Direct Acrylation of Soybean Oil and the Influence of the Acrylation Degree on Waterborne Acrylic Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
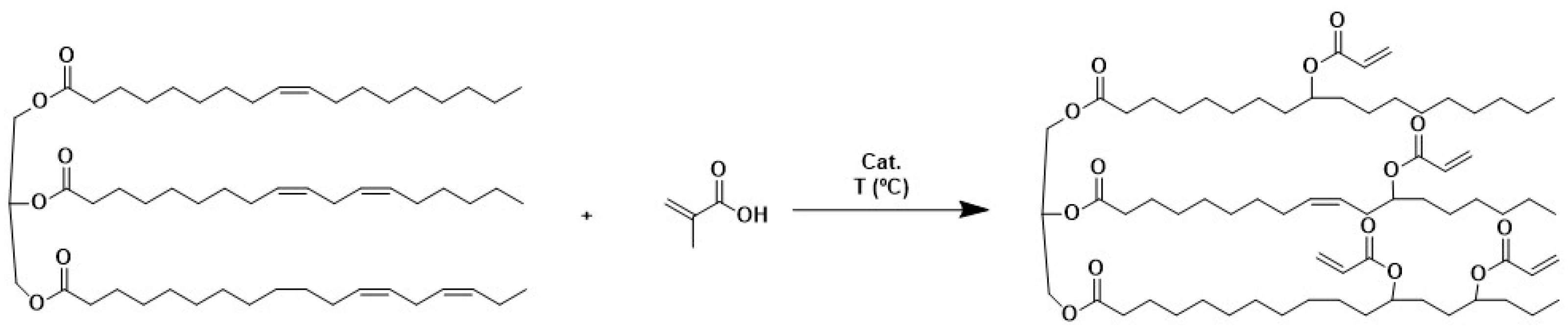
2.2. Synthesis of Acrylated Monomers (ASO) in a One-Step Process
2.3. Copolymerization of ASO and Acrylic Monomers by Miniemulsion Polymerization
2.4. Characterization Techniques
3. Results and Discussion
3.1. ASO Monomer
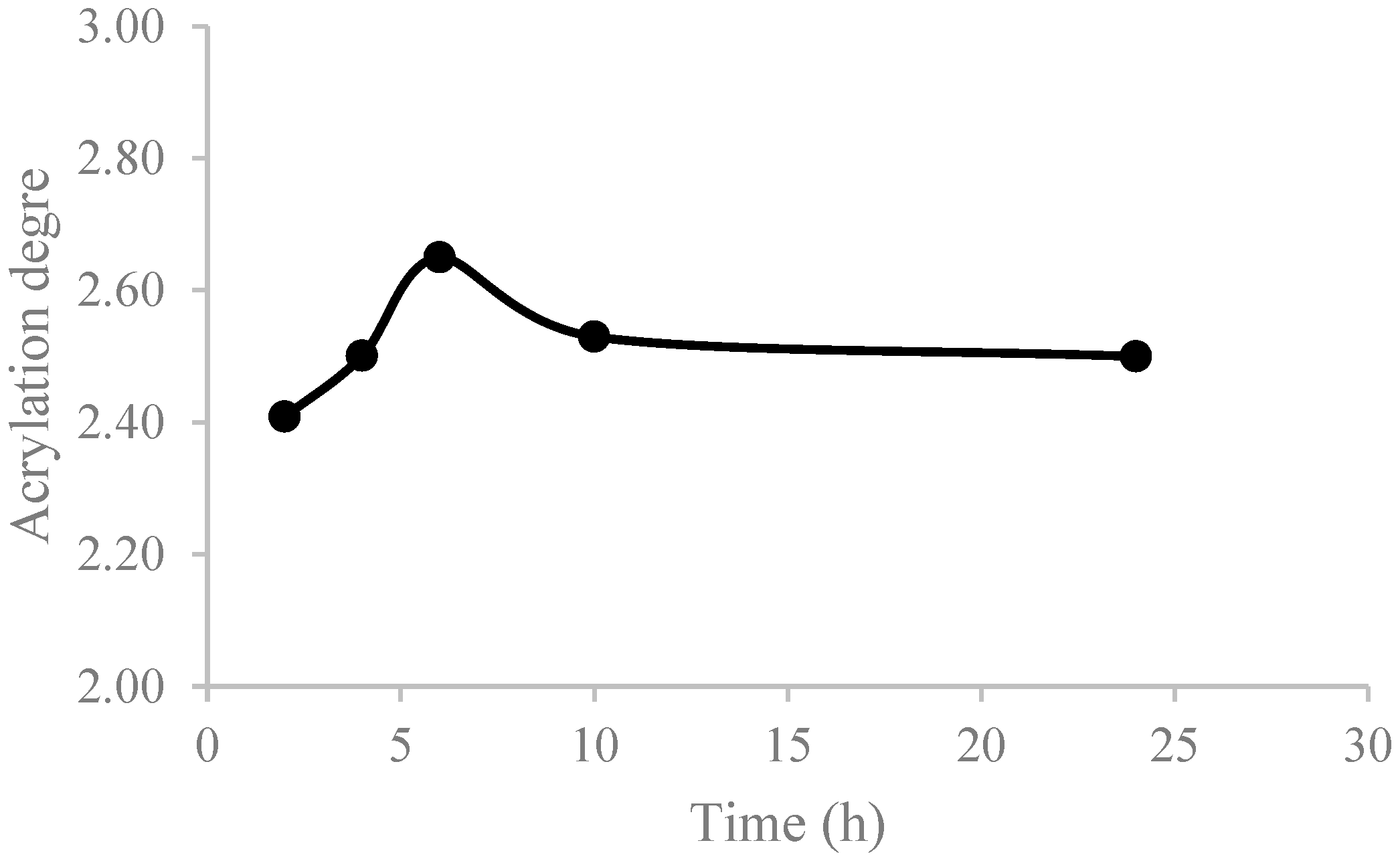
3.1.1. One-Step Process
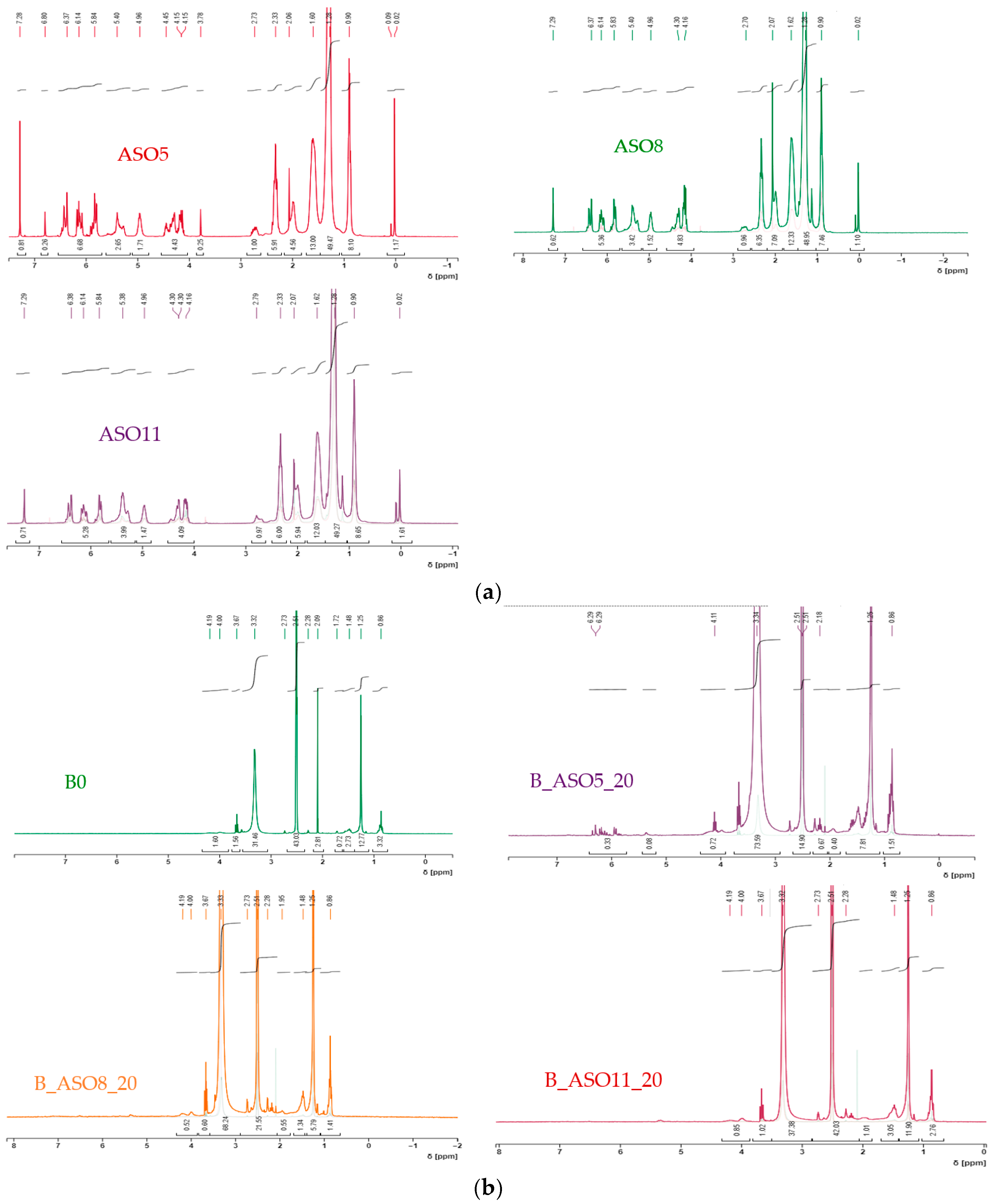
3.1.2. Characterization
3.2. ASO–Acrylic Copolymers
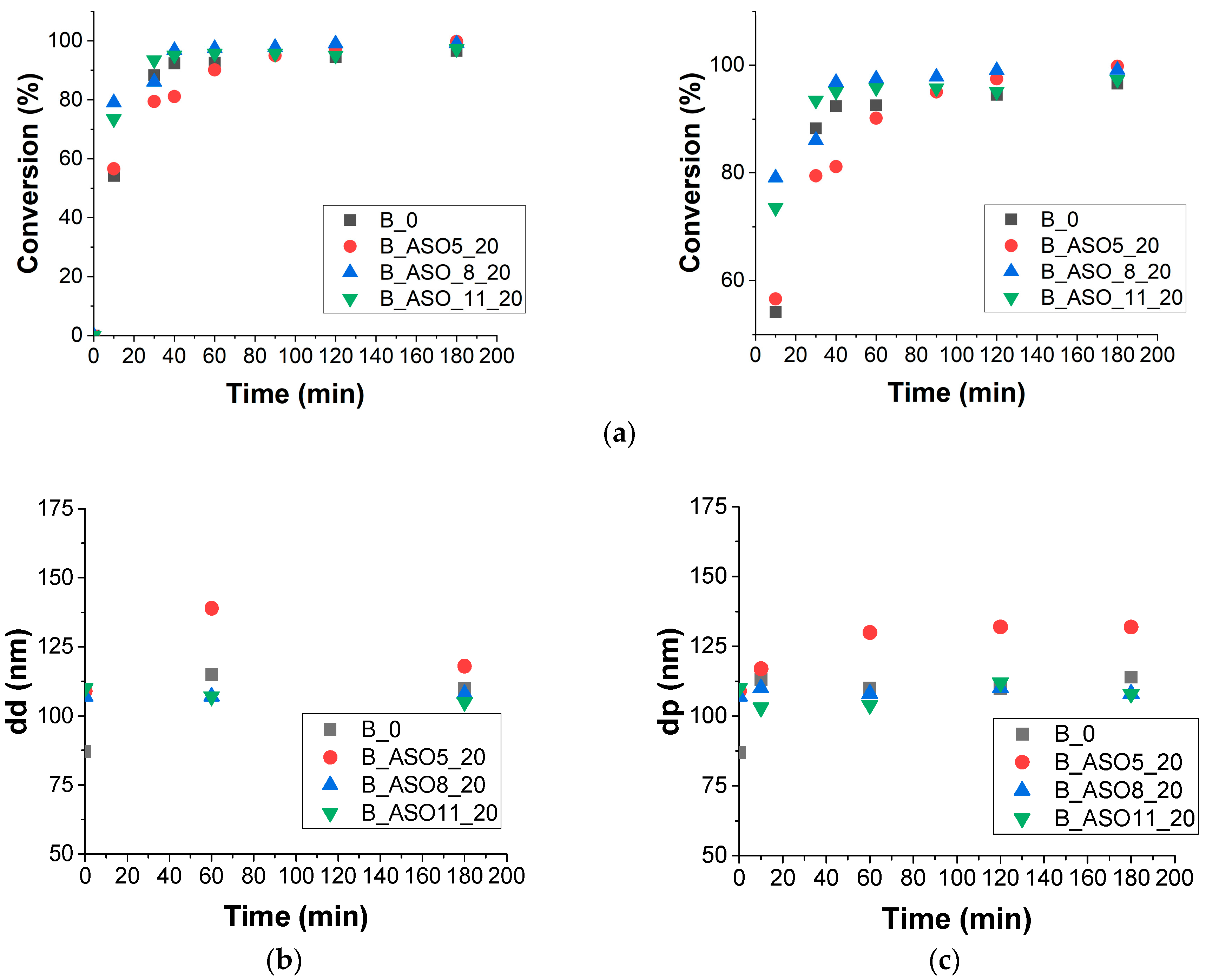
3.2.1. Batch Polymerization
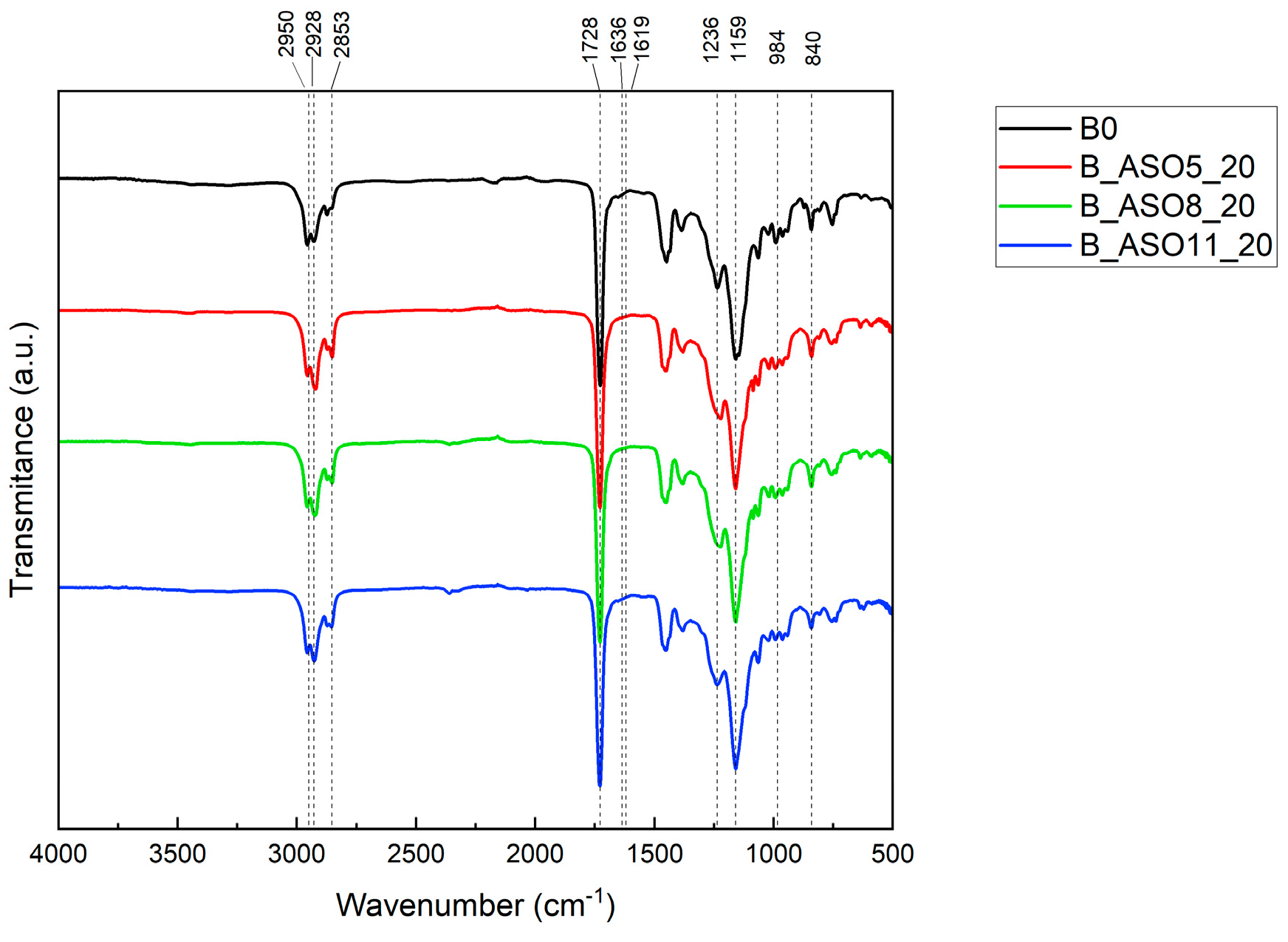
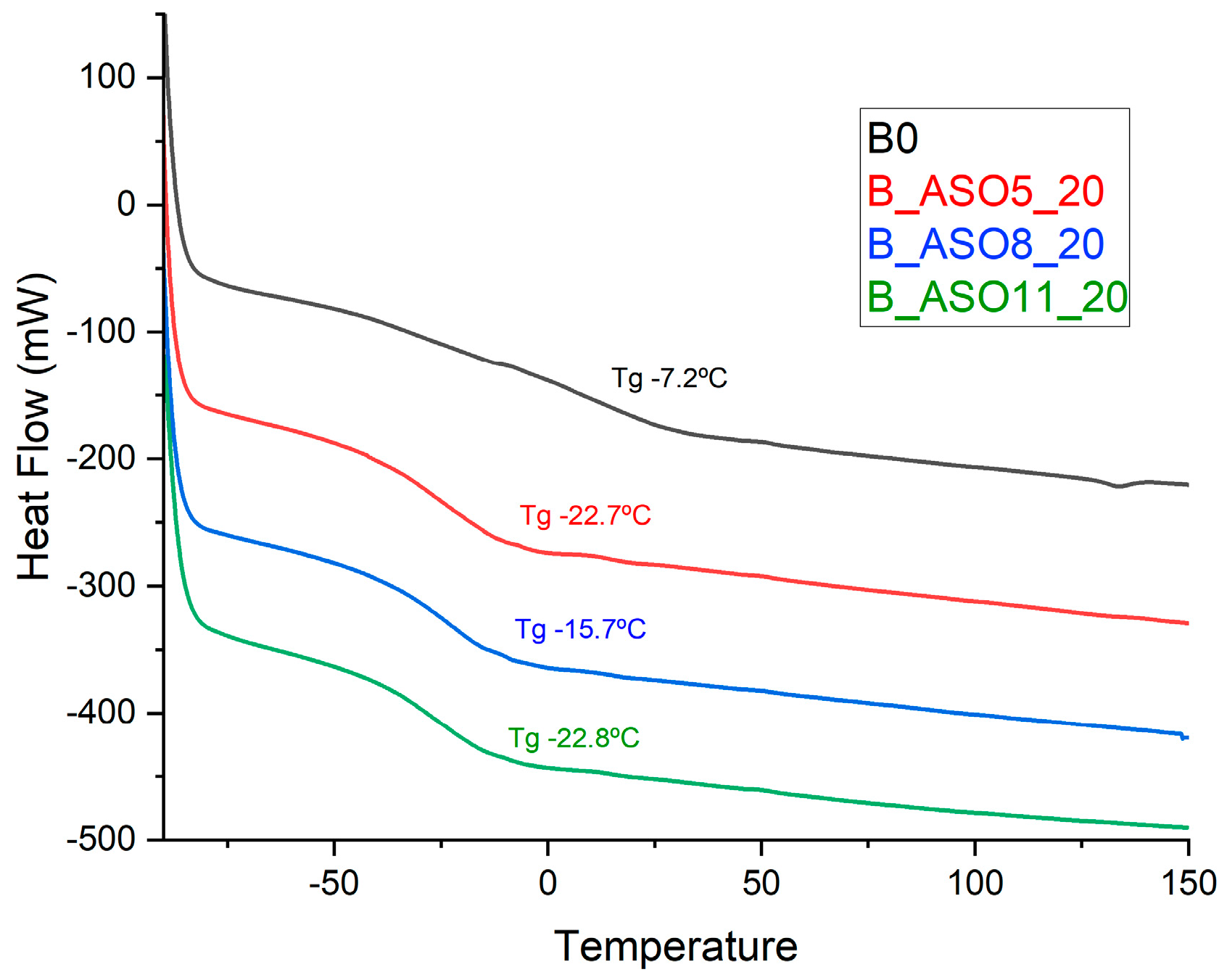
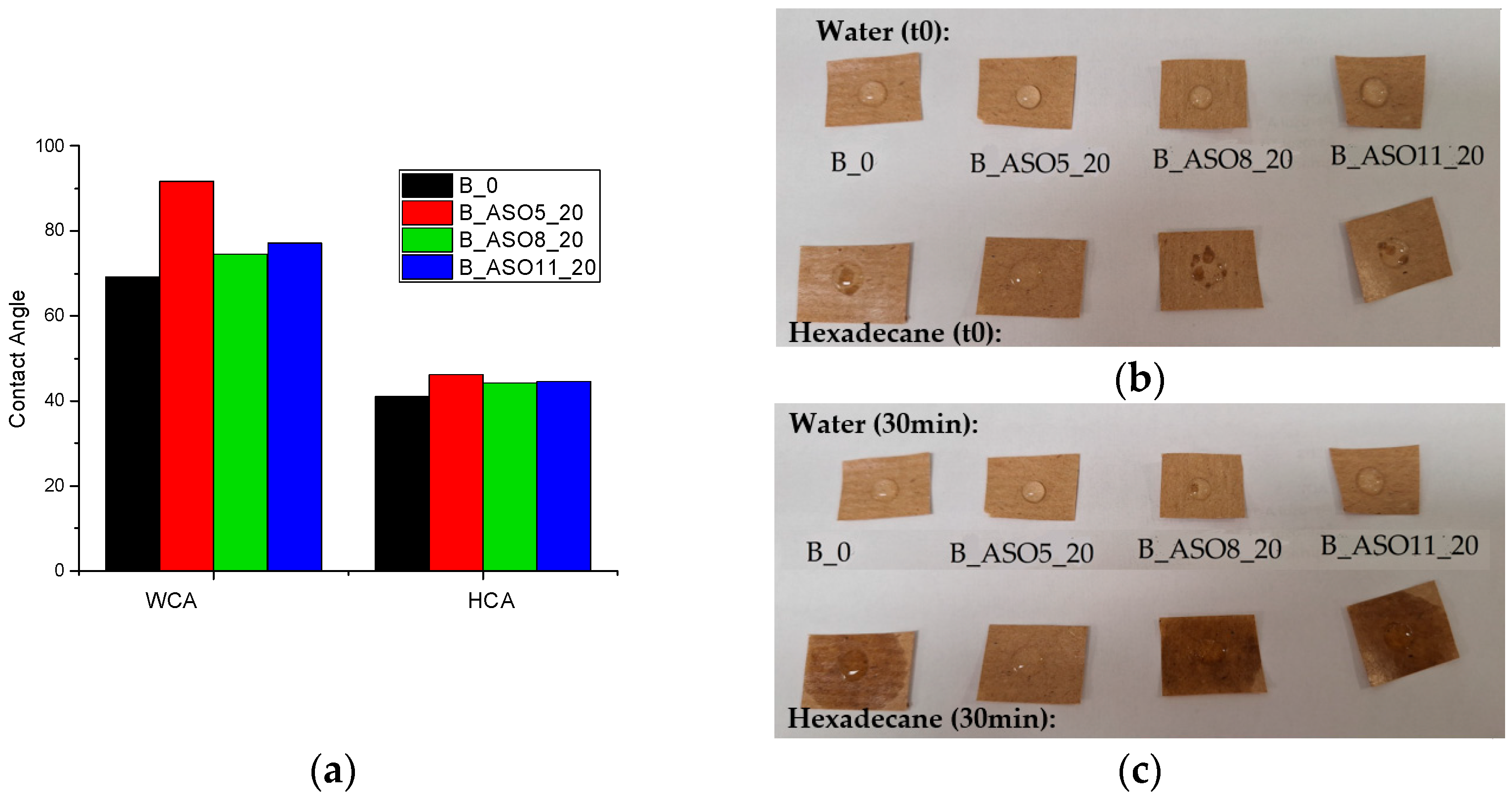
3.2.2. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Gallezot, P. Conversion of biomass to selected chemical products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef]
- Güner, F.S.; Yağcı, Y.; Erciyes, A.T. Polymers from triglyceride oils. Prog. Polym. Sci. 2006, 31, 633–670. [Google Scholar] [CrossRef]
- Wool, R.P.; Sun, X.S. Bio-Based Polymers and Composites; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Gunstone, F.D. The Lipid Handbook; Taylor & Francis LTF: Abingdon, UK, 2004. [Google Scholar]
- Gandini, A.; Lacerda, T.M. From monomers to polymers from renewable resources: Recent advances. Prog. Polym. Sci. 2015, 48, 1–39. [Google Scholar] [CrossRef]
- Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Renewable polymeric materials from vegetable oils: A perspective. Mater. Today 2013, 16, 337–343. [Google Scholar] [CrossRef]
- Belgacem, M.N.; Gandini, A. Monomers, Polymers and Composites from Re-Newable Resources; Elsevier Ltd.: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Xia, Y.; Larock, R.C. Vegetable oil-based polymeric materials: Synthesis, properties, and applications. Green Chem. 2010, 12, 1893–1909. [Google Scholar] [CrossRef]
- Malburet, S.; Di Mauro, C.; Noè, C.; Mija, A.; Sangermano, M.; Graillot, A. Sustainable access to fully biobased epoxidized vegetable oil thermoset materials prepared by thermal or UV-cationic processes. RSC Adv. 2020, 10, 41954–41966. [Google Scholar] [CrossRef]
- Danov, S.M.; Kazantsev, O.A.; Esipovich, A.L.; Belousov, A.S.; Rogozhin, A.E.; Kanakov, E.A. Recent advances in the field of selective epoxidation of vegetable oils and their derivatives: A review and perspective. Catal. Sci. Technol. 2017, 7, 3659–3675. [Google Scholar] [CrossRef]
- Droesbeke, M.A.; Aksakal, R.; Simula, A.; Asua, J.M.; Du Prez, F.E. Biobased acrylic pressure-sensitive adhesives. Prog. Polym. Sci. 2021, 117, 101396. [Google Scholar] [CrossRef]
- Mendes-Felipe, C.; Isusi, I.; Gómez-Jiménez-Aberasturi, O.; Prieto-Fernandez, S.; Ruiz-Rubio, L.; Sangermano, M.; Vilas-Vilela, J.L. One-Step Method for Direct Acrylation of Vegetable Oils: A Biobased Material for 3D Printing. Polymers 2023, 15, 3136. [Google Scholar] [CrossRef]
- Khot, S.N.; Lascala, J.J.; Can, E.; Morye, S.S.; Williams, G.I.; Palmese, G.R.; Kusefoglu, S.H.; Wool, R.P. Delopment and application of triglyceride-based polymers and composites. J. Appl. Polym. Sci. 2001, 82, 703. [Google Scholar] [CrossRef]
- Öztürk, C.; Mutlu, H.; Meier, M.A.; Küsefoğlu, S.H. 4-Vinylbenzenesulfonic acid adduct of epoxidized soybean oil: Synthesis, free radical and ADMET polymerizations. Eur. Polym. J. 2011, 47, 1467–1476. [Google Scholar] [CrossRef]
- Galià, M.; de Espinosa, L.M.; Ronda, J.C.; Lligadas, G.; Cádiz, V. Vegetable oil-based thermosetting polymers. Eur. J. Lipid Sci. Technol. 2010, 112, 87–96. [Google Scholar] [CrossRef]
- de Espinosa, L.M.; Meier, M.A. Plant oils: The perfect renewable resource for polymer science?! Eur. Polym. J. 2011, 47, 837–852. [Google Scholar] [CrossRef]
- Saithai, P.; Lecomte, J.; Dubreucq, E.; Tanrattanakul, V. Effects of different epoxidation methods of soybean oil on the characteristics of acrylated epoxidized soybean oil-co-poly(methyl methacrylate) copolymer. Express Polym. Lett. 2013, 7, 910–924. [Google Scholar] [CrossRef]
- Guo, J.; Schork, F.J. Hybrid Miniemulsion Polymerization of Acrylate/Oil and Acrylate/Fatty Acid Systems. Macromol. React. Eng. 2008, 2, 265–276. [Google Scholar] [CrossRef]
- Asua, J.M. Miniemulsion polymerization. Prog. Polym. Sci. 2002, 27, 1283. [Google Scholar] [CrossRef]
- El-Aasser, M.S.; Sudol, E. Miniemulsions: Overview of research and applications. JCT Res. 2004, 1, 21. [Google Scholar]
- Moreno, M.; Miranda, J.I.; Goikoetxea, M.; Barandiaran, M.J. Sustainable polymer latexes based on linoleic acid for coatings applications. Prog. Org. Coat. 2014, 77, 1709–1714. [Google Scholar] [CrossRef]
- Thames, S.F.; Smith, O.W.; Evans, J.M.; Dutta, S.; Chen, L. Functionalized Vegetable Oil Derivatives, Latex Compositions and Coatings. U.S. Patent US20050203246A1, 15 September 2005. [Google Scholar]
- Quintero, C.; Mendon, S.K.; Smith, O.W.; Thames, S.F. Miniemulsion polymerization of vegetable oil macromonomers. Prog. Org. Coat. 2006, 57, 195–201. [Google Scholar] [CrossRef]
- Bunker, S.; Staller, C.; Willenbacher, N.; Wool, R. Miniemulsion polymerization of acrylated methyl oleate for pressure sensitive adhesives. Int. J. Adhes. Adhes. 2003, 23, 29–38. [Google Scholar] [CrossRef]
- Medeiros, A.M.M.S.; Machado, F.; Rubim, J.C.; McKenna, T.F.L. Bio-based copolymers obtained through miniemulsion copolymerization of methyl esters of acrylated fatty acids and styrene. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1422–1432. [Google Scholar] [CrossRef]
- Laurentino, L.S.; Medeiros, A.M.; Machado, F.; Costa, C.; Araújo, P.H.; Sayer, C. Synthesis of a biobased monomer derived from castor oil and copolymerization in aqueous medium. Chem. Eng. Res. Des. 2018, 137, 213–220. [Google Scholar] [CrossRef]
- Jensen, A.T.; Sayer, C.; Araújo, P.H.H.; Machado, F. Emulsion copolymerization of styrene and acrylated methyl oleate. Eur. J. Lipid Sci. Technol. 2013, 116, 37–43. [Google Scholar] [CrossRef]
- Klaas, M.R.G.; Warwel, S. Complete and partial epoxidation of plant oils by lipase-catalyzed perhydrolysis. Ind. Crop. Prod. 1999, 9, 125–132. [Google Scholar] [CrossRef]
- Tanabe, K.; Misono, M.; Ono, Y.; Hattori, H. Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1989; pp. 27–213. [Google Scholar]
- Nordin, N.A.M.; Adnan, N.F.; Alias, A.; Isahak, W.N.R.W.; Salimon, J.; Yarmo, M.A.; Kamaruddin, R.A. New Silica Supported HClO4 as Efficient Catalysts for Estolide Synthesis from Oleic Acid. Adv. Mater. Res. 2010, 173, 140–145. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, J. One-step acrylation of soybean oil (SO) for the preparation of SO-based macromonomers. Green Chem. 2013, 15, 641–645. [Google Scholar] [CrossRef]
- Zhang, P.; Xin, J.; Zhang, J. Effects of Catalyst Type and Reaction Parameters on One-Step Acrylation of Soybean Oil. ACS Sustain. Chem. Eng. 2013, 2, 181–187. [Google Scholar] [CrossRef]
- Meier, M.A.R.; Metzger, J.O.; Schubert, U.S. Plant oil renewable resources as green alternatives in polymer science. Chem. Soc. Rev. 2007, 36, 1788–1802. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, J.F.; Fernando, S.; Jagodzinski, K. Soy-based, high biorenewable content UV curable coatings. Prog. Org. Coat. 2011, 71, 98–109. [Google Scholar] [CrossRef]
- McCutcheon, W. Wijs iodine method. Ind. Eng. Chem. Anal. 1940, 12, 465. [Google Scholar] [CrossRef]
- Capek, I. Miniemulsion Polymerization of Styrene Initiated by Tween-surfinit. Des. Monomers Polym. 2010, 13, 349–368. [Google Scholar] [CrossRef]
- Demchuk, Z.; Mora, A.-S.; Choudhary, S.; Caillol, S.; Voronov, A. Biobased latexes from natural oil derivatives. Ind. Crops Prod. 2021, 162, 113237. [Google Scholar] [CrossRef]



| Reference | MMA (g) | BA (g) | AA (g) | SA (g) | Biomonomer (g) | SDS (g) | H2O (g) |
|---|---|---|---|---|---|---|---|
| B_0 | 45 | 54 | 1 | 4 | - | 2 | 150 |
| B_ASO5_20 | 25 | 54 | 1 | 4 | 20 ASO5 | 2 | 150 |
| B_ASO8_20 | 25 | 54 | 1 | 4 | 20 ASO8 | 2 | 150 |
| B_ASO11_20 | 25 | 54 | 1 | 4 | 20 ASO11 | 2 | 150 |
| Reference | MR Cat/C=C | MR AA/C=C | Acrylation Degree | Process |
|---|---|---|---|---|
| ASO5 | 0.1 | 6 | 2.5 | Batch |
| ASO8 | 0.1 | 4 | 1.6 | Batch |
| ASO11 | 0.1 | 4 | 1.9 | Semicontinuous |
| Reference | dd (nm) | dp (nm) | Nd | Np | Np/Nd |
|---|---|---|---|---|---|
| B0 | 87 | 114 | 2.3 × 1017 | 1.0 × 1017 | 0.43 |
| B_ASO5_20 | 110 | 132 | 1.1 × 1017 | 6.3 × 1016 | 0.6 |
| B_ASO8_20 | 107 | 108 | 1.2 × 1017 | 1.2 × 1017 | 1 |
| B_ASO11_20 | 110 | 108 | 1.1 × 1017 | 1.3 × 1017 | 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez, B.; Blanco, N.; Villaverde, H.; Echeverria, O.; Gomez de Miranda, O.; Rodriguez, R. Direct Acrylation of Soybean Oil and the Influence of the Acrylation Degree on Waterborne Acrylic Systems. Polymers 2024, 16, 2355. https://doi.org/10.3390/polym16162355
Perez B, Blanco N, Villaverde H, Echeverria O, Gomez de Miranda O, Rodriguez R. Direct Acrylation of Soybean Oil and the Influence of the Acrylation Degree on Waterborne Acrylic Systems. Polymers. 2024; 16(16):2355. https://doi.org/10.3390/polym16162355
Chicago/Turabian StylePerez, Beatriz, Noelia Blanco, Haizea Villaverde, Oihane Echeverria, Olga Gomez de Miranda, and Raquel Rodriguez. 2024. "Direct Acrylation of Soybean Oil and the Influence of the Acrylation Degree on Waterborne Acrylic Systems" Polymers 16, no. 16: 2355. https://doi.org/10.3390/polym16162355
APA StylePerez, B., Blanco, N., Villaverde, H., Echeverria, O., Gomez de Miranda, O., & Rodriguez, R. (2024). Direct Acrylation of Soybean Oil and the Influence of the Acrylation Degree on Waterborne Acrylic Systems. Polymers, 16(16), 2355. https://doi.org/10.3390/polym16162355







