; Omotosho, T.F.; Adediran, A.A. Polymer-Based Composites: An Indispensable Material for Present and Future Applications. Int. J. Polym. Sci. 2020, 2020, 8834518. [Google Scholar] [CrossRef]
- Utracki, L.A.; Sepher, M.; Li, J. Melt Compounding of Polymeric Nanocomposites. Int. Polym. Process 2006, 21, 3–15. [Google Scholar] [CrossRef]
- Giannelis, E.P.; Krishnamoorti, R.; Manias, E. Polymer–silicate nanocomposites: Model systems for confined polymers and polymer brushes. Adv. Polym. Sci. 1999, 138, 107–147. [Google Scholar]
- LeBaron, P.C.; Wang, Z.; Pinnavaia, T.J. Polymer-layered silicate nanocomposites: An overview. Appl. Clay Sci. 1999, 15, 11–29. [Google Scholar] [CrossRef]
- Durmuş, A.; Woo, M.; Kaşgöz, A.; Macosko, C.W.; Tsapatsis, M. Intercalated linear low density polyethylene (LLDPE)/clay nanocomposites prepared with oxidized polyethylene as a new type compatibilizer: Structural; mechanical and barrier properties. Eur. Polym. J. 2007, 43, 3737–3749. [Google Scholar] [CrossRef]
- Shebani, A.; Elhrari, W.; Klash, A.; Aswei, A.; Omran, K.; Rhab, A. High Density Polyethylene/Libyan Kaolin Clay Nanocomposites: Effect of Clay Particle Size on Rheological; Surface and Mechanical Properties. AIJR Proc. 2018, 4, 529–539. [Google Scholar]
- Donchak, V.; Stetsyshyn, Y.; Bratychak, M.; Broza, G.; Harhay, K.; Stepina, N.; Kostenko, M.; Voronov, S. Nanoarchitectonics at surfaces using multifunctional initiators of surface-initiated radical polymerization for fabrication of the nanocomposites. Appl. Surf. Sci. Adv. 2021, 5, 100104. [Google Scholar] [CrossRef]
- Liu, S.P.; Hwang, S.S.; Yeh, J.M.; Hung, C.C. Mechanical properties of polyamide-6/montmorillonite nanocomposites—Prepared by the twin-screw extruder mixed technique. Int. Commun. Heat Mass Transf. 2011, 38, 37–43. [Google Scholar] [CrossRef]
- Tyan, H.L.; Liu, Y.C.; Wei, K.H. Effect of reactivity of organics-modified montmorillonite on the thermal and mechanical properties of montmorillonite/polyimide nanocomposites. Chem. Mater. 2001, 13, 222–226. [Google Scholar] [CrossRef]
- Gilman, J.W.; Jackson, C.L.; Morgan, A.B.; Hayis, R.J.; Manias, E.; Giannelis, E.P.; Hilton, M.; Wuthenow, D.; Phillips, S.H. Flammability properties of polymer-layered-silicate nanocomposites. Polypropylene and polystyrene nanocomposites. Chem. Mater. 2000, 12, 1866–1873. [Google Scholar] [CrossRef]
- Lan, T.; Kaviratna, P.D.; Pinnavaia, T.J. On the nature of polyimide–clay hybridcomposites. Chem. Mater. 1994, 6, 573–575. [Google Scholar] [CrossRef]
- Yu, Y.H.; Yeh, J.M.; Liou, S.J.; Chen, C.L.; Liaw, D.J.; Lu, H.Y. Preparation and properties of polyimide–clay nanocomposite materials for anticorrosion application. J. App. Polym. Sci. 2004, 92, 3573–3582. [Google Scholar] [CrossRef]
- Liu, S.P.; Tu, L.C. Studies on mechanical properties of dispersing intercalated silane montmorillonite in low density polyethylene matrix. Int. Commun. Heat Mass Transfer 2011, 38, 879–886. [Google Scholar] [CrossRef]
- Pandit, P. Advanced applications of green materials in textile. In Applications of Advanced Green Materials, 1st ed.; Ahmed, S., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2021; pp. 131–150. [Google Scholar]
- Zhang, H.; Lu, Y.; Zhang, Q.; Yang, F.; Hui, A.; Wang, A. Structural evolution of palygorskite-rich clay as the nanocarriers of silver nanoparticles for efficient improving antibacterial activity. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129885. [Google Scholar] [CrossRef]
- Mokhtar, A.; Ahmed, A.B.; Asli, B.; Boukoussa, B.; Hachemaoui, M.; Sassi, M.; Abboud, M. Recent Advances in Antibacterial Metallic Species Supported on Montmorillonite Clay Mineral: A Review. Minerals 2023, 13, 1268. [Google Scholar] [CrossRef]
- Brigatti, M.F.; Galan, E.; Theng, B.K.G. Structures and Mineralogy of Clay Minerals. In Handbook of Clay Science, 1st ed.; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2006; Volume 1, pp. 19–86. [Google Scholar]
- Caillère, S.; Hénin, S.; Rautureau, M.; Minéralogie des Argiles, I. Structure et Propriétés Physico-Chimiques; Masson: Paris, France, 1982. [Google Scholar]
- Luckhamu, P.F.; Rossi, S. The colloidal and rheological properties of bentonite suspension. Adv. Colloid Interface Sci. 1999, 82, 43–92. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Van Meerbeck, A. Clay Mineral and Organoclay-polymer Nanocomposite. In Handbook of Clay Science, 1st ed.; Bergaya, F., Theng, B.K.G., Lagaly, G., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2006; Volume 1, pp. 583–622. [Google Scholar]
- Thuc, C.-N.H.; Grillet, A.-C.; Reinert, L.; Ohashi, F.; Thuc, H.H.; Duclaux, L. Separation and purification of montmorillonite and polyethylene oxide modified montmorillonite from Vietnamese bentonites. Appl. Clay Sci. 2010, 49, 229–238. [Google Scholar] [CrossRef]
- Padil, V.V.T.; Kumar, K.P.A.; Murugesan, S.; Torres-Mendieta, R.; Wacławek, S.; Cheong, J.Y.; Černík, M.; Varma, R.S. Sustainable and safer nanoclay composites for multifaceted applications. Green Chem. 2022, 24, 3081–3114. [Google Scholar] [CrossRef]
- Gul, S.; Kausar, A.; Muhammad, B.; Jabeen, S. Research Progress on Properties and Applications of Polymer/Clay Nanocomposite. Polym. Plast. Technol. Eng. 2016, 55, 684–703. [Google Scholar] [CrossRef]
- Utracki, L.A. Clay-Containing Polymeric Nanocomposites, 1st ed.; Rapra Techn Ltd.: Shawbury, UK, 2004; Volume 1. [Google Scholar]
- Krishnamoorti, R.; Vaia, R.A.; Giannelis, E.P. Microstructural evolution of melt intercalated polymer-organically modified layered silicates nanocomposites. Chem. Mater. 1996, 8, 1728–1734. [Google Scholar] [CrossRef]
- Guégan, R. Organoclay applications and limits in the environment. C. R. Chimie 2019, 22, 132–141. [Google Scholar] [CrossRef]
- Yahya, K.; Hamdi, W.; Hamdi, N. Organoclay Nano-Adsorbent: Preparation, Characterization and Applications. In Nanoclay-Recent Advances, New Perspectives and Applications, 1st ed.; Oueslati, W., Ed.; IntechOpen: Rijeka, Crotia, 2022; pp. 1–25. [Google Scholar]
- Gürses, A.; Ejder Korucu, M.; Karaca, S. Clay-Organoclay and Organoclay/Polymer Nanocomposites. In Clay: Types; Properties and Uses, 1st ed.; Humphrey, J.P., Boyd, D.E., Eds.; Nova Science Publishers: New York, NY, USA, 2011; pp. 155–191. [Google Scholar]
- Vaia, R.A.; Teukolsky, R.K.; Giannelis, E.P. Interlayer structure and molecular environment of alkylammonium layered silicates. Chem. Mater. 1994, 6, 1017–1022. [Google Scholar] [CrossRef]
- Zhang, J.; Gupta, R.K.; Wilkie, C.A. Controlled silylation of montmorillonite and its polyethylene nanocomposites. Polymer 2006, 47, 4537–4543. [Google Scholar] [CrossRef]
- Graziano, A.; Jaffer, S.; Sain, M. Graphene oxide modification for enhancing high-density polyethylene properties: A comparison between solvent reaction and melt mixing. J. Polym. Eng. 2019, 39, 85–93. [Google Scholar] [CrossRef]
- Wójcik-Bania, M.; Matusik, J. The Effect of Surfactant-Modified Montmorillonite on the Cross-Linking Efficiency of Polysiloxanes. Materials 2021, 14, 2623–2639. [Google Scholar] [CrossRef]
- Sharudin, R.W.; Azmi, N.S.M.; Hanizan, A.; Akhbar, S.; Ahmad, Z.; Ohshima, M. Dynamic Molecular Simulation of Polyethylene/Organoclay Nanocomposites for Their Physical Properties and Foam Morphology. Materials 2023, 16, 3122. [Google Scholar] [CrossRef]
- Kato, A.; Usuki, A.; Hasegawa, N.; Okamoto, H.; Kawasumi, M. Development and applications of polyolefin–and rubber–clay nanocomposites. Polym. J. 2011, 43, 583–593. [Google Scholar] [CrossRef]
- Gürses, A. Introduction to Polymer-Clay Nanocomposites; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Pettarin, V.; Rodriguez Pita, V.J.R.; Valenzuela-Díaz, F.R.; Moschiar, S.; Fasce, L.; Seltzer, R.; Lopes Dias, M.; Frontini, P. Preparation, physical and mechanical characterization of montmorillonite/polyethylene nanocomposites. Key Eng. Mater. 2006, 312, 205–210. [Google Scholar] [CrossRef]
- ASTM C837-81; Standard Test Method for Methylene Bue Index of Clay. ASTM: West Conshohocken, PA, USA, 1989.
- Carrado, K.A.; Decarreau, A.; Petıt, S.; Bergaya, F.; Lagaly, G. Handbook of Clay Science; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- ASTM D 2294-96; Creep Properties of Adhesives in Shear by Tension Loading (Metal to Metal). ASTM International: West Conshohocken, PA, USA, 1996.
- Uthirakumar, P.; Song, M.-K.; Nah, C.; Lee, Y.-S. Preparation and characterization of exfoliated polystyrene/clay nanocomposites using a cationic radical initiator-MMT hybrid. Eur. Polym. J. 2005, 41, 211–217. [Google Scholar] [CrossRef]
- Abdullah, M.A.A.; Mamat, M.; Awang, M.; Kusrini, E.; Mubin, F.N.A.; Sudin, N.H. Effect of Trihexyltetradecylphosphonium On Thermal Degradation Properties of Low Linear Density Polyethylene/Montmorillonite Nanocomposites. Int. J. Technol. 2013, 2, 129–135. [Google Scholar] [CrossRef]
- Pérez, M.A.; Rivas, B.L.; Garrido-Miranda, K.A.; Requena, V.H.C.; Martínez, M.; Castaño, J.; Maldonado, Á. Low Density Polyethylene (LDPE) Nanocomposites with Passive and Active Barrier Properties. J. Chil. Chem. Soc. 2014, 59, 2442–2446. [Google Scholar]
- Khezri, K.; Haddadi-Asl, V.; Roghani-Mamaqani, H.; Salami-Kalajahi, M. Effect of MCM-41 nanoparticles on ARGET ATRP of styrene: Investigating thermal properties. J. Compos. Mater. 2015, 49, 1525–1535. [Google Scholar] [CrossRef]
- Dadbin, S.; Noferesti, M.; Frounchi, M. Oxygen barrier LDPE/LLDPE/organo clay nano-composite films for food packaging. Macromol. Symp. 2008, 274, 22–27. [Google Scholar] [CrossRef]
- Khalili, S.; Masoomi, M.; Bagheri, R. The effect of organo-modified montmorillonite on mechanical and barrier properties of linear low-density polyethylene/low-density polyethyleneblend films. J. Plast. Film Sheeting 2012, 29, 39–55. [Google Scholar] [CrossRef]
- Lee, W.G.; Zheng, Y.H.; Park, C.B.; Kontopoulou, M. Effects of Clay Dispersion on the Mechanical Properties and Flammability of Polyethylene/Clay Nanocomposites. SPE ANTEC Technol. Pap. 2005, 63, 1428–1432. [Google Scholar]
- López-Quintanilla, M.; Sánchez-Valdés, S.; Ramos de Valle, L.; Miranda, R.G. Preparation and mechanical properties of PP/PP-g-MA/Org-MMT nanocomposites with different MA content. Polym. Bull. 2006, 57, 385–393. [Google Scholar] [CrossRef]
- Pegoretti, A.; Dorigato, A.; Penati, A. Tensile mechanical response of polyethylene—Clay nanocomposites. EXPRESS Polym. Lett. 2007, 1, 123–131. [Google Scholar] [CrossRef]
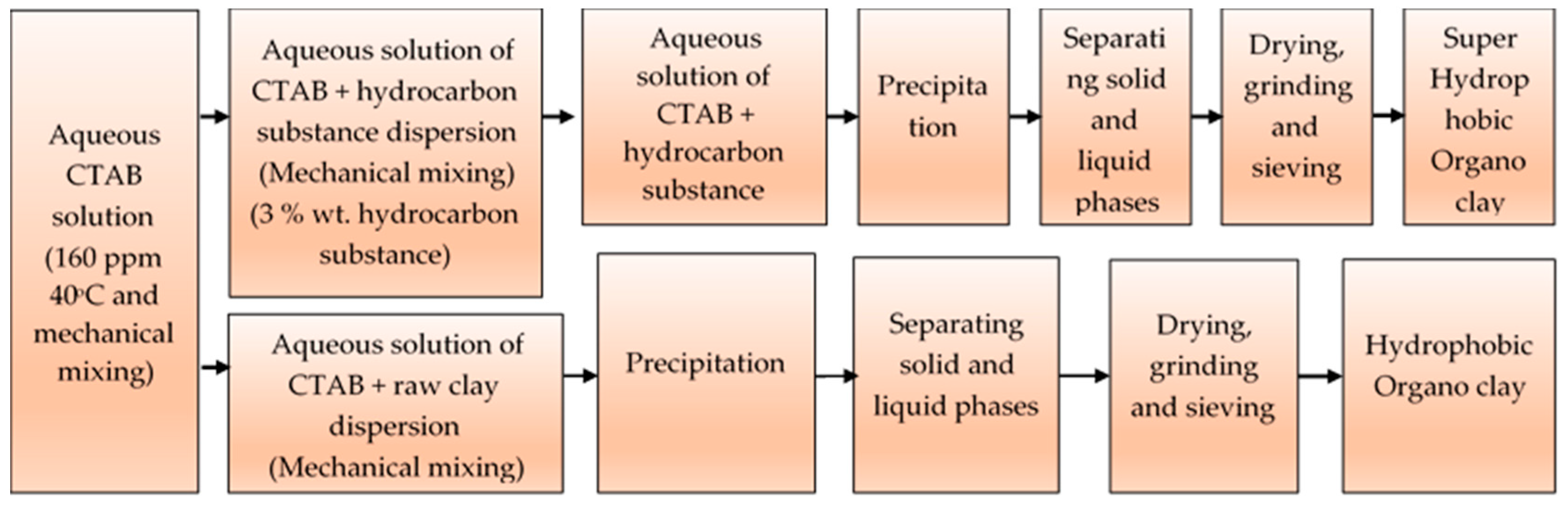
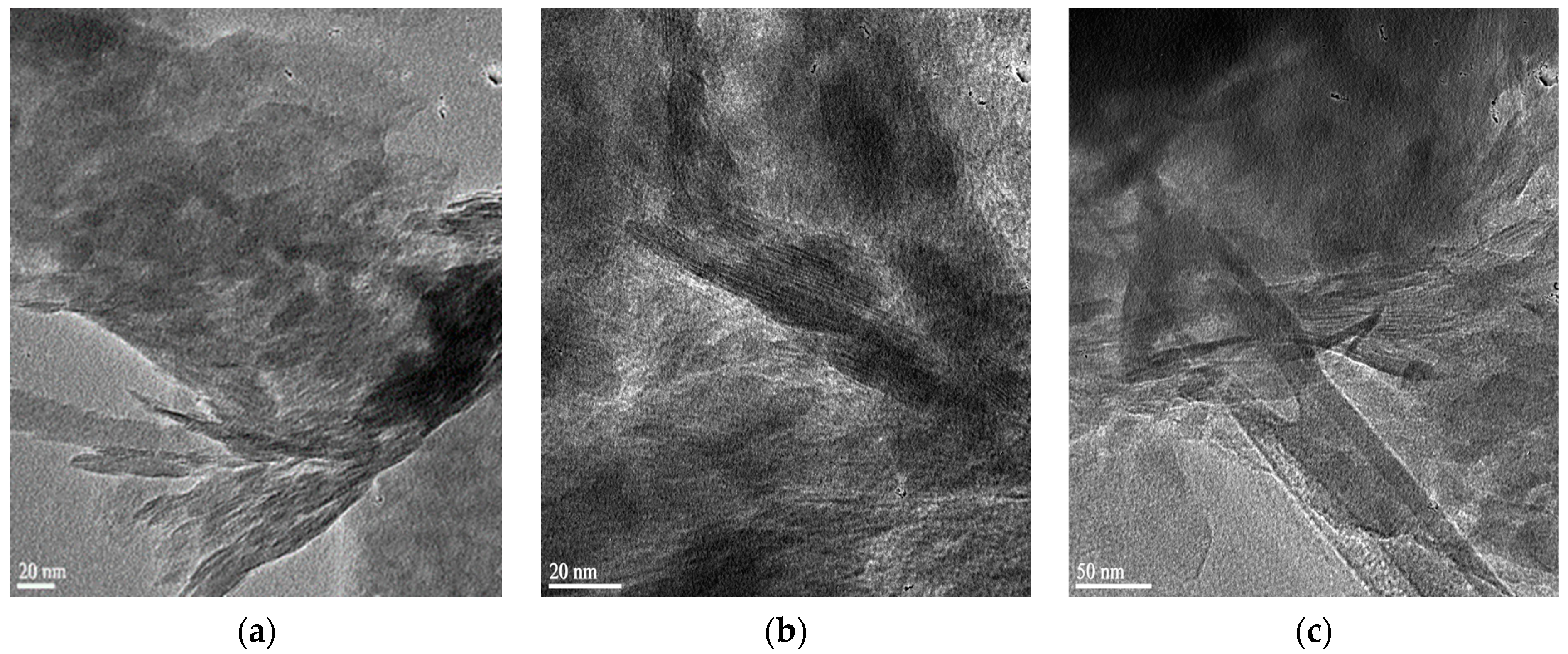
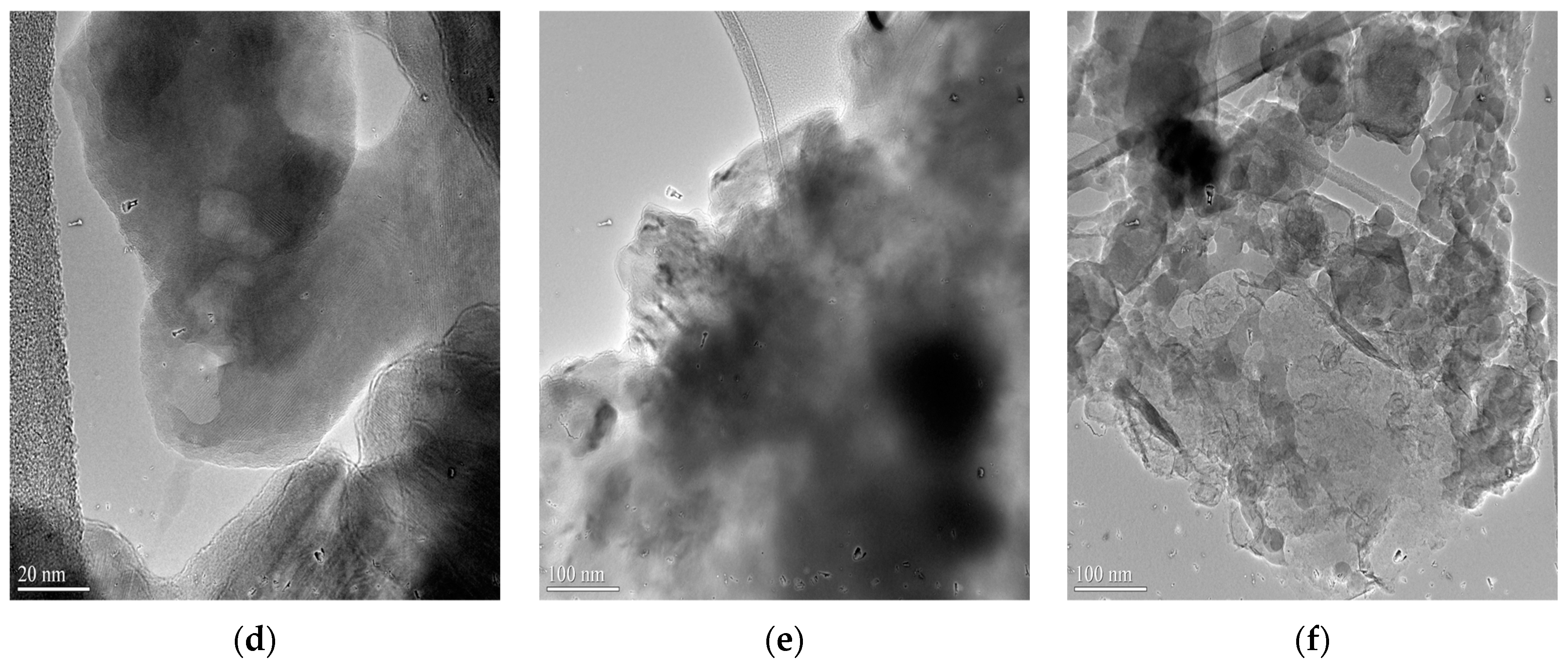


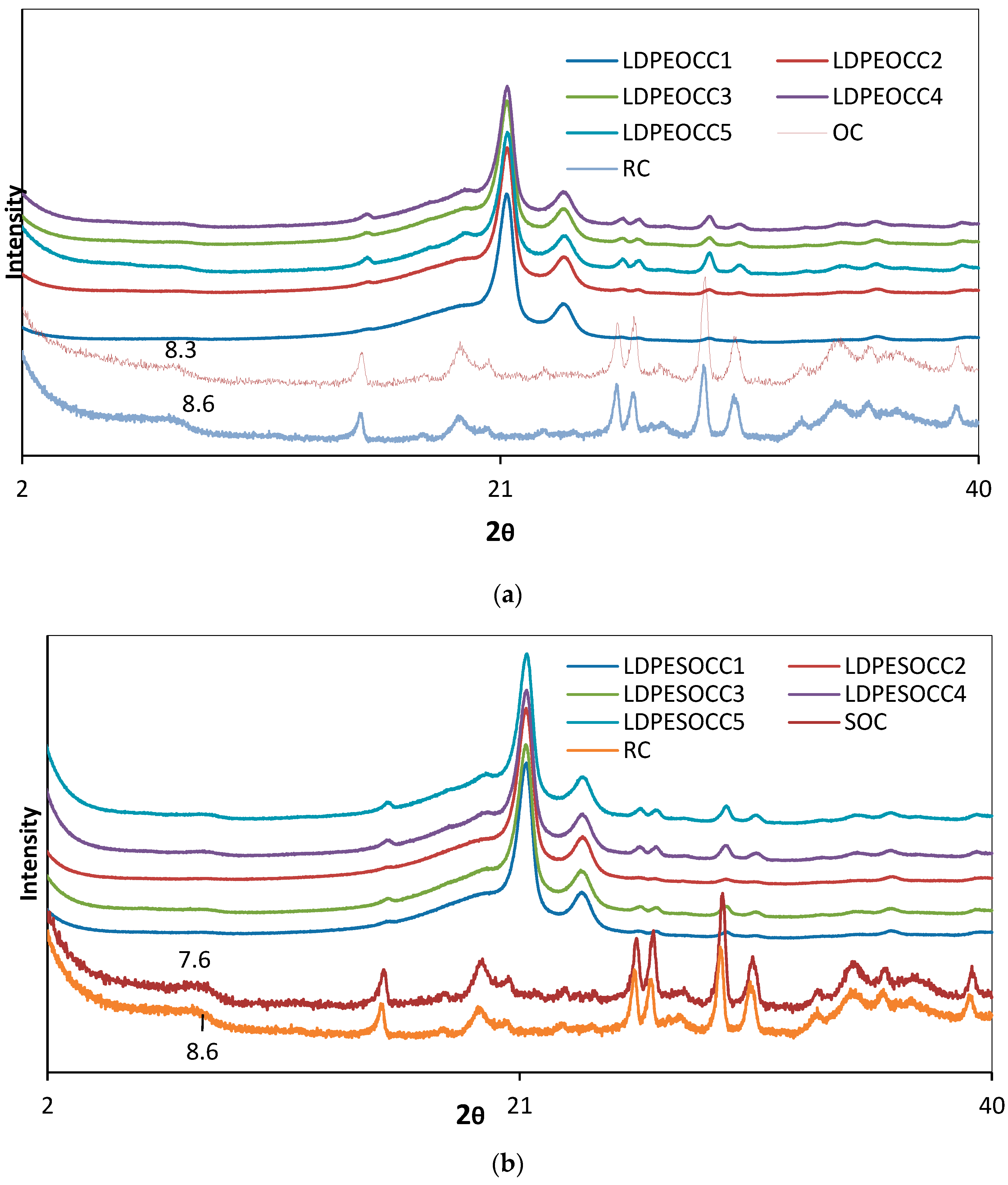

| Components (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | CaO | MgO | Fe2O3 | K2O | Na2O | TiO2 | SO3 | P2O5 |
| 56.77 | 15.16 | 8.44 | 8.79 | 0.48 | 4.04 | 4.20 | 0.84 | 0.91 | 0.37 |
| CEC a | d b | OMC c | Liquid Limit, | Plastic Limit, | Plasticity | a d |
|---|---|---|---|---|---|---|
| (meq/100 g) | (g/cm3) | (%) | wL (%) | wP (%) | index, Ip | (m2/g) |
| 48.90 | 2.61 | 5.10 | 102.00 | 35.00 | 67.00 | 64.20 |
| Density | Calorific Value | Flash Point °C | Water by Distillation | C | H | N | S | Ash |
|---|---|---|---|---|---|---|---|---|
| (15 °C), kg/m3 | MJ/kg | wt.% | ||||||
| 990.7 | 42.74 | 105.8 | 0.1 | 83.4 | 11.9 | 0.8 | 1.5 | 0.03 |
| Specimen Code | Nano Filler | (%wt.) |
|---|---|---|
| LDPE | - | - |
| (Low-Density Polyethylene) | ||
| LDPEOCC1 | Hydrophobic organoclay | 5.0 |
| (Hydrophobic organoclay and low-density polyethylene composite 1) | ||
| LDPEOCC2 | Hydrophobic organoclay | 10.0 |
| (Hydrophobic organoclay and low-density polyethylene composite 2) | ||
| LDPEOCC3 | Hydrophobic organoclay | 15.0 |
| (Hydrophobic organoclay and low-density polyethylene composite 3) | ||
| LDPEOCC4 | Hydrophobic organoclay | 20.0 |
| (Hydrophobic organoclay and low-density polyethylene composite 4) | ||
| LDPEOCC5 | Hydrophobic organoclay | 25.0 |
| (Hydrophobic organoclay and low-density polyethylene composite 5) | ||
| LDPESOCC1 | ||
| (Superhydrophobic organoclay and low-density polyethylene composite 1) | Superhydrophobic organoclay | 5.0 |
| LDPESOCC2 | ||
| (Superhydrophobic organoclay and low-density polyethylene composite 2) | Superhydrophobic organoclay | 10.0 |
| LDPESOCC3 | ||
| (Superhydrophobic organoclay and low-density polyethylene composite 3) | Superhydrophobic organoclay | 15.0 |
| LDPESOCC4 | ||
| (Superhydrophobic organoclay and low-density polyethylene composite 4) | Superhydrophobic organoclay | 20.0 |
| LDPESOCC5 | ||
| (Superhydrophobic organoclay and low-density polyethylene composite 5) | Superhydrophobic organoclay | 25.0 |
| Clay Ratio (%) | Hardness (Shore D) | Scratch Hardness (MPa) | Tensile Strength (MPa) | Yield Strength (MPa) | Elasticity Module (MPa) | Elongation (%) | |
|---|---|---|---|---|---|---|---|
| LDPE | 0 | 55.00 | 16,616 | 9.17 | 4.36 | 2.16 | 256.94 |
| LDPEOCC | 5 | 55.00 | 23,291 | 15.70 | 3.65 | 2.61 | 721.05 |
| 10 | 56.33 | 15,000 | 12.41 | 5.28 | 2.61 | 336.66 | |
| 15 | 57.00 | 16,421 | 12.58 | 4.81 | 2.44 | 463.96 | |
| 20 | 56.40 | 18,051 | 7.14 | 3.74 | 1.83 | 141.05 | |
| 25 | 57.16 | 9527 | 7.83 | 3.81 | 0.69 | 232.51 | |
| LDPESOCC | 5 | 56.16 | 14,317 | 11.52 | 3.45 | 1.70 | 510.39 |
| 10 | 55.50 | 15,621 | 11.37 | 2.58 | 2.29 | 536.29 | |
| 15 | 55.83 | 16,041 | 12.96 | 4.83 | 1.64 | 582.92 | |
| 20 | 56.83 | 13,862 | 10.61 | 4.45 | 1.88 | 476.42 | |
| 25 | 56.33 | 7579 | 7.53 | 3.31 | 1.91 | 78.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gürses, A.; Güneş, K. Preparation of Polyethylene Clay Composites via Melt Intercalation Using Hydrophobic and Superhydrophobic Organoclays and Comparison of Their Textural, Mechanical and Thermal Properties. Polymers 2024, 16, 272. https://doi.org/10.3390/polym16020272
Gürses A, Güneş K. Preparation of Polyethylene Clay Composites via Melt Intercalation Using Hydrophobic and Superhydrophobic Organoclays and Comparison of Their Textural, Mechanical and Thermal Properties. Polymers. 2024; 16(2):272. https://doi.org/10.3390/polym16020272
Chicago/Turabian StyleGürses, Ahmet, and Kübra Güneş. 2024. "Preparation of Polyethylene Clay Composites via Melt Intercalation Using Hydrophobic and Superhydrophobic Organoclays and Comparison of Their Textural, Mechanical and Thermal Properties" Polymers 16, no. 2: 272. https://doi.org/10.3390/polym16020272
APA StyleGürses, A., & Güneş, K. (2024). Preparation of Polyethylene Clay Composites via Melt Intercalation Using Hydrophobic and Superhydrophobic Organoclays and Comparison of Their Textural, Mechanical and Thermal Properties. Polymers, 16(2), 272. https://doi.org/10.3390/polym16020272








