Preparation and Characterization of Perfluoropolyether-Silane@Ethye Cellulose Polymeric Microcapsules
Abstract
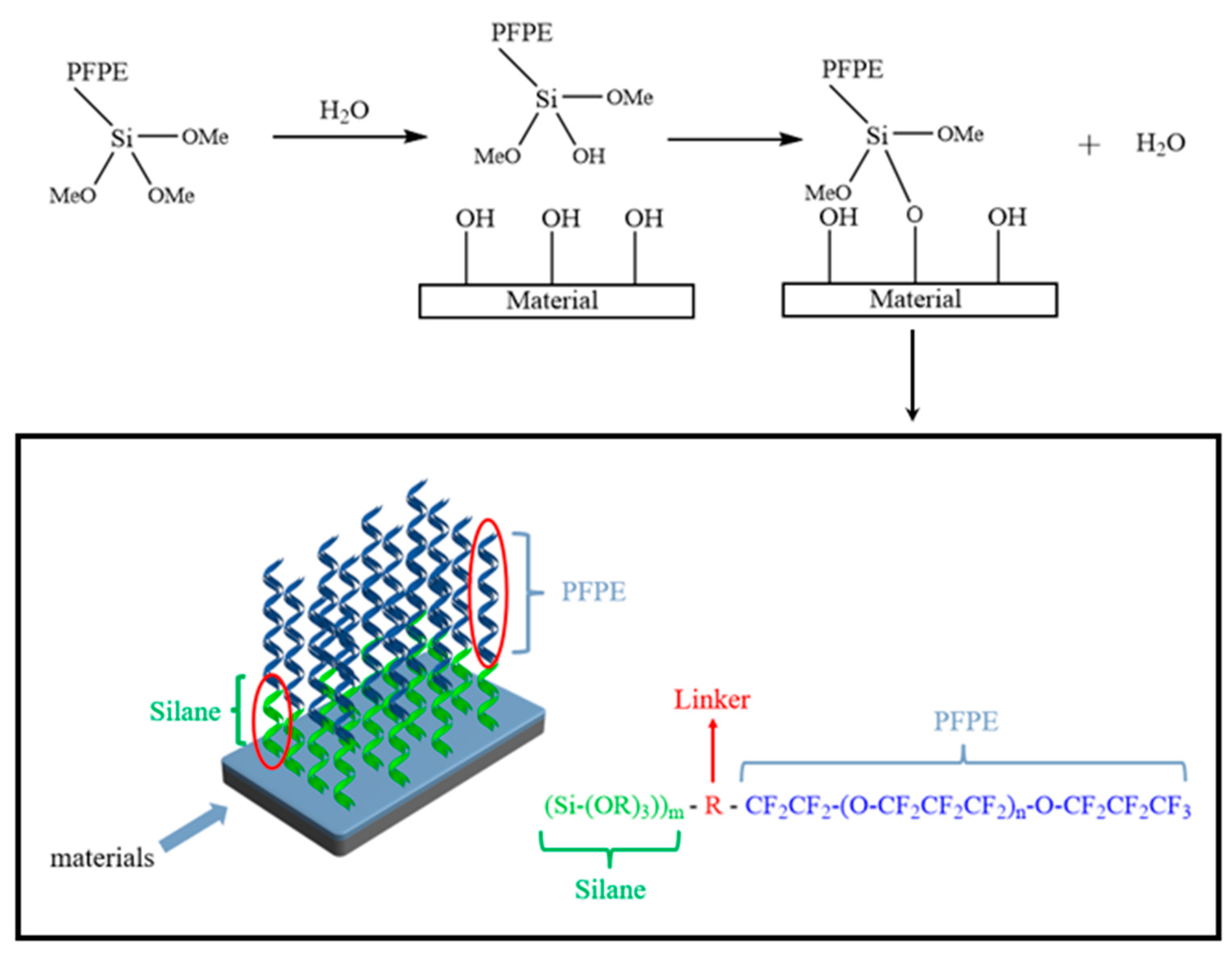
1. Introduction
2. Experimental Section
2.1. Materials
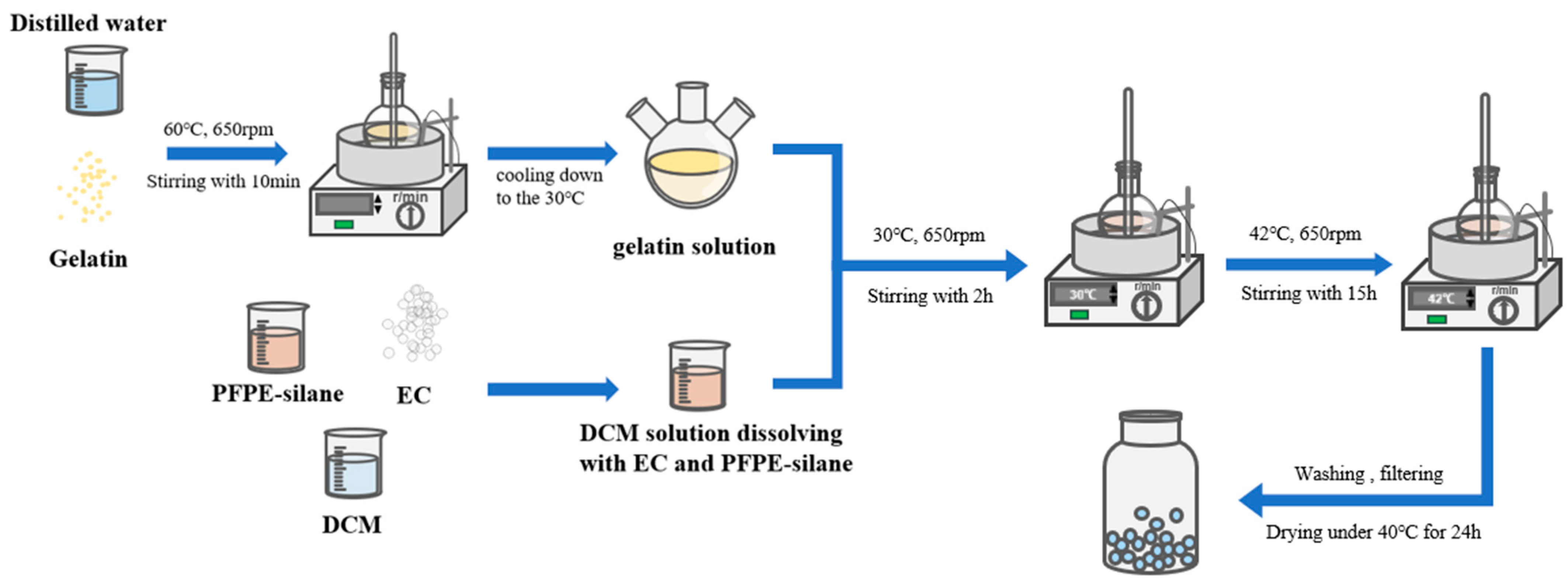
2.2. Preparation of Microcapsules
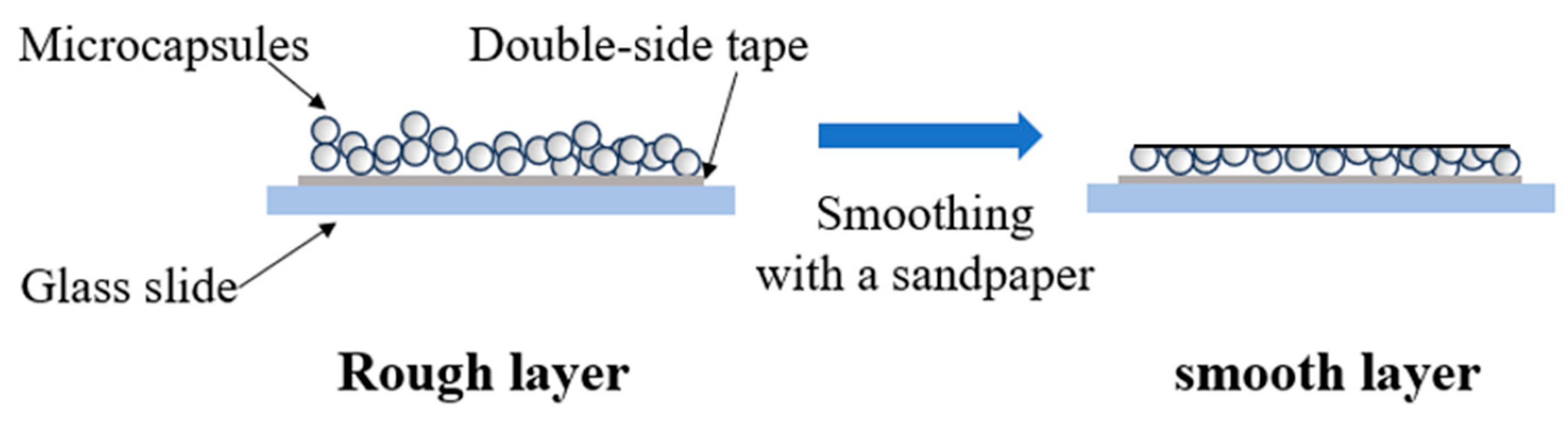
2.3. Preparation of Capsule Layers
2.4. Characterization of Microcapsules
2.4.1. Preparation Process Analysis
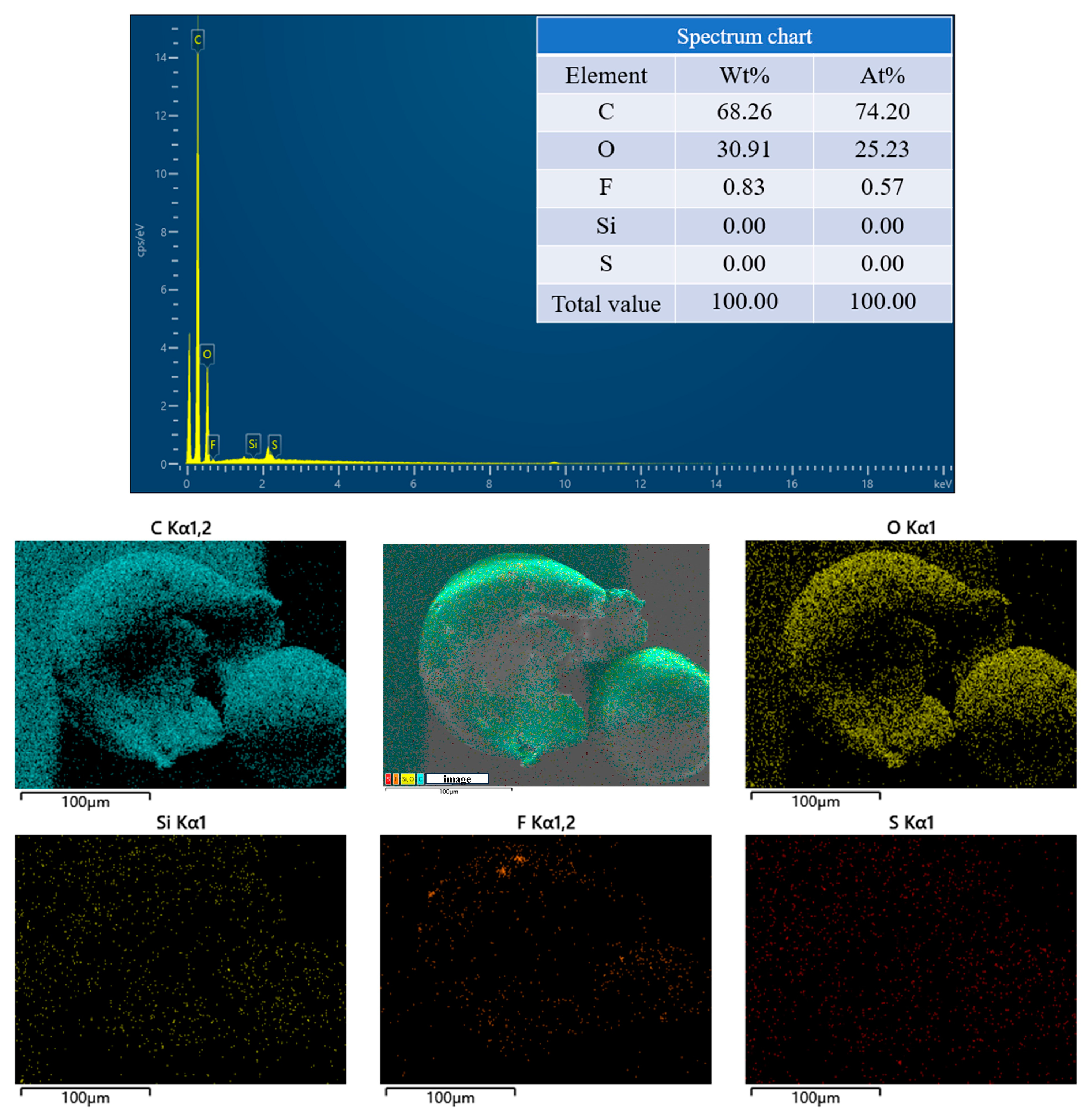
2.4.2. SEM-EDS Analysis
2.4.3. Particle Size Analysis
2.4.4. FT-IR Analysis
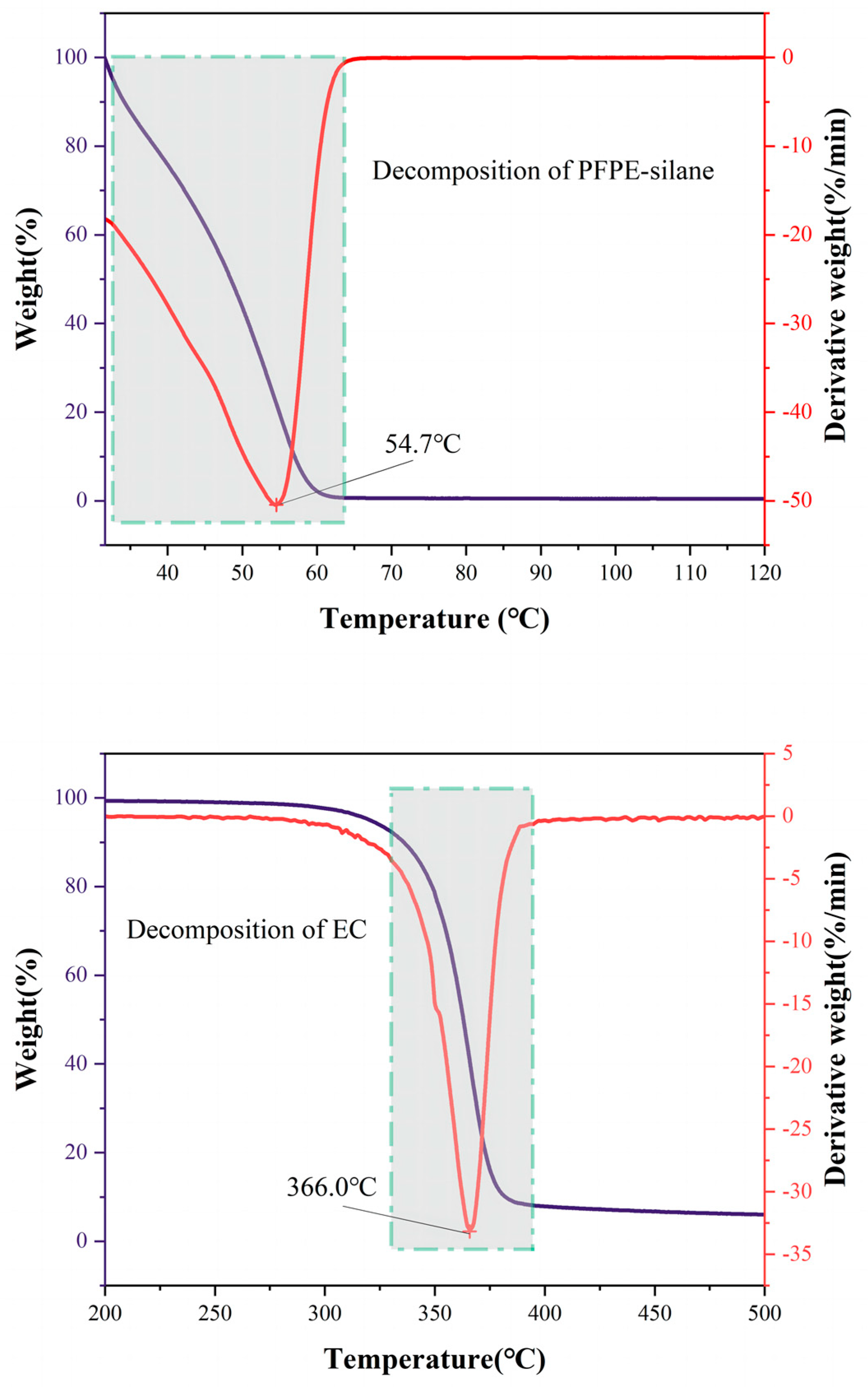
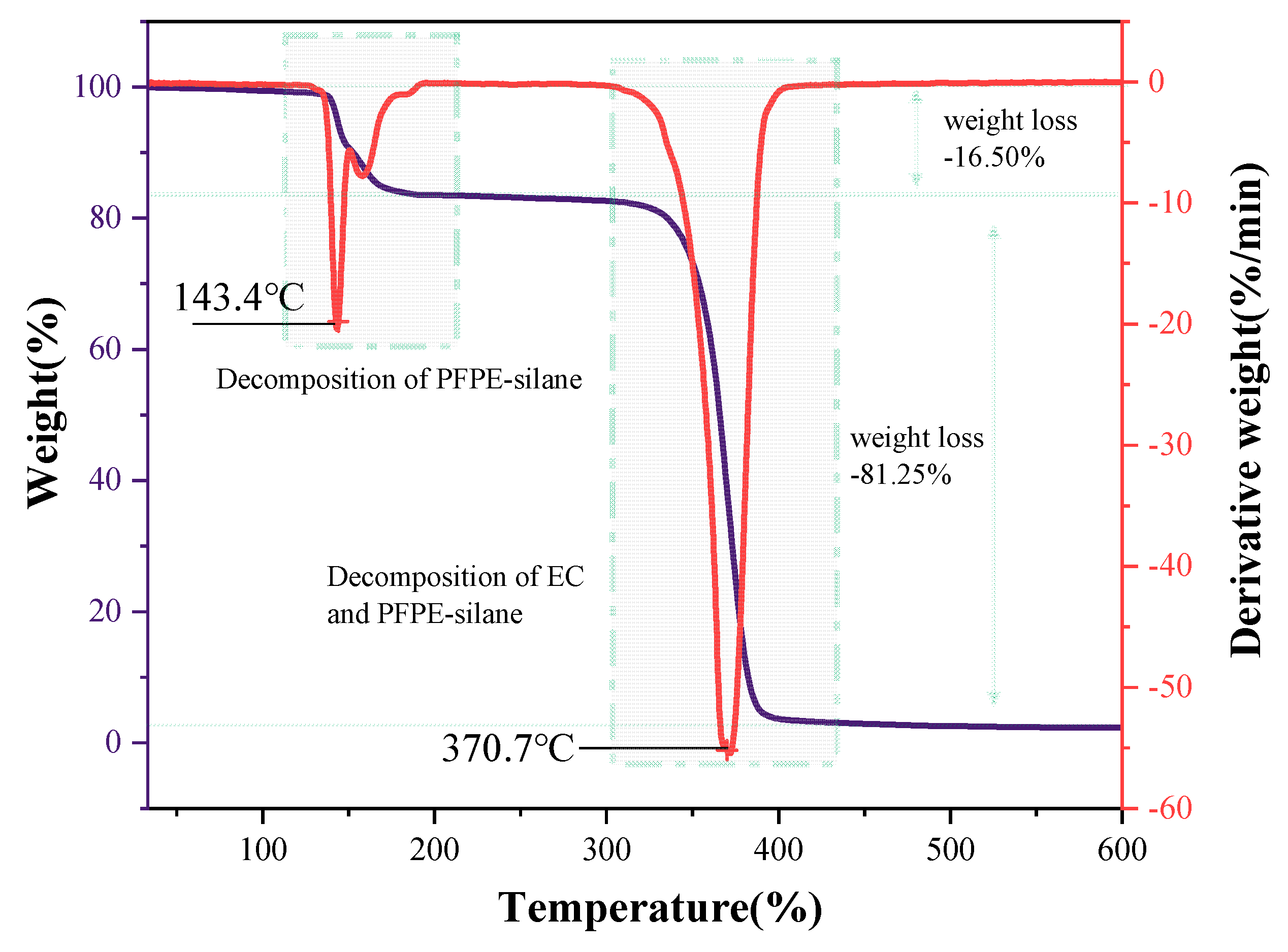
2.4.5. TG-DTG Analysis
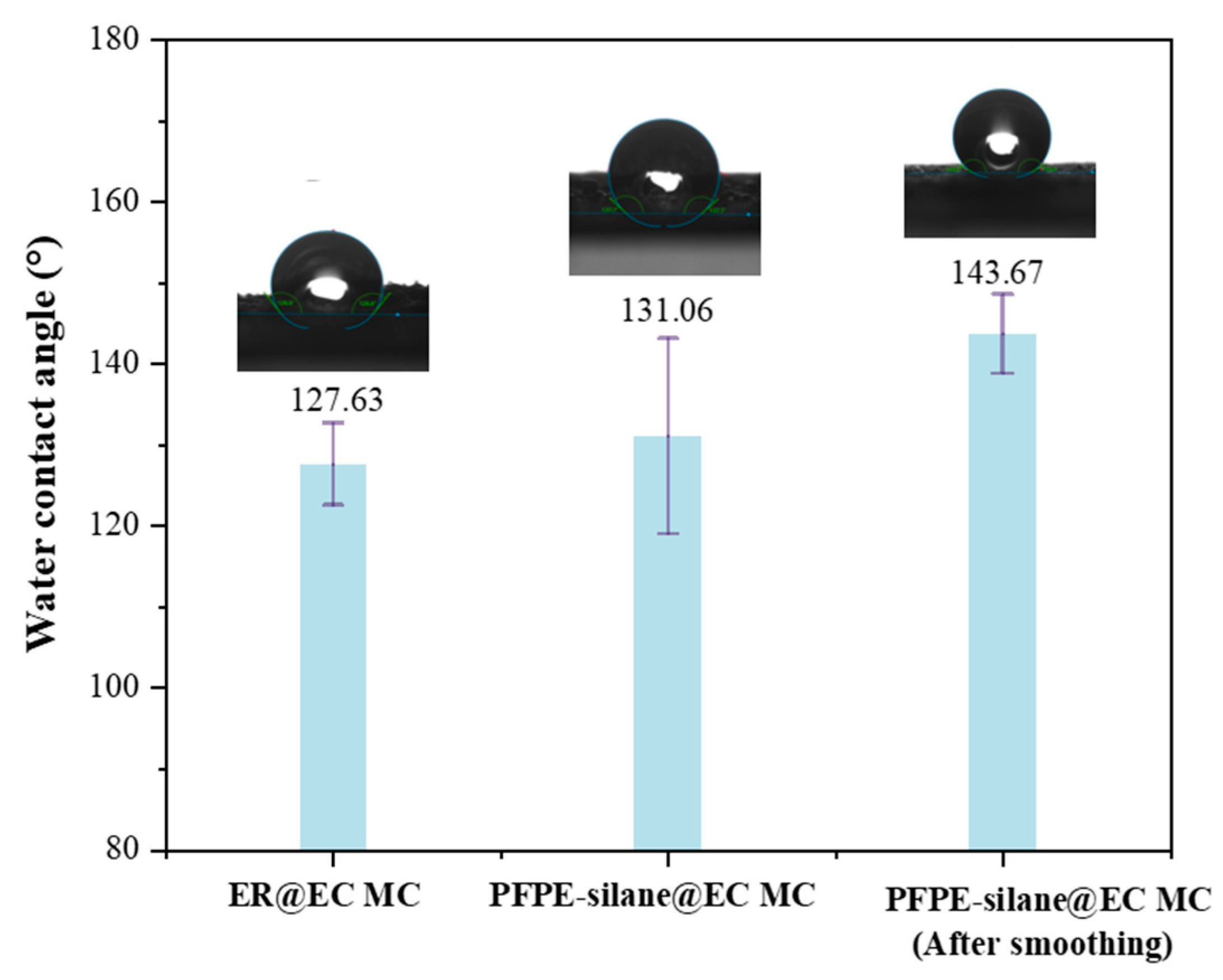
2.4.6. Contact Angle Measurements
3. Results and Discussion
3.1. Optimization of Preparation Process
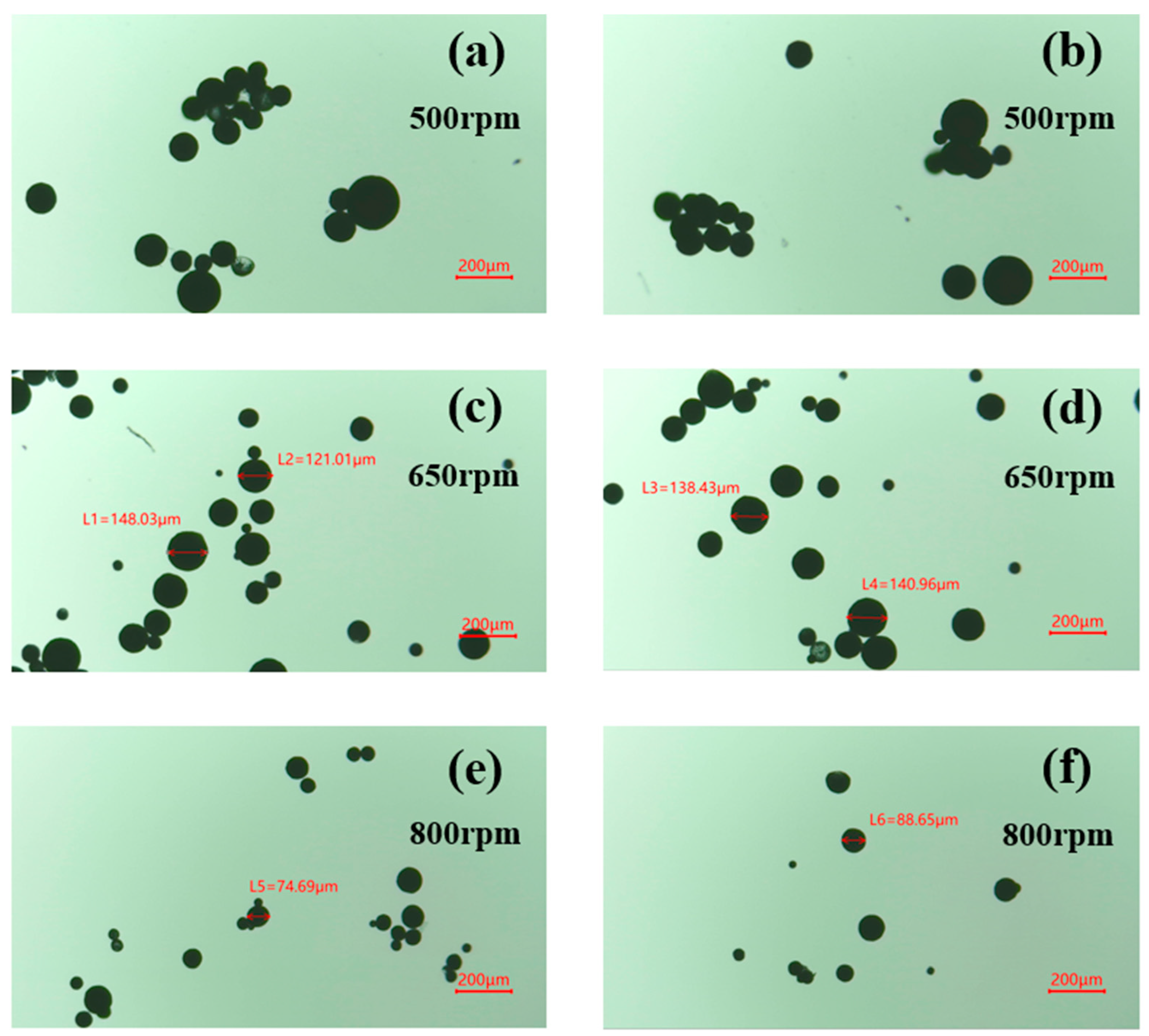
3.1.1. Stirring Rate
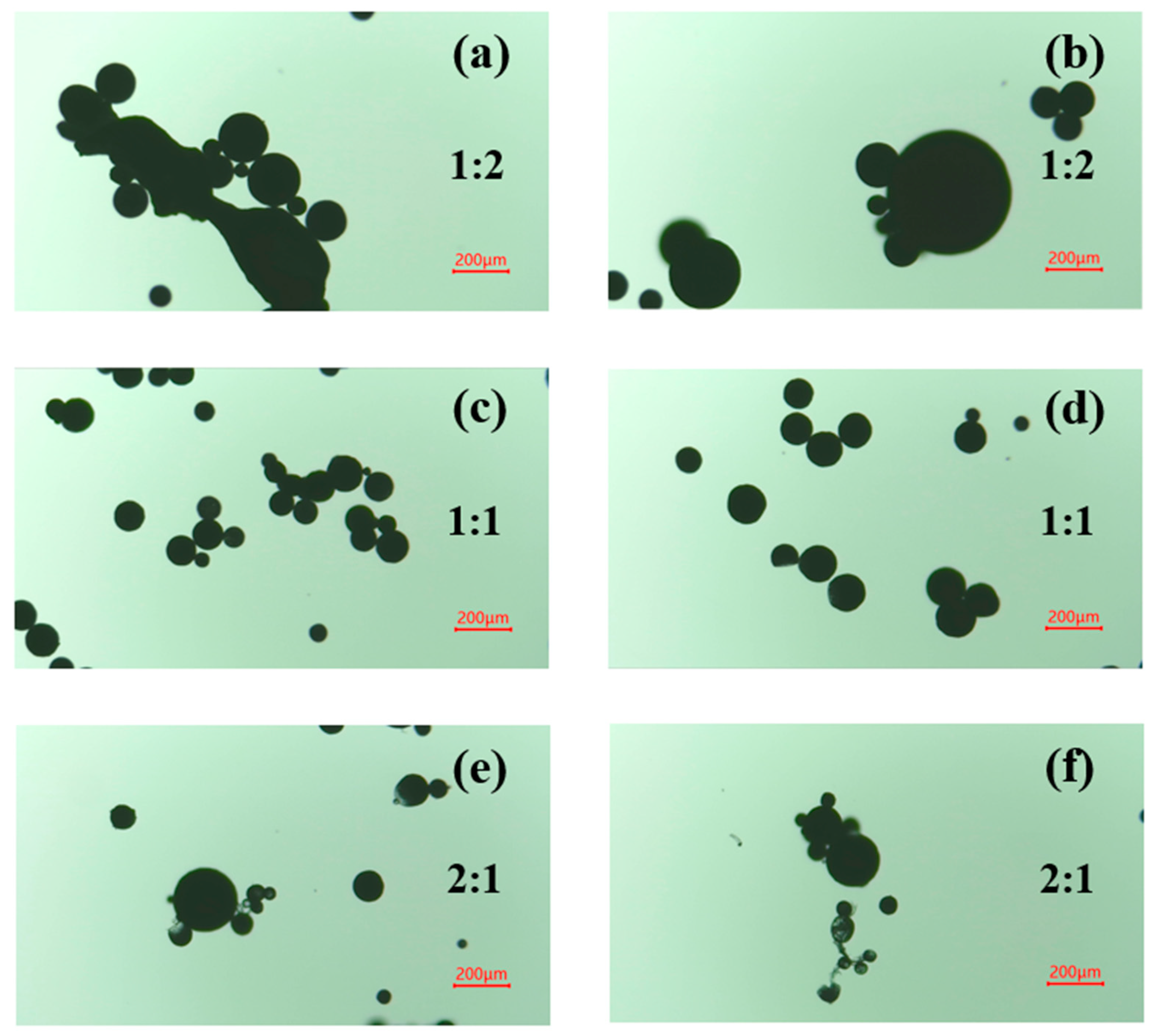
3.1.2. Core-to-Shell Ratio
3.2. Morphology and Structure
3.3. Size Distribution
3.4. Chemical Properties
3.5. Thermal Stability
3.6. Hydrophobicity of Microcapsules in Layers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, P.; Cheng, X.; Qian, J.; Zhang, R.; Cao, W.; Shah, S.P. Characteristics of surface-treatment of nano-SiO2 on the transport properties of hardened cement pastes with different water-to-cement ratios. Cem. Concr. Compos. 2015, 55, 26–33. [Google Scholar] [CrossRef]
- Xu, S.; Xie, N.; Cheng, X.; Huang, S.; Feng, L.; Hou, P.; Zhu, Y. Environmental resistance of cement concrete modified with low dosage nano particles. Constr. Build. Mater. 2018, 164, 535–553. [Google Scholar] [CrossRef]
- Wu, Y.; Dong, L.; Shu, X.; Yang, Y.; She, W.; Ran, Q. A review on recent advances in the fabrication and evaluation of superhydrophobic concrete. Compos. Part B Eng. 2022, 237, 109867. [Google Scholar] [CrossRef]
- Yao, H.; Xie, Z.; Huang, C.; Yuan, Q.; Yu, Z. Recent progress of hydrophobic cement-based materials: Preparation, characterization and properties. Constr. Build. Mater. 2021, 299, 124255. [Google Scholar] [CrossRef]
- Li, Y.; Gou, L.; Wang, H.; Wang, Y.; Zhang, J.; Li, N.; Hu, S.; Yang, J. Fluorine-free superhydrophobic carbon-based coatings on the concrete. Mater. Lett. 2019, 244, 31–34. [Google Scholar] [CrossRef]
- Tonelli, M.; Peppou-Chapman, S.; Ridi, F.; Neto, C. Effect of Pore Size, Lubricant Viscosity, and Distribution on the Slippery Properties of Infused Cement Surfaces. J. Phys. Chem. C 2019, 123, 2987–2995. [Google Scholar] [CrossRef]
- Zhang, H.; Mu, S.; Cai, J.; Ma, Q.; Hong, J.; Wang, J.; Du, F. Moisture diffusion in cement pastes with hydrophobic agent. Constr. Build. Mater. 2022, 319, 125596. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Zhang, C.; Zhang, X. Visualization and quantification of self-healing behaviors of microcracks in cement-based materials incorporating fluorescence-labeled self-healing microcapsules. Constr. Build. Mater. 2021, 315, 125668. [Google Scholar] [CrossRef]
- Du, J.; Ibaseta, N.; Guichardon, P. Characterization of polyurea microcapsules synthesized with an isocyanate of low toxicity and eco-friendly esters via microfluidics: Shape, shell thickness, morphology and encapsulation efficiency. Chem. Eng. Res. Des. 2022, 182, 256–272. [Google Scholar] [CrossRef]
- Hong, S.; Qin, S.; Dong, B.; Xing, F. Corrosion Features of the Reinforcing Bar in Concrete with Intelligent OH Regulation of Microcapsules. Materials 2019, 12, 3966. [Google Scholar] [CrossRef]
- Liu, S.L.; Chen, C.Y.; Chen, Y.S. Characteristic properties of spray-drying Bifidobacterium adolescentis microcapsules with biosurfactant. J. Biosci. Bioeng. 2022, 133, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.D.; Shen, X.C.; Tao, L. Modified emulsion solvent evaporation method for fabricating core-shell microspheres. Int. J. Pharm. 2013, 452, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Teo, A.; Lee, S.J.; Goh, K.K.; Wolber, F.M. Kinetic stability and cellular uptake of lutein in WPI-stabilised nanoemulsions and emulsions prepared by emulsification and solvent evaporation method. Food Chem. 2017, 221, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Sheen, Y.C.; Huang, Y.C.; Liao, C.S.; Chou, H.Y.; Chang, F.C. New approach to fabricate an extremely super-amphiphobic surface based on fluorinated silica nanoparticles. J. Polym. Sci. Part B Polym. Phys. 2010, 46, 1984–1990. [Google Scholar] [CrossRef]
- Cao, Y.; Salvini, A.; Camaiti, M. One-step Fabrication of Robust and Durable Superamphiphobic, Self-cleaning Surface for Outdoor and in Situ Application on Building Substrates. J. Colloid Interface Sci. 2021, 591, 239–252. [Google Scholar] [CrossRef]
- Phanthong, P.; Guan, G.; Karnjanakom, S.; Hao, X.; Wang, Z.; Kusakabe, K.; Abudula, A. Amphiphobic nanocellulose-modified paper: Fabrication and evaluation. RSC Adv. 2016, 6, 13328–13334. [Google Scholar] [CrossRef]
- Kim, J.; Cho, J.; Seidler, P.M.; Kurland, N.E.; Yadavalli, V.K. Investigations of chemical modifications of amino-terminated organic films on silicon substrates and controlled protein immobilization. Langmuir ACS J. Surf. Colloids 2010, 26, 2599–2608. [Google Scholar] [CrossRef]
- Pasternack, R.M.; Rivillon Amy, S.; Chabal, Y.J. Attachment of 3-(Aminopropyl)triethoxysilane on silicon oxide surfaces: Dependence on solution temperature. Langmuir ACS J. Surf. Colloids 2008, 24, 12963. [Google Scholar] [CrossRef]
- Qin, M.; Hou, S.; Wang, L.; Feng, X.; Wang, R.; Yang, Y.; Wang, C.; Yu, L.; Shao, B.; Qiao, M. Two methods for glass surface modification and their application in protein immobilization. Colloids Surf. B Biointerfaces 2007, 60, 243–249. [Google Scholar] [CrossRef]
- Roscioni, O.M.; Muccioli, L.; Mityashin, A.; Cornil, J.; Zannoni, C. Structural characterization of alkylsilane and fluoroalkylsilane self-assembled monolayers on SiO2 by molecular dynamics simulations. J. Phys. Chem. C 2016, 120, 14652–14662. [Google Scholar] [CrossRef]
- Kim, H.; Saha, J.K.; Zhang, Z.; Jang, J.; Matin, M.A.; Jang, J. Molecular Dynamics Study on the Self-Assembled Monolayer Grown from a Droplet of Alkanethiol. J. Phys. Chem. C 2014, 118, 11149–11157. [Google Scholar] [CrossRef]
- Ahn, Y.N.; Lee, S.H.; Oh, S.Y. Adsorption characteristics of silane-functionalized perfluoropolyether on hydroxylated glassy silica surfaces: A multiscale approach. Appl. Surf. Sci. 2019, 496, 143699. [Google Scholar] [CrossRef]
- Honda, K.; Morita, M.; Sakata, O.; Sasaki, S.; Takahara, A. Effect of Surface Molecular Aggregation State and Surface Molecular Motion on Wetting Behavior of Water on Poly (fluoroalkyl methacrylate) Thin Films. Trans. Mater. Res. Soc. Jpn. 2010, 43, 454–460. [Google Scholar] [CrossRef]
- Xu, N.; Song, Z.; Guo, M.Z.; Jiang, L.; Chu, H.; Pei, C.; Yu, P.; Liu, Q.; Li, Z. Employing ultrasonic wave as a novel trigger of microcapsule self-healing cementitious materials. Cem. Concr. Compos. 2021, 118, 103951. [Google Scholar] [CrossRef]
- Wang, X.; Xing, F.; Zhang, M.; Han, N.; Qian, Z. Experimental study on cementitious composites embedded with organic microcapsules. Materials 2013, 6, 4064–4081. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Fang, G.; Wang, Y.; Liu, Y.; Hong, S.; Zhang, J.; Lin, S.; Xing, F. Performance recovery concerning the permeability of concrete by means of a microcapsule based self-healing system. Cem. Concr. Compos. 2017, 78, 84–96. [Google Scholar] [CrossRef]
- Criado, M.; Sobrados, I.; Sanz, J. Polymerization of hybrid organic–inorganic materials from several silicon compounds followed by TGA/DTA, FTIR and NMR techniques. Prog. Org. Coat. 2014, 77, 880–891. [Google Scholar] [CrossRef]
- Saputra, R.E.; Astuti, Y.; Darmawan, A. Hydrophobicity of silica thin films: The deconvolution and interpretation by Fourier-transform infrared spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 199, 12–20. [Google Scholar] [CrossRef]
- Zhou, G.; Jiang, W.; Li, S.; Liu, R.; Zhang, Q.; Qi, G.; He, Z. Preparation and performance analysis of dopamine hydrochloride functionalized E-51@MPF/SiO2 double-wall microcapsules for microcracks self-healing in cement-based materials. Constr. Build. Mater. 2022, 325, 126622. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Zou, F.; Zhu, J. Self-healing microcapsules modified by montmorillonite for modulating slow-release properties. Mater. Chem. Phys. 2022, 291, 126688. [Google Scholar] [CrossRef]
- Es-Haghi, H.; Mirabedini, S.M.; Imani, M.; Farnood, R.R. Preparation and characterization of pre-silane modified ethyl cellulose-based microcapsules containing linseed oil. Colloids Surf. A Physicochem. Eng. Asp. 2014, 447, 71–80. [Google Scholar] [CrossRef]
- Aggour, Y.A. Thermal decomposition behaviour of ethyl cellulose grafted copolymers in homogeneous media. J. Mater. Sci. 2000, 35, 1623–1627. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Z.; Chen, R.; Huang, Z.; Gong, Y.; Zhao, H. Preparation and Characterization of Perfluoropolyether-Silane@Ethye Cellulose Polymeric Microcapsules. Polymers 2024, 16, 169. https://doi.org/10.3390/polym16020169
Song Z, Chen R, Huang Z, Gong Y, Zhao H. Preparation and Characterization of Perfluoropolyether-Silane@Ethye Cellulose Polymeric Microcapsules. Polymers. 2024; 16(2):169. https://doi.org/10.3390/polym16020169
Chicago/Turabian StyleSong, Zijian, Ruijie Chen, Zilang Huang, Yucheng Gong, and Haitao Zhao. 2024. "Preparation and Characterization of Perfluoropolyether-Silane@Ethye Cellulose Polymeric Microcapsules" Polymers 16, no. 2: 169. https://doi.org/10.3390/polym16020169
APA StyleSong, Z., Chen, R., Huang, Z., Gong, Y., & Zhao, H. (2024). Preparation and Characterization of Perfluoropolyether-Silane@Ethye Cellulose Polymeric Microcapsules. Polymers, 16(2), 169. https://doi.org/10.3390/polym16020169






