Delamination and Evaluation of Multilayer PE/Al/PET Packaging Waste Separated Using a Hydrophobic Deep Eutectic Solvent
Abstract
1. Introduction
2. Materials and Methods
2.1. Solvent
2.2. Packaging Laminate
2.3. Delamination
2.4. Design of Experiments
2.5. Fourier-Transform Infrared Spectroscopy (FT-IR)
2.6. Differential Scanning Calorimetry (DSC)
2.7. Thermogravimetric Analysis (TGA)
3. Results and Discussion
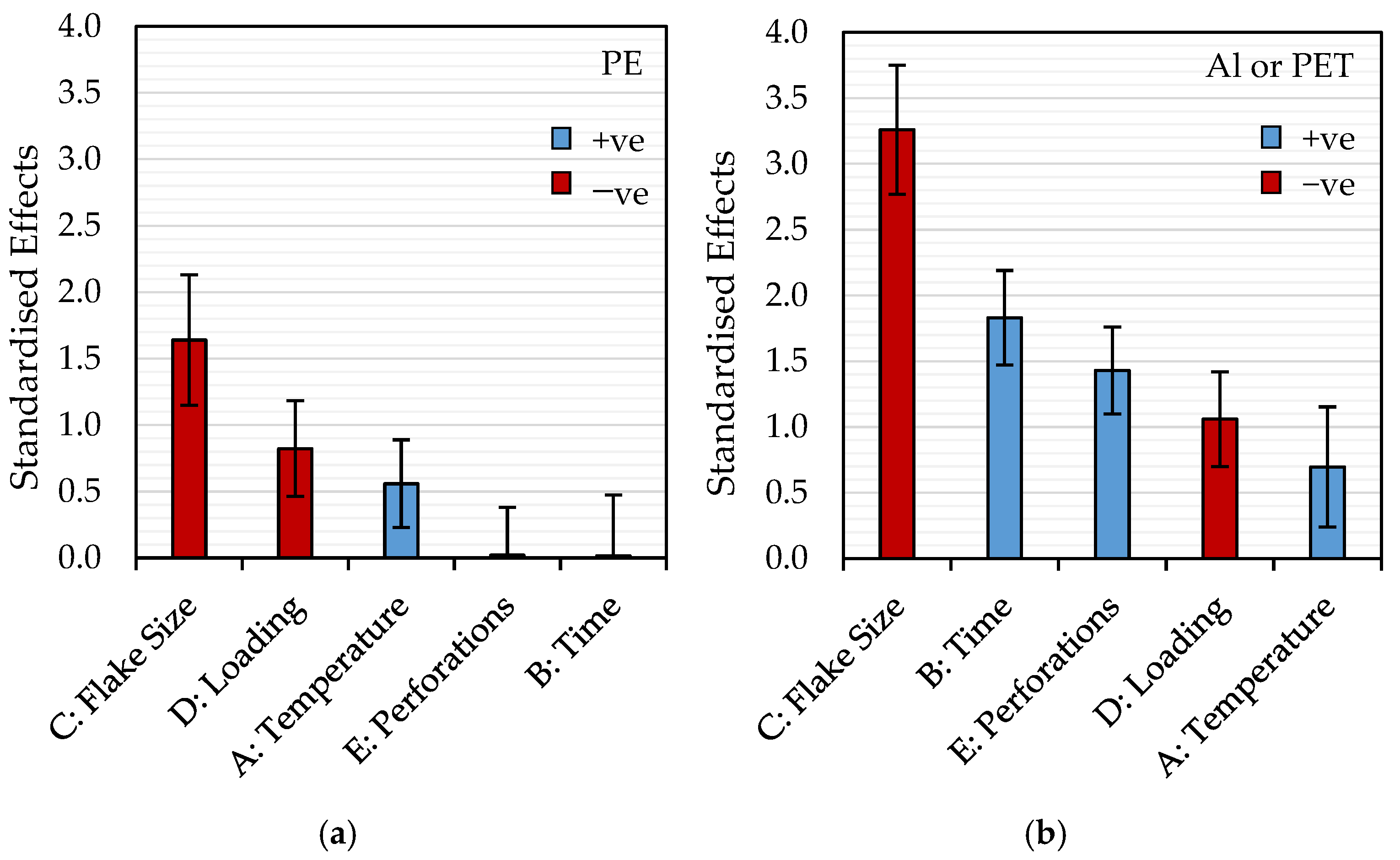
3.1. Effect of Temperature, Time, Loading, Flake Size, and Perforations
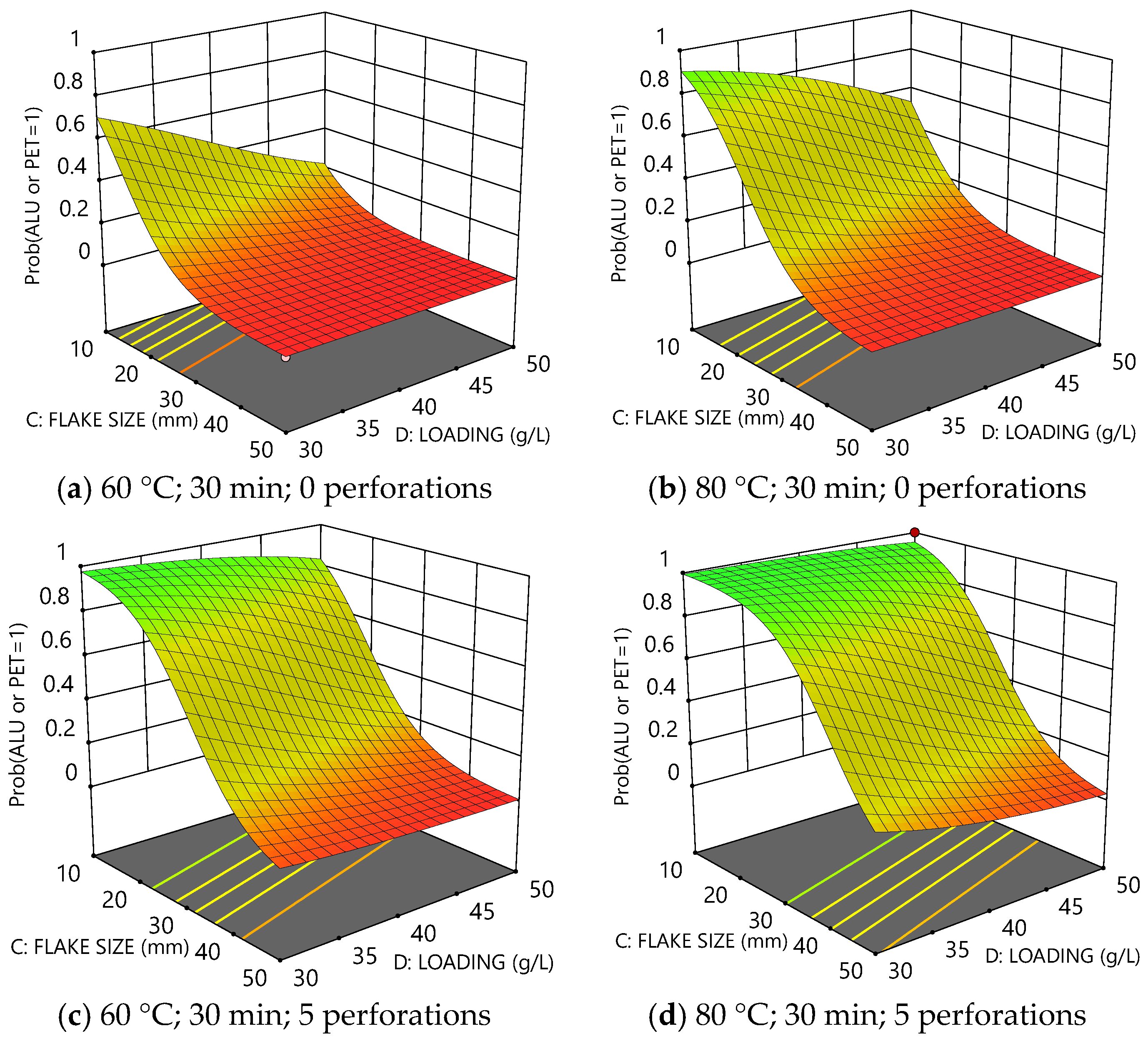
3.2. Analysis of Delamination Models
3.2.1. Model for PE Delamination
3.2.2. Model for Al or PET Delamination
3.2.3. Optimisation of PE/Al/PET Delamination
3.3. Recovery of Solvents and Materials
3.4. FTIR Spectroscopy
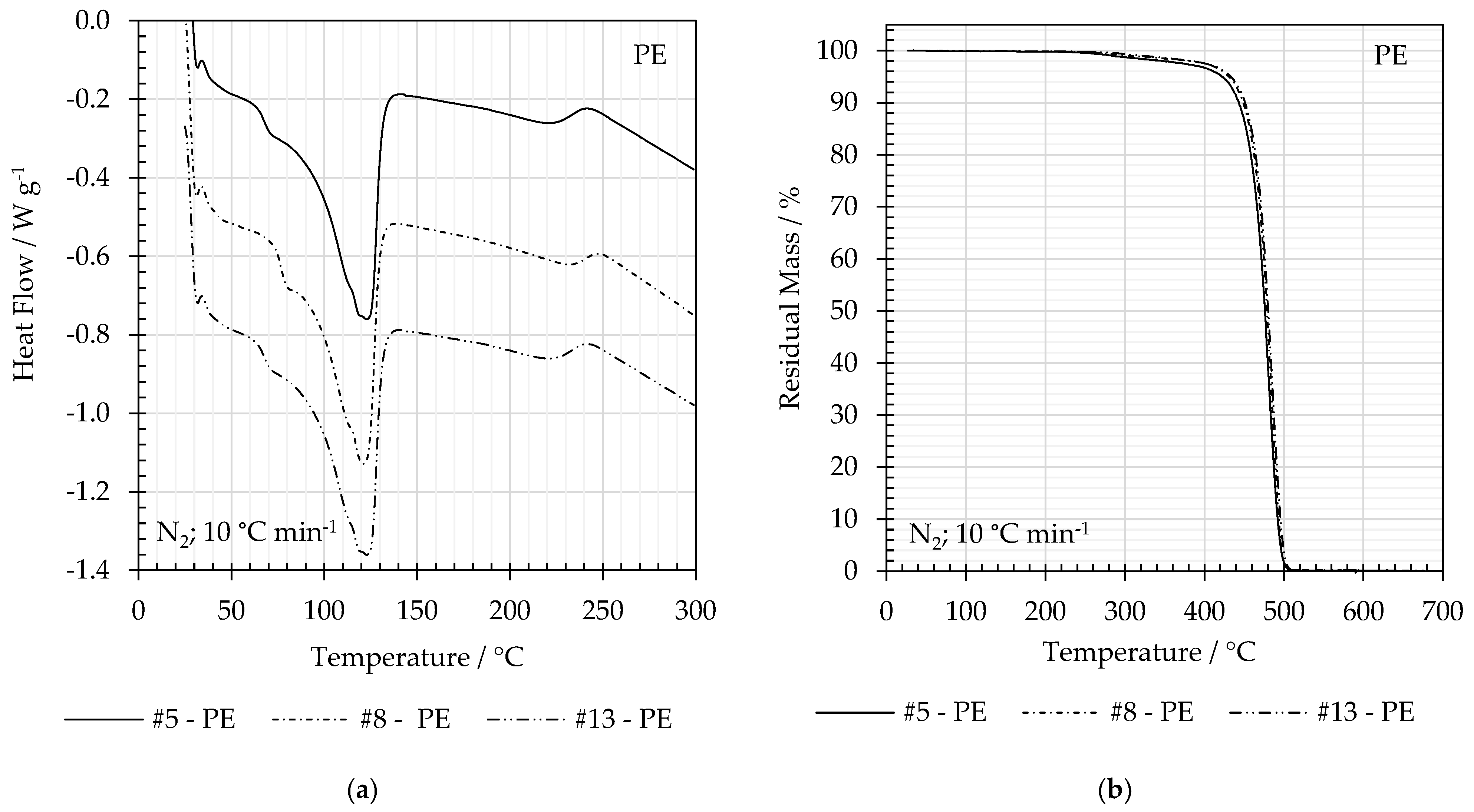
3.5. Thermal Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jung, H.; Shin, G.; Kwak, H.; Hao, L.T.; Jegal, J.; Kim, H.J.; Jeon, H.; Park, J.; Oh, D.X. Review of polymer technologies for improving the recycling and upcycling efficiency of plastic waste. Chemosphere 2023, 320, 138089. [Google Scholar] [CrossRef] [PubMed]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef] [PubMed]
- Hopewell, J.; Dvorak, R.; Kosior, E. Plastics recycling: Challenges and opportunities. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 2115–2126. [Google Scholar] [CrossRef] [PubMed]
- Ügdüler, S.; De Somer, T.; Van Geem, K.M.; Roosen, M.; Kulawig, A.; Leineweber, R.; De Meester, S. Towards a Better Understanding of Delamination of Multilayer Flexible Packaging Films by Carboxylic Acids. ChemSusChem 2021, 14, 4198–4213. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, J.; Anugwom, I.; Kallioinen, M.; Mänttäri, M. Green solvents in recovery of aluminium and plastic from waste pharmaceutical blister packaging. Waste Manag. 2020, 107, 20–27. [Google Scholar] [CrossRef]
- Li, T.; Theodosopoulos, G.; Lovell, C.; Loukodimou, A.; Maniam, K.K.; Paul, S. Progress in Solvent-Based Recycling of Polymers from Multilayer Packaging. Polymers 2024, 16, 1670. [Google Scholar] [CrossRef]
- Ritchie, H.; Samborska, V.; Roser, M. Plastic Pollution. 2023. Available online: https://ourworldindata.org/plastic-pollution (accessed on 1 June 2024).
- Plastics Europe. Plastics—The Fast Facts 2023. Available online: https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2023/ (accessed on 1 June 2024).
- Shogren, R.; Wood, D.; Orts, W.; Glenn, G. Plant-based materials and transitioning to a circular economy. Sustain. Prod. Consum. 2019, 19, 194–215. [Google Scholar] [CrossRef]
- The Circular Economy for Plastics, A European Analysis; Plastics Europe: Brussels, Belgium, 2024.
- Wu, W.-M.; Yang, J.; Criddle, C.S. Microplastics pollution and reduction strategies. Front. Environ. Sci. Eng. 2016, 11, 6. [Google Scholar] [CrossRef]
- Kaiser, K.; Schmid, M.; Schlummer, M. Recycling of Polymer-Based Multilayer Packaging: A Review. Recycling 2017, 3, 1. [Google Scholar] [CrossRef]
- Collins, D.M. Separating Polymer from Composite Structures. WO2018035565A1, 1 March 2018. [Google Scholar]
- Lovis, F. Method and Apparatus for Recycling Packaging Material. GB2557682A, 5 May 2015. [Google Scholar]
- Font, A.F. Method for Removing Adhesives and/or Interlaminar Inks on Laminated Plastic Material. WO/2021/089895, 14 May 2021. [Google Scholar]
- Berkane, I.; Cabanes, A.; Horodytska, O.; Aracil, I.; Fullana, A. The delamination of metalized multilayer flexible packaging using a microperforation technique. Resour. Conserv. Recycl. 2023, 189, 106744. [Google Scholar] [CrossRef]
- Allen, K.W. A Review of Contemporary Views of Theories of Adhesion. J. Adhes. 1987, 21, 261–277. [Google Scholar] [CrossRef]
- Fowkes, F.M. Acid-Base Interactions in Polymer Adhesion. Tribol. Ser. 1981, 7, 119–137. [Google Scholar]
- Olafsson, G.; Jägerstad, M.; Öste, R.; Wesslén, B. Delamination of Polyethylene and Aluminum Foil Layers of Laminated Packaging Material by Acetic Acid. J. Food Sci. 1993, 58, 215–219. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 39, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Hou, Y.; Yao, C.; Wu, W. Deep Eutectic Solvents: Green Solvents for Separation Applications. Acta Phys.-Chim. Sin. 2018, 34, 873–885. [Google Scholar] [CrossRef]
- Makoś, P.; Słupek, E.; Gębicki, J. Hydrophobic deep eutectic solvents in microextraction techniques—A review. Microchem. J. 2020, 152, 104384. [Google Scholar] [CrossRef]
- van Osch, D.J.G.P.; Dietz, C.H.J.T.; Warrag, S.E.E.; Kroon, M.C. The Curious Case of Hydrophobic Deep Eutectic Solvents: A Story on the Discovery, Design, and Applications. ACS Sustain. Chem. Eng. 2020, 8, 10591–10612. [Google Scholar] [CrossRef]
- Omar, K.A.; Sadeghi, R. Physicochemical properties of deep eutectic solvents: A review. J. Mol. Liq. 2022, 360, 119524. [Google Scholar] [CrossRef]
- Abbott, A.P.; Al-Murshedi, A.Y.; Alshammari, O.A.; Harris, R.C.; Kareem, J.H.; Qader, I.B.; Ryder, K. Thermodynamics of phase transfer for polar molecules from alkanes to deep eutectic solvents. Fluid Phase Equilibria 2017, 448, 99–104. [Google Scholar] [CrossRef]
- Häkkinen, R.; Abbott, A. Solvation of carbohydrates in five choline chloride-based deep eutectic solvents and the implication for cellulose solubility. Green Chem. 2019, 21, 4673–4682. [Google Scholar] [CrossRef]
- Zainal-Abidin, M.H.; Hayyan, M.; Wong, W.F. Hydrophobic deep eutectic solvents: Current progress and future directions. J. Ind. Eng. Chem. 2021, 97, 142–162. [Google Scholar] [CrossRef]
- Definitive Screening Design (DSD): Characteristics and Analysis Methods. Cited 29 August 2024. Available online: https://www.statease.com/docs/latest/contents/response-surface-designs/definitive-screening-design-dsd-analysis-methods/ (accessed on 23 August 2024).
- Definitive Screening Designs. Cited 29 August 2024. Available online: https://support.minitab.com/en-us/minitab/help-and-how-to/statistical-modeling/doe/supporting-topics/factorial-and-screening-designs/definitive-screening-designs/ (accessed on 23 August 2024).
- Gulmine, J.V.; Janissek, P.R.; Heise, H.M.; Akcelrud, L. Polyethylene characterization by FTIR. Polym. Test. 2002, 21, 557–563. [Google Scholar] [CrossRef]
- Chen, Z.; Hay, J.N.; Jenkins, M.J. FTIR spectroscopic analysis of poly(ethylene terephthalate) on crystallization. Eur. Polym. J. 2012, 48, 1586–1610. [Google Scholar] [CrossRef]

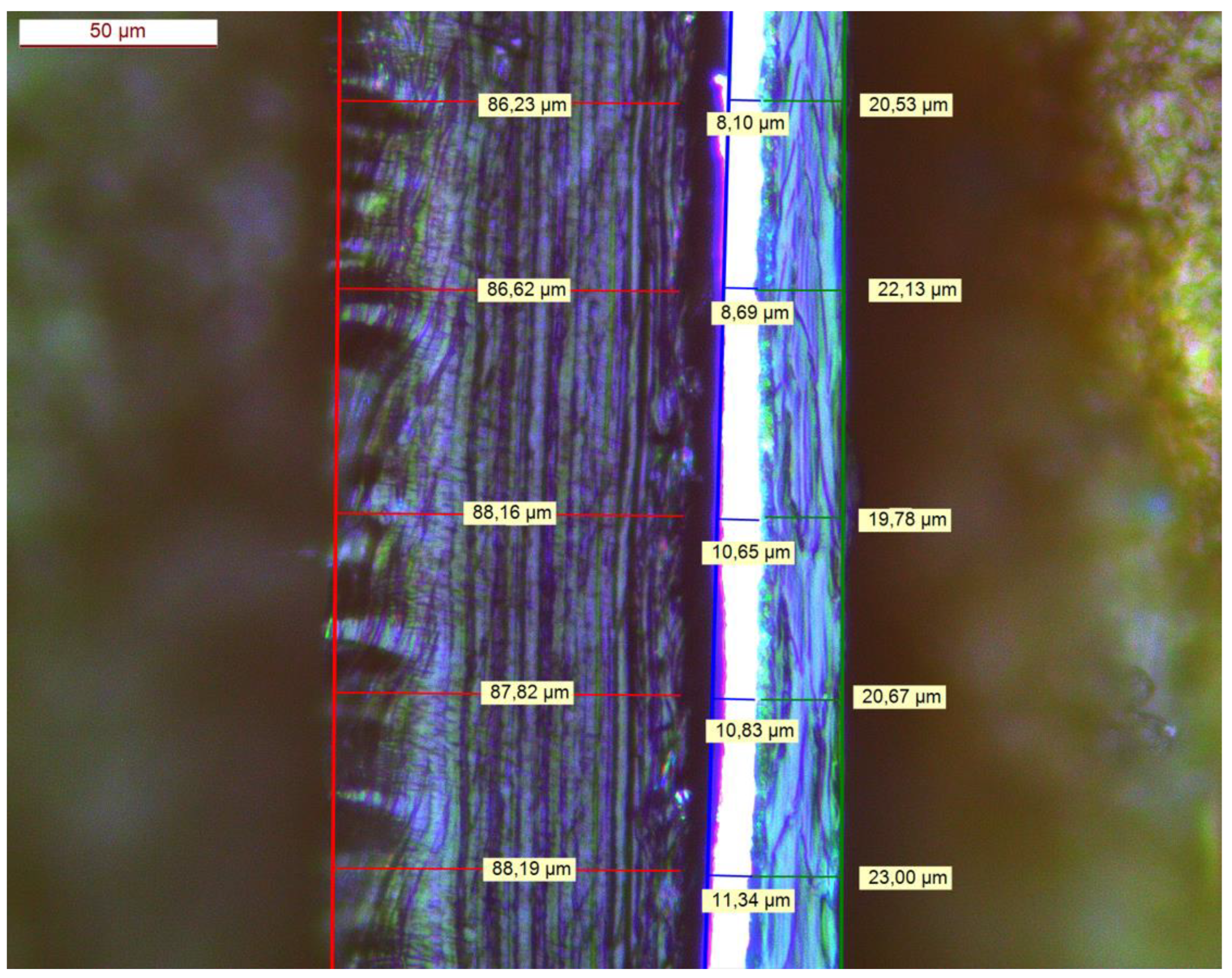





| Parameters | Units | Type | Low | Centre | High |
|---|---|---|---|---|---|
| Temperature | °C | Numeric | 60 | 70 | 80 |
| Flakes Size | mm | Numeric | 10 | 30 | 50 |
| Loading | g | Numeric | 30 | 40 | 50 |
| Perforation | pins cm−1 | Categoric | 0 | n/a | 7 |
| Time | min | Numeric | 15 | 30 | 45 |
| Factors | Delamination Count | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| # | A: Temperature (°C) | B: Time (min) | C: Flake Size (mm) | D: Loading (g) | E: Perforations (pins cm−2) | PE/Al/PET | Al/PET | Al or PET | PE |
| 1 | 60 | 30 | 50 | 30 | 0 | 51 | 49 | 0 | 49 |
| 2 | 60 | 15 | 10 | 50 | 0 | 12 | 87 | 1 | 88 |
| 3 | 60 | 15 | 50 | 40 | 5 | 65 | 27 | 8 | 35 |
| 4 | 60 | 45 | 30 | 50 | 0 | 53 | 35 | 2 | 37 |
| 5 | 60 | 45 | 10 | 30 | 5 | 1 | 1 | 98 | 99 |
| 6 | 70 | 30 | 30 | 40 | 0 | 28 | 62 | 10 | 72 |
| 7 | 70 | 45 | 50 | 50 | 5 | 78 | 20 | 2 | 22 |
| 8 | 70 | 30 | 30 | 40 | 5 | 30 | 10 | 60 | 70 |
| 9 | 70 | 15 | 10 | 30 | 0 | 0 | 57 | 43 | 100 |
| 10 | 80 | 45 | 10 | 40 | 0 | 0 | 0 | 100 | 100 |
| 11 | 80 | 15 | 50 | 50 | 0 | 62 | 38 | 0 | 38 |
| 12 | 80 | 45 | 50 | 30 | 5 | 17 | 21 | 62 | 83 |
| 13 | 80 | 15 | 30 | 30 | 5 | 20 | 40 | 40 | 80 |
| 14 | 80 | 30 | 10 | 50 | 5 | 1 | 1 | 98 | 99 |
| Factor p-Values | ||||||
|---|---|---|---|---|---|---|
| Material | Model | A: Temperature (°C) | B: Time (min) | C: Flake Size (mm) | D: Loading (g) | E: Perforations (pins cm−2) |
| PE | <0.0001 | <0.0001 | 0.855 | <0.0001 | <0.0001 | 0.782 |
| Al or PET | <0.0001 | 0.0036 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Factors and Responses | Criteria | Accepted Range | Optimal | |
|---|---|---|---|---|
| Min | Max | |||
| A: Temperature (°C) | Minimise | 60 | 80 | 72 |
| B: Time (min) | Minimise | 15 | 45 | 33 |
| C: Flake size (mm) | Maximise | 10 | 50 | 14 |
| D: Loading (g) | Maximise | 30 | 50 | 36 |
| E: Perforations | Any | 0 | 5 | 5 |
| Probability of PE Delamination | Maximise | 0.9 | 0.999 | 0.96 ± 0.02 |
| Probability of Al or PET Delamination | Maximise | 0.9 | 0.999 | 0.95 ± 0.03 |
| # | Batch | Recovered Solvent (g) | Mass of Delaminated Materials Recovered (g) |
|---|---|---|---|
| 1 | 60 °C | 890.8 | 30.1 |
| 2 | 865.7 | 49.8 | |
| 3 | 869.2 | 41.4 | |
| 4 | 885.3 | 50.7 | |
| 5 | - | 29.8 | |
| 6 | 70 °C | 850.0 | 40.5 |
| 7 | 867.5 | 50.3 | |
| 8 | 884.2 | 39.7 | |
| 9 | - | 27.4 | |
| 10 | 80 °C | 863.9 | 40.3 |
| 11 | 831.0 | 50.6 | |
| 12 | 916.5 | 31.4 | |
| 13 | 843.4 | 29.9 | |
| 14 | - | 49.7 |
| Wavenumber/cm−1 | Group | Mode | Ref. | |
|---|---|---|---|---|
| PE | 2913 | C-H | stretch | [24] |
| 2848 | ||||
| 1470 | CH2 | bending | ||
| 718 | CH2 | rocking | ||
| PET | 1713 | C=O | stretch | [25] |
| 1409 | benzene ring | stretch | ||
| 1340 | CH2, trans | wagging | ||
| 1241 | C-C-O | asymmetric stretch | ||
| 1092 | C-O-C | stretch | ||
| 1016 | C-H, aromatic | bending | ||
| 723 | benzene ring | bending |
| # | Material | Tm/°C | ΔHm/°C | Δχc/% | Td/°C |
|---|---|---|---|---|---|
| 5 | PE | 123 | −138 | 47.5 | 458 |
| 8 | 121 | −140 | 48.3 | 461 | |
| 13 | 121 | −134 | 46.2 | 462 | |
| 5 | PET | 257 | −44 | 31.4 | 416 |
| 8 | 256 | −40 | 28.5 | 417 | |
| 13 | 260 | −45 | 32.1 | 416 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loukodimou, A.; Lovell, C.; Theodosopoulos, G.; Maniam, K.K.; Paul, S. Delamination and Evaluation of Multilayer PE/Al/PET Packaging Waste Separated Using a Hydrophobic Deep Eutectic Solvent. Polymers 2024, 16, 2718. https://doi.org/10.3390/polym16192718
Loukodimou A, Lovell C, Theodosopoulos G, Maniam KK, Paul S. Delamination and Evaluation of Multilayer PE/Al/PET Packaging Waste Separated Using a Hydrophobic Deep Eutectic Solvent. Polymers. 2024; 16(19):2718. https://doi.org/10.3390/polym16192718
Chicago/Turabian StyleLoukodimou, Adamantini, Christopher Lovell, George Theodosopoulos, Kranthi Kumar Maniam, and Shiladitya Paul. 2024. "Delamination and Evaluation of Multilayer PE/Al/PET Packaging Waste Separated Using a Hydrophobic Deep Eutectic Solvent" Polymers 16, no. 19: 2718. https://doi.org/10.3390/polym16192718
APA StyleLoukodimou, A., Lovell, C., Theodosopoulos, G., Maniam, K. K., & Paul, S. (2024). Delamination and Evaluation of Multilayer PE/Al/PET Packaging Waste Separated Using a Hydrophobic Deep Eutectic Solvent. Polymers, 16(19), 2718. https://doi.org/10.3390/polym16192718







