Performance of Recycled Opaque PET Modified by Reactive Extrusion
Abstract
1. Introduction
2. Experimental Details
2.1. Materials
2.2. Preparation of Formulations and Specimen Obtaining
2.3. Differential Scanning Calorimetry
2.4. Rheological Dynamic Analysis
2.5. Tensile Mechanical Properties
2.6. Impact Fracture Behavior
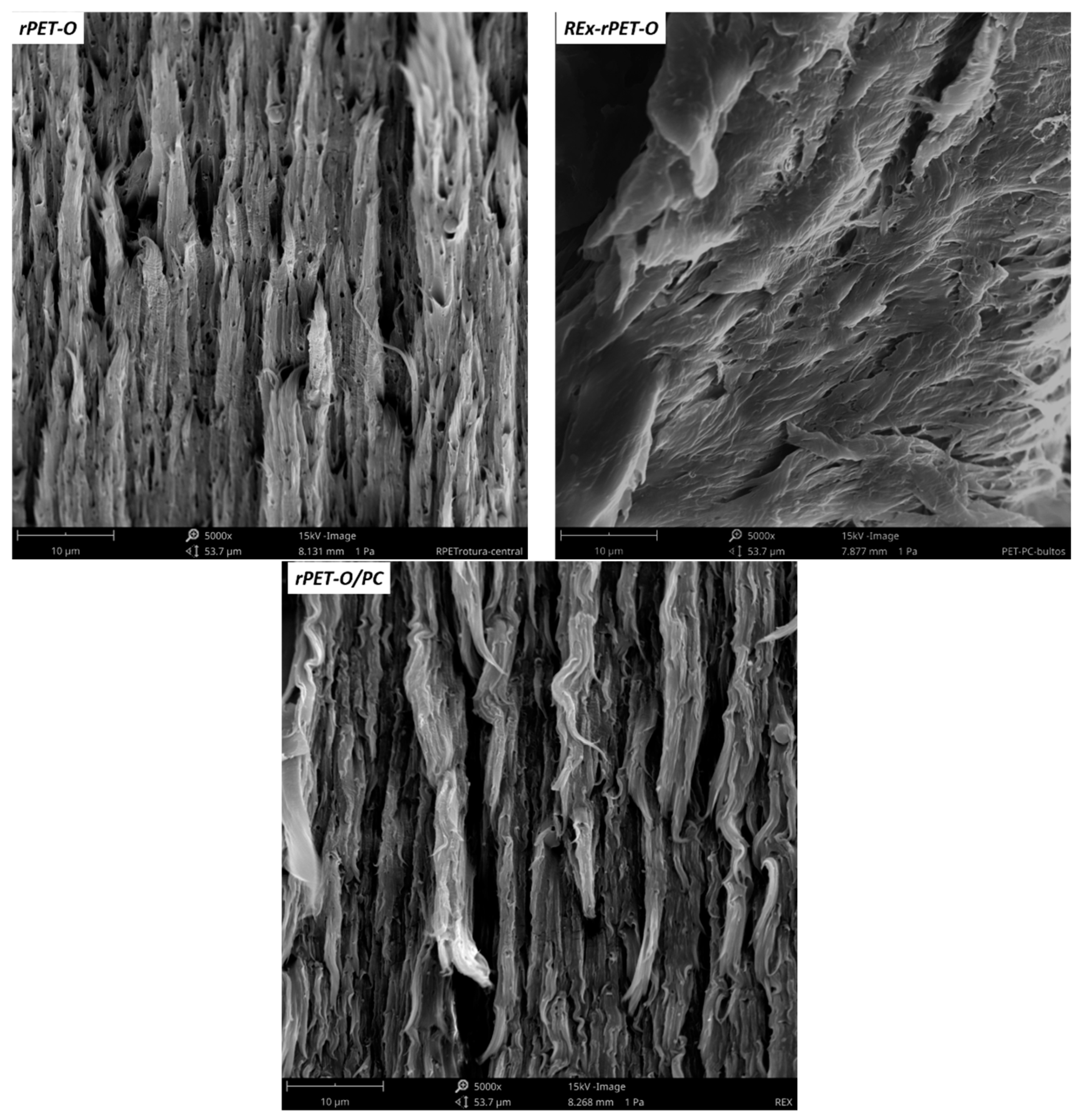
2.7. “Post-Mortem” Fractographic Analysis
3. Results and Discussion
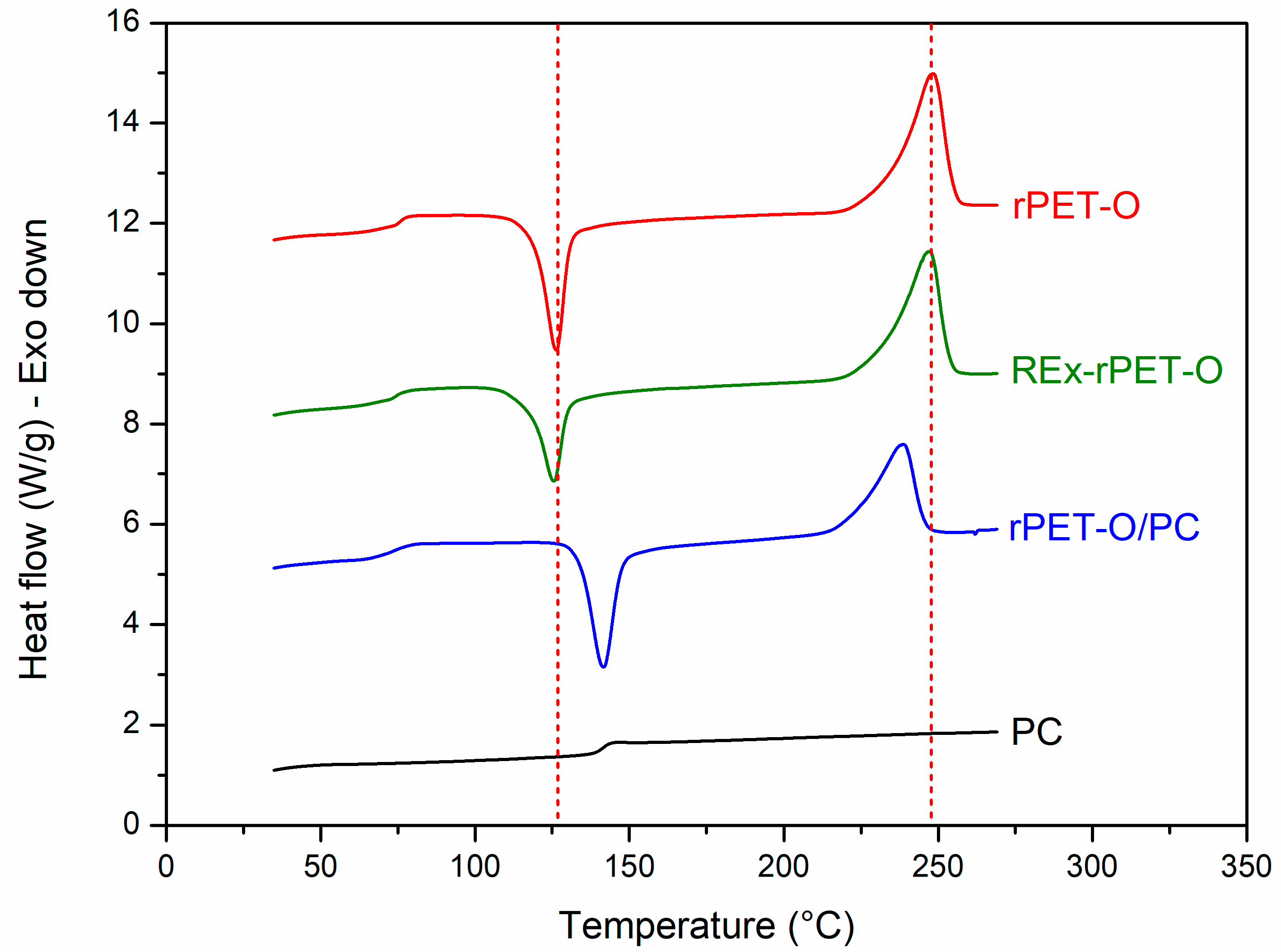
3.1. Verification of Structural Modifications
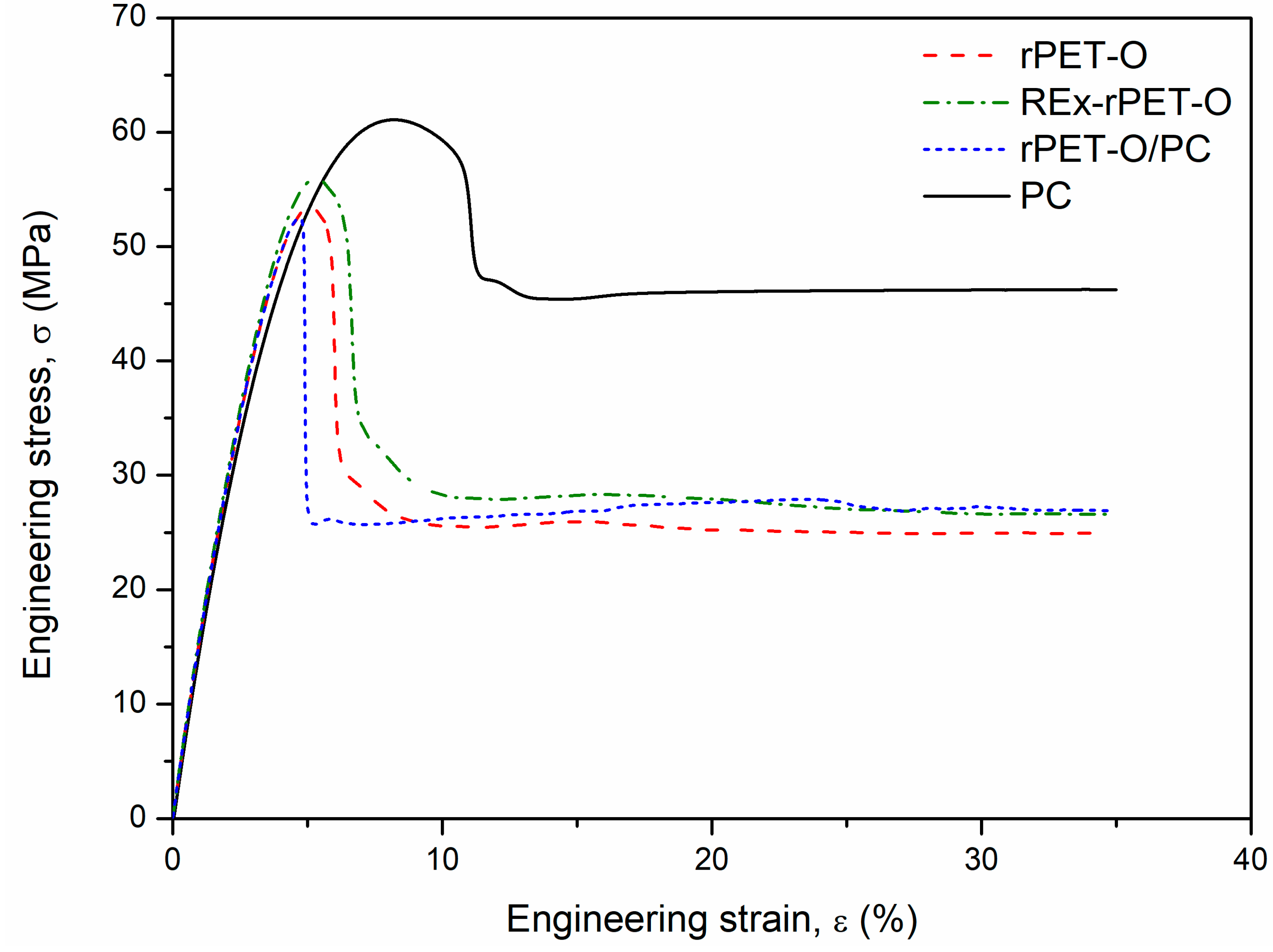
3.2. Tensile Mechanical Behavior
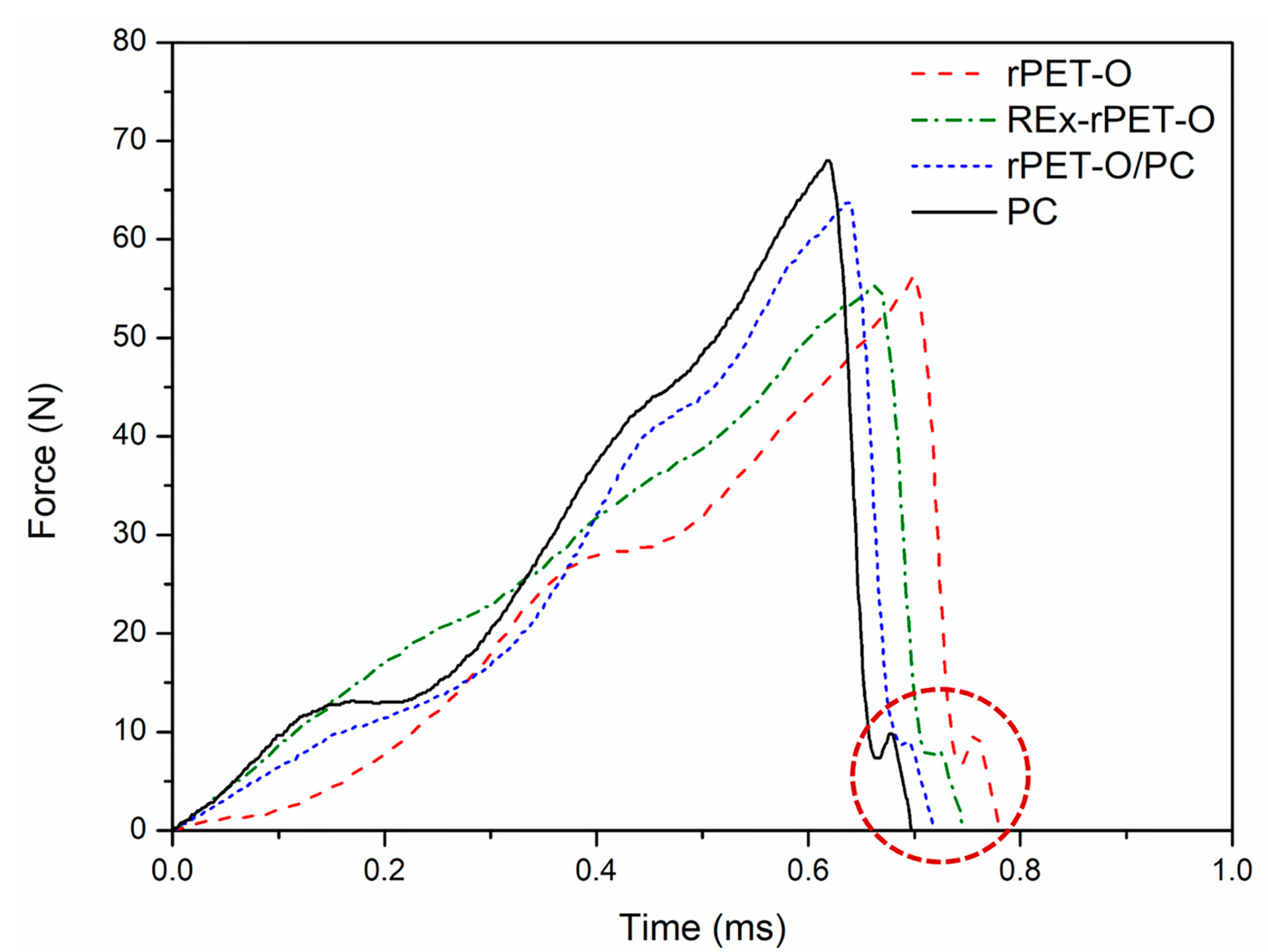
3.3. Fracture Behavior at High Strain Rate
- (a)
- The initial tear region (1) corresponds to the beginning of crack propagation within the previously formed craze and is caused by the decohesion of the fibrils of the active zone of the craze. In this case, the crack advancement speed (Vcrack) is lower than the longitudinal growth speed of craze formation (Vcraze);
- (b)
- The fast propagation region within the craze (2) occurs when the Vcrack will equate with the Vcraze, the rupture pivots between the upper and lower faces of the craze;
- (c)
- For the region of uncontrolled propagation (3), in this stage the Vcrack exceeds the Vcraze and this leads to the generation of the typical river pattern.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Welle, F. Twenty years of PET bottle to bottle recycling—An overview. Resour. Conserv. Recycl. 2011, 55, 865–875. [Google Scholar] [CrossRef]
- Karanan, A.D.; Özer, B.; Pascall, M.A.; Alvarez, V. Recent advances in dairy packaging. Food Rev. Int. 2015, 31, 295–318. [Google Scholar] [CrossRef]
- Mestdagh, F.; De Meulenaer, B.; De Clippeller, J.; Deulieghere, F.; Huyghebaert, A. Protective influence of several packaging materials on light oxidation of milk. J. Dairy Sci. 2005, 88, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Lago, E.D.; Boaretti, C.; Piovesan, F.; Ruso, M.; Lorenzetti, A.; Modesti, M. The effect of different compatibilizers on the properties of a post-industrial PC/PET blend. Materials 2019, 12, 49. [Google Scholar] [CrossRef]
- Candal, M.V.; Safari, M.; Fernández, M.; Otagi, I.; Múgica, A.; Zubitur, M.; Gerrica-echevarria, G.; Sebastián, V.; Irusta, S.; Laoeza, D.; et al. Structure and properties of reactively extruded opaque post-consumer recycled PET. Polymers 2021, 13, 3531. [Google Scholar] [CrossRef]
- Taniguchi, A.; Cakmak, M. The suppression of strain induced crystallization in PET through sub micron TiO2 particle incorporation. Polym. J. 2004, 45, 6647–6654. [Google Scholar] [CrossRef]
- Loaeza, D.; Cailloux, J.; Santana, O.O.; Sanchez-Soto, M.; Maspoch, M.L. Impact of titanium dioxide in the mechanical recycling of post-consumer polyethylene terephthalate bottle waste: Tensile and fracture behavior. Polymers 2021, 13, 310. [Google Scholar] [CrossRef]
- Kaseem, M.; Deri, F.; Hamad, K. Recycling of waste from polymer materials: An overview of the recent works. Polym. Degrad. Stabil. 2013, 98, 2801–2812. [Google Scholar]
- Akkapeddi, M.K.; Van Buskirk, B.; Mason, C.D.; Chung, S.S.; Swamikann, X. Performance blends based on recycled polymer. Polym. Eng. Sci. 1995, 35, 72–78. [Google Scholar]
- Fraïsse, F.; Verney, V.; Commerevc, S.; Obadal, M. Recycling of poly(ethylene terephthalate/polycarbonate blends. Polym. Degrad. Stab. 2005, 90, 250–255. [Google Scholar] [CrossRef]
- Lotfi, M. Optimization of catalyst content for recycled polyethylene terephthalate (PET) and polycarbonate (PC) blending. Polym. Bull. 2023, 80, 12319–12331. [Google Scholar] [CrossRef]
- Sanchez, J.J.; Santana, O.O.; Gordillo, A.; Maspoch, M.L.; Martínez, A.B. Essential work of fracture of injection moulded samples of PET and PET/PC blends. Eur. Struct. Integr. Soc. (ESIS) 2003, 32, 77–88. [Google Scholar]
- Sanchez, J.J. Comportamiento Térmico y Mecánico del Poli (Etilén Tereftalato) (PET) Modificado con Resinas Poliméricas Basadas en Bisfenol-A. Ph.D. Thesis, Universitat Politècnica de Catalunya, Barcelona, Spain, 2003. [Google Scholar]
- Carrot, C.; Mbarek, S.; Jaziri, M.; Chalamet, Y.; Raveyre, C.; Prochazka, F. Inmiscible blends of PC and PET, current knowledge and new results: Rheological properties. Macromol. Mater. Eng. 2007, 292, 693–706. [Google Scholar] [CrossRef]
- Duarte, I.S.; Tavares, A.A.; Lima, P.S.; Andrade, D.L.A.C.S.; Carvalho, L.H.; Canedo, E.L. Chain extension of virgin and recycled poly(ethylene terephthalate): Effect of processing conditions and reprocessing. Polym. Degrad. Stab. 2016, 124, 26–34. [Google Scholar] [CrossRef]
- Mestry, J.; Abdelwahab, M.A.; Elkholy, H.M.; Rabnawaz, M. Superior glycidol-free chain extenders post-consumer PET bottles and PET thermoform blends. Resourc. Conserv. Recycl. 2024, 203, 107420. [Google Scholar] [CrossRef]
- Jang, J.Y.; Sadeghi, K.; Seo, J. Chain-extending modification for value-added recycled PET: A review. Polym. Rev. 2022, 62, 860–879. [Google Scholar] [CrossRef]
- Nofar, M.; Oguz, H. Development of PBT/Recycled-PET blends and the influence of using chain extender. J. Polym. Envirom. 2019, 27, 1404–1417. [Google Scholar] [CrossRef]
- Guclu, M.; Göksu, Y.A.; Özdemir, B.; Ghanbari, A.; Nofar, M. Thermal stabilization of recycled PET through chain extension and blending with PBT. J. Polym. Environ. 2022, 30, 719–727. [Google Scholar] [CrossRef]
- Kruse, M.; Wagner, M.H. Rheological and molecular characterization of long-chain branched Poly(Ethylene Terephthalate). Rheol. Acta 2017, 56, 887–904. [Google Scholar] [CrossRef]
- Kruse, M.; Wang, P.; Shah, R.S.; Wagner, M.H. Analysis of high melt-strength Poly(Ethylene Terephthalate) produced by reactive processing by shear and elongational rheology. J. Polym. Eng. Sci. 2019, 59, 396–410. [Google Scholar] [CrossRef]
- Qin, D.; Wang, C.; Wang, H.; Jian, X. Chain extension and thermal behavior of recycled Poly(Ethylene Terephthalate) modified by reactive extrusion with Triphenyl Phosphite. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2016; Volume 67, pp. 06052–06057. [Google Scholar]
- Vozniak, A.; Hosseinnezhad, R.; Vozniak, I.; Galeski, A. PET mechanical recycling. A new principle for chain extender introduction. Sustain. Mater. Technol. 2024, 40, e00886. [Google Scholar]
- Odet, F.; Ylla, N.; Delage, K.; Cassagnau, P. Influence of chain extenders on recycled standard and Opaque PET rheology and melt-spun filament properties. ACS Appl. Polym. Mater. 2022, 4, 8290–8302. [Google Scholar] [CrossRef]
- Standau, T.; Nofar, M.; Dörr, D.; Ruckdäschel, H.; Altstädt, V. A review of multifunctional epoxy-based Joncryl® ADR chain extended thermoplastics. Polym. Rev. 2021, 62, 296–350. [Google Scholar] [CrossRef]
- Härth, M.; Dörnhöfer, A. Film blowing of linear and long-chain branched Poly(Ethylene-Terephthalate). Polymers 2020, 12, 1605. [Google Scholar] [CrossRef]
- Härth, M.; Dörnhöfer, A.; Kaschta, J.; Münstedt, H.; Schubert, D.W. Molecular structure and rheological properties of a Poly(Ethylene Terephthalate) modified by two different chain extenders. J. Appl. Polym. Sci. 2021, 138, 50110. [Google Scholar] [CrossRef]
- Bhander, K.K.; Joshi, J.R.; Patel, J.V. Recycling of polyethylene terephthalate (PET or PETE) plastics—An alternative to obtain value added products: A review. J. Indian Chem. 2023, 100, 100843. [Google Scholar] [CrossRef]
- Joseph, T.M.; Azat, S.; Ahmadi, Z.; Jazani, O.M.; Esmaeili, A.; Kianfar, E.; Hapomiuk, J.; Thomas, S. Polyethylene therephthalate (PET) recycling: A review. Case Stud. Chem. Environ. Eng. 2024, 9, 100673. [Google Scholar] [CrossRef]
- Babaei, M.; Jalilian, M.; Shahbaz, K. Chemical recycling of polyethylene terephthalate: A mini-review. J. Environ. Chem. Eng. 2024, 12, 112507. [Google Scholar] [CrossRef]
- Bohre, A.; Jadhao, P.R.; Tripathi, K.; Pant, K.K.; Lilozar, B.; Saha, B. Chemical recycling processes of waste polyethylene terephthalate using solid catalysts. ChemSusChem 2023, 16, e202300142. [Google Scholar] [CrossRef]
- Kirshanov, K.; Toms, R.; Melnikou, P.; Gervald, A. Unsaturaded polyester resin nanocomposites based on post-consumer polyethylene. Polymers 2022, 14, 1602. [Google Scholar] [CrossRef]
- Kirshanov, K.A.; Gervald, A.Y.; Toms, R.V.; Lobanov, A.N. Obtaining phthalate substituted post-consumer polyethylene terephthalate and its isothermal crystallization. Fine Chem. Tech. 2022, 17, 164–171. [Google Scholar] [CrossRef]
- Martínez, A.B.; Salazar, A.; León, N.; Illescas, S.; Rodríguez, J. Influence of the notch sharpening technique on styrene-acrylonitrile fracture behavior. J. Appl. Polym. Sci. 2016, 133, 43775. [Google Scholar] [CrossRef]
- Martínez, A.B.; León, N.; Segovia, A.; Cailloux, J.; Martínez, P.P. Effect of specimen notch quality on the essential work of fracture of ductile polymer films. Eng. Fract. Mech. 2017, 180, 296–314. [Google Scholar] [CrossRef]
- León, N.; Martínez, A.B.; Castejón, P.; Arencón, D.; Martínez, P.P. The fracture testing of ductile polymer films: Effect of the specimen notching. Polym. Test. 2017, 63, 180–193. [Google Scholar] [CrossRef]
- Moreno, P.; Méndez, C.; García, A.; Arias, I.; Roso, L. Femtosecond laser ablation of carbon reinforced polymers. Appl. Surf. Sci. 2006, 252, 4110–4119. [Google Scholar] [CrossRef]
- León, N.; Martínez, A.B.; Castejón, P.; Martínez, P.P.; Arencón, D. Notch effect on the fracture of a polymeric film. Theor. Appl. Fract. Mec. 2018, 95, 270–282. [Google Scholar] [CrossRef]
- León, N.; Martínez, A.B.; Maspoch, M. Notch effect on the linear elastic fracture mechanics values of a polysulfone thermoplastic polymer. Theor. Appl. Fract. Mech. 2021, 114, 102995. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Karayannidis, G.P. Chain extension of polyesters PET and PBT with two new diimidodiepoxides. J. Polym. Sci. Pol. Chem. 1996, 34, 1337–1342. [Google Scholar] [CrossRef]
- Yuan-Hsiang, W.; Cheng-Chien, W.; Chuh-Yung, C. Nucleation effect of aliphatic polycarbonate in its blends with poly(ethylene terephthalate). Mater. Chem. Phys. 2020, 248, 122920. [Google Scholar]
- Meziane, O.; Bensedira, A.R.; Guessoum, M. Morphological and thermal characterization of an immiscible catalyzed polymer blends (PC/PET). Polym. Polym. Compos. 2021, 29, S937–S948. [Google Scholar] [CrossRef]
- Srithep, Y.; Pholharn, D.; Dassakorn, A.; Morris, J. Effect of chain extenders on mechanical and thermal properties of recycled poly(ethylene terephthalate) and polycarbonate blends. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 213, p. 012008. [Google Scholar]
- Liu, J.; Zhao, X.; Ye, L. Compatibility and toughening mechanism of poly(ethylene terephthalate)/polycarbonate blends. Polym. Int. 2020, 69, 1297–1307. [Google Scholar] [CrossRef]
- Karsli, N.G.; Yilmaz, T. From polymeric waste to potential industrial product: Modification of recycled polycarbonate. J. Elastom. Plast. 2022, 54, 857–876. [Google Scholar] [CrossRef]



| Material | Temperature Profile [C] | Screw-Rotation Speed [rpm] |
|---|---|---|
| Rex-rPET-O | 180/215/235/240/240/245/245 | 40 |
| rPET-O/PC | 180/215/235/260/260/270/270 | 125 |
| Material | Temperature Profile [C] | Inj. Velocity [cm3/s] | Injection Pressure [bar] | Holding Pressure [bar] |
|---|---|---|---|---|
| Rex-rPET-O | 265/270/270/270/260 | 60 | 780 | 650 |
| rPET-O/PC | 295/290/285/280/260 | 60 | 520 | 450 |
| Material | Tcc [C] | Tm [C] | Xc-m [%] | Xc [%] |
|---|---|---|---|---|
| rPET-O | 126.2 | 248.2 | 32 | 17 |
| Rex-rPET-O | 123.7 | 246.8 | 29 | 18 |
| rPET-O/PC | 141.6 | 238.9 | 19 | 4 |
| Material | E [GPa] | σy [MPa] | εy [%] | σf [MPa] | εb [%] |
|---|---|---|---|---|---|
| rPET-O | 2.28 ± 0.06 | 54.9 ± 0.9 | 3.9 ± 0.1 | 25.9 ± 0.8 | 93.9 ± 28.5 |
| Rex-rPET-O | 2.42 ± 0.2 | 56.1 ± 0.4 | 3.8 ± 0.1 | 26.8 ± 0.4 | 95.3 ± 13.6 |
| rPET-O/PC | 2.30 ± 0.04 | 51.5 ± 1.1 | 3.3 ± 0.1 | 26.4 ± 0.6 | 245.1 ± 11.1 |
| PC | 2.29 ± 0.02 | 61.2 ± 0.5 | 5.7 ± 0.01 | 46.4 ± 0.3 | 91.9 ± 11.3 |
| Material | KQ [MPa.m]1/2 | wf [kJ/m2] |
|---|---|---|
| rPET-O | 1.27 ± 0.15 | 0.46 ± 0.24 |
| Rex-rPET-O | 1.47 ± 0.20 | 0.51 ± 0.17 |
| rPET-O/PC | 1.52 ± 0.14 | 0.74 ± 0.20 |
| PC | 1.55 ± 0.12 | 0.61 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
León-Albiter, N.; Santana, O.O.; Martinez Orozco, L.; Candau, N.; Maspoch, M.L. Performance of Recycled Opaque PET Modified by Reactive Extrusion. Polymers 2024, 16, 2843. https://doi.org/10.3390/polym16192843
León-Albiter N, Santana OO, Martinez Orozco L, Candau N, Maspoch ML. Performance of Recycled Opaque PET Modified by Reactive Extrusion. Polymers. 2024; 16(19):2843. https://doi.org/10.3390/polym16192843
Chicago/Turabian StyleLeón-Albiter, Noel, Orlando O. Santana, Leandro Martinez Orozco, Nicolas Candau, and Maria Lluïsa Maspoch. 2024. "Performance of Recycled Opaque PET Modified by Reactive Extrusion" Polymers 16, no. 19: 2843. https://doi.org/10.3390/polym16192843
APA StyleLeón-Albiter, N., Santana, O. O., Martinez Orozco, L., Candau, N., & Maspoch, M. L. (2024). Performance of Recycled Opaque PET Modified by Reactive Extrusion. Polymers, 16(19), 2843. https://doi.org/10.3390/polym16192843








