Optimized Drop-Casted Polyaniline Thin Films for High-Sensitivity Electrochemical and Optical pH Sensors
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. PANI Synthesis
2.3. Sample Preparation and Structural Characterisation
2.4. Electrochemical Proprieties and Electrochemical Sensor Characterizations
2.5. Optical Proprieties and Optical Sensor Characterizations
3. Results and Discussion
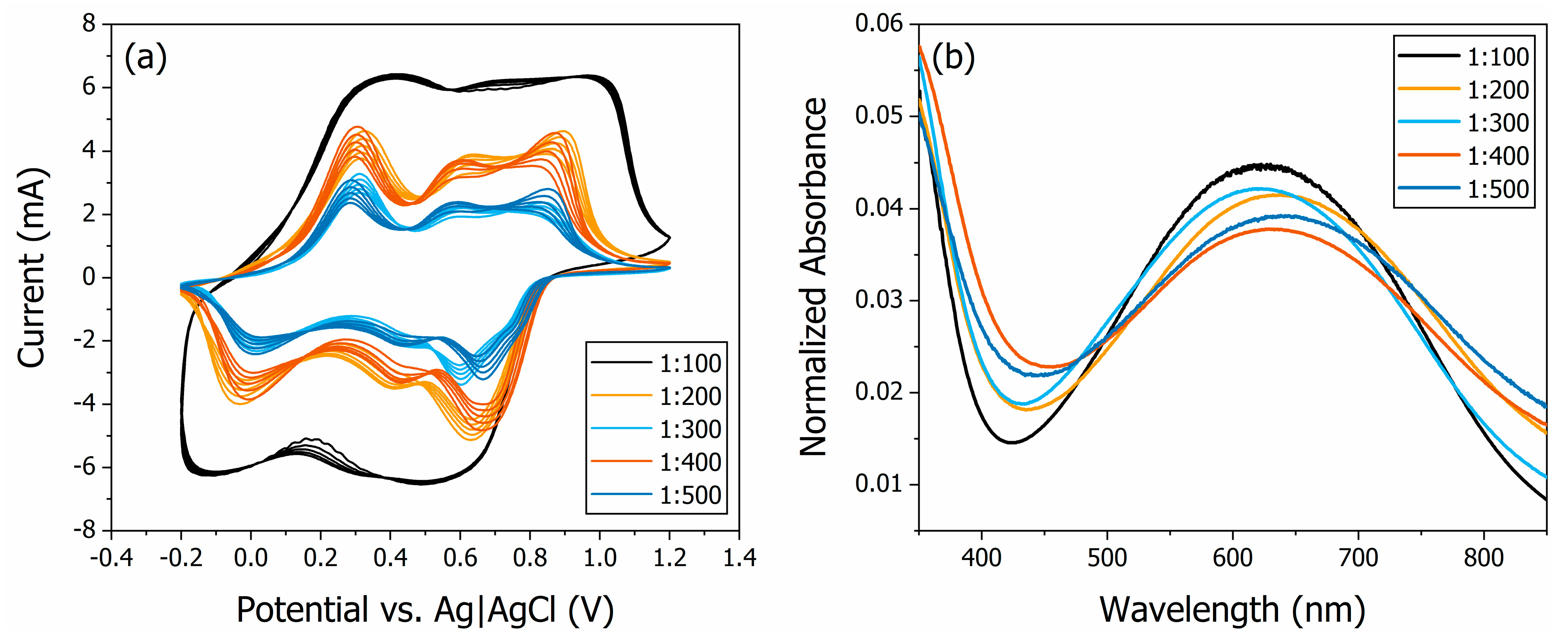
3.1. Electrochemical and Spectroscopic Characterisation
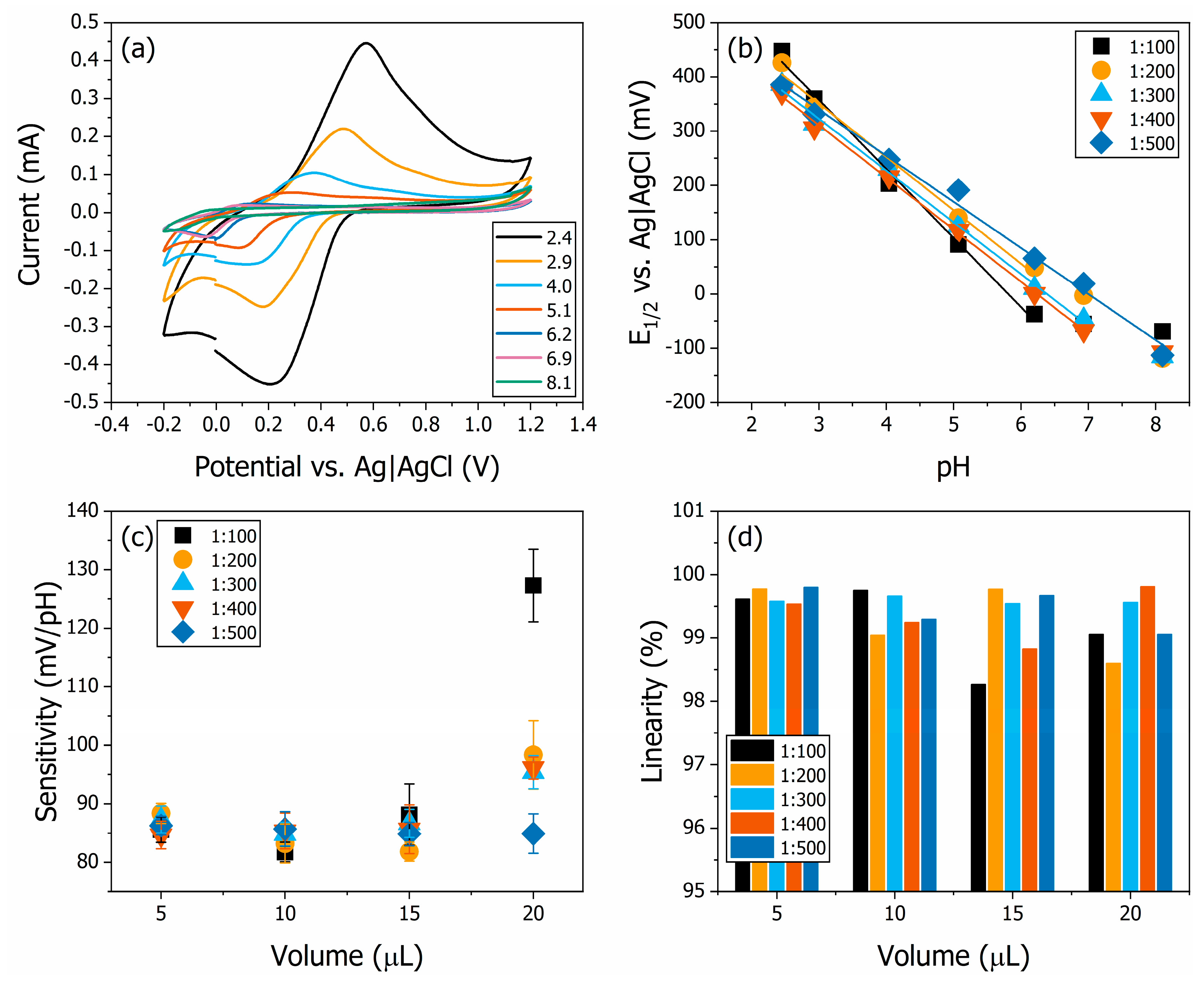
3.2. Electrochemical and Optical Sensors
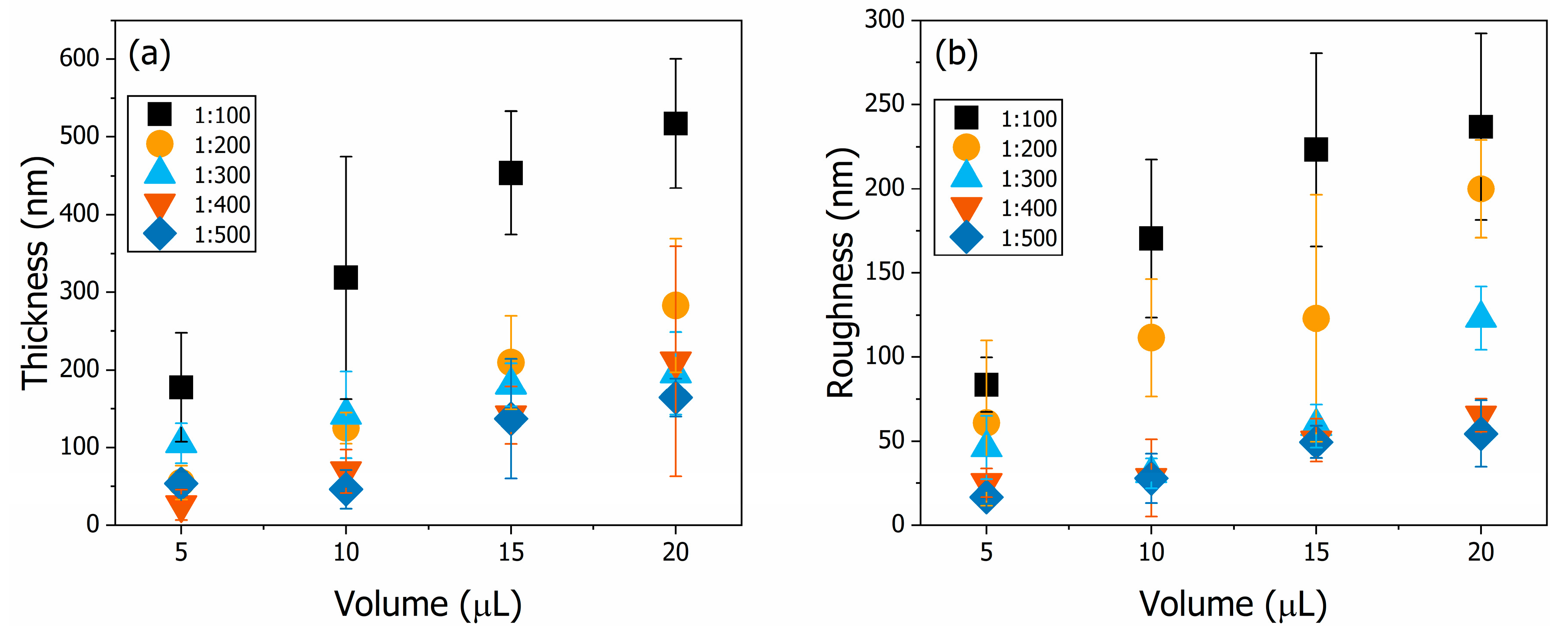
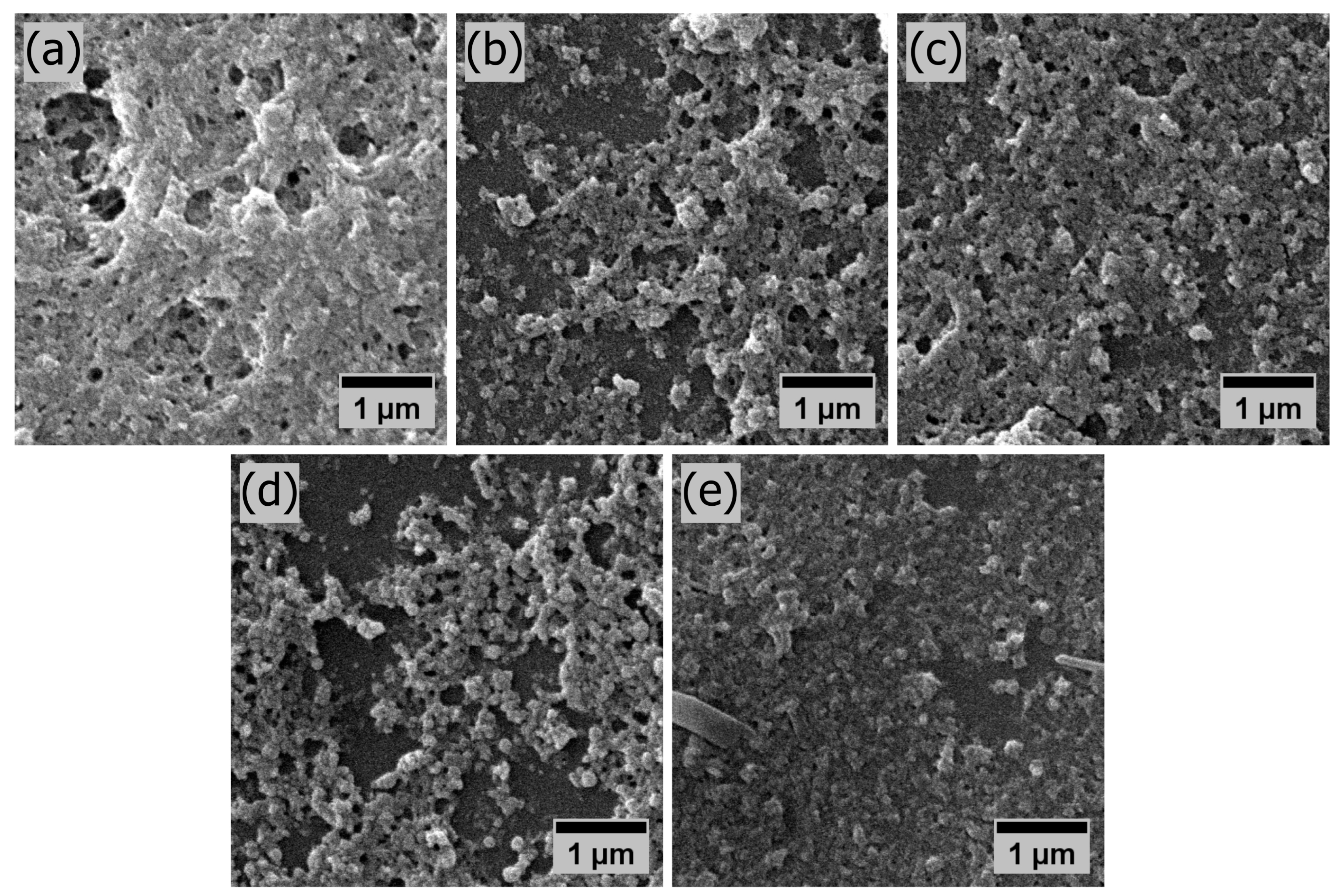
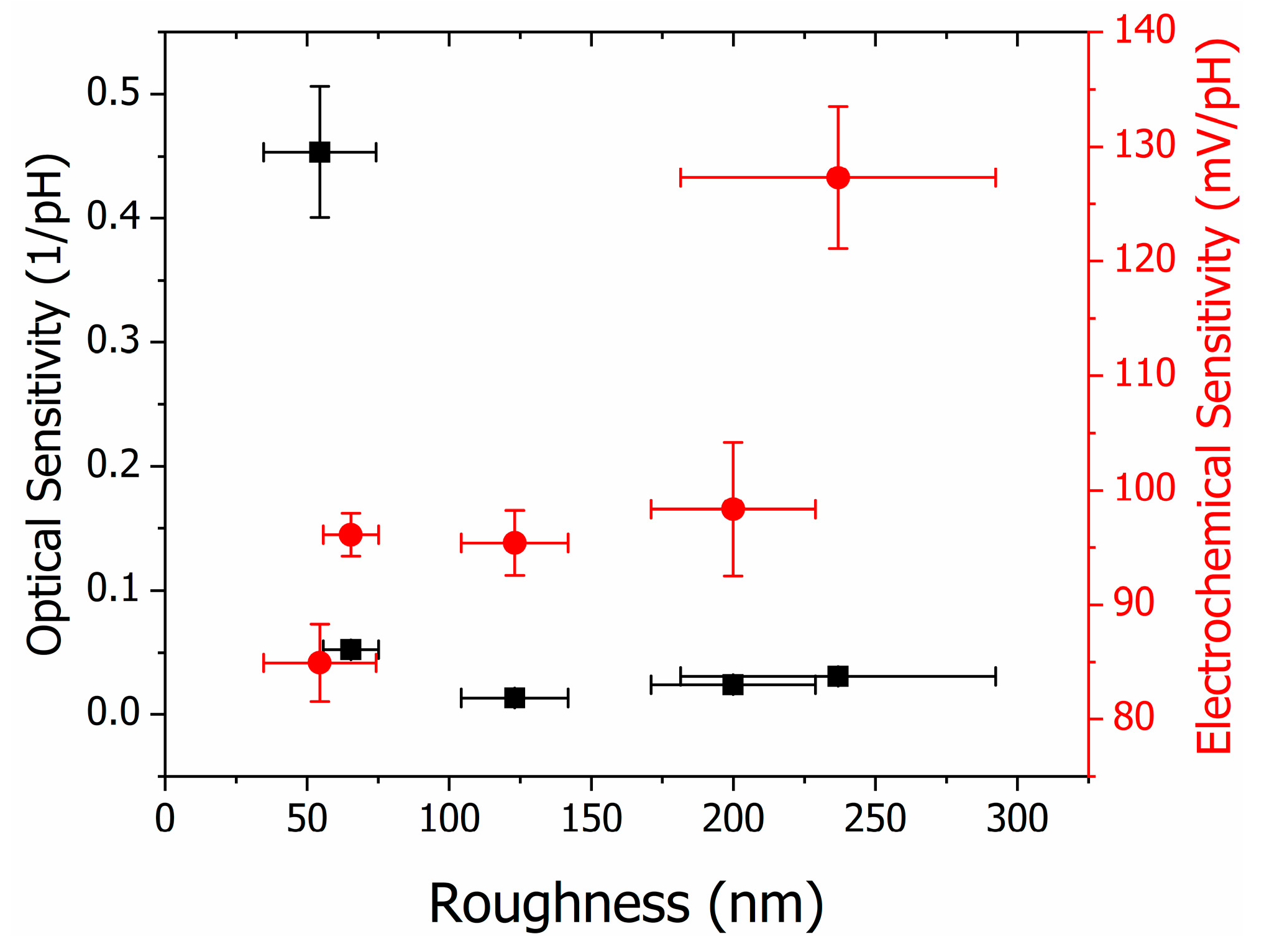
3.3. Thin Film Structural Characteriation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aydemir, N.; Malmström, J.; Travas-Sejdic, J. Conducting Polymer Based Electrochemical Biosensors. Phys. Chem. Chem. Phys. 2016, 18, 8264–8277. [Google Scholar] [CrossRef]
- Chiang, J.-C.; MacDiarmid, A.G. Polyaniline: Protonic Acid Doping of the Emeraldine Form to the Metallic Regime. Synth. Met. 1986, 13, 193–205. [Google Scholar] [CrossRef]
- Pile, D.L.; Hillier, A.C. Electrochemically Modulated Transport through a Conducting Polymer Membrane. J. Membr. Sci. 2002, 208, 119–131. [Google Scholar] [CrossRef]
- Fu, X.; Jia, C.; Wan, Z.; Weng, X.; Xie, J.; Deng, L. Hybrid Electrochromic Film Based on Polyaniline and TiO2 Nanorods Array. Org. Electron. 2014, 15, 2702–2709. [Google Scholar] [CrossRef]
- Ping, Z.; Nauer, B.G.E.; Neugebauer, H.; Theiner, J.; Neckel, A. Protonation and Electrochemical Redox Doping Processes of Polyaniline in Aqueous Solutions: Investigations Using in-Situ FTIR-ATR Spectroscopy and a New Doping System. J. Chem. Soc. Faraday Trans. 1997, 93, 121–129. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Y. Synthesis and Applications of One-Dimensional Nano-Structured Polyaniline: An Overview. Mater. Sci. Eng. B 2006, 134, 9–19. [Google Scholar] [CrossRef]
- Wang, H.; Lin, J.; Shen, Z.X. Polyaniline (PANi) Based Electrode Materials for Energy Storage and Conversion. J. Sci. Adv. Mater. Devices 2016, 1, 225–255. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Gicevicius, M.; Ramanaviciene, A.; Shirsat, M.D.; Viter, R.; Ramanavicius, A. Hybrid Electrochemical/Electrochromic Cu(II) Ion Sensor Prototype Based on PANI/ITO-Electrode. Sens. Actuators B Chem. 2017, 248, 527–535. [Google Scholar] [CrossRef]
- Mashkour, M.; Rahimnejad, M.; Mashkour, M.; Soavi, F. Electro-Polymerized Polyaniline Modified Conductive Bacterial Cellulose Anode for Supercapacitive Microbial Fuel Cells and Studying the Role of Anodic Biofilm in the Capacitive Behavior. J. Power Sources 2020, 478, 228822. [Google Scholar] [CrossRef]
- Mello, H.J.N.P.D.; Mulato, M. Impedimetric and Capacitive Transducer Platform for Chemical Sensors Based on Electrodeposited Polyaniline Thin Films. J. Phys. Chem. C 2022, 126, 12222–12229. [Google Scholar] [CrossRef]
- Vieira, N.C.S.; Fernandes, E.G.R.; Faceto, A.D.; Zucolotto, V.; Guimarães, F.E.G. Nanostructured Polyaniline Thin Films as pH Sensing Membranes in FET-Based Devices. Sens. Actuators B Chem. 2011, 160, 312–317. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Nguyen, T.H.; Hoang, N.V.; Le, N.N.; Nguyen, T.N.N.; Doan, D.C.T.; Dang, M.C. pH Sensitivity of Emeraldine Salt Polyaniline and Poly (Vinyl Butyral) Blend. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 045001. [Google Scholar] [CrossRef][Green Version]
- Tang, Y.; Zhong, L.; Wang, W.; He, Y.; Han, T.; Xu, L.; Mo, X.; Liu, Z.; Ma, Y.; Bao, Y.; et al. Recent Advances in Wearable Potentiometric pH Sensors. Membranes 2022, 12, 504. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, M.T.; Nguyen, A.; Dereje, N.; Huang, J.; Moore, G.C.; Murzynowski, P.J.; Dagdeviren, C. Recent Progress in Electrochemical pH-Sensing Materials and Configurations for Biomedical Applications. Chem. Rev. 2019, 119, 5248–5297. [Google Scholar] [CrossRef] [PubMed]
- Faqir, Y.; Qayoom, A.; Erasmus, E.; Schutte-Smith, M.; Visser, H.G. A Review on the Application of Advanced Soil and Plant Sensors in the Agriculture Sector. Comput. Electron. Agric. 2024, 226, 109385. [Google Scholar] [CrossRef]
- Zhou, B.; Bian, C.; Tong, J.; Xia, S. Fabrication of a Miniature Multi-Parameter Sensor Chip for Water Quality Assessment. Sensors 2017, 17, 157. [Google Scholar] [CrossRef]
- Habel, W.R.; Krebber, K. Fiber-Optic Sensor Applications in Civil and Geotechnical Engineering. Photonic Sens. 2011, 1, 268–280. [Google Scholar] [CrossRef]
- Staudinger, C.; Strobl, M.; Breininger, J.; Klimant, I.; Borisov, S.M. Fast and Stable Optical pH Sensor Materials for Oceanographic Applications. Sens. Actuators B Chem. 2019, 282, 204–217. [Google Scholar] [CrossRef]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. Chemiresistive Polyaniline-Based Gas Sensors: A Mini Review. Sens. Actuators B Chem. 2015, 220, 534–548. [Google Scholar] [CrossRef]
- Bhadra, S.; Khastgir, D.; Singha, N.K.; Lee, J.H. Progress in preparation, processing and applications of polyaniline. Prog. Polym. Sci. 2009, 34, 783–810. [Google Scholar] [CrossRef]
- Elanjeitsenni, V.P.; Vadivu, K.S.; Prasanth, B.M. A Review on Thin Films, Conducting Polymers as Sensor Devices. Mater. Res. Express 2022, 9, 022001. [Google Scholar] [CrossRef]
- Beygisangchin, M.; Abdul Rashid, S.; Shafie, S.; Sadrolhosseini, A.R.; Lim, H.N. Preparations, Properties, and Applications of Polyaniline and Polyaniline Thin Films—A Review. Polymers 2021, 13, 2003. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.K.S.; Zhang, Y.; Li, D.; Compton, R.G. A Mini-Review: How Reliable Is the Drop Casting Technique? Electrochem. Commun. 2020, 121, 106867. [Google Scholar] [CrossRef]
- Zhao, C.; Xing, L.; Xiang, J.; Cui, L.; Jiao, J.; Sai, H.; Li, Z.; Li, F. Formation of Uniform Reduced Graphene Oxide Films on Modified PET Substrates Using Drop-Casting Method. Particuology 2014, 17, 66–73. [Google Scholar] [CrossRef]
- Wang, C.; Chen, X.; Chen, F.; Shao, J. Organic Photodetectors Based on Copper Phthalocyanine Films Prepared by a Multiple Drop Casting Method. Org. Electron. 2019, 66, 183–187. [Google Scholar] [CrossRef]
- Gettler, R.C.; Kinlen, P.J.; Renfroe, E.; Xing, Y.; Young, M.J. Postprocessing of Solution-Cast Polyaniline for Enhanced Electrochemical Properties. J. Power Sources 2023, 583, 233557. [Google Scholar] [CrossRef]
- Arshak, A.; Gill, E.I.; Arshak, K.; Korostynska, O.; Cunniffe, C. Drop-Coated Polyaniline Composite Conductimetric pH Sensors. In Proceedings of the 2007 30th International Spring Seminar on Electronics Technology (ISSE), Cluj-Napoca, Romania, 9–13 May 2007; pp. 213–218. [Google Scholar]
- Hansen, B.; Hocevar, M.A.; Ferreira, C.A. A Facile and Simple Polyaniline-Poly (Ethylene Oxide) Based Glucose Biosensor. Synth. Met. 2016, 222, 224–231. [Google Scholar] [CrossRef]
- Lange, U.; Roznyatovskaya, N.V.; Mirsky, V.M. Conducting Polymers in Chemical Sensors and Arrays. Anal. Chim. Acta 2008, 614, 1–26. [Google Scholar] [CrossRef]
- Mihai, I.; Addiego, F.; Ruch, D.; Ball, V. Composite and Free Standing PANI-PVA Membranes as Flexible and Stable Optical pH Sensors. Sens. Actuators B Chem. 2014, 192, 769–775. [Google Scholar] [CrossRef]
- Wencel, D.; Abel, T.; McDonagh, C. Optical Chemical pH Sensors. Anal. Chem. 2014, 86, 15–29. [Google Scholar] [CrossRef]
- Jin, Z.; Su, Y.; Duan, Y. An Improved Optical pH Sensor Based on Polyaniline. Sens. Actuators B Chem. 2000, 71, 118–122. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- van Hal, R.E.G.; Eijkel, J.C.T.; Bergveld, P. A General Model to Describe the Electrostatic Potential at Electrolyte Oxide Interfaces. Adv. Colloid Interface Sci. 1996, 69, 31–62. [Google Scholar] [CrossRef]
- Park, H.J.; Yoon, J.H.; Lee, K.G.; Choi, B.G. Potentiometric Performance of Flexible pH Sensor Based on Polyaniline Nanofiber Arrays. Nano Converg. 2019, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Fraga, V.M.; Lovi, I.T.; Abegão, L.M.G.; Mello, H.J.N.P.D. Understanding the Effect of Deposition Technique on the Structure–Property Relationship of Polyaniline Thin Films Applied in Potentiometric pH Sensor. Polymers 2023, 15, 3450. [Google Scholar] [CrossRef]
- Li, Y.; Mao, Y.; Xiao, C.; Xu, X.; Li, X. Flexible pH Sensor Based on a Conductive PANI Membrane for pH Monitoring. RSC Adv. 2019, 10, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Hossain, S.; Padmanathan, N.; Badal, M.R.; Razeeb, K.M.; Jamal, M. Highly Sensitive Potentiometric pH Sensor Based on Polyaniline Modified Carbon Fiber Cloth for Food and Pharmaceutical Applications. ACS Omega 2024, 9, 40122–40133. [Google Scholar] [CrossRef] [PubMed]
- Winquist, F. Voltammetric Electronic Tongues—Basic Principles and Applications. Microchim. Acta 2008, 163, 3–10. [Google Scholar] [CrossRef]
- Watanabe, K.; Sugiyama, K.; Komatsu, S.; Yoshida, K.; Ono, T.; Fujimura, T.; Kashiwagi, Y.; Sato, K. Voltammetric pH Measurements Using Azure A-Containing Layer-by-Layer Film Immobilized Electrodes. Polymers 2020, 12, 2328. [Google Scholar] [CrossRef]
- Amiri, M.; Amali, E.; Nematollahzadeh, A.; Salehniya, H. Poly-Dopamine Films: Voltammetric Sensor for pH Monitoring. Sens. Actuators B Chem. 2016, 228, 53–58. [Google Scholar] [CrossRef]
- Su, W.; Xu, J.; Ding, X. An Electrochemical pH Sensor Based on the Amino-Functionalized Graphene and Polyaniline Composite Film. IEEE Trans. NanoBioscience 2016, 15, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Takehara, H.; Ichiki, T. A Polyaniline/Polyvinyl Acetate Composite Film Electrode for Highly Sensitive Electrochemical Sensing of pH. Synth. Met. 2023, 297, 117380. [Google Scholar] [CrossRef]
- Marmisollé, W.A.; Florit, M.I.; Posadas, D. A Formal Representation of the Anodic Voltammetric Response of Polyaniline. J. Electroanal. Chem. 2011, 655, 17–22. [Google Scholar] [CrossRef]
- Pöller, S.; Schuhmann, W. A Miniaturized Voltammetric pH Sensor Based on Optimized Redox Polymers. Electrochim. Acta 2014, 140, 101–107. [Google Scholar] [CrossRef]
- Mostafaei, A.; Zolriasatein, A. Synthesis and Characterization of Conducting Polyaniline Nanocomposites Containing ZnO Nanorods. Prog. Nat. Sci. Mater. Int. 2012, 22, 273–280. [Google Scholar] [CrossRef]
- Deshmukh, P.R.; Shinde, N.M.; Patil, S.V.; Bulakhe, R.N.; Lokhande, C.D. Supercapacitive Behavior of Polyaniline Thin Films Deposited on Fluorine Doped Tin Oxide (FTO) Substrates by Microwave-Assisted Chemical Route. Chem. Eng. J. 2013, 223, 572–577. [Google Scholar] [CrossRef]
- Pruneanu, S.; Veress, E.; Marian, I.; Oniciu, L. Characterization of Polyaniline by Cyclic Voltammetry and UV-Vis Absorption Spectroscopy. J. Mater. Sci. 1999, 34, 2733–2739. [Google Scholar] [CrossRef]
- Huerta-Vilca, D.; de Moraes, S.R.; Motheo, A.d.J. Aspects of Polyaniline Electrodeposition on Aluminium. J. Solid State Electrochem. 2004, 9, 416–420. [Google Scholar] [CrossRef]
- Tarver, J.; Yoo, J.E.; Dennes, T.J.; Schwartz, J.; Loo, Y.-L. Polymer Acid Doped Polyaniline Is Electrochemically Stable Beyond pH 9. Chem. Mater. 2009, 21, 280–286. [Google Scholar] [CrossRef]
- Park, H.-W.; Kim, T.; Huh, J.; Kang, M.; Lee, J.E.; Yoon, H. Anisotropic Growth Control of Polyaniline Nanostructures and Their Morphology-Dependent Electrochemical Characteristics. ACS Nano 2012, 6, 7624–7633. [Google Scholar] [CrossRef]
- Albuquerque, J.E.; Mattoso, L.H.C.; Balogh, D.T.; Faria, R.M.; Masters, J.G.; MacDiarmid, A.G. A Simple Method to Estimate the Oxidation State of Polyanilines. Synth. Met. 2000, 113, 19–22. [Google Scholar] [CrossRef]
- Mello, H.J.N.P.D.; Mulato, M. Optochemical Sensors Using Electrodeposited Polyaniline Films: Electrical Bias Enhancement of Reflectance Response. Sens. Actuators B Chem. 2015, 213, 195–201. [Google Scholar] [CrossRef]
- Mello, H.J.N.P.D.; Mulato, M. Influence of Galvanostatic Electrodeposition Parameters on the Structure-Property Relationships of Polyaniline Thin Films and Their Use as Potentiometric and Optical pH Sensors. Thin Solid Films 2018, 656, 14–21. [Google Scholar] [CrossRef]
- Mello, H.J.N.P.D.; Mulato, M. Effect of Aniline Monomer Concentration on PANI Electropolymerization Process and Its Influence for Applications in Chemical Sensors. Synth. Met. 2018, 239, 66–70. [Google Scholar] [CrossRef]
- Kim, J.; Sohn, J.; Jo, Y.; Woo, H.; Han, J.; Cho, S.; Inamdar, A.I.; Kim, H.; Im, H. Drop-Casted Polyaniline Thin Films on Flexible Substrates for Supercapacitor Applications. J. Korean Phys. Soc. 2014, 65, 1320–1323. [Google Scholar] [CrossRef]
- Mello, H.J.N.P.D.; Heimfarth, T.; Mulato, M. Influence of the Physical–Chemical Properties of Polyaniline Thin Films on the Final Sensitivity of Varied Field Effect Sensors. Mater. Chem. Phys. 2015, 160, 257–263. [Google Scholar] [CrossRef]
- Madeira, G.D.M.; Mello, H.J.N.P.D.; Faleiros, M.C.; Mulato, M. Model Improvement for Super-Nernstian pH Sensors: The Effect of Surface Hydration. J. Mater. Sci. 2020, 56, 2738–2747. [Google Scholar] [CrossRef]
- Lakard, B.; Herlem, G.; Lakard, S.; Guyetant, R.; Fahys, B. Potentiometric pH Sensors Based on Electrodeposited Polymers. Polymer 2005, 46, 12233–12239. [Google Scholar] [CrossRef]

| Deposition | Transduction | Sensitivity | pH Range | Linearity | Reference |
|---|---|---|---|---|---|
| Electrodeposition | Electrochemical | 81 ± 1 mV/pH | 2–8 | 99.9% | [54] |
| Electrodeposition | Electrochemical | 70 ± 1 mV/pH | 2–8 | 99.6% | [55] |
| Electrodeposition | Electrochemical | 76 mV/pH | 2–8 | 99.9% | [57] |
| Electrodeposition | Electrochemical | 66 ± 1 mV/pH | 2–8 | 99.0% | [58] |
| Electrodeposition | Electrochemical | 74.2 ± 2.2 mV/pH | 2–7 | 99.2% | [36] |
| Electrodeposition | Electrochemical | 52 mV/pH | 2–9 | 95.7% | [59] |
| Spin-coated | Electrochemical | 56 ± 2 mV/pH | 2–7 | 99.0% | [58] |
| Spin-coated | Electrochemical | 62 ± 3 mV/pH | 2–8 | 98.7% | [36] |
| Drop-casted | Electrochemical | 127.3 ± 6.2 mV/pH | 2–6 | 99.1% | This work |
| Chem. oxidation | Optical | 0.09 1/pH | 5–8 | - | [32] |
| Electrodeposition | Optical | 25 1/pH | 2–8 | 97.4% | [53] |
| Electrodeposition | Optical | 30 ± 3 1/pH | 2–6 | 92.0% | [54] |
| Drop-casted | Optical | 0.45 ± 0.05 1/pH | 2–7 | 95.2% | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mücke, B.E.D.; Rossignatti, B.C.; Abegão, L.M.G.; Barbosa, M.S.; Mello, H.J.N.P.D. Optimized Drop-Casted Polyaniline Thin Films for High-Sensitivity Electrochemical and Optical pH Sensors. Polymers 2024, 16, 2789. https://doi.org/10.3390/polym16192789
Mücke BED, Rossignatti BC, Abegão LMG, Barbosa MS, Mello HJNPD. Optimized Drop-Casted Polyaniline Thin Films for High-Sensitivity Electrochemical and Optical pH Sensors. Polymers. 2024; 16(19):2789. https://doi.org/10.3390/polym16192789
Chicago/Turabian StyleMücke, Bruna Eduarda Darolt, Beatriz Cotting Rossignatti, Luis Miguel Gomes Abegão, Martin Schwellberger Barbosa, and Hugo José Nogueira Pedroza Dias Mello. 2024. "Optimized Drop-Casted Polyaniline Thin Films for High-Sensitivity Electrochemical and Optical pH Sensors" Polymers 16, no. 19: 2789. https://doi.org/10.3390/polym16192789
APA StyleMücke, B. E. D., Rossignatti, B. C., Abegão, L. M. G., Barbosa, M. S., & Mello, H. J. N. P. D. (2024). Optimized Drop-Casted Polyaniline Thin Films for High-Sensitivity Electrochemical and Optical pH Sensors. Polymers, 16(19), 2789. https://doi.org/10.3390/polym16192789






