Manufacture of Bioplastics Prepared from Chitosan Functionalized with Callistemon citrinus Extract
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Methods
2.2.1. Enriched Fraction of Acidified Ethanol Extract of Callistemon citrinus Flowers (CCE) Preparation and Characterization
Preparation of Fortified Extract of Callistemon citrinus Flowers (CCE)
CCE Profile Characterization by Reverse Phase High-Performance Liquid Chromatography Coupled with Diode Array and Electrospray Mass Spectrometry Detection (RP-HPLC-DAD-ESI-MS/MS) Analysis
Determination of Total Phenolic Content
DPPH Radical Scavenging Activity
2.2.2. Film-Forming Solutions (FFSs) Preparation and Derived Films Characterization
Zeta Potential and Particle Size Measurements of the Film Forming Solutions (FFSs)
Casting of the Film-Forming Solutions (FFSs)
Film Mechanical Properties and Sealing Strength
Film Moisture Content and Uptake
Film Water Contact Angle (WCA)
Film Barrier Properties
Fourier Transform Infrared Spectroscopy (FTIR-ATR)
Film Antioxidant Properties
DPPH Assay
ABTS Radical Scavenging Assay
Ferric-Reducing Antioxidant Power (FRAP)
Total Antioxidant Capacity Assay (TAC)
2.2.3. Statistical Analysis
3. Results
3.1. Preparation, Identification, and Quantification of CCE by RP-HPLC-DAD-ESI-MS/MS
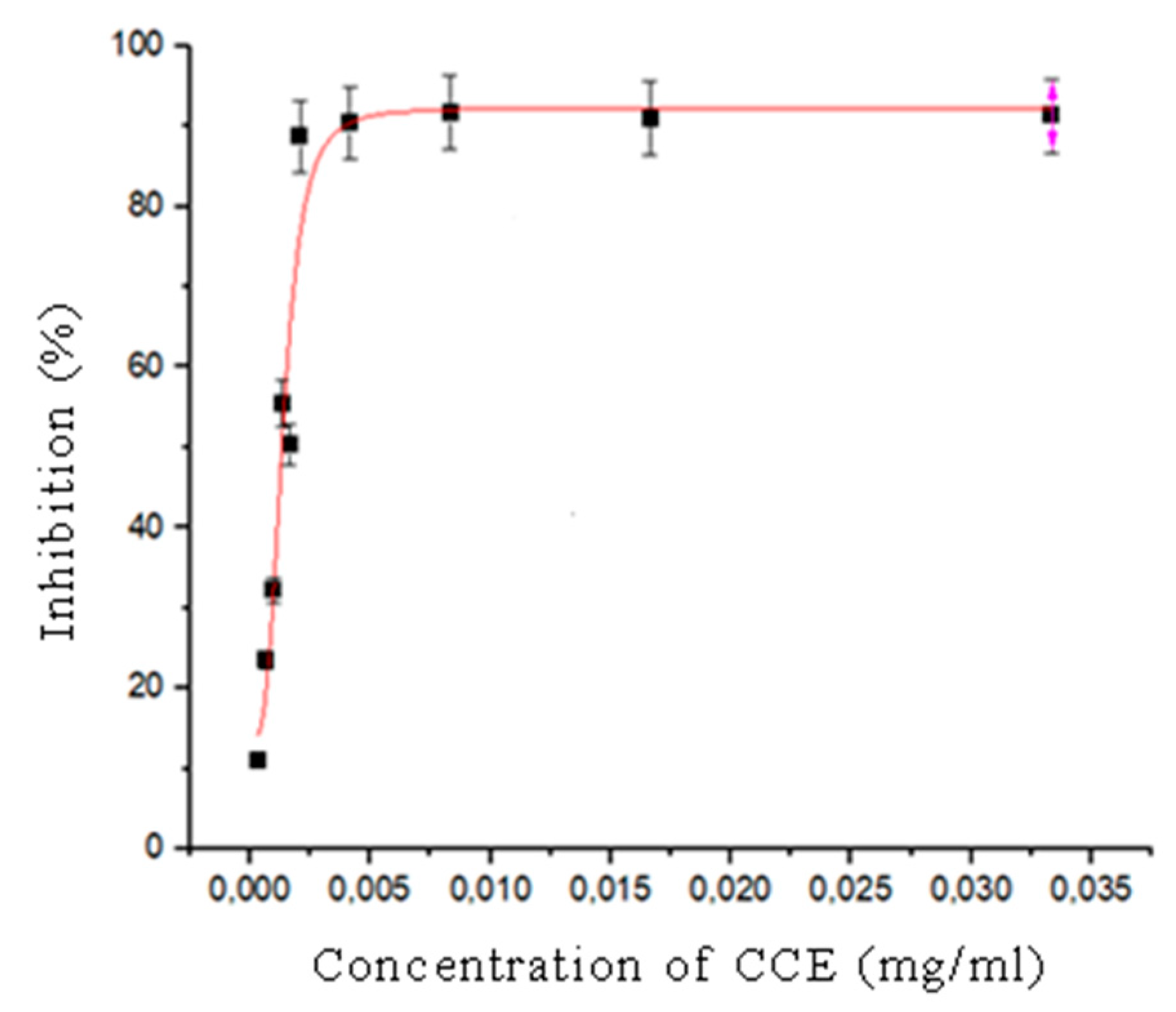
3.2. DPPH Radical Scavenging Activity of Callistemon citrinus Flower Extract
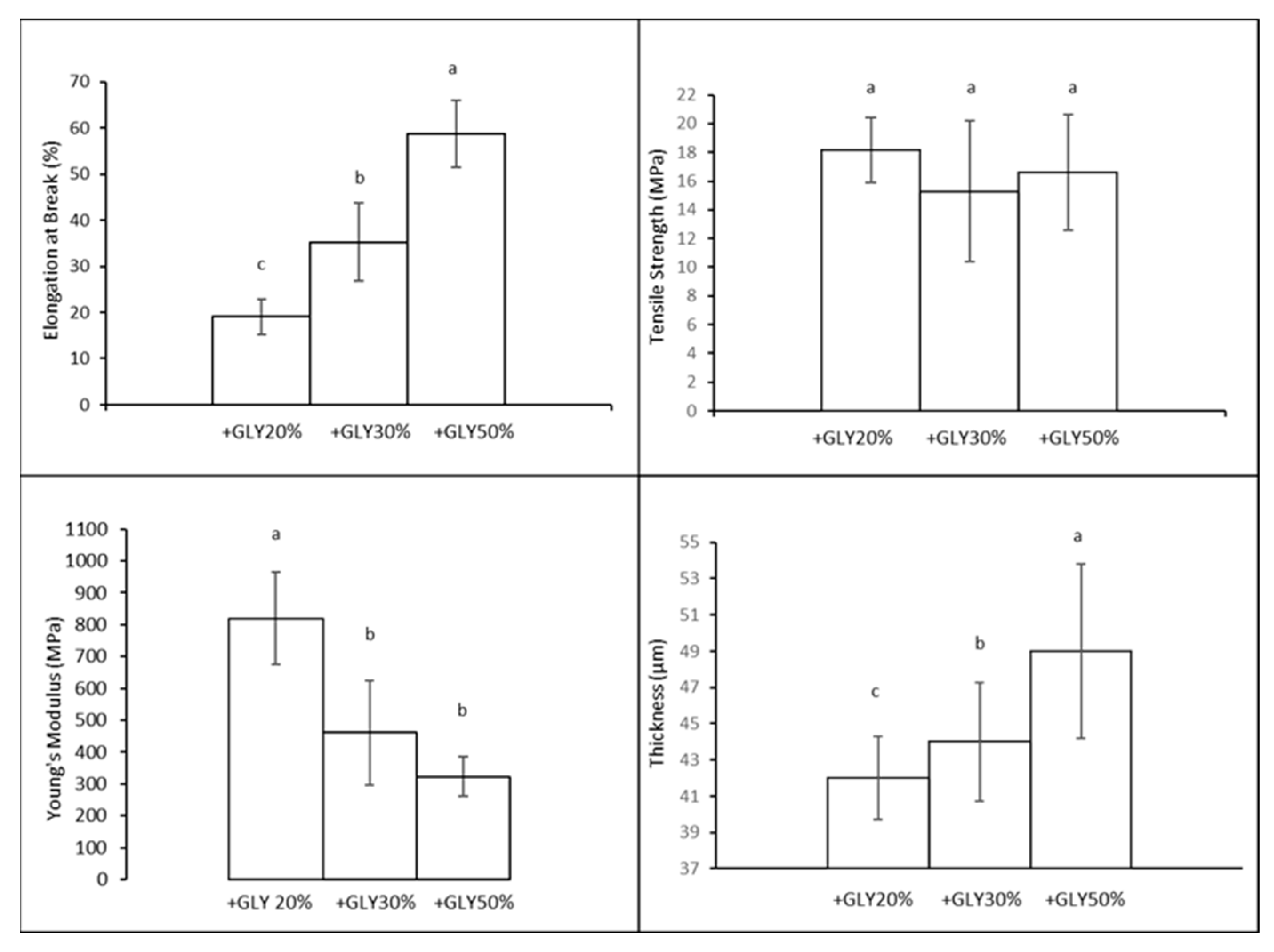
3.3. Manufacture of Films without CCE Prepared as Function of Different Concentrations of GLY
3.4. Characterization of Films
3.4.1. Mechanical Properties
3.4.2. Moisture Content and Moisture Uptake
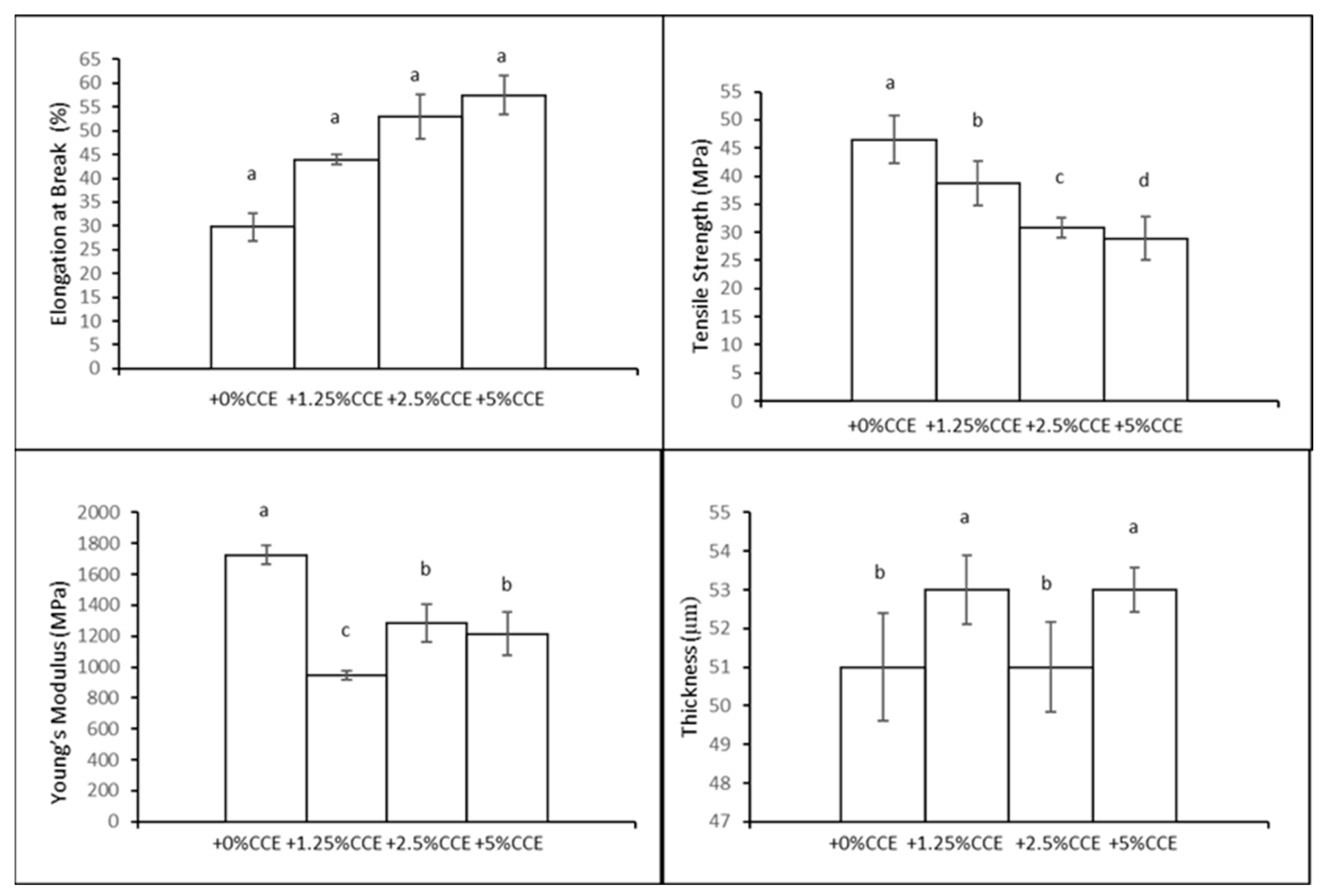
3.5. Manufacture of Films with CCE Prepared with 30% of Glycerol (GLY, w/w of CH)
3.5.1. Zeta Size and Zeta Potential of the Film-Forming Solutions (FFSs)
3.5.2. FT-IR Film
3.5.3. Film Mechanical Properties and Sealing Strength
3.5.4. Film Water Contact Angle (WCA)
3.5.5. Film Barrier Properties
3.5.6. Films Antioxidant Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reddy, R.L.; Reddy, V.S.; Gupta, G.A. Study of Bioplastics as Green and Sustainable Alternative to Plastics. Int. J. Emerg. Technol. Adv. Eng. 2013, 3, 82–89. [Google Scholar]
- Farajinejad, Z.; Karimi Sani, I.; Alizadeh, M.; Amiri, S. A Review of Recent Advances in the Photocatalytic Activity of Protein and Polysaccharide-Based Nanocomposite Packaging Films: Antimicrobial, Antioxidant, Mechanical, and Strength Properties. J. Polym. Environ. 2024, 32, 3437–3447. [Google Scholar] [CrossRef]
- MacLeod, M.; Arp, H.P.H.; Tekman, M.B.; Jahnke, A. The global threat from plastic pollution. Science 2021, 373, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cornish, K.; Vodovotz, Y. Narrowing the Gap for Bioplastic Use in Food Packaging: An update. Environ. Sci. Technol. 2020, 54, 4712–4732. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; Morici, E. Recovery of rose flower waste to formulate eco-friendly biopolymer packaging films. Molecules 2023, 28, 3165. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Klunklin, W.; Jantrawut, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Seesuriyachan, P.; Leksawasdi, N.; Chaiyaso, T.; Ruksiriwanich, W.; Phongthai, S.; et al. Characterization of chitosan film incorporated with curcumin extract. Polymers 2021, 13, 963. [Google Scholar] [CrossRef]
- Xie, C.; Wang, F.; He, Z.; Tang, H.; Li, H.; Hou, J.; Liu, Y.; Jiang, L. Development and characterization of active packaging based on chitosan/chitin nanofibers incorporated with scallion flower extract and its preservation in fresh-cut bananas. Int. J. Biol. Macromol. 2023, 242, 125045. [Google Scholar] [CrossRef]
- Dìaz-Montes, E.; Castro-Munoz, R. Edible films and coating as food-quality preserves: An overview. Foods 2021, 10, 249. [Google Scholar] [CrossRef]
- Jorda, J.J.; Casem, D.T.; Bradley, J.M.; Dwivedi, A.K.; Brown, E.N.; Jordan, C.W. Mechanical properties of low density polyethylene. J. Dyn. Behav. Mater. 2016, 2, 411–420. [Google Scholar] [CrossRef]
- Shebani, A.; Klash, A.; Elhabishi, R.; Abdsalam, S.; Elbreki, H.; Elhrari, W. The influence of LDPE content on the mechanical properties of HDPE/LDPE blends. Res. Dev. Mater. Sci. 2018, 7, 791–797. [Google Scholar] [CrossRef]
- Phan The, D.; Debeaufort, F.; Voilley, A.; Luu, D. Biopolymer interactions affect the functional properties of edible films based on agar, cassava starch and arabinoxylan blends. J. Food Eng. 2009, 90, 548–558. [Google Scholar] [CrossRef]
- Fiallos-Nunez, J.; Cardero, Y.; Cabrera-Barjas, G.; Garcìa-Herrera, C.M.; Inostroza, M.; Estevez, M.; Espana-Sànchez, B.L.; Valenzuela, L.M. Eco-friendly design of chitosan-based films with biodegradable properties as an alternative to low-density polyethylene packaging. Polymers 2024, 16, 2471. [Google Scholar] [CrossRef] [PubMed]
- Xuan Tan, S.; Andriyana, A.; Chyuan Ong, H.; Lim, S.; Ling Pang, Y.; Cheng Ngoh, G. A comprehensive review on the emerging roles of nanofillers and plasticizers towards sustainable starch-based bioplastics fabrication. Polymers 2022, 14, 664. [Google Scholar] [CrossRef] [PubMed]
- Ling Tan, Y.; Peng Teoh, Y.; Xian Ooi, Z.; Hoong Shuit, S.; Hwa Ng, Q.; Yong Hoo, P.; Siong Leong, S.; Yu Low, C. Effect of glycerol as Plasticizing Agent on the Mechanical Properties of Polyvinyl Alcohol/Banana Peel Powder Blended Film. Emerg. Technol. Future Sustain. 2023, 2, 375–389. [Google Scholar] [CrossRef]
- Nechita, P.; Roman, M.; Nastac, S.M. Green approaches on modification of xylene hemicellulose to enhance the functional properties for food packaging materials—A review. Polymers 2023, 15, 2088. [Google Scholar] [CrossRef] [PubMed]
- Sanyang, M.L.; Sapuan, M.S.; Jawaid, M.; Ishak, M.R.; Sahari, J. Effect of plasticizer type and concentration on tensile, thermal and barrier properties of biodegradable films based on sugar palm (Arenga pinnata) starch. Polymers 2015, 7, 1106–1124. [Google Scholar] [CrossRef]
- Suyatma, N.E.; Tighzert, L.; Copinet, A. Effect of hydrophilic plasticizers on mechanical, thermal and surface properties of chitosan films. J. Agric. Food Chem. 2005, 53, 3950–3957. [Google Scholar] [CrossRef]
- Calva-Estrada, S.; Jimenez, M.; Lugo, E. Protein-Based Films: Advances in the Development of Biomaterials Applicable to Food Packaging. Food Eng. Rev. 2019, 11, 78–92. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Pramanik, S.; Abdelgawad, M.A.; Abualsoud, B.M.; Kadu, A.; Ansari, M.J.; Deepak, A. Recent Advances of Chitosan Formulations in Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 10975. [Google Scholar] [CrossRef]
- Fayemi, P.O.; Öztürk, I.; Özcan, C.; Muguruma, M.; Yetim, H.; Sakata, R.; Ahhmed, A. Antimicrobial activity of extracts of Callistemon citrinus flowers and leaves against Listeria monocytogenes in beef burger. J. Food Meas. Charact. 2017, 11, 924–929. [Google Scholar] [CrossRef]
- Laganà, G.; Barreca, D.; Smeriglio, A.; Germanò, M.P.; D’Angelo, V.; Calderaro, A.; Bellocco, E.; Trombetta, D. Evaluation of Anthocyanin Profile, Antioxidant, Cytoprotective, and Anti-Angiogenic Properties of Callistemon citrinus Flowers. Plants 2020, 9, 1045. [Google Scholar] [CrossRef] [PubMed]
- Altemini, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods. 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Mirpoor, S.F.; Giosafatto, C.V.L.; Di Girolamo, R.; Famiglietti, M.; Porta, R. Hemp (Cannabis sativa) seed oilcake as a promising by-product for developing protein-based films: Effect of transglutaminase-induced crosslinking. Food Packag. Shelf Life 2022, 31, 100779. [Google Scholar] [CrossRef]
- ASTM D882-18; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 1997.
- Giosafatto, C.V.L.; Di Pierro, P.; Gunning, P.; Mackie, A.; Porta, R.; Mariniello, L. Characterization of Citrus pectin edible films containing transglutaminase-modified phaseolin. Carbohydr. Polym. 2014, 106, 200–208. [Google Scholar] [CrossRef]
- Al-Asmar, A.; Giosafatto, C.V.L.; Sabbah, M.; Sanchez, A.; Villalonga Santana, R.; Mariniello, L. Effect of Mesoporous Silica Nanoparticles on The Physicochemical Properties of Pectin Packaging Material for Strawberry Wrapping. Nanomaterials 2020, 10, 52. [Google Scholar] [CrossRef]
- Mirpoor, S.F.; Zannini, D.; Santagata, G.; Giosafatto, C.V.L. Cardoon seed oil cake proteins ad substrate for microbial transglutaminase: Their application as matrix for bio-based packaging to extend the shelf-life of peanuts. Food Hydrocoll. 2024, 147, 109339. [Google Scholar] [CrossRef]
- ASTM D3985-05; Standard Test Method for Oxygen Gas Transmission Rate Through Plastic Film and Sheeting Using a Coulometric Sensor. ASTM International: West Conshohocken, PA, USA, 2010.
- ASTM F2476-05; Standard Test Method for the Determination of Carbon Dioxide Gas Transmission Rate Through Barrier Materials Using an Infrared Detector. ASTM International: West Conshohocken, PA, USA, 2005.
- ASTM F1249-13; Standard Test Method for Water Vapor Transmission Rate Through Plastic Film and Sheeting Using a Modulated Infrared Sensor. ASTM International: West Conshohocken, PA, USA, 2013.
- Barreca, D.; Laganà, G.; Leuzzi, U.; Smeriglio, A.; Trombetta, D.; Bellocco, E. Evaluation of the nutraceutical, antioxidant and cytoprotective properties of ripe pistachio (Pistacia vera L., variety Bronte) hulls. Food Chem. 2016, 196, 493–502. [Google Scholar] [CrossRef]
- Farahnaky, A.; Saberi, B.; Majzoobi, M. Effect of Glycerol on Physical and Mechanical Properties of Wheat Starch Edible Films. J. Texture Stud. 2013, 44, 176–186. [Google Scholar] [CrossRef]
- Tan, X.T.; Ong, H.C.; Andriyana, A.; Lim, S.; Pang, Y.L.; Kusumo, F.; Ngoh, G.C. Characterization and parametric study on mechanical properties enhancement in biodegradable chitosan-reinforced starch-based bioplastic film. Polymers 2022, 14, 278. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Wronska, N.; Katir, N.; Nowak-Lange, M.; El Kadib, A.; Lisowska, K. Biodegradable chitosan-based films as an alternative to plastic packaging. Foods 2023, 12, 3519. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y. Development of chitosan-based films incorporated with chestnut flower essential oil that possess good anti-ultraviolet radiation and antibacterial effects for banana storage. Coatings 2024, 15, 548. [Google Scholar] [CrossRef]
- Lòpez-Mata, M.A.; Ruiz-Cruz, S.; Ornelas-Paz, J.J.; Del Toro-Sànchez, C.L.; Màrquez-Rìos, E.; Silva-Beltràn, N.P.; Cira-Chàvez, L.A.; Burruel-Ibarra, S.E. Mechanical, barrier and antioxidant properties of chitosan films incorporating cinnamaldehyde. J. Polym. Environ. 2018, 26, 452–461. [Google Scholar] [CrossRef]
- Azanha de Carvalho, F.; Moreira, A.A.; Martinez de Oliveria, A.L.; Yamashita, F. Biodegradation of poly(lactic acid)-cassava bagasse composites produced by injection molding. J. Appl. Polym. Sci. 2021, 138, 50667. [Google Scholar] [CrossRef]
- Prus-Walendziak, W.; Kozlowska, J. Design of sodium alginate/gelatin-based emulsion film fused with polylactide microparticles charged with plant extract. Materials 2021, 14, 745. [Google Scholar] [CrossRef]
- Goudar, N.; Vanjeri, V.N.; Masti, S.P.; Chougale, R.B. Spathodea campanulata bud fluid reinforced mechanical, hydrophilicity and degradation studies of poly (vinyl alcohol) matrix. Appl. Sci. 2020, 2, 568. [Google Scholar] [CrossRef]
- Raza, Z.A.; Khatoon, R.; Banat, I.M. Altering the Hydrophobic/Hydrophilic Nature of Bioplastic Surfaces for Biomedical Applications. Bioplastics Sustain. Dev. 2021, 15, 431–466. [Google Scholar] [CrossRef]
- Laboulfie, F.; Hèmati, M.; Lamure, A.; Diguet, S. Effect of the plasticizer on permeability, mechanical resistance and thermal behavior of composite coating films. Powder Technol. 2013, 238, 14–19. [Google Scholar] [CrossRef]
- Subramani, G.; Manian, R. Bioactive chitosan films: Integrating antibacterial, antioxidant, and antifungal properties in food packaging. Int. J. Biol. Macromol. 2024, 278, 134596. [Google Scholar] [CrossRef]





| Rt | Compounds | Fragmentation Pattern | Quantification |
|---|---|---|---|
| 17.8 | Cyanidin 3,5-O-diglucoside | MS:611; MS:611, 449, 287 | 255.27 ± 2.39 |
| 20.4 | Peonidin-3,5-O-diglucoside | MS:625; MS:625, 463, 301 | 187.22 ± 2.35 |
| 22.7 | Cyanidin-3-O-glucoside | MS:449; MS:449, 287 | 33.13 ± 0.85 |
| 25.1 | Cyanidin-coumaroylglucoside-pyruvic acid | MS:661; MS:661, 595, 482 | 10.81 ± 0.78 |
| Moisture Content (%) | Moisture Uptake (%) | |
|---|---|---|
| +20% GLY | 16.57 ± 0.002 c | 12.44 ± 0.002 a |
| +30% GLY | 20.19 ± 0.003 b | 8.98 ± 0.001 b |
| +50% GLY | 39.90 ± 0.009 a | 6.98 ± 0.001 c |
| T0 | Z-Size (d.nm) | Z-Potential (mV) | PDI |
| FFS + 0% CCE | 1239 ±166 a | 36.2 ± 3.1 a | 0.52 ± 0.02 a |
| FFS + 1.25% CCE | 1221 ± 91 a | 35.0 ± 0.4 a | 0.47 ± 0.04 b |
| FFS + 2.5% CCE | 995 ± 12 b | 35.7 ± 1.9 a | 0.54 ± 0.01 a,b |
| FFS + 5% CCE | 992 ± 33 b | 40.1 ± 3.7 a | 0.53 ± 0.02 a,b |
| T2 | Z-Size (d.nm) | Z-Potential (mV) | PDI |
| FFS + 0% CCE | 1189 ± 96 a | 41.6 ± 5.0 a | 0.73 ± 0.06 b,c |
| FFS + 1.25% CCE | 985 ± 192 a,b | 34.7 ± 3.9 b | 0.72 ± 0.10 b |
| FFS + 2.5% CCE | 758 ± 250 b | 42.1 ± 1.6 a | 0.55 ± 0.01 a |
| FFS + 5% CCE | 754 ± 87 b | 41.7 ± 0.6 a | 0.67 ± 0.05 c |
| T4 | Z-Size (d.nm) | Z-Potential (mV) | PDI |
| FFS + 0% CCE | 1060 ± 47 a | 45.2 ± 2.6 a,b | 0.65 ± 0.12 a |
| FFS + 1.25% CCE | 851 ± 107 b | 33.5 ± 18.0 c | 0.61 ± 0.06 b |
| FFS + 2.5% CCE | 828 ± 112 b | 40.3 ± 4.9 b | 0.69 ± 0.09 a,b |
| FFS + 5% CCE | 810 ± 6 b | 46.1 ± 0.9 a | 0.64 ± 0.13 a,b |
| T6 | Z-Size (d.nm) | Z-Potential (mV) | PDI |
| FFS + 0% CCE | 954 ± 52 a,b | 40.3 ± 5.7 a | 0.52 ± 0.03 a |
| FFS + 1.25% CCE | 1069 ± 43 a | 39.9 ± 2.0 a | 0.59 ± 0.03 a |
| FFS + 2.5% CCE | 886 ± 57 b,c | 41.3 ± 4.4 a | 0.57 ± 0.01 a |
| FFS + 5% CCE | 772 ± 98 c | 46.4 ± 3.2 a | 0.56 ± 0.06 a |
| Film | Sealing Strength (N/cm) |
|---|---|
| +0% CCE | 0.82 ± 0.04 a |
| +1.25% CCE | 0.73 ± 0.03 b |
| +2.5% CCE | 0.73 ± 0.05 b |
| +5% CCE | 1.20 ± 0.1 a |
| LDPE | 5.35 ± 0.13 c |
| Film Type | 0 s | 30 s |
|---|---|---|
| +0% CCE | 90.48 ± 3.4 b | n.a. |
| +1.25% CCE | 106.3 ± 4.3 a | n.a. |
| +2.5% CCE | 86.52 ± 3.7 b | n.a. |
| +5% CCE | 114.80 ± 14.3 a | 101.22 ± 7.3 a |
| CO2 (cm3 mm m−2 d −1 kPa−1) | WVP (g mm m−2 d −1 kPa−1) | |
|---|---|---|
| LDPE | 14.09 ± 0.05 a | 0.06 ± 0.01 b |
| +0% CCE | 7.26 ± 0.5 b | 10.85 ± 0.50 b |
| +1.25% CCE | 0.57 ± 0.01 c | 14.09 ± 0.61 a |
| +2.5% CCE | 0.27 ± 0.02 d | 16.01 ± 0.40 a |
| +5% CCE | 0.16 ± 0.01 d | 13.64 ± 0.52 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avitabile, M.; Mirpoor, S.F.; Esposito, S.; Merola, G.; Mariniello, L.; Patanè, G.T.; Barreca, D.; Giosafatto, C.V.L. Manufacture of Bioplastics Prepared from Chitosan Functionalized with Callistemon citrinus Extract. Polymers 2024, 16, 2693. https://doi.org/10.3390/polym16192693
Avitabile M, Mirpoor SF, Esposito S, Merola G, Mariniello L, Patanè GT, Barreca D, Giosafatto CVL. Manufacture of Bioplastics Prepared from Chitosan Functionalized with Callistemon citrinus Extract. Polymers. 2024; 16(19):2693. https://doi.org/10.3390/polym16192693
Chicago/Turabian StyleAvitabile, Marika, Seyedeh Fatemeh Mirpoor, Sefora Esposito, Giusi Merola, Loredana Mariniello, Giuseppe Tancredi Patanè, Davide Barreca, and Concetta Valeria Lucia Giosafatto. 2024. "Manufacture of Bioplastics Prepared from Chitosan Functionalized with Callistemon citrinus Extract" Polymers 16, no. 19: 2693. https://doi.org/10.3390/polym16192693
APA StyleAvitabile, M., Mirpoor, S. F., Esposito, S., Merola, G., Mariniello, L., Patanè, G. T., Barreca, D., & Giosafatto, C. V. L. (2024). Manufacture of Bioplastics Prepared from Chitosan Functionalized with Callistemon citrinus Extract. Polymers, 16(19), 2693. https://doi.org/10.3390/polym16192693










