Organic–Inorganic Hybrid Nanoparticles for Enhancing Adhesion of 2K Polyurethane to Steel and Their Performance Optimization Using Response Surface Methodology
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of AFAP
2.3. Synthesis of O-I Hybrid NPs Dispersed in BG
2.4. Preparation of SB/EB PUs Containing O-I Hybrid NPs
2.5. Multiparameter Experimental Design Model
2.6. Characteristics
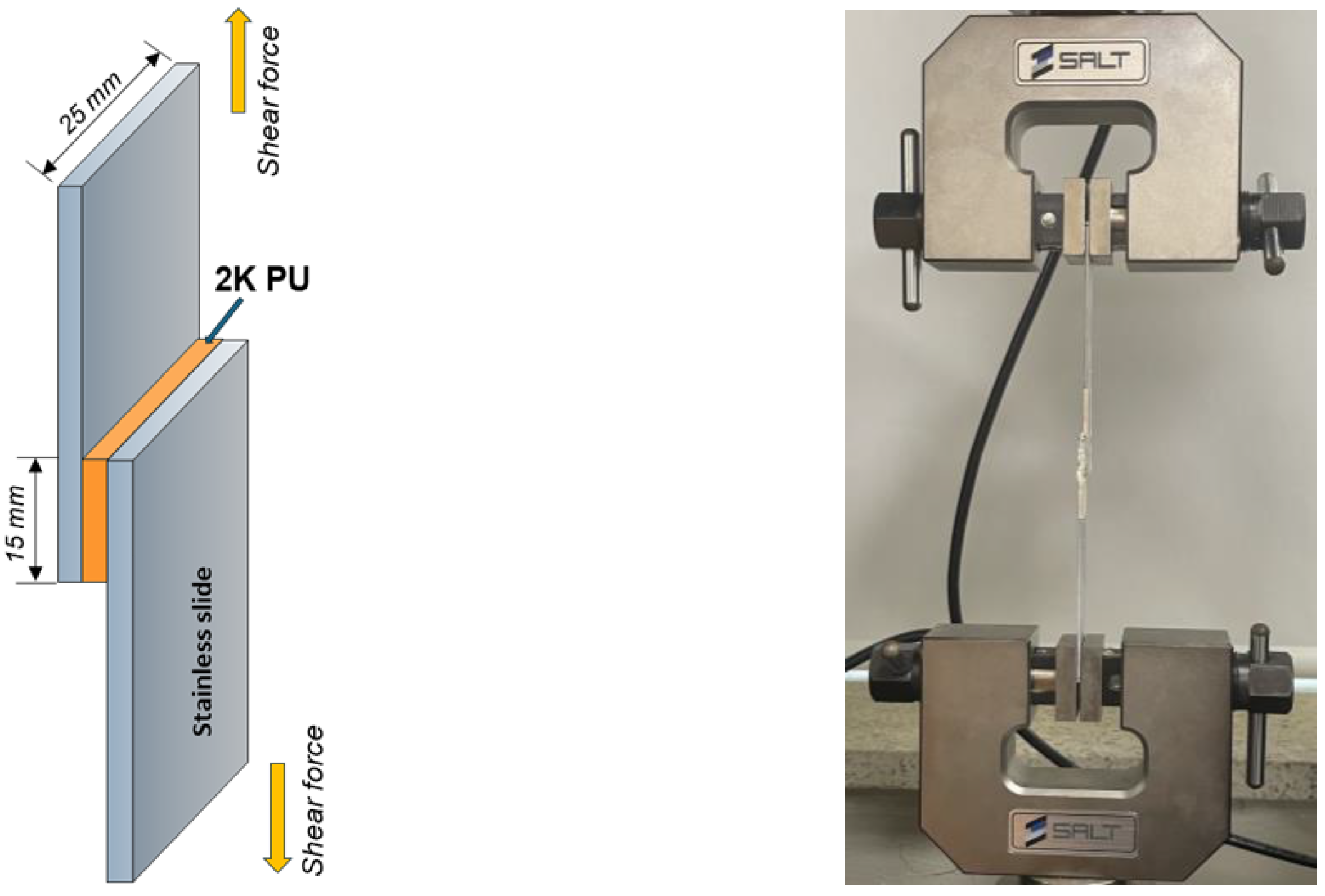
2.7. Adhesion Strength Test
3. Results and Discussion
3.1. Characteristic of O-I Hybrid NPs
3.2. Adhesion Performance
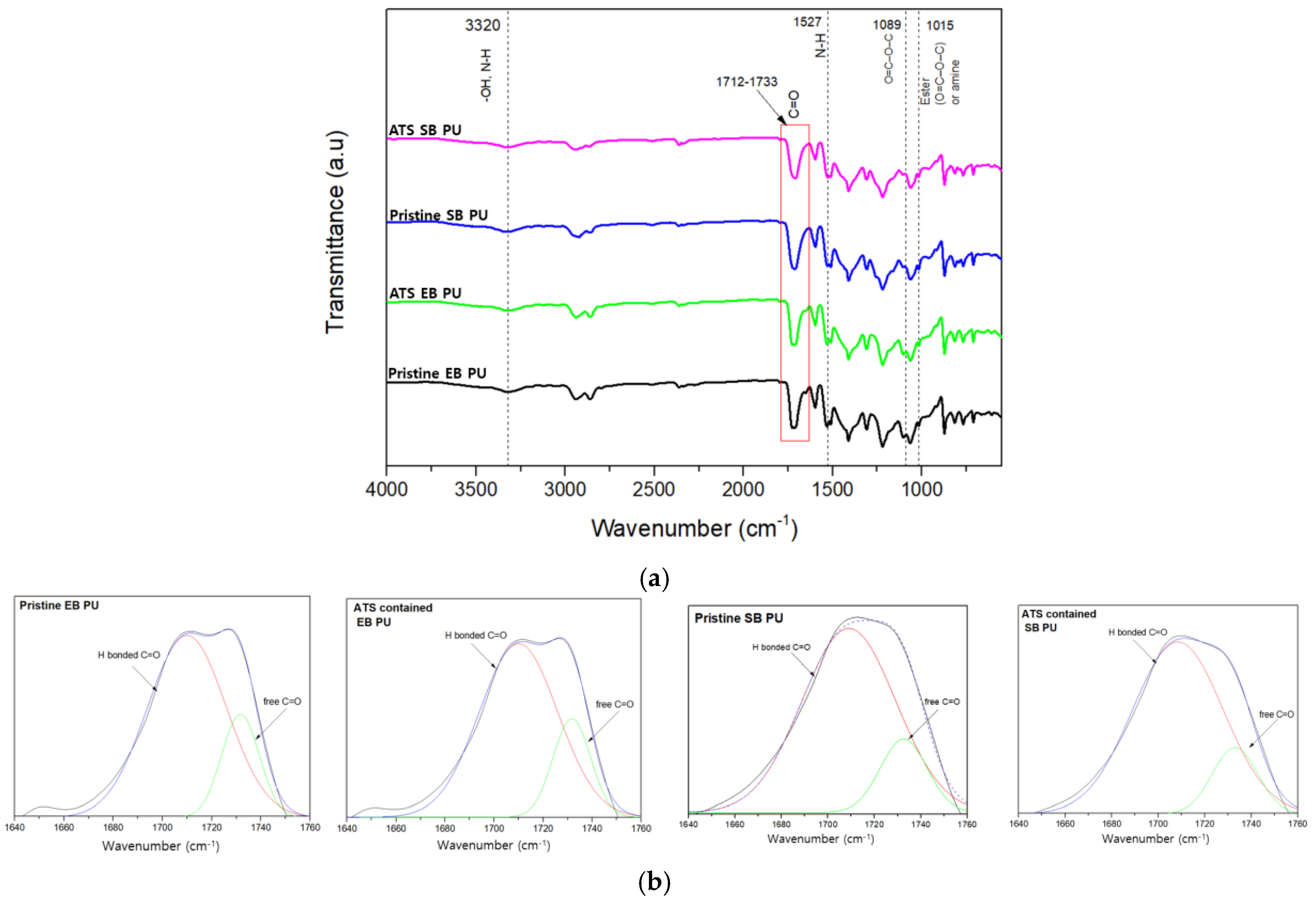
3.3. FT-IR
3.4. Morphology SB PU containing ATS
3.5. Optimization Adhesion Strength
3.5.1. Development of Regression Model Equation and Optimization
−5.69973 + 0.113088 × AFAP + 1.3691 × APTES + 0.158998 × BG + 0.349081 × Mixing mass ratio
+ 0.062894 × AFAP × APTES + 0.007095 × AFAP × BG + 0.011137 × APTES × BG − 0.008235 × AFAP × Mixing mass ratio + 0.018290 × APTES × Mixing mass ratio − 0.000388 × BG × Mixing mass ratio
−0.115125 × AFAP2 − 0.104397 × APTES2 − 0.004112 × BG2 − 0.07092 × [Mixing mass ratio]2
3.5.2. Three-Dimensional Surface Plots and the Effects of Variables on the Adhesion Strength
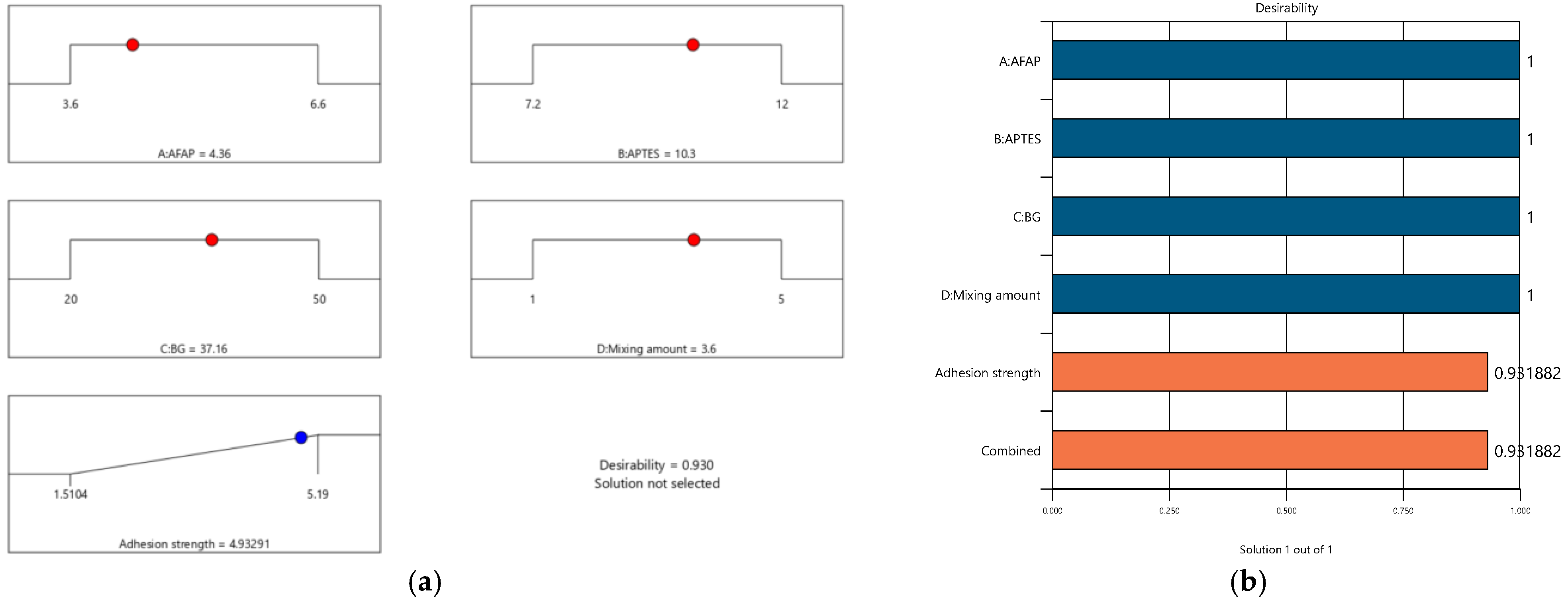
3.5.3. Confirmation Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Golling, F.E.; Pires, R.; Hecking, A.; Weikard, J.; Richter, F.; Danielmeier, K.; Dijkstra, D. Polyurethanes for coatings and adhesives—Chemistry and applications. Polym. Int. 2019, 68, 848–855. [Google Scholar] [CrossRef]
- Paulina, K.; Adam, O.; Nowak, P.; Łukasz, P. Polyurethanes for Coating, Adhesives, and Other Applications. In Polyurethane Chemistry: Renewable Polyols and Isocyanates; American Chemical Society: Washington, DC, USA, 2021; pp. 305–326. [Google Scholar] [CrossRef]
- Le, M.L.; Jung, M.C.; Kim, H.W.; Kim, J. Preparation of organic-inorganic hybrid adhesion promoters for polyurethane-metal adhesion and their properties. Compos. Interfaces 2022, 29, 1619–1635. [Google Scholar] [CrossRef]
- Somarathna, H.M.C.C.; Raman, S.N.; Mohotti, D.; Mutalib, A.A.; Badri, K.H. The use of polyurethane for structural and infrastructural engineering applications: A state-of-the-art review. Constr. Build. Mater. 2018, 190, 995–1014. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, J. Advanced lightweight materials for Automobiles: A review. Mater. Des. 2022, 221, 110994. [Google Scholar] [CrossRef]
- Marques, A.C.; Mocanu, A.; Tomić, N.Z.; Balos, S.; Stammen, E.; Lundevall, A.; Abrahami, S.T.; Günther, R.; de Kok, J.M.M.; de Freitas, S.T. Review on Adhesives and Surface Treatments for Structural Applications: Recent Developments on Sustainability and Implementation for Metal and Composite Substrates. Materials 2020, 13, 5590. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, K.; Łopiński, J.; Kowalczyk, A. Preparation and characterisation of montmorillonite-ammonium silane surface layers promoting adhesion between steel and a polyurethane adhesive. Int. J. Adhes. Adhes. 2018, 82, 153–159. [Google Scholar] [CrossRef]
- Meier, T.; Choffat, F.; Montalbano, A. Toughened 2K-epoxy adhesives: Structural strengthening of steel structures. In Proceedings of the IABSE Congress: Resilient Technologies for Sustainable Infrastructure, Christchurch, New Zealand, 3–5 February 2021; pp. 898–902. [Google Scholar] [CrossRef]
- Rohart, V.; Lebel, L.L.; Dubé, M. Improved adhesion between stainless steel heating element and PPS polymer in resistance welding of thermoplastic composites. Compos. B Eng. 2020, 188, 107876. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Bertels, E.; Goderis, B.; Smet, M.; Van Hemelrijck, D.; Van Mele, B. Optimisation of wet chemical silane deposition to improve the interfacial strength of stainless steel/epoxy. Appl. Surf. Sci. 2015, 324, 134–142. [Google Scholar] [CrossRef]
- GBox, E.P.; Wilson, K.B. On the Experimental Attainment of Optimum Conditions. J. R. Stat. Soc. Ser. B Stat. Methodol. 1951, 13, 1–38. [Google Scholar] [CrossRef]
- Kim, N.; Li, X.; Kim, S.H.; Kim, J. Colloidally stable organic–inorganic hybrid nanoparticles prepared using alkoxysilane-functionalized amphiphilic polymer precursors and mechanical properties of their cured coating film. J. Ind. Eng. Chem. 2018, 68, 209–219. [Google Scholar] [CrossRef]
- Kim, J.; Wainaina, J.; Na, J.S. Synthesis of amphiphilic silica/polymer composite nanoparticles as water-dispersible nano-absorbent for hydrophobic pollutants. J. Ind. Eng. Chem. 2011, 17, 681–690. [Google Scholar] [CrossRef]
- Cho, S.; Kim, N.; Lee, S.; Lee, H.; Lee, S.-H.; Kim, J.; Choi, J.-W. Use of hybrid composite particles prepared using alkoxysilane-functionalized amphiphilic polymer precursors for simultaneous removal of various pollutants from water. Chemosphere 2016, 156, 302–311. [Google Scholar] [CrossRef]
- Atiqah, A.; Mastura, M.; Ali, B.; Jawaid, M.; Sapuan, S. A Review on Polyurethane and its Polymer Composites. Curr. Org. Synth. 2017, 14, 233–248. [Google Scholar] [CrossRef]
- Beaud, F.; Niemz, P.; Pizzi, A. Structure–property relationships in one-component polyurethane adhesives for wood: Sensitivity to low moisture content. J. Appl. Polym. Sci. 2006, 101, 4181–4192. [Google Scholar] [CrossRef]
- Santamaria-Echart, A.; Fernandes, I.; Barreiro, F.; Corcuera, M.A.; Eceiza, A. Advances in Waterborne Polyurethane and Polyurethane-Urea Dispersions and Their Eco-friendly Derivatives: A Review. Polymers 2021, 13, 409. [Google Scholar] [CrossRef]
- Shokrolahi, F.; Yeganeh, H. Soft segment composition and its influence on phase-separated morphology of PCL/PEG-based poly(urethane urea)s. Iran. Polym. J. 2014, 23, 505–512. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, K.; Li, Z.; Wu, Y.; Zhu, B. Hydrogen-bonded supramolecular adhesives: Synthesis, responsiveness, and application. Supramol. Mater. 2023, 2, 100032. [Google Scholar] [CrossRef]
- Nakamura, S.; Tsuji, Y.; Yoshizawa, K. Role of Hydrogen-Bonding and OH−π Interactions in the Adhesion of Epoxy Resin on Hydrophilic Surfaces. ACS Omega 2020, 5, 26211–26219. [Google Scholar] [CrossRef]
- Fu, B.X.; Hsiao, B.S.; White, H.; Rafailovich, M.; Mather, P.T.; Jeon, H.G.; Phillips, S.; Lichtenhan, J.; Schwab, J. Nanoscale reinforcement of polyhedral oligomeric silsesquioxane (POSS) in polyurethane elastomer. Polym. Int. 2000, 49, 437–440. [Google Scholar] [CrossRef]
- Smith, M.E.; Holland, D. Atomic-Scale Structure of Gel Materials by Solid-State NMR. In Handbook of Sol-Gel Science and Technology; Springer International Publishing: Cham, Switzerland, 2018; pp. 1281–1322. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, T.-H.; Shim, K.-S.; Park, J.-W.; Kim, H.-J.; Kim, Y.; Jung, S. Effect of crosslinking density on adhesion performance and flexibility properties of acrylic pressure sensitive adhesives for flexible display applications. Int. J. Adhes. Adhes. 2017, 74, 137–143. [Google Scholar] [CrossRef]
- Semsarzadeh, M.A.; Navarchian, A.H. Effects of NCO/OH ratio and catalyst concentration on structure, thermal stability, and crosslink density of poly(urethane-isocyanurate). J. Appl. Polym. Sci. 2003, 90, 963–972. [Google Scholar] [CrossRef]
- Poh, A.K.; Sin, L.C.; Foon, C.S.; Hock, C.C. Polyurethane wood adhesive from palm oil-based polyester polyol. J. Adhes. Sci. Technol. 2014, 28, 1020–1033. [Google Scholar] [CrossRef]
- Ti, Y.; Wen, Q.; Chen, D. Characterization of the hydrogen bond in polyurethane/attapulgite nanocomposites. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Infrared and Raman Characteristic Group Frequencies: Tables and Charts. 3rd ed By George Socrates (The University of West London, Middlesex, U.K.). J. Wiley and Sons: Chichester. 2001. xviii + 348 pp. $185.00. ISBN: 0-471-85298-8. J. Am. Chem. Soc. 2002, 124, 1830. [CrossRef]
- Painter, P.C.; Coleman, M.M. Fundamentals of Polymer Science; Routledge: London, UK, 2019. [Google Scholar] [CrossRef]











| Solution | Ingredients | |||
|---|---|---|---|---|
| AFAP | APTES | Ethanol | HCl (0.1 M) | |
| ATS 1-1 | 5 g | 5 g | 40 g | 1.54 g |
| ATS 1-2 | 5 g | 10 g | 40 g | 2.77 g |
| ATS 2-1 | 5 g | 2.5 g | 40 g | 0.93 g |
| No | Sample | Ingredients | |
|---|---|---|---|
| ATS | BG | ||
| 1 | ATS (1-1)–BG (30) | 50 g | 30 g |
| 2 | ATS (2-1)–BG (30) | 50 g | 30 g |
| 3 | ATS (1-2)–BG (30) | 50 g | 30 g |
| 4 | ATS (1-2)–BG (40) | 50 g | 40 g |
| 5 | ATS (1-2)–BG (50) | 50 g | 50 g |
| No | Ingredients | Resin | Hardener | ||
|---|---|---|---|---|---|
| wt% | g | wt% | g | ||
| 1 | SB/EB polyol | 40.60% | 2.029 | 80.70% | 2.738 |
| 2 | Moisture absorbent | 5.04% | 0.252 | - | |
| 3 | Filler | 54.40% | 2.72 | 19.40% | 0.66 |
| Total weight | 5.001 | 3.398 | |||
| Weight ratio | 1: 0.68 | ||||
| No | Ingredient | Resin (g) | Hardener (g) | |||
|---|---|---|---|---|---|---|
| 1 wt% | 3 wt% | 5 wt% | ||||
| 1 | SB/EB polyol containing ATS–BG | SB/EB polyol | 2.029 | 2.029 | 2.029 | 2.738 |
| ATS (*)–BG (**) | 0.02 | 0.061 | 0.101 | 0 | ||
| 2 | Moisture absorbent | 0.252 | 0.252 | 0.252 | 0 | |
| 3 | Filler | 2.72 | 2.72 | 2.72 | 0.66 | |
| Total weight (g)/ | 5.021 | 5.062 | 5.102 | 3.398 | ||
| Mass difference (%) compared to pristine PU | 0.24% | 0.72% | 1.19% | |||
| Independent Variable | Symbol | Unit | Range and Level of Coded Variables | ||||
|---|---|---|---|---|---|---|---|
| −α (−1.667) | Coded Low (−1) | Mean (0) | Coded High (+1) | +α (+1.682) | |||
| AFAP | X1 | g | 2.58 | 3.6 | 5.1 | 6.6 | 7.62 |
| APTES | X2 | g | 5.56 | 7.2 | 9.6 | 12 | 13.64 |
| BG | X3 | g | 9.77 | 20 | 35 | 50 | 60.23 |
| Mixing ratio | X4 | % | 0 | 1 | 3 | 5 | 6.36 |
| O-I Hybrid Solutions | Particle Size (nm) a | Moisture Content (wt%) | Assigned Structures SixO2x+1(C2H5)x+2(C3H6NH2)x b | DOC c | |
|---|---|---|---|---|---|
| 1 | ATS (1-1) | 11.66 | - | Si3 → Si6 | 97.19% |
| 2 | ATS (2-1) | 32.86 | - | Si3 → Si6 | 93.59% |
| 3 | ATS (1-2) | 14.32 | - | Si3 → Si9 | 98.74% |
| 4 | ATS (1-1)–BG (30) | 30.78 | 0.04 | - | 94.86% |
| 5 | ATS (2-1)–BG (30) | 38.51 | 0.08 | - | 90.88% |
| 6 | ATS (1-2)–BG (30) | 30.86 | 0.06 | - | 97.06% |
| 7 | ATS (1-2)–BG (40) | 25.10 | 0.07 | - | 97.02% |
| 8 | ATS (1-2)–BG (50) | 22.80 | 0.08 | - | 97.59% |
| Sample | A1733 * | A1710 ** | R *** | DPS | DPM |
|---|---|---|---|---|---|
| Pristine EB PU | 7.26 | 2.02 | 3.59 | 0.78 | 0.22 |
| ATS EB PU | 7.2 | 1.68 | 4.29 | 0.81 | 0.19 |
| Pristine SB PU | 9.28 | 1.87 | 4.96 | 0.83 | 0.17 |
| ATS SB PU | 7.62 | 1.3 | 5.86 | 0.85 | 0.15 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Contribution (%) |
|---|---|---|---|---|---|---|
| Model | 33.35 | 14 | 2.38 | 13.55 | <0.0001 (s) | 92.66% |
| A-AFAP | 2.66 | 1 | 2.66 | 15.14 | 0.0014 | 7.39% |
| B-APTES | 2.11 | 1 | 2.11 | 12.01 | 0.0035 | 5.86% |
| C-BG | 0.8358 | 1 | 0.8358 | 4.75 | 0.0456 | 2.32% |
| D-Mixing amount | 0.1600 | 1 | 0.1600 | 0.9099 | 0.3553 | 0.44% |
| AB | 0.8202 | 1 | 0.8202 | 4.66 | 0.0474 | 2.28% |
| AC | 0.4078 | 1 | 0.4078 | 2.32 | 0.1486 | 1.13% |
| AD | 0.0098 | 1 | 0.0098 | 0.0555 | 0.8169 | 0.03% |
| BC | 2.57 | 1 | 2.57 | 14.63 | 0.0017 | 7.14% |
| BD | 0.1233 | 1 | 0.1233 | 0.7013 | 0.4155 | 0.34% |
| CD | 0.0022 | 1 | 0.0022 | 0.0123 | 0.9131 | 0.01% |
| A2 | 1.09 | 1 | 1.09 | 6.22 | 0.0248 | 3.03% |
| B2 | 5.90 | 1 | 5.90 | 33.55 | <0.0001 | 16.39% |
| C2 | 13.97 | 1 | 13.97 | 79.41 | <0.0001 | 38.82% |
| D2 | 1.13 | 1 | 1.13 | 6.44 | 0.0228 | 3.14% |
| Residual | 2.64 | 15 | 0.1759 | 7.34% | ||
| Lack of Fit | 2.04 | 10 | 0.2042 | 1.71 | 0.2871 (ns) | |
| Pure Error | 0.5958 | 5 | 0.1192 | |||
| Cor Total | 35.99 | 29 | 100% |
| Std. Dev. | 0.4162 | R2 | 0.9267 |
| Mean | 3.26 | Adjusted R2 | 0.8583 |
| C.V. % | 12.75 | Predicted R2 | 0.6597 |
| Adeq. Precision | 10.2524 | ||
| Parameters | AFAP (g) | APTES (g) | BG (g) | ATS Mixing Ratio (%) | Adhesion Strength Mpa | |
|---|---|---|---|---|---|---|
| Predicted | Experimental | |||||
| Optimum conditions | 4.36 | 10.25 | 37.16 | 3.6 | 4.94 | 4.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duong, T.T.; Le, M.L.; Lee, C.; Kim, J. Organic–Inorganic Hybrid Nanoparticles for Enhancing Adhesion of 2K Polyurethane to Steel and Their Performance Optimization Using Response Surface Methodology. Polymers 2024, 16, 2816. https://doi.org/10.3390/polym16192816
Duong TT, Le ML, Lee C, Kim J. Organic–Inorganic Hybrid Nanoparticles for Enhancing Adhesion of 2K Polyurethane to Steel and Their Performance Optimization Using Response Surface Methodology. Polymers. 2024; 16(19):2816. https://doi.org/10.3390/polym16192816
Chicago/Turabian StyleDuong, Thu Thuy, Manh Linh Le, Changhoon Lee, and Juyoung Kim. 2024. "Organic–Inorganic Hybrid Nanoparticles for Enhancing Adhesion of 2K Polyurethane to Steel and Their Performance Optimization Using Response Surface Methodology" Polymers 16, no. 19: 2816. https://doi.org/10.3390/polym16192816
APA StyleDuong, T. T., Le, M. L., Lee, C., & Kim, J. (2024). Organic–Inorganic Hybrid Nanoparticles for Enhancing Adhesion of 2K Polyurethane to Steel and Their Performance Optimization Using Response Surface Methodology. Polymers, 16(19), 2816. https://doi.org/10.3390/polym16192816






