Biomedical Composites of Polycaprolactone/Hydroxyapatite for Bioplotting: Comprehensive Interpretation of the Reinforcement Course
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Information
2.2. Composite Preparation, Mixtures, Filaments and Pellets, and Testing of Filaments
2.3. Manufacturing of Three-Dimensional Printing Samples
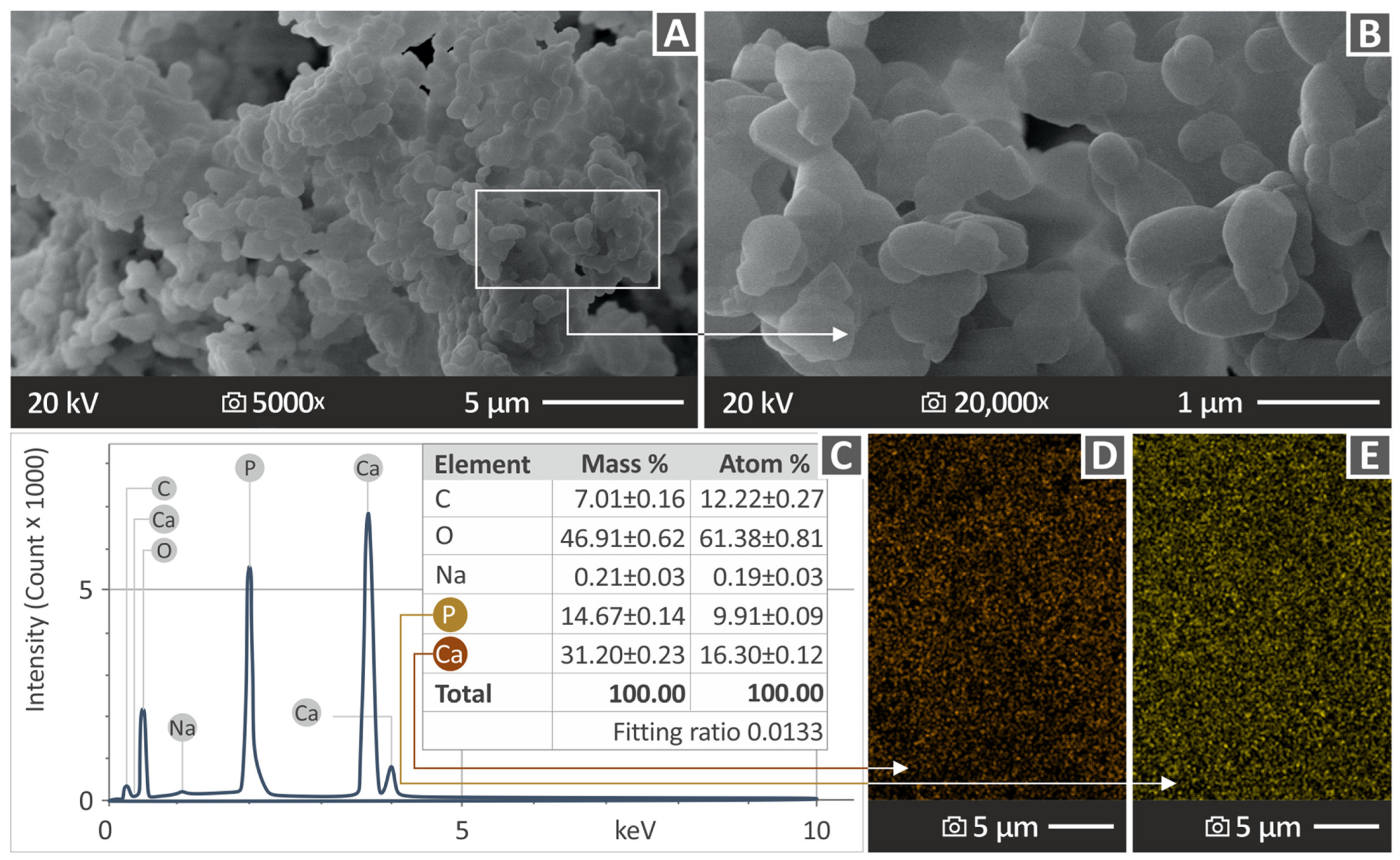
2.4. Morphological and Elemental Examination of the Parts
2.5. Raman Spectral Evaluation
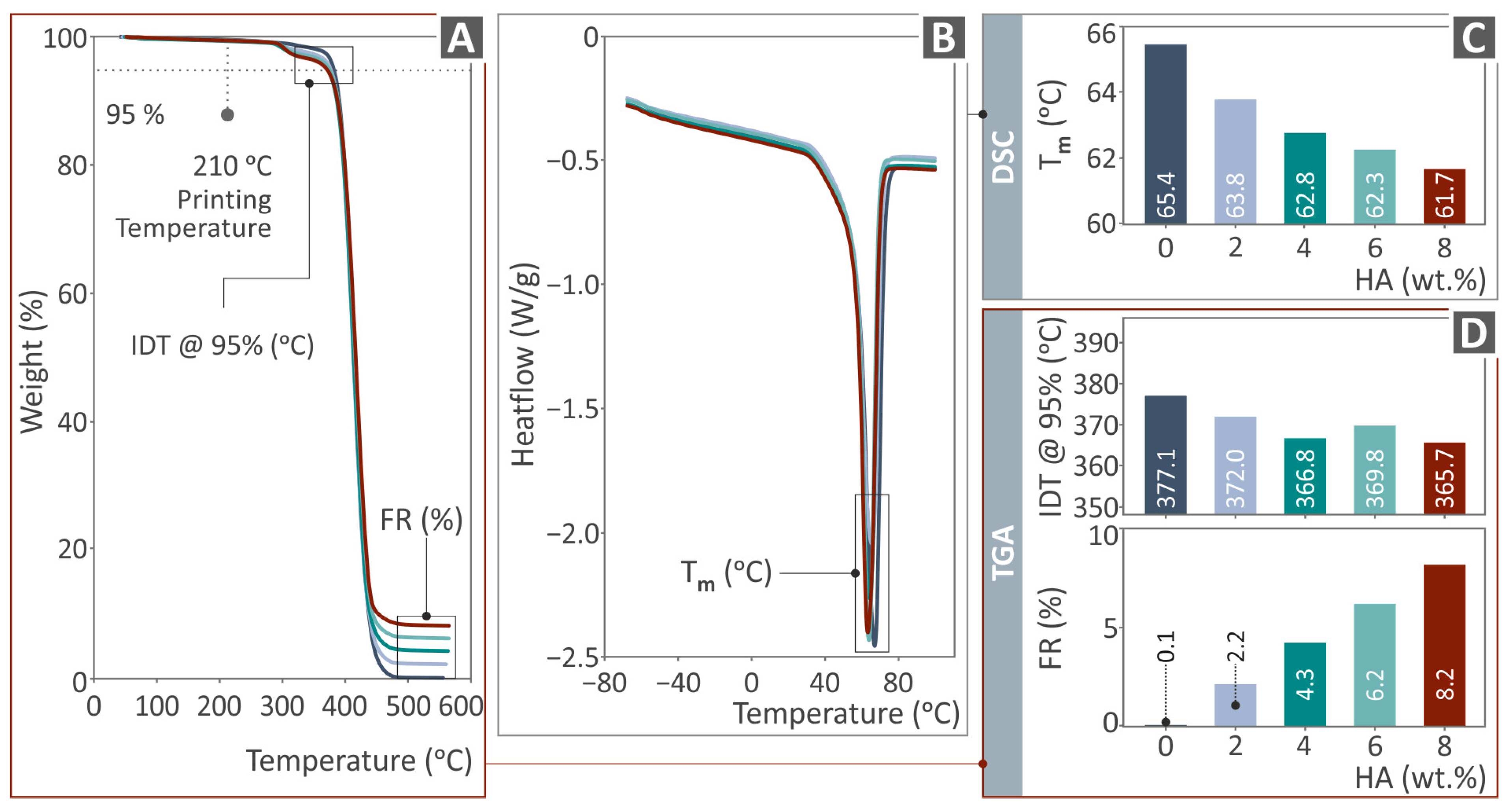
2.6. Conduction of Thermal Analysis
2.7. μ-CT Analysis
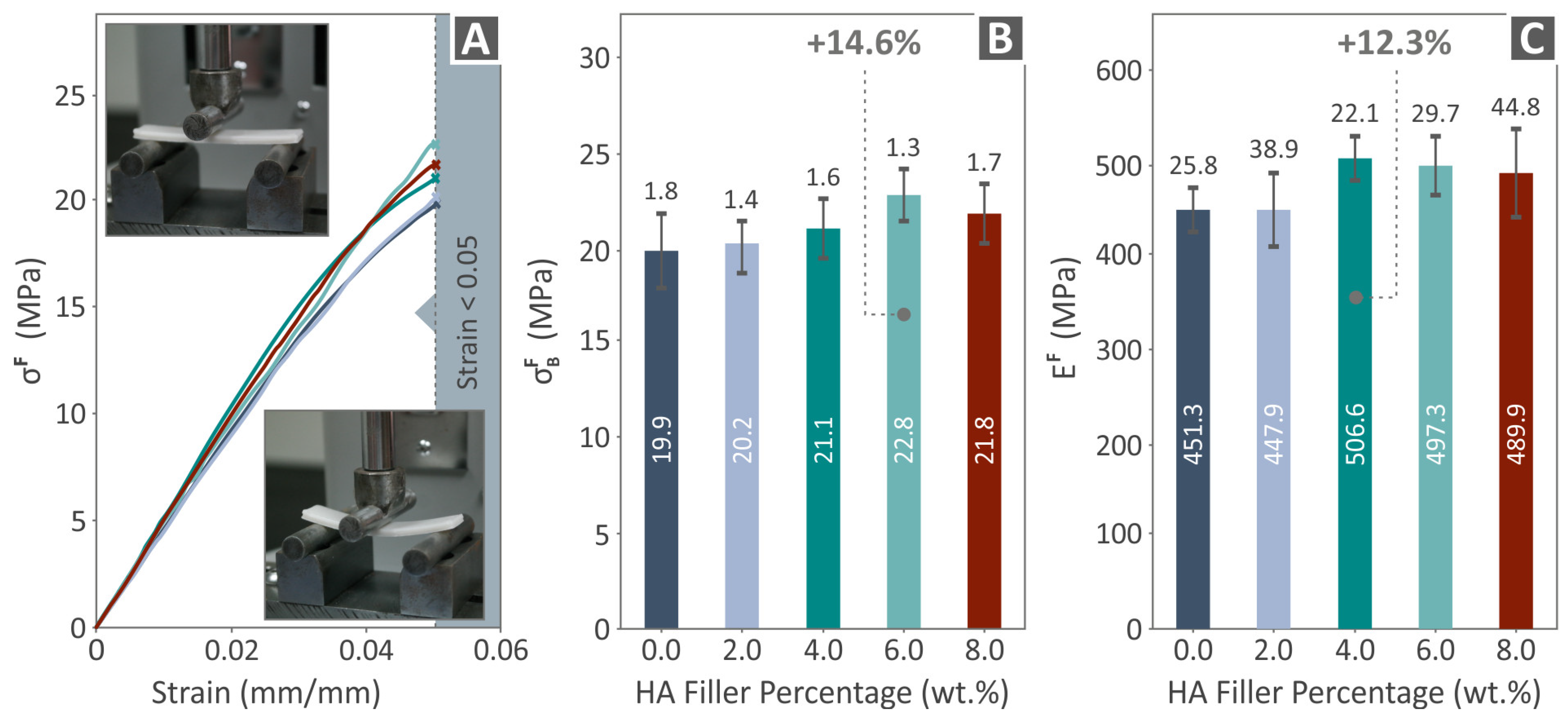
2.8. Mechanical Equipment and Settings
3. Results
3.1. Raman Spectroscopy and Spectral Differences
3.2. Thermal Characterization
3.3. Viscosity and MFR (Rheological Properties)
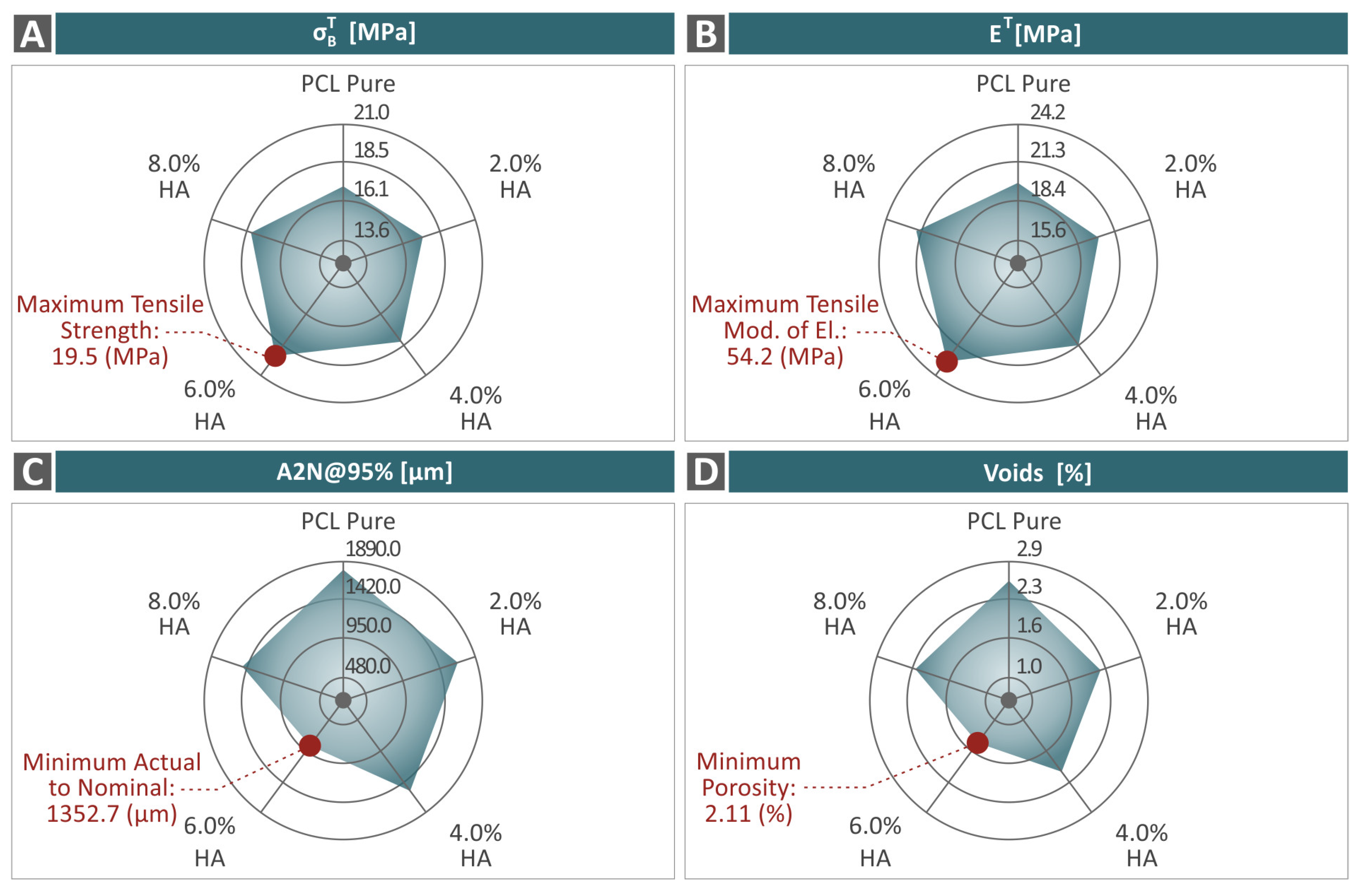
3.4. Mechanical Response of 3D-Printed Examples
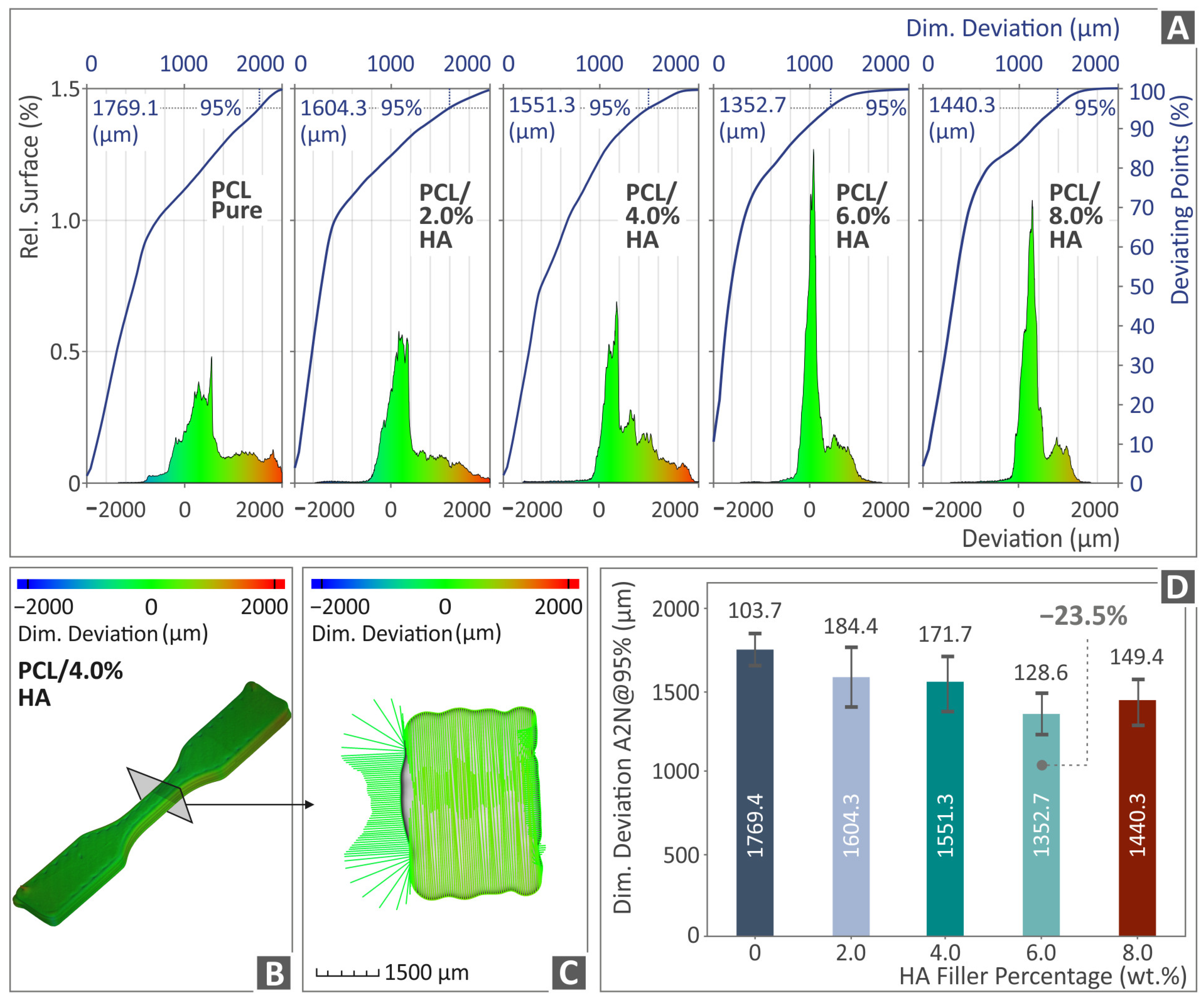
3.5. Tomography Results of 3D-Bioplotted Specimens
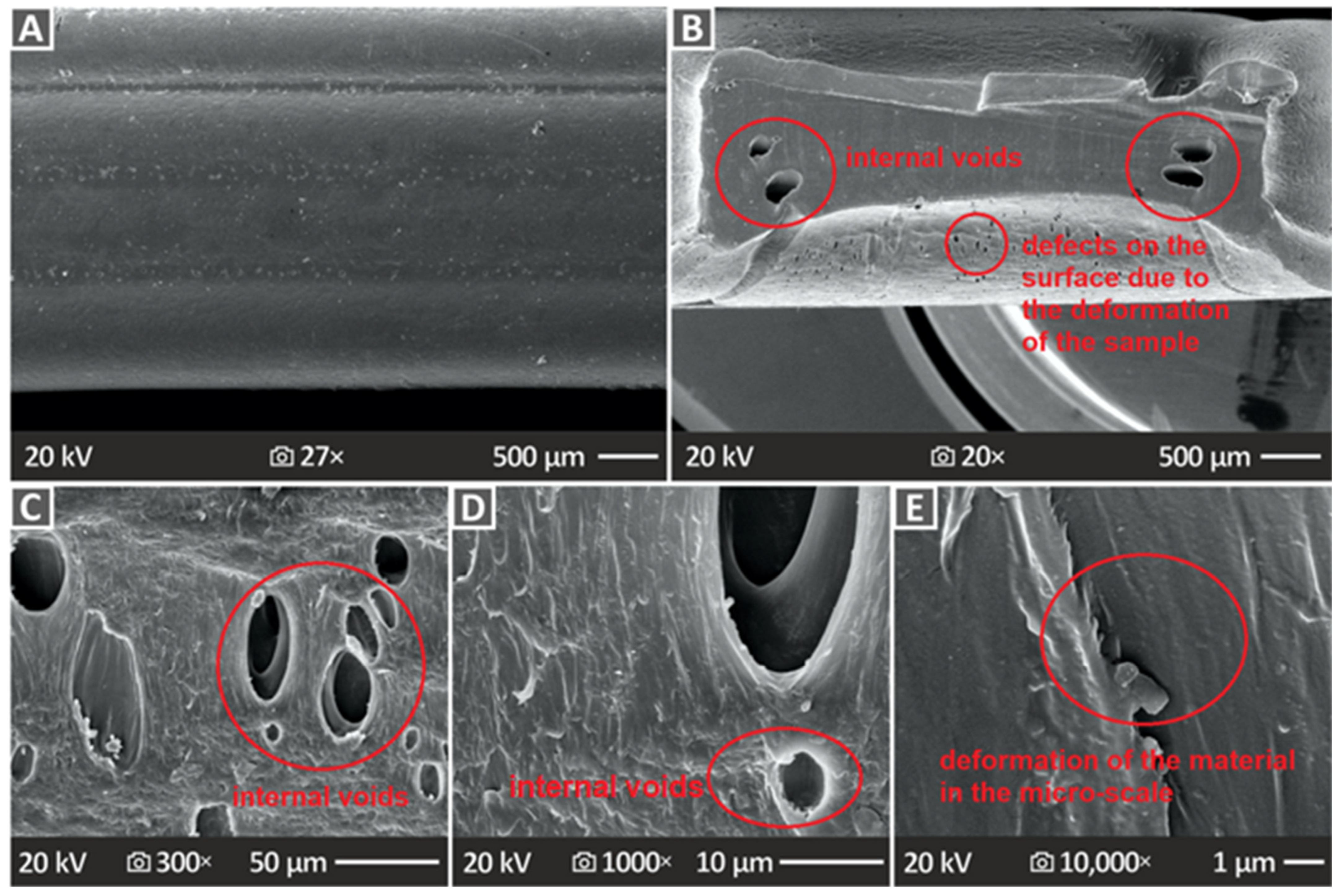
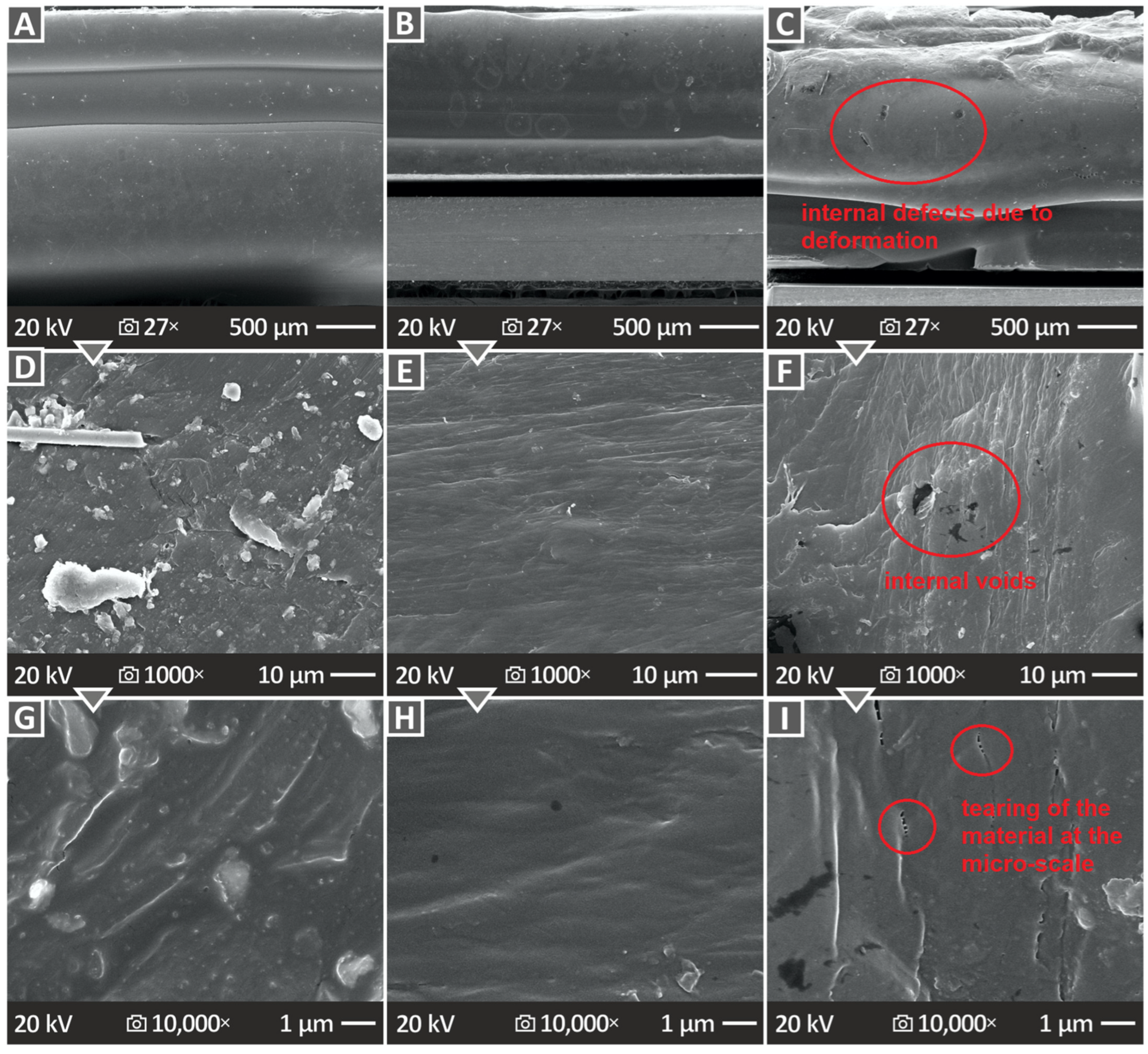
3.6. Three-Dimensional-Printed Samples’ SEM Morphological Analysis
4. Discussion
5. Conclusions
- The introduction of HAp in the PCL matrix did not negatively affect the behavior of the polymer, as the characterization process revealed;
- The 6.0 wt. % composite overall was the optimum loading in the study in terms of mechanical reinforcement;
- There was a connection found between the mechanical performance and both the porosity and dimensional accuracy of the samples;
- The study proposed a biocompatible PCL/HAp composite for bioplotting, with robust mechanical performance, which can be an asset for medical-related applications in tissue engineering and scaffolding.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gharibshahian, M.; Salehi, M.; Beheshtizadeh, N.; Kamalabadi-Farahani, M.; Atashi, A.; Nourbakhsh, M.-S.; Alizadeh, M. Recent Advances on 3D-Printed PCL-Based Composite Scaffolds for Bone Tissue Engineering. Front. Bioeng. Biotechnol. 2023, 11, 1168504. [Google Scholar] [CrossRef] [PubMed]
- Haffner, M.; Quinn, A.; Hsieh, T.; Strong, E.B.; Steele, T. Optimization of 3D Print Material for the Recreation of Patient-Specific Temporal Bone Models. Ann. Otol. Rhinol. Laryngol. 2018, 127, 338–343. [Google Scholar] [CrossRef] [PubMed]
- El-Habashy, S.E.; El-Kamel, A.H.; Essawy, M.M.; Abdelfattah, E.-Z.A.; Eltaher, H.M. Engineering 3D-Printed Core–Shell Hydrogel Scaffolds Reinforced with Hybrid Hydroxyapatite/Polycaprolactone Nanoparticles for in Vivo Bone Regeneration. Biomater. Sci. 2021, 9, 4019–4039. [Google Scholar] [CrossRef]
- Michailidis, N.; Petousis, M.; Moutsopoulou, A.; Argyros, A.; Ntintakis, I.; Papadakis, V.; Nasikas, N.K.; Vidakis, N. Engineering Response of Biomedical Grade Isotactic Polypropylene Reinforced with Titanium Nitride Nanoparticles for Material Extrusion Three-Dimensional Printing. Eur. J. Mater. 2024, 4, 2340944. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Michailidis, N.; Mountakis, N.; Papadakis, V.; Argyros, A.; Charou, C. Medical Grade Polyamide 12 Silver Nanoparticle Filaments Fabricated with In-Situ Reactive Reduction Melt-Extrusion: Rheological, Thermomechanical, and Bactericidal Performance in MEX 3D Printing. Appl. Nanosci. 2024, 14, 69–88. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Michailidis, N.; Mountakis, N.; Papadakis, V.; Argyros, A.; Charou, C. Polyethylene Glycol and Polyvinylpyrrolidone Reduction Agents for Medical Grade Polyamide 12/Silver Nanocomposites Development for Material Extrusion 3D Printing: Rheological, Thermomechanical, and Biocidal Performance. React. Funct. Polym. 2023, 190, 105623. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Michailidis, N.; Grammatikos, S.; David, C.N.; Mountakis, N.; Argyros, A.; Boura, O. Development and Optimization of Medical-Grade MultiFunctional Polyamide 12-Cuprous Oxide Nanocomposites with Superior Mechanical and Antibacterial Properties for Cost-Effective 3D Printing. Nanomaterials 2022, 12, 534. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Michailidis, N.; Papadakis, V.; Mountakis, N.; Argyros, A.; Dimitriou, E.; Charou, C.; Moutsopoulou, A. Polylactic Acid/Silicon Nitride Biodegradable and Biomedical Nanocomposites with Optimized Rheological and Thermomechanical Response for Material Extrusion Additive Manufacturing. Biomed. Eng. Adv. 2023, 6, 100103. [Google Scholar] [CrossRef]
- Vidakis, N.; Moutsopoulou, A.; Petousis, M.; Michailidis, N.; Charou, C.; Mountakis, N.; Argyros, A.; Papadakis, V.; Dimitriou, E. Medical-Grade PLA Nanocomposites with Optimized Tungsten Carbide Nanofiller Content in MEX Additive Manufacturing: A Rheological, Morphological, and Thermomechanical Evaluation. Polymers 2023, 15, 3883. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Michailidis, N.; David, C.; Saltas, V.; Sagris, D.; Spiridaki, M.; Argyros, A.; Mountakis, N.; Papadakis, V. Interpretation of the Optimization Course of Silicon Nitride Nano-Powder Content in Biomedical Resins for Vat Photopolymerization Additive Manufacturing. Ceram. Int. 2024, 50, 14919–14935. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Moutsopoulou, A.; Mountakis, N.; Grammatikos, S.; Papadakis, V.; Tsikritzis, D. Biomedical Engineering Advances Cost-Effective Bi-Functional Resin Reinforced with a Nano-Inclusion Blend for Vat Photopolymerization Additive Manufacturing: The Effect of Multiple Antibacterial Nanoparticle Agents. Biomed. Eng. Adv. 2023, 5, 100091. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Mountakis, N.; Papadakis, V.; Moutsopoulou, A. Mechanical Strength Predictability of Full Factorial, Taguchi, and Box Behnken Designs: Optimization of Thermal Settings and Cellulose Nanofibers Content in PA12 for MEX AM. J. Mech. Behav. Biomed. Mater. 2023, 142, 105846. [Google Scholar] [CrossRef] [PubMed]
- Vidakis, N.; Petousis, M.; David, C.N.; Sagris, D.; Mountakis, N. Biomedical Resin Reinforced with Cellulose Nanofibers (CNF) in VAT Photopolymerization (VPP) Additive Manufacturing (AM): The Effect of Filler Loading and Process Control Parameters on Critical Quality Indicators (CQIs). J. Manuf. Process 2023, 101, 755–769. [Google Scholar] [CrossRef]
- Liu, F.; Vyas, C.; Poologasundarampillai, G.; Pape, I.; Hinduja, S.; Mirihanage, W.; Bartolo, P. Structural Evolution of PCL during Melt Extrusion 3D Printing. Macromol. Mater. Eng. 2018, 303, 1700494. [Google Scholar] [CrossRef]
- Sharifi, F.; Atyabi, S.M.; Norouzian, D.; Zandi, M.; Irani, S.; Bakhshi, H. Polycaprolactone/Carboxymethyl Chitosan Nanofibrous Scaffolds for Bone Tissue Engineering Application. Int. J. Biol. Macromol. 2018, 115, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Thadavirul, N.; Pavasant, P.; Supaphol, P. Development of polycaprolactone porous scaffolds by combining solvent casting, particulate leaching, and polymer leaching techniques for bone tissue engineering. J. Biomed. Mater. Res. A 2013, 102, 3379–3392. [Google Scholar] [CrossRef]
- Carter, P.; Rahman, S.M.; Bhattarai, N. Facile Fabrication of Aloe Vera Containing PCL Nanofibers for Barrier Membrane Application. J. Biomater. Sci. Polym. Ed. 2016, 27, 692–708. [Google Scholar] [CrossRef]
- Rashidi, H.; Yang, J.; Shakesheff, K.M. Surface Engineering of Synthetic Polymer Materials for Tissue Engineering and Regenerative Medicine Applications. Biomater. Sci. 2014, 2, 1318–1331. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The Return of a Forgotten Polymer—Polycaprolactone in the 21st Century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Okamoto, M.; John, B. Synthetic Biopolymer Nanocomposites for Tissue Engineering Scaffolds. Prog. Polym. Sci. 2013, 38, 1487–1503. [Google Scholar] [CrossRef]
- Guo, B.; Ma, P.X. Synthetic Biodegradable Functional Polymers for Tissue Engineering: A Brief Review. Sci. China Chem. 2014, 57, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Backes, E.H.; Harb, S.V.; Beatrice, C.A.G.; Shimomura, K.M.B.; Passador, F.R.; Costa, L.C.; Pessan, L.A. Polycaprolactone Usage in Additive Manufacturing Strategies for Tissue Engineering Applications: A Review. J. Biomed. Mater. Res. B Appl. Biomater. 2022, 110, 1479–1503. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, J.; Liu, J.; Zhou, W.; Peng, S. Recent Progress of Preparation of Branched Poly(Lactic Acid) and Its Application in the Modification of Polylactic Acid Materials. Int. J. Biol. Macromol. 2021, 193, 874–892. [Google Scholar] [CrossRef] [PubMed]
- Arif, Z.U.; Khalid, M.Y.; Noroozi, R.; Sadeghianmaryan, A.; Jalalvand, M.; Hossain, M. Recent Advances in 3D-Printed Polylactide and Polycaprolactone-Based Biomaterials for Tissue Engineering Applications. Int. J. Biol. Macromol. 2022, 218, 930–968. [Google Scholar] [CrossRef] [PubMed]
- Haryńska, A.; Kucinska-Lipka, J.; Sulowska, A.; Gubanska, I.; Kostrzewa, M.; Janik, H. Medical-Grade PCL Based Polyurethane System for FDM 3D Printing—Characterization and Fabrication. Materials 2019, 12, 887. [Google Scholar] [CrossRef]
- Mochane, M.J.; Motsoeneng, T.S.; Sadiku, E.R.; Mokhena, T.C.; Sefadi, J.S. Morphology and Properties of Electrospun PCL and Its Composites for Medical Applications: A Mini Review. Appl. Sci. 2019, 9, 2205. [Google Scholar] [CrossRef]
- Espinoza, S.M.; Patil, H.I.; San Martin Martinez, E.; Casañas Pimentel, R.; Ige, P.P. Poly-ε-Caprolactone (PCL), a Promising Polymer for Pharmaceutical and Biomedical Applications: Focus on Nanomedicine in Cancer. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 85–126. [Google Scholar] [CrossRef]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-Based Materials in Biomedical Applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef]
- Augustine, R.; Nethi, S.K.; Kalarikkal, N.; Thomas, S.; Patra, C.R. Electrospun Polycaprolactone (PCL) Scaffolds Embedded with Europium Hydroxide Nanorods (EHNs) with Enhanced Vascularization and Cell Proliferation for Tissue Engineering Applications. J. Mater. Chem. B 2017, 5, 4660–4672. [Google Scholar] [CrossRef]
- Sayyar, S.; Murray, E.; Thompson, B.C.; Gambhir, S.; Officer, D.L.; Wallace, G.G. Covalently Linked Biocompatible Graphene/Polycaprolactone Composites for Tissue Engineering. Carbon 2013, 52, 296–304. [Google Scholar] [CrossRef]
- Kamath, M.S.; Ahmed, S.S.; Dhanasekaran, M.; Santosh, S.W. Polycaprolactone Scaffold Engineered for Sustained Release of Resveratrol: Therapeutic Enhancement in Bone Tissue Engineering. Int. J. Nanomed. 2013, 9, 183–195. [Google Scholar] [CrossRef]
- Plivelic, T.S.; Cassu, S.N.; do Carmo Gonçalves, M.; Torriani, I.L. Structure and Morphology of Poly(ε-Caprolactone)/Chlorinated Polyethylene (PCL/PECl) Blends Investigated by DSC, Simultaneous SAXS/WAXD, and Elemental Mapping by ESI-TEM. Macromolecules 2007, 40, 253–264. [Google Scholar] [CrossRef]
- Guo, T.; Holzberg, T.R.; Lim, C.G.; Gao, F.; Gargava, A.; Trachtenberg, J.E.; Mikos, A.G.; Fisher, J.P. 3D Printing PLGA: A Quantitative Examination of the Effects of Polymer Composition and Printing Parameters on Print Resolution. Biofabrication 2017, 9, 024101. [Google Scholar] [CrossRef] [PubMed]
- Bas, O.; De-Juan-Pardo, E.M.; Meinert, C.; D’Angella, D.; Baldwin, J.G.; Bray, L.J.; Wellard, R.M.; Kollmannsberger, S.; Rank, E.; Werner, C.; et al. Biofabricated Soft Network Composites for Cartilage Tissue Engineering. Biofabrication 2017, 9, 025014. [Google Scholar] [CrossRef]
- Koch, F.; Thaden, O.; Conrad, S.; Tröndle, K.; Finkenzeller, G.; Zengerle, R.; Kartmann, S.; Zimmermann, S.; Koltay, P. Mechanical Properties of Polycaprolactone (PCL) Scaffolds for Hybrid 3D-Bioprinting with Alginate-Gelatin Hydrogel. J. Mech. Behav. Biomed. Mater. 2022, 130, 105219. [Google Scholar] [CrossRef]
- Elomaa, L.; Keshi, E.; Sauer, I.M.; Weinhart, M. Development of GelMA/PCL and DECM/PCL Resins for 3D Printing of Acellular in Vitro Tissue Scaffolds by Stereolithography. Mater. Sci. Eng. C 2020, 112, 110958. [Google Scholar] [CrossRef] [PubMed]
- Eosoly, S.; Vrana, N.E.; Lohfeld, S.; Hindie, M.; Looney, L. Interaction of Cell Culture with Composition Effects on the Mechanical Properties of Polycaprolactone-Hydroxyapatite Scaffolds Fabricated via Selective Laser Sintering (SLS). Mater. Sci. Eng. C 2012, 32, 2250–2257. [Google Scholar] [CrossRef]
- Bruyas, A.; Lou, F.; Stahl, A.M.; Gardner, M.; Maloney, W.; Goodman, S.; Yang, Y.P. Systematic Characterization of 3D-Printed PCL/β-TCP Scaffolds for Biomedical Devices and Bone Tissue Engineering: Influence of Composition and Porosity. J. Mater. Res. 2018, 33, 1948–1959. [Google Scholar] [CrossRef]
- Cengiz, B.; Gokce, Y.; Yildiz, N.; Aktas, Z.; Calimli, A. Synthesis and Characterization of Hydroxyapatite Nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2008, 322, 29–33. [Google Scholar] [CrossRef]
- Lara-Ochoa, S.; Ortega-Lara, W.; Guerrero-Beltrán, C.E. Hydroxyapatite Nanoparticles in Drug Delivery: Physicochemistry and Applications. Pharmaceutics 2021, 13, 1642. [Google Scholar] [CrossRef]
- Hassan, I.; Sajad, P.; Majid, S.; Hassan, T. Serum Antioxidant Status in Patients with Systemic Sclerosis. Indian. J. Dermatol. 2013, 58, 239. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Xu, W.; Guo, X.; Zhang, Y.; Wei, Q.; Du, B. Synthesis of Strontium Hydroxyapatite Embedding Ferroferric Oxide Nano-Composite and Its Application in Pb2+ Adsorption. J. Mol. Liq. 2014, 197, 40–47. [Google Scholar] [CrossRef]
- Kong, L.; Gao, Y.; Lu, G.; Gong, Y.; Zhao, N.; Zhang, X. A Study on the Bioactivity of Chitosan/Nano-Hydroxyapatite Composite Scaffolds for Bone Tissue Engineering. Eur. Polym. J. 2006, 42, 3171–3179. [Google Scholar] [CrossRef]
- Degirmenbasi, N.; Kalyon, D.M.; Birinci, E. Biocomposites of Nanohydroxyapatite with Collagen and Poly(Vinyl Alcohol). Colloids Surf. B Biointerfaces 2006, 48, 42–49. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, C.; Li, Y.; Yan, Y.; Hou, C.; Wang, H.; Yang, Y.; Guan, G.; Feng, Q. The Preparation and In Vitro Evaluations of a Nanoscaled Injectable Bone Repair Material. J. Nanomater. 2015, 2015, 858493. [Google Scholar] [CrossRef]
- Marchi, J.; Dantas, A.C.S.; Greil, P.; Bressiani, J.C.; Bressiani, A.H.A.; Müller, F.A. Influence of Mg-Substitution on the Physicochemical Properties of Calcium Phosphate Powders. Mater. Res. Bull. 2007, 42, 1040–1050. [Google Scholar] [CrossRef]
- Koutsopoulos, S. Synthesis and Characterization of Hydroxyapatite Crystals: A Review Study on the Analytical Methods. J. Biomed. Mater. Res. 2002, 62, 600–612. [Google Scholar] [CrossRef] [PubMed]
- Ramay, H.R.; Zhang, M. Preparation of Porous Hydroxyapatite Scaffolds by Combination of the Gel-Casting and Polymer Sponge Methods. Biomaterials 2003, 24, 3293–3302. [Google Scholar] [CrossRef]
- Parhi, P.; Ramanan, A.; Ray, A.R. A Convenient Route for the Synthesis of Hydroxyapatite through a Novel Microwave-Mediated Metathesis Reaction. Mater. Lett. 2004, 58, 3610–3612. [Google Scholar] [CrossRef]
- Rodriguez, G.; Dias, J.; d’Ávila, M.A.; Bártolo, P. Influence of Hydroxyapatite on Extruded 3D Scaffolds. Procedia Eng. 2013, 59, 263–269. [Google Scholar] [CrossRef]
- Farag, M.M.; Yun, H. Effect of Gelatin Addition on Fabrication of Magnesium Phosphate-Based Scaffolds Prepared by Additive Manufacturing System. Mater. Lett. 2014, 132, 111–115. [Google Scholar] [CrossRef]
- Leukers, B.; Gülkan, H.; Irsen, S.H.; Milz, S.; Tille, C.; Schieker, M.; Seitz, H. Hydroxyapatite Scaffolds for Bone Tissue Engineering Made by 3D Printing. J. Mater. Sci. Mater. Med. 2005, 16, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.C.; Thornby, J.A.; Gibbons, G.J.; Williams, M.A.; Mallick, K.K. 3D Printing of Porous Hydroxyapatite Scaffolds Intended for Use in Bone Tissue Engineering Applications. Mater. Sci. Eng. C 2015, 47, 237–247. [Google Scholar] [CrossRef]
- Bogala, M.R. Three-Dimensional (3D) Printing of Hydroxyapatite-Based Scaffolds: A Review. Bioprinting 2022, 28, e00244. [Google Scholar] [CrossRef]
- Han, Y.; Wei, Q.; Chang, P.; Hu, K.; Okoro, O.V.; Shavandi, A.; Nie, L. Three-Dimensional Printing of Hydroxyapatite Composites for Biomedical Application. Crystals 2021, 11, 353. [Google Scholar] [CrossRef]
- Li, X.; Zhang, S.; Zhang, X.; Xie, S.; Zhao, G.; Zhang, L. Biocompatibility and Physicochemical Characteristics of Poly(Ɛ-Caprolactone)/Poly(Lactide-Co-Glycolide)/Nano-Hydroxyapatite Composite Scaffolds for Bone Tissue Engineering. Mater. Des. 2017, 114, 149–160. [Google Scholar] [CrossRef]
- Lee, J.-S.; Seol, Y.-J.; Sung, M.; Moon, W.; Kim, S.W.; Oh, J.-H.; Cho, D.-W. Development and Analysis of Three-Dimensional (3D) Printed Biomimetic Ceramic. Int. J. Precis. Eng. Manuf. 2016, 17, 1711–1719. [Google Scholar] [CrossRef]
- Jakus, A.E.; Shah, R.N. Multi and Mixed 3D-printing of Graphene-hydroxyapatite Hybrid Materials for Complex Tissue Engineering. J. Biomed. Mater. Res. A 2017, 105, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Bendtsen, S.T.; Quinnell, S.P.; Wei, M. Development of a Novel Alginate-polyvinyl Alcohol-hydroxyapatite Hydrogel for 3D Bioprinting Bone Tissue Engineered Scaffolds. J. Biomed. Mater. Res. A 2017, 105, 1457–1468. [Google Scholar] [CrossRef]
- Slots, C.; Jensen, M.B.; Ditzel, N.; Hedegaard, M.A.B.; Borg, S.W.; Albrektsen, O.; Thygesen, T.; Kassem, M.; Andersen, M.Ø. Simple Additive Manufacturing of an Osteoconductive Ceramic Using Suspension Melt Extrusion. Dent. Mater. 2017, 33, 198–208. [Google Scholar] [CrossRef]
- Wu, H.; Cheng, Y.; Liu, W.; He, R.; Zhou, M.; Wu, S.; Song, X.; Chen, Y. Effect of the Particle Size and the Debinding Process on the Density of Alumina Ceramics Fabricated by 3D Printing Based on Stereolithography. Ceram. Int. 2016, 42, 17290–17294. [Google Scholar] [CrossRef]
- Kim, I.-S.; Kumta, P.N. Sol–Gel Synthesis and Characterization of Nanostructured Hydroxyapatite Powder. Mater. Sci. Eng. B 2004, 111, 232–236. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, G.; Wang, Z.; Guo, H. Synthesis of Single-Crystal HAP Nanorods. Ceram. Int. 2006, 32, 951–954. [Google Scholar] [CrossRef]
- Koumoulidis, G.C.; Katsoulidis, A.P.; Ladavos, A.K.; Pomonis, P.J.; Trapalis, C.C.; Sdoukos, A.T.; Vaimakis, T.C. Preparation of Hydroxyapatite via Microemulsion Route. J. Colloid. Interface Sci. 2003, 259, 254–260. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, S.; Wei, K.; Zhao, N.; Chen, J.; Wang, X. Hydrothermal Synthesis of Hydroxyapatite Nanopowders Using Cationic Surfactant as a Template. Mater. Lett. 2006, 60, 1484–1487. [Google Scholar] [CrossRef]
- Han, J.-K.; Song, H.-Y.; Saito, F.; Lee, B.-T. Synthesis of High Purity Nano-Sized Hydroxyapatite Powder by Microwave-Hydrothermal Method. Mater. Chem. Phys. 2006, 99, 235–239. [Google Scholar] [CrossRef]
- Wang, F.; Li, M.-S.; Lu, Y.-P.; Qi, Y.-X.; Liu, Y.-X. Synthesis and Microstructure of Hydroxyapatite Nanofibers Synthesized at 37°C. Mater. Chem. Phys. 2006, 95, 145–149. [Google Scholar] [CrossRef]
- Cüneyt Tas, A. Synthesis of Biomimetic Ca-Hydroxyapatite Powders at 37°C in Synthetic Body Fluids. Biomaterials 2000, 21, 1429–1438. [Google Scholar] [CrossRef]
- Jiao, Z.; Luo, B.; Xiang, S.; Ma, H.; Yu, Y.; Yang, W. 3D Printing of HA / PCL Composite Tissue Engineering Scaffolds. Adv. Ind. Eng. Polym. Res. 2019, 2, 196–202. [Google Scholar] [CrossRef]
- Guerra, A.J.; Cano, P.; Rabionet, M.; Puig, T.; Ciurana, J. 3D-Printed PCL/PLA Composite Stents: Towards a New Solution to Cardiovascular Problems. Materials 2018, 11, 1679. [Google Scholar] [CrossRef]
- Ródenas-Rochina, J.; Ribelles, J.L.G.; Lebourg, M. Comparative Study of PCL-HAp and PCL-Bioglass Composite Scaffolds for Bone Tissue Engineering. J. Mater. Sci. Mater. Med. 2013, 24, 1293–1308. [Google Scholar] [CrossRef]
- Banimohamad-Shotorbani, B.; Rahmani Del Bakhshayesh, A.; Mehdipour, A.; Jarolmasjed, S.; Shafaei, H. The Efficiency of PCL/HAp Electrospun Nanofibers in Bone Regeneration: A Review. J. Med. Eng. Technol. 2021, 45, 511–531. [Google Scholar] [CrossRef]
- Hamlekhan, A.; Mozafari, M.; Nezafati, N.; Azami, M.; Hadipour, H. A Proposed Fabrication Method of Novel PCL-GEL-HAp Nanocomposite Scaffolds for Bone Tissue Engineering Applications. Adv. Compos. Lett. 2010, 19, 096369351001900401. [Google Scholar] [CrossRef]
- Qi, H.; Ye, Z.; Ren, H.; Chen, N.; Zeng, Q.; Wu, X.; Lu, T. Bioactivity Assessment of PLLA/PCL/HAP Electrospun Nanofibrous Scaffolds for Bone Tissue Engineering. Life Sci. 2016, 148, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Uma Maheshwari, S.; Samuel, V.K.; Nagiah, N. Fabrication and Evaluation of (PVA/HAp/PCL) Bilayer Composites as Potential Scaffolds for Bone Tissue Regeneration Application. Ceram. Int. 2014, 40, 8469–8477. [Google Scholar] [CrossRef]
- Lebourg, M.; Antón, J.S.; Ribelles, J.L.G. Hybrid Structure in PCL-HAp Scaffold Resulting from Biomimetic Apatite Growth. J. Mater. Sci. Mater. Med. 2010, 21, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Chuenjitkuntaworn, B.; Inrung, W.; Damrongsri, D.; Mekaapiruk, K.; Supaphol, P.; Pavasant, P. Polycaprolactone/Hydroxyapatite Composite Scaffolds: Preparation, Characterization, and in Vitro and in Vivo Biological Responses of Human Primary Bone Cells. J. Biomed. Mater. Res. A 2010, 94A, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Shokrollahi, P.; Mehmanchi, M.; Atai, M.; Omidian, H.; Shokrolahi, F. Effect of Interface on Mechanical Properties and Biodegradation of PCL HAp Supramolecular Nano-Composites. J. Mater. Sci. Mater. Med. 2014, 25, 23–35. [Google Scholar] [CrossRef]
- Motloung, M.P.; Mofokeng, T.G.; Ray, S.S. Viscoelastic, Thermal, and Mechanical Properties of Melt-Processed Poly (ε-Caprolactone) (PCL)/Hydroxyapatite (HAP) Composites. Materials 2022, 15, 104. [Google Scholar] [CrossRef]
- Petretta, M.; Gambardella, A.; Desando, G.; Cavallo, C.; Bartolotti, I.; Shelyakova, T.; Goranov, V.; Brucale, M.; Dediu, V.A.; Fini, M.; et al. Multifunctional 3D-Printed Magnetic Polycaprolactone/Hydroxyapatite Scaffolds for Bone Tissue Engineering. Polymers 2021, 13, 3825. [Google Scholar] [CrossRef]
- Mystiridou, E.; Patsidis, A.C.; Bouropoulos, N. Development and Characterization of 3D Printed Multifunctional Bioscaffolds Based on PLA/PCL/HAp/BaTiO3 Composites. Appl. Sci. 2021, 11, 4253. [Google Scholar] [CrossRef]
- Ebrahimi, Z.; Irani, S.; Ardeshirylajimi, A.; Seyedjafari, E. Enhanced Osteogenic Differentiation of Stem Cells by 3D Printed PCL Scaffolds Coated with Collagen and Hydroxyapatite. Sci. Rep. 2022, 12, 12359. [Google Scholar] [CrossRef]
- Kwon, B.-J.; Kim, J.; Kim, Y.H.; Lee, M.H.; Baek, H.S.; Lee, D.H.; Kim, H.-L.; Seo, H.J.; Lee, M.H.; Kwon, S.-Y.; et al. Biological Advantages of Porous Hydroxyapatite Scaffold Made by Solid Freeform Fabrication for Bone Tissue Regeneration. Artif. Organs 2013, 37, 663–670. [Google Scholar] [CrossRef]
- Fox, K.; Tran, P.A.; Tran, N. Recent Advances in Research Applications of Nanophase Hydroxyapatite. ChemPhysChem 2012, 13, 2495–2506. [Google Scholar] [CrossRef] [PubMed]
- Macuvele, D.L.P.; Nones, J.; Matsinhe, J.V.; Lima, M.M.; Soares, C.; Fiori, M.A.; Riella, H.G. Advances in Ultra High Molecular Weight Polyethylene/Hydroxyapatite Composites for Biomedical Applications: A Brief Review. Mater. Sci. Eng. C 2017, 76, 1248–1262. [Google Scholar] [CrossRef] [PubMed]
- Jouault, N.; Dalmas, F.; Boué, F.; Jestin, J. Multiscale Characterization of Filler Dispersion and Origins of Mechanical Reinforcement in Model Nanocomposites. Polymer 2012, 53, 761–775. [Google Scholar] [CrossRef]
- Kamatchi, T.; Saravanan, R.; Rangappa, S.M.; Siengchin, S. Effect of Filler Content and Size on the Mechanical Properties of Graphene-Filled Natural Fiber-Based Nanocomposites. Biomass Convers. Biorefin 2023, 13, 11311–11320. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Michailidis, N.; David, C.; Mountakis, N.; Papadakis, V.; Sfakiotakis, E.; Sagris, D.; Argyros, A. Optimization of Cellulose Nanocrystal (CNC) Concentration in Polycaprolactone Bio-Composites for Bio-Plotting: A Robust Interpretation of the Reinforcement Mechanisms. Cellulose 2024, 31, 3657–3680. [Google Scholar] [CrossRef]
- ASTM D638-02; Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D790-17; Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. ASTM International: West Conshohocken, PA, USA, 2017.
- Lin, Z.; Guo, X.; He, Z.; Liang, X.; Wang, M.; Jin, G. Thermal Degradation Kinetics Study of Molten Polylactide Based on Raman Spectroscopy. Polym. Eng. Sci. 2020, 61, 201–210. [Google Scholar] [CrossRef]
- Stuart, B.H. Temperature Studies of Polycarbonate Using Fourier Transform Raman Spectroscopy. Polym. Bull. 1996, 36, 341–346. [Google Scholar] [CrossRef]
- Camerlingo, C.; Zenone, F.; Delfino, I.; Diano, N.; Mita, D.G.; Lepore, M. Investigation on Clarified Fruit Juice Composition by Using Visible Light Micro-Raman Spectroscopy. Sensors 2007, 7, 2049–2061. [Google Scholar] [CrossRef] [PubMed]
- Veluthandath, A.V.; Bisht, P.B. Identification of Whispering Gallery Mode (WGM) Coupled Photoluminescence and Raman Modes in Complex Spectra of MoS2 in Polymethyl Methacrylate (PMMA) Microspheres. J. Lumin. 2017, 187, 255–259. [Google Scholar] [CrossRef]
- Makarem, M.; Lee, C.M.; Kafle, K.; Huang, S.; Chae, I.; Yang, H.; Kubicki, J.D.; Kim, S.H. Probing Cellulose Structures with Vibrational Spectroscopy. Cellulose 2019, 26, 35–79. [Google Scholar] [CrossRef]
- ASTM D1238-13; Standard Test Method for Melt Flow Rates of Thermoplastics by Extrusion Plastometer. ASTM International: West Conshohocken, PA, USA, 2020.
- Feng, P.; He, J.; Peng, S.; Gao, C.; Zhao, Z.; Xiong, S.; Shuai, C. Characterizations and Interfacial Reinforcement Mechanisms of Multicomponent Biopolymer Based Scaffold. Mater. Sci. Eng. C 2019, 100, 809–825. [Google Scholar] [CrossRef] [PubMed]
- Roeder, R.K.; Converse, G.L.; Kane, R.J.; Yue, W. Hydroxyapatite-Reinforced Polymer Biocomposites for Synthetic Bone Substitutes. JOM 2008, 60, 38–45. [Google Scholar] [CrossRef]
- Zhang, Y.; Tanner, K.E. Impact Behavior of Hydroxyapatite Reinforced Polyethylene Composites. J. Mater. Sci. Mater. Med. 2003, 14, 63–68. [Google Scholar] [CrossRef]
- Ramier, J.; Bouderlique, T.; Stoilova, O.; Manolova, N.; Rashkov, I.; Langlois, V.; Renard, E.; Albanese, P.; Grande, D. Biocomposite Scaffolds Based on Electrospun Poly(3-Hydroxybutyrate) Nanofibers and Electrosprayed Hydroxyapatite Nanoparticles for Bone Tissue Engineering Applications. Mater. Sci. Eng. C 2014, 38, 161–169. [Google Scholar] [CrossRef]
- Sopyan, I.; Mel, M.; Ramesh, S.; Khalid, K.A. Porous Hydroxyapatite for Artificial Bone Applications. Sci. Technol. Adv. Mater. 2007, 8, 116–123. [Google Scholar] [CrossRef]
- Ma, P.X.; Choi, J.-W. Biodegradable Polymer Scaffolds with Well-Defined Interconnected Spherical Pore Network. Tissue Eng. 2001, 7, 23–33. [Google Scholar] [CrossRef]
- Yuan, H.; Fernandes, H.; Habibovic, P.; de Boer, J.; Barradas, A.M.C.; de Ruiter, A.; Walsh, W.R.; van Blitterswijk, C.A.; de Bruijn, J.D. Osteoinductive Ceramics as a Synthetic Alternative to Autologous Bone Grafting. Proc. Natl. Acad. Sci. USA 2010, 107, 13614–13619. [Google Scholar] [CrossRef]
- ISO 10993; Biocompatibility testing of medical devices. International Organization for Standardization: Geneva, Switzerland, 2018.
- Xu, C.; Liu, Z.; Chen, X.; Gao, Y.; Wang, W.; Zhuang, X.; Zhang, H.; Dong, X. Bone Tissue Engineering Scaffold Materials: Fundamentals, Advances, and Challenges. Chin. Chem. Lett. 2024, 35, 109197. [Google Scholar] [CrossRef]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for Bone Tissue Engineering Scaffolds: A Review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, B.; Li, M.; Li, J.; Zhang, C.; Han, Y.; Wang, L.; Wang, K.; Zhou, C.; Liu, L.; et al. 3D Printing of PLA/n-HA Composite Scaffolds with Customized Mechanical Properties and Biological Functions for Bone Tissue Engineering. Compos. B Eng. 2021, 224, 109192. [Google Scholar] [CrossRef]
- Feuerbach, T.; Callau-Mendoza, S.; Thommes, M. Development of Filaments for Fused Deposition Modeling 3D Printing with Medical Grade Poly(Lactic-Co-Glycolic Acid) Copolymers. Pharm. Dev. Technol. 2019, 24, 487–493. [Google Scholar] [CrossRef]
- Topsakal, A.; Midha, S.; Yuca, E.; Tukay, A.; Sasmazel, H.T.; Kalaskar, D.M.; Gunduz, O. Study on the Cytocompatibility, Mechanical and Antimicrobial Properties of 3D Printed Composite Scaffolds Based on PVA/ Gold Nanoparticles (AuNP)/ Ampicillin (AMP) for Bone Tissue Engineering. Mater. Today Commun. 2021, 28, 102458. [Google Scholar] [CrossRef]
- Statnik, E.S.; Sorokina, E.A.; Larin, I.I.; Yu, K.; Salimon, A.I.; Kalyaev, V.Y.; Zherebtsov, D.D.; Zadorozhnyy, M.Y.; Korsunsky, A.M. The Characterization of PVA/PHY Hydrogels for 3D Printing Fabrication of Organ Phantoms. Mater. Today Proc. 2020, 33, 1874–1879. [Google Scholar] [CrossRef]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D Printing of Composite Calcium Phosphate and Collagen Scaffolds for Bone Regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef]
- Abifarin, J.K.; Prakash, C.; Singh, S. Optimization and Significance of Fabrication Parameters on the Mechanical Properties of 3D Printed Chitosan/PLA Scaffold. Mater. Today Proc. 2022, 50, 2018–2025. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Michailidis, N.; David, C.; Mountakis, N.; Papadakis, V.; Sfakiotakis, E.; Sagris, D.; Spiridaki, M.; Argyros, A. Optimized PCL/CNF Bio-Nanocomposites for Medical Bio-Plotted Applications: Rheological, Structural, and Thermomechanical Aspects. Bioprinting 2023, 36, e00311. [Google Scholar] [CrossRef]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in Modern Medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Bhat, A.H.; Abu Bakar, A.; Tahir, P.M.; Zaidul, I.S.M.; Jawaid, M. Cellulosic Nanocomposites from Natural Fibers for Medical Applications: A Review. In Handbook of Polymer Nanocomposites. Processing, Performance and Application: Volume C: Polymer Nanocomposites of Cellulose Nanoparticles; Pandey, J.K., Takagi, H., Nakagaito, A.N., Kim, H.-J., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 475–511. ISBN 978-3-642-45232-1. [Google Scholar]
- Cherian, B.M.; Leão, A.L.; de Souza, S.F.; Costa, L.M.M.; de Olyveira, G.M.; Kottaisamy, M.; Nagarajan, E.R.; Thomas, S. Cellulose Nanocomposites with Nanofibres Isolated from Pineapple Leaf Fibers for Medical Applications. Carbohydr. Polym. 2011, 86, 1790–1798. [Google Scholar] [CrossRef]
- Zimmerer, C.; Matulaitiene, I.; Niaura, G.; Reuter, U.; Janke, A.; Boldt, R.; Sablinskas, V.; Steiner, G. Nondestructive Characterization of the Polycarbonate - Octadecylamine Interface by Surface Enhanced Raman Spectroscopy. Polym. Test 2019, 73, 152–158. [Google Scholar] [CrossRef]
- Gatin, E.; Iordache, S.-M.; Matei, E.; Luculescu, C.-R.; Iordache, A.-M.; Grigorescu, C.; Ilici, R. Raman Spectroscopy as Spectral Tool for Assessing the Degree of Conversion after Curing of Two Resin-Based Materials Used in Restorative Dentistry. Diagnostics 2022, 12, 1993. [Google Scholar] [CrossRef] [PubMed]
- Resta, V.; Quarta, G.; Lomascolo, M.; Maruccio, L.; Calcagnile, L. Raman and Photoluminescence Spectroscopy of Polycarbonate Matrices Irradiated with Different Energy 28Si+ Ions. Vacuum 2015, 116, 82–89. [Google Scholar] [CrossRef]
- Luiz, B.K.M.; Amboni, R.D.M.C.; Prates, L.H.M.; Roberto Bertolino, J.; Pires, A.T.N. Influence of Drinks on Resin Composite: Evaluation of Degree of Cure and Color Change Parameters. Polym. Test 2007, 26, 438–444. [Google Scholar] [CrossRef]
- Peris-Díaz, M.D.; Łydżba-Kopczyńska, B.; Sentandreu, E. Raman Spectroscopy Coupled to Chemometrics to Discriminate Provenance and Geological Age of Amber. J. Raman Spectrosc. 2018, 49, 842–851. [Google Scholar] [CrossRef]
- Liu, X.; Zou, Y.; Li, W.; Cao, G.; Chen, W. Kinetics of Thermo-Oxidative and Thermal Degradation of Poly(d,l-Lactide) (PDLLA) at Processing Temperature. Polym. Degrad Stab. 2006, 91, 3259–3265. [Google Scholar] [CrossRef]

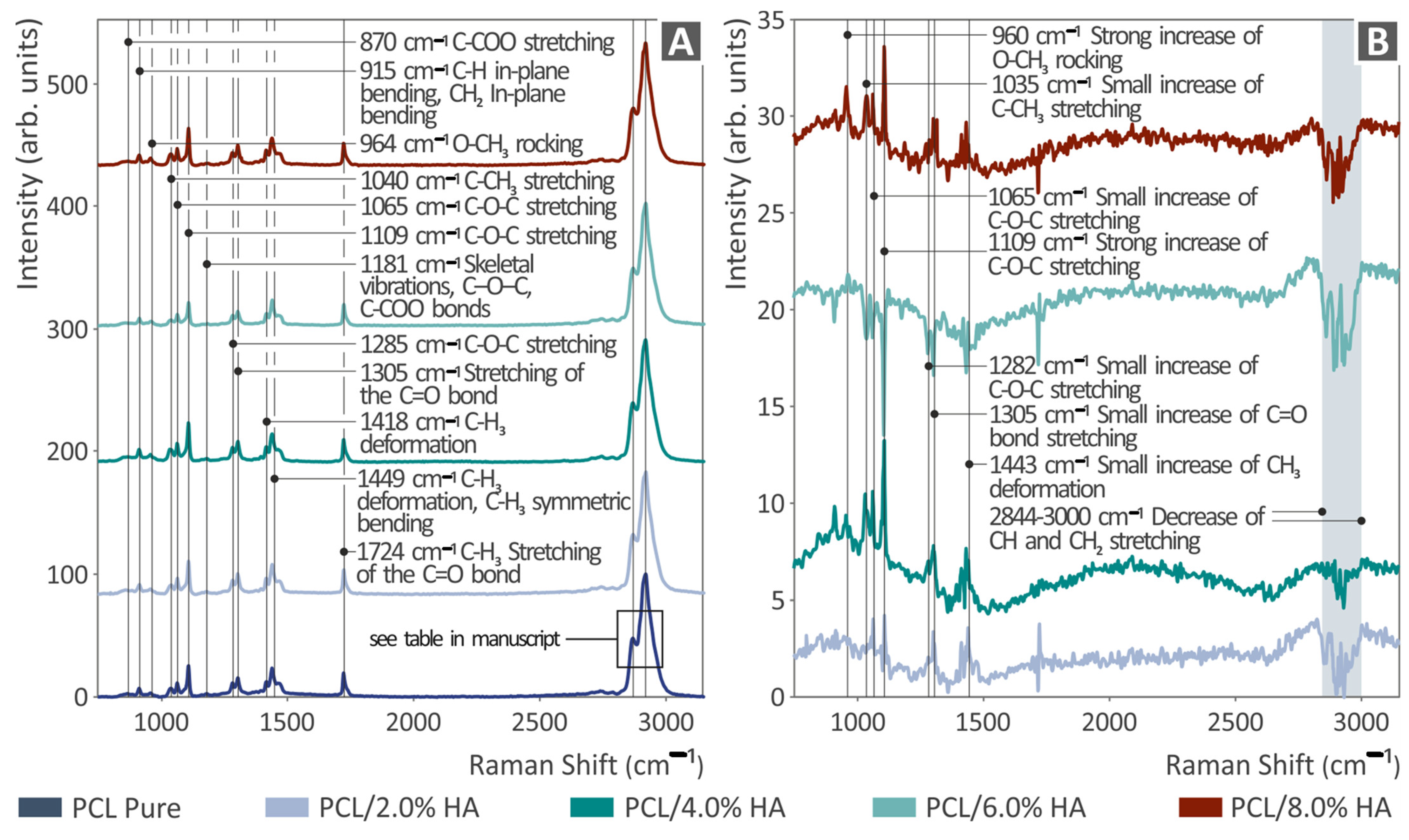




| Wavenumber (cm−1) | Change | Raman Peak Assignment |
|---|---|---|
| 960 | Gradual increase | Strong increase in O-CH3 rocking |
| 1035 | Gradual increase | Small increase in C-CH3 stretching |
| 1065 | Gradual increase | Small increase in C-O-C stretching |
| 1109 | Gradual increase | Strong increase in C-O-C stretching |
| 1282 | increase | Small increase in C-O-C stretching |
| 1305 | increase | Small increase in C=O bond stretching |
| 1443 | Increase | Small increase in CH3 deformation |
| 2844–3000 | Gradual decrease | Decrease in CH and CH2 stretching |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petousis, M.; Michailidis, N.; Korlos, A.; Papadakis, V.; David, C.; Sagris, D.; Mountakis, N.; Argyros, A.; Valsamos, J.; Vidakis, N. Biomedical Composites of Polycaprolactone/Hydroxyapatite for Bioplotting: Comprehensive Interpretation of the Reinforcement Course. Polymers 2024, 16, 2400. https://doi.org/10.3390/polym16172400
Petousis M, Michailidis N, Korlos A, Papadakis V, David C, Sagris D, Mountakis N, Argyros A, Valsamos J, Vidakis N. Biomedical Composites of Polycaprolactone/Hydroxyapatite for Bioplotting: Comprehensive Interpretation of the Reinforcement Course. Polymers. 2024; 16(17):2400. https://doi.org/10.3390/polym16172400
Chicago/Turabian StylePetousis, Markos, Nikolaos Michailidis, Apostolos Korlos, Vassilis Papadakis, Constantine David, Dimitrios Sagris, Nikolaos Mountakis, Apostolos Argyros, John Valsamos, and Nectarios Vidakis. 2024. "Biomedical Composites of Polycaprolactone/Hydroxyapatite for Bioplotting: Comprehensive Interpretation of the Reinforcement Course" Polymers 16, no. 17: 2400. https://doi.org/10.3390/polym16172400
APA StylePetousis, M., Michailidis, N., Korlos, A., Papadakis, V., David, C., Sagris, D., Mountakis, N., Argyros, A., Valsamos, J., & Vidakis, N. (2024). Biomedical Composites of Polycaprolactone/Hydroxyapatite for Bioplotting: Comprehensive Interpretation of the Reinforcement Course. Polymers, 16(17), 2400. https://doi.org/10.3390/polym16172400










