(Bio)degradable Biochar Composites of PLA/P(3HB-co-4HB) Commercial Blend for Sustainable Future—Study on Degradation and Electrostatic Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Composites
2.3. Characterization Studies
2.3.1. Thermal Properties
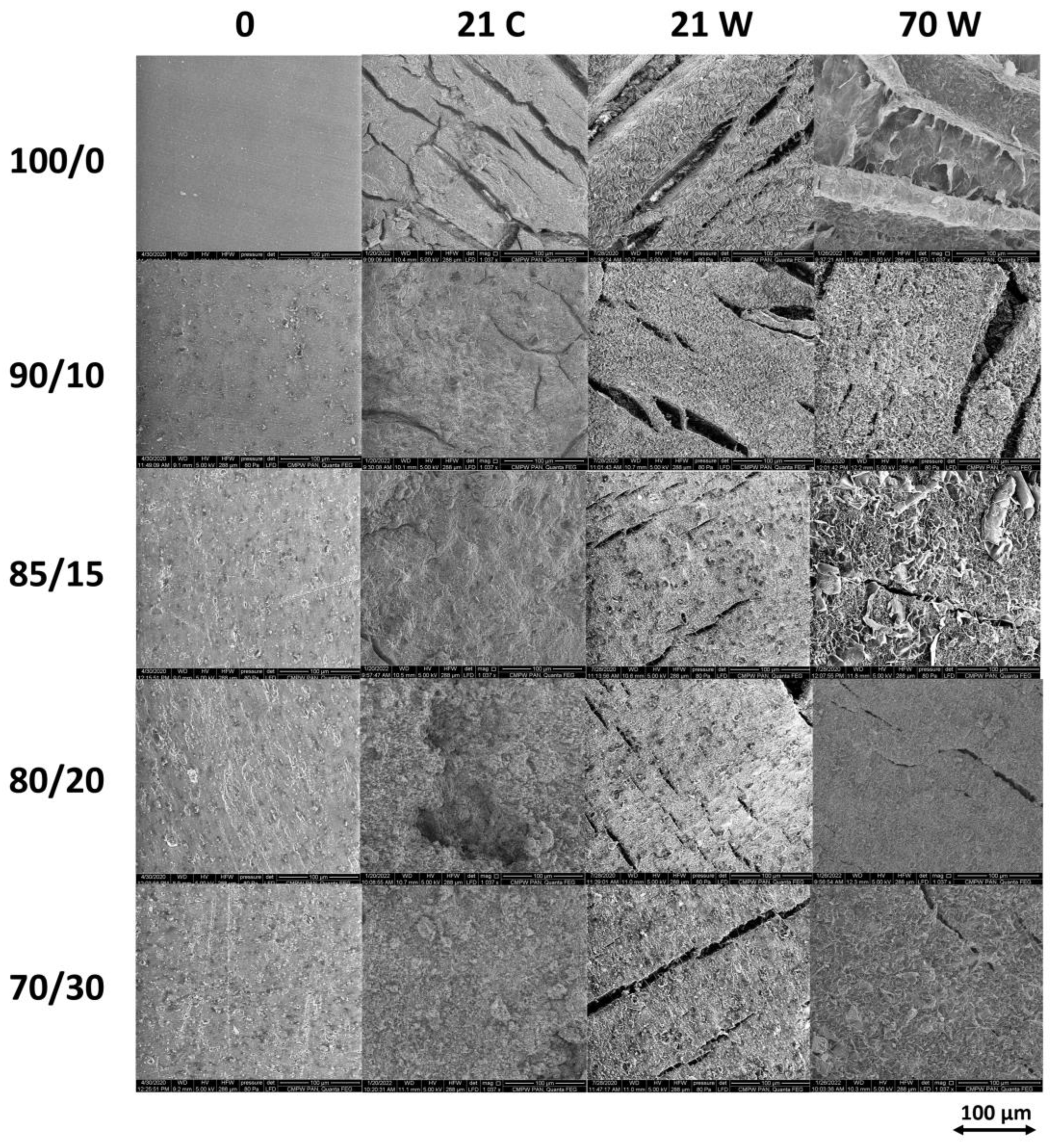
2.3.2. Visual Examination
2.3.3. Surface Resistivity Measurement
- l—length of line electrodes;
- g—distance between the lines (gap);
- Rs—surface resistance.
2.3.4. Nuclear Magnetic Resonance (NMR) Measurements
2.4. Degradation Environments
2.4.1. Test under Composting Conditions
2.4.2. Abiotic Degradation
3. Results
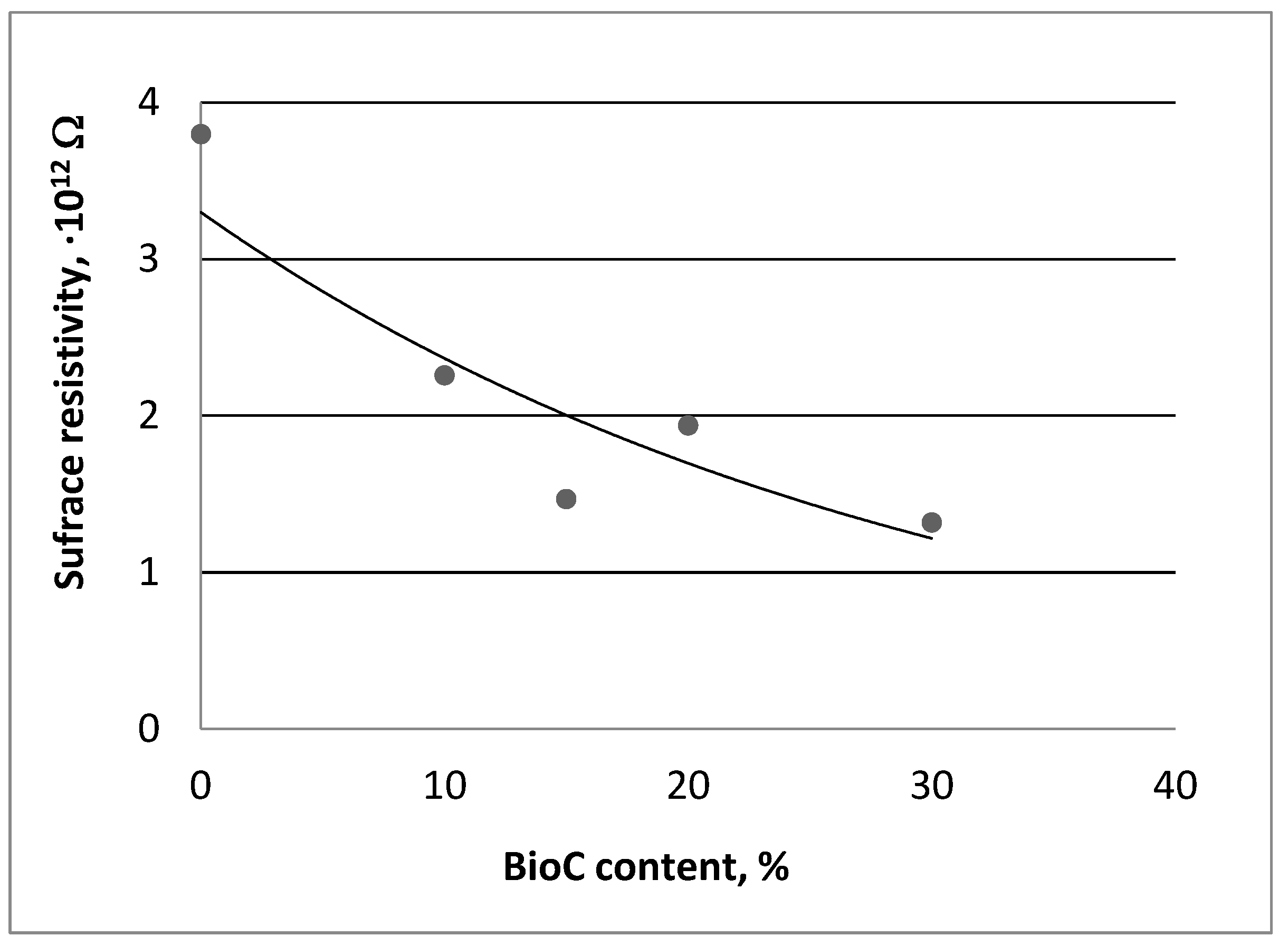
3.1. Electrostatic Properties
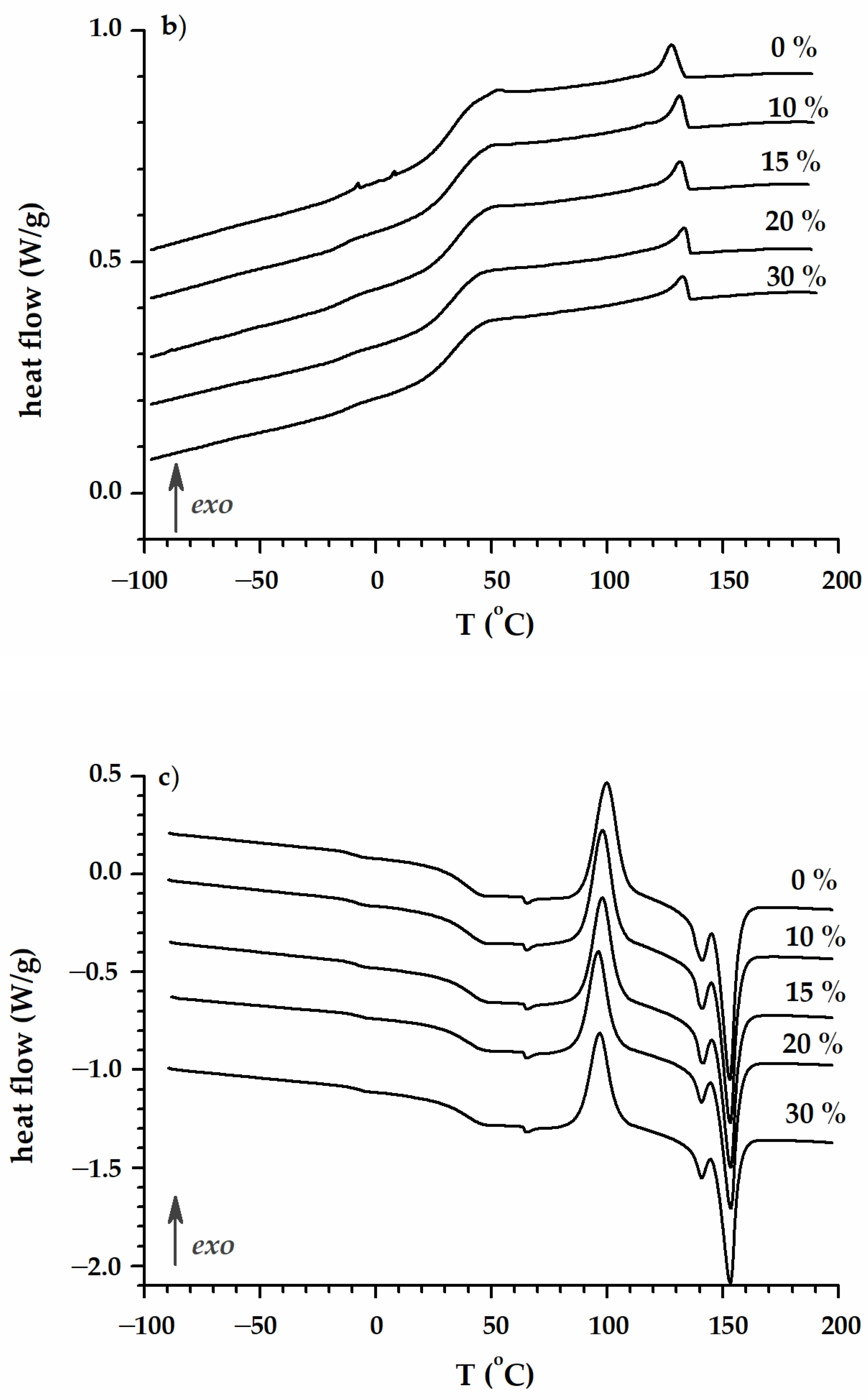
3.2. Degradation Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission. A European Strategy for Plastics in a Circular Economy. Available online: https://www.europarc.org/wp-content/uploads/2018/01/Eu-plastics-strategy-brochure.pdf (accessed on 7 September 2023).
- ResearchAndMarkets.com. Biodegradable Polymers Global Market Report 2022–2030: Increasing Use of Biodegradable Products and Public Concern for the Environment Boosting Sector. Available online: https://www.globenewswire.com/en/news-release/2022/07/26/2485592/28124/en/Biodegradable-Polymers-Global-Market-Report-2022-2030-Increasing-Use-of-Biodegradable-Products-and-Public-Concern-for-the-Environment-Boosting-Sector.html (accessed on 8 September 2023).
- Silva, M.; Ferreira, F.N.; Alves, N.M.; Paiva, M.C. Biodegradable polymer nanocomposites for ligament/tendon tissue engineering. J. Nanobiotechnol. 2020, 23, 18–23. [Google Scholar] [CrossRef]
- Sikorska, W.; Musioł, M.; Duale, K.; Rydz, J.; Zawidlak-Węgrzyńska, B. Biodegradable Polymers. Value Chain in the Circular Economy; Rydz, J., Ed.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2024; ISBN 9780367370671. [Google Scholar]
- Chavan, S.; Yadav, B.; Tyagi, R.D.; Drogui, P. A review on production of polyhydroxyalkanoate (PHA) biopolyesters by thermophilic microbes using waste feedstocks. Bioresour. Technol. 2021, 341, 125900. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.; Jang, Y.; Lee, E.; Shin, S.; Kang, H.J. The modification of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) by melt blending. Polymers 2022, 14, 1725. [Google Scholar] [CrossRef]
- Li, X.; Liu, K.; Liu, Z.; Lu, X.; Li, Y.; Wang, H.; Jia, L.; Tong, Y.; Qu, J. Poly (ethylene-butylacrylate-glycidyl methacrylate) reaction compatibilized poly (lactic acid)/poly(3-hydroxybutyrate-4-hydroxybutyrate) blends with enhanced mechanical property, biodegradability and thermal stability. Polym. Test. 2022, 111, 107610. [Google Scholar] [CrossRef]
- Li, X.; Zhu Ch Wang, H.; Xiao, Y.; Lu, X.; Li, Y.; Liu, Z.; Tong, Y.; Qu, J. A novel PLA/P(3HB-co-4HB)/MWCNT composite featuring enhanced mechanical properties and excellent thermal stability based on elongational rheology. Polym. Test. 2022, 114, 107700. [Google Scholar] [CrossRef]
- Guo, J.; Liu, M.; Liu, Y.; Hu Ch Xia, Y.; Zhang, H.; Gong, Y. Rheological, thermal, and mechanical properties of P (3HB-co-4HB) and P (3HB-co-4HB)/EVA blends. J. Appl. Polym. Sci. 2014, 131, 41206. [Google Scholar] [CrossRef]
- Kovalčík, A.; Smilek, J.; Machovský, M.; Kalina, M.; Enev, V.; Dugová, H.; Černeková, N.; Kováčova, M.; Špitálský, Z. Properties and structure of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) filaments for fused deposition modelling. Int. J. Biol. Macromol. 2021, 183, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Acharjee, S.A.; Bharali, P.; Gogoi, B.; Sorhie, V.; Walling, B.; Alemtoshi. PHA-based bioplastic: A potential alternative to address microplastic pollution. Water Air Soil Pollut. 2023, 234, 21. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, X.; Wu, J.; Zhou, T.; Nguyen, T.T.; Wang, Y. Biodegradable polylactic acid and its composites: Characteristics, processing, and sustainable applications in sports. Polymers 2023, 15, 3096. [Google Scholar] [CrossRef]
- Kuciel, S.; Rydarowski, H. (Eds.) Wprowadzenie. In Biokompozyty z Surowców Odnawialnych; Collegium Columbium: Kraków, Poland, 2012; pp. 9–11. (In Polish) [Google Scholar]
- Musioł, M.; Rydz, J.; Janeczek, H.; Kordyka, A.; Andrzejewski, J.; Sterzyński, T.; Jurczyk, S.; Cristea, M.; Musioł, K.; Kampik, M.; et al. (Bio)degradable biochar composites—Studies on degradation and electrostatic properties. Mater. Sci. Eng. B 2022, 275, 115515. [Google Scholar] [CrossRef]
- Saif, M.J.; Ali, N.A. Properties characterization of plasticized polylactic acid/Biochar (bio carbon) nano-composites for antistatic packaging. Iraqi J. Appl. Phys. 2019, 17, 13–26. [Google Scholar]
- Tolvanen, J.; Hannu, J.; Hietal, M.; Kordas, K.; Jantunen, H. Biodegradable multiphase poly(lactic acid)/biochar/graphite composites forelectromagnetic interference shielding. Compos. Sci. Technol. 2019, 181, 107704. [Google Scholar] [CrossRef]
- Zhang, X.; Hao, X.; Hao, J.; Wang, Q. Effect of the addition of carbon nanomaterials on electrical and mechanical properties of wood plastic composites. Polymers 2017, 9, 620. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Jung, D.; Bhattacharyya, D. Improvement of electrical and mechanical properties of PLA/PBAT composites using coconut shell biochar for antistatic applications. Appl. Sci. 2023, 13, 902. [Google Scholar] [CrossRef]
- Jurczyk, S.; Andrzejewski, J.; Piasecki, A.; Musioł, M.; Rydz, J.; Kowalczuk, M. Mechanical and rheological evaluation of polyester-based composites containing biochar. Polymers 2024, 16, 1231. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, W.; Wang, X.; Chen, X.; Chen, G.Q.; Xu, K. Processability modifications of poly(3-hydroxybutyrate) by plasticizing, blending, and stabilizing. J. Appl. Polym. Sci. 2008, 107, 166–173. [Google Scholar] [CrossRef]
- Andrzejewski, J.; Aniśko, J.; Szulc, J. A comparative study of biocarbon reinforced polyoxymethylene and polyamide: Materials performance and durability. Compos. A Appl. Sci. 2022, 152, 106715. [Google Scholar] [CrossRef]
- ISO 527-1:2019; Plastics—Determination of Tensile Properties. ISO: Geneva, Switzerland, 2019; p. 26.
- IEC 62631-3-2:2015; Dielectric and Resistive Properties of Solid Insulating Materials—Part 3-2: Determination of Resistive Properties (DC Methods)—Surface Resistance and Surface Resistivity. International Standard: Geneva, Switzerland, 2015.
- Columbus Instruments. Available online: http://www.colinst.com (accessed on 17 October 2023).
- ISO 13781; Implants for Surgery—Homopolymers, Copolymers and Blends Based on Polylactide—In Vitro Degradation Testing. International Organization for Standardization: Vernier, Switzerland, 2017.
- Sikorska, W.; Richert, J.; Rydz, J.; Musioł, M.; Adamus, G.; Janeczek, H.; Kowalczuk, M. Degradability studies of poly(L-lactide) after multi-reprocessing experiments in extruder. Polym. Degrad. Stab. 2012, 97, 1891–1897. [Google Scholar] [CrossRef]
- García-Campo, M.J.; Boronat, T.; Quiles-Carrillo, L.; Balart, R.; Montanes, N. Manufacturing and characterization of toughened poly(lactic acid) (PLA) formulations by ternary blends with polyesters. Polymers 2018, 10, 3. [Google Scholar] [CrossRef]
- Guessasma, S.; Belhabib, S.; Nouri, H. Microstructure and mechanical performance of 3D printed wood-PLA/PHA using fused deposition modeling: Effect of printing temperature. Polymers 2019, 11, 1778. [Google Scholar] [CrossRef]
- Spyros, A.; Marchessault, R.H. Segmental dynamics of poly(3-hydroxybutyrate-co-4-hydroxybutyrate)s above the glass transition temperature: 13C Nuclear Magnetic Relaxation in the amorphous phase. Macromolecules 1996, 29, 2479–2486. [Google Scholar] [CrossRef]
- Li, Y.; Han, C.; Yu, Y.; Huang, D. Uniaxial stretching and properties of fully biodegradable poly(lactic ac-id)/poly(3-hydroxybutyrate-co-4-hydroxybutyrate) blends. Int. J. Biol. Macromol. 2019, 129, 1–12. [Google Scholar] [CrossRef]
- Zhao, H.; Bian, Y.; Li, Y.; Dong, Q.; Han, C.; Dong, L. Bioresource-based blends of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) and stereocomplex polylactide with improved rheological and mechanical properties of enzymatic hydrolysis. J. Mater. Chem. A 2014, 2, 8881–8892. [Google Scholar] [CrossRef]
- Wang, G.; Chi, X. Ductile poly[(3-hydroxybutyrate)-co-(4-hydroxybutyrate)]-based blends derived from biomass. Polym. Int. 2024, 73, 425–434. [Google Scholar] [CrossRef]
- Musioł, M.; Rydz, J.; Sikorska, W.; Janeczek, H.; Jurczyk, S. Organic recycling challenges of (bio)degradable packages: Degradation studies of polylactide/cork composites. Express Polym. Lett. 2024, 18, 868–880. [Google Scholar] [CrossRef]
- de Souza Reis, G.A.; Michels, M.H.A.; Fajardo, G.L.; Lamot, I.; de Best, J.H. Optimization of green extraction and purification of PHA produced by mixed microbial cultures from sludge. Water 2020, 12, 1185. [Google Scholar] [CrossRef]
- Michalski, A.; Łapienis, G. Synthesis and characterization of high-molar-mass star-shaped poly(L-lactide)s. Polimery 2018, 63, 488–494. [Google Scholar] [CrossRef]
- Carrasco, F.; Pagès, P.; Gámez-Pérez, J.; Santana, O.O.; Maspoch, M.L. Processing of poly(lactic acid): Characterization of chemical structure, thermal stability and mechanical properties. Polym. Degrad. Stab. 2010, 95, 116–125. [Google Scholar] [CrossRef]
- Bai, L.; Zhang, Z.M.; Pu, J.H.; Feng, C.P.; Zhao, X.; Bao, R.Y.; Liu, Z.Y.; Yang, M.B.; Yang, W. Highly thermally conductive electrospun stereocomplex polylactide fibrous film dip-coated with silver nanowires. Polymer 2020, 194, 122390. [Google Scholar] [CrossRef]
- Haeldermans, T.; Samyn, P.; Cardinaels, R.; Vandamme, D.; Vanreppelen, K.; Cuypers, A.; Schreurs, S. Bio-based poly(3-hydroxybutyrate)/thermoplastic starch composites as a host matrix for biochar fillers. J. Polym. Environ. 2021, 29, 2478–2491. [Google Scholar] [CrossRef]
- Yazdaninia, A.; Khonakdar, H.A.; Jafari, S.H.; Asadi, V. Influence of trifluoropropyl-POSS nanoparticles on microstructure, rheological, thermal and thermomechanical properties of PLA. RSC Adv. 2016, 6, 37149–37159. [Google Scholar] [CrossRef]
- Cristea, M.; Ionita, D.; Iftime, M.M. Dynamic mechanical analysis investigations of PLA-based renewable materials: How are they useful? Materials 2020, 13, 5302. [Google Scholar] [CrossRef] [PubMed]
- Starkweather, H.W., Jr.; Avakian, P.; Fontanella, J.J.; Wintersgill, M.G. Internal motions in polylactide and related polymers. Macromolecules 1993, 26, 5084–5087. [Google Scholar] [CrossRef]
- Lee, C.Y.C.; Goldfarb, I.J. The glass transition temperature of partially cured polymers as a non-equilibrium parameter and its effect on observed dynamic mechanical behavior. Polym. Eng. Sci. 1981, 21, 951–957. [Google Scholar] [CrossRef]
- Cristea, M. Dynamic mechanical analysis in polymeric multiphase systems. In Multiphase Polymer Systems. Micro- to Nanostructural Evolution in Advanced Technologies; Barzic, A.I., Ioan, S., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 173–191. [Google Scholar]
- Cristea, M.; Ionita, D.; Simionescu, B.C. A new insight in the dynamo-mechanical behavior of poly(ethylene terephthalate). Eur. Polym. J. 2010, 46, 2005–2012. [Google Scholar] [CrossRef]
- Xia, Z.; Zhao, H.; Wang, Y.; Ma, Y.; Wang, X.; Meng, L.; Wang, D.; Sheng, J.; Chen, W. Chain dynamics and crystalline network structure of poly[R-3-hydroxybutyrate-co-4-hydroxybutyrate] as revealed by solid-state NMR. Soft Matter 2021, 17, 4195–4203. [Google Scholar] [CrossRef] [PubMed]
- Jachowicz, M. Electrostatic properties of selected personal protective equipment regarding explosion hazard, in terms of explosion hazard. J. Sust. Min. 2013, 12, 27–33. [Google Scholar]
- Mojzes, Á.; Tóth, B.S.; Csavajda, P. Investigation of an electrostatic discharge protective biodegradable packaging foam in the logistic chain. JSDTL 2014, 5, 25–33. [Google Scholar] [CrossRef][Green Version]
- Dan, L.; Pope, M.A.; Elias, A.L. Solution-processed conductive biocomposites based on polyhydroxybutyrate and reduced graphene oxide. Phys. Chem. C 2018, 122, 17490–17500. [Google Scholar] [CrossRef]
- Vieira, L.S.; Oyama, I.C.; Montagna, L.S.; Rezende, M.C.; Passador, F.R. Green composites for application in antistatic packaging. In Green Composites. Materials Horizons: From Nature to Nanomaterials; Thomas, S., Balakrishnan, P., Eds.; Springer: Singapore, 2021; pp. 429–453. [Google Scholar]
- Musioł, M.; Janeczek, H.; Jurczyk, S.; Kwiecień, I.; Sobota, M.; Marcinkowski, A.; Rydz, J. (Bio)degradation studies of degradable polymer composites with jute in different environments. Fiber Polym. 2015, 16, 1362–1369. [Google Scholar] [CrossRef]
- EN 13432:2000; Packaging. Reguirements for Packaging Recoverable Throu Composting and Biodegradation. Test Scheme and Evaluation Criteria for the Final Acceptance of Packaging. European Standards s.r.o.: Plzen, Czech Republic, 2007.
- Barczewski, M.; Hejna, A.; Aniśko, J.; Andrzejewski, J.; Piasecki, A.; Mysiukiewicz, O.; Bąk, M.; Gapiński, B.; Ortega, Z. Rotational molding of polylactide (PLA) composites filled with copper slag as a waste filler from metallurgical industry. Polym. Test. 2022, 106, 107449. [Google Scholar] [CrossRef]
- Navrátilová, N.; Náplava, A. Study of Biodegradable Plastics Produced by Injection Molding. 2011. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=94fd4cc58cdabdd1fe588daf51e73ddaa35d79bd (accessed on 10 October 2023).
- Ameli, A.; Jahani, D.; Nofar, M.; Jung, P.U.; Park, C.B. Processing and characterization of solid and foamed injection-molded polylactide with talc. J. Cell. Plast. 2013, 49, 351–374. [Google Scholar] [CrossRef]
- Noh, S.; Kim, D.; Jeong, G.; Koo, J.M.; Koo, J. Highly dispersed biochar as a sustainable filler for enhancing mechanical performance and biodegradation of polybutylene succinate. J. Appl. Polym. Sci. 2024, 141, e55539. [Google Scholar] [CrossRef]
- Papadopoulou, K.; Klonos, P.A.; Kyritsis, A.; Mašek, O.; Wurzer, C.; Tsachouridis, K.; Anastasiou, A.D.; Bikiaris, D.N. Synthesis and study of fully biodegradable composites based on poly(butylene succinate) and biochar. Polymers 2023, 15, 1049. [Google Scholar] [CrossRef] [PubMed]
- Jurczyk, S.; Musioł, M.; Sobota, M.; Klim, M.; Hercog, A.; Kurcok PJaneczek, H.; Rydz, J. (Bio)degradable polymeric materials for sustainable future—Part 2: Degradation studies of polymer-cork composites in different environments. Polymers 2019, 11, 547. [Google Scholar] [CrossRef] [PubMed]
- Edeh, I.G.; Mašek, O. The role of biochar particle size and hydrophobicity in improving soil hydraulic properties. Eur. J. Soil Sci. 2022, 73, e13138. [Google Scholar] [CrossRef]
- Papadopoulou, K.; Tarani, E.; Ainali, N.M.; Chrissafis, K.; Wurzer, C.; Mašek, O.; Bikiaris, D.N. The effect of biochar addition on thermal stability and decomposition mechanism of poly(butylene succinate) bionanocomposites. Molecules 2023, 28, 5330. [Google Scholar] [CrossRef] [PubMed]
- Berthé, V.; Ferry, L.; Bénézet, J.C.; Bergeret, A. Ageing of different biodegradable polyesters blends mechanical and hygrothermal behavior. Polym. Degrad. Stab. 2010, 95, 262–269. [Google Scholar] [CrossRef]
- Bautista Quispe, J.I.; Campos, L.C.; Mašek, O.; Bogush, A. Use of biochar-based column filtration systems for greywater treatment: A systematic literature review. J. Water Process Eng. 2022, 48, 102908. [Google Scholar] [CrossRef]
- Maiza, M.; Benaniba, M.T.; Massardier-Nageotte, V. Plasticizing effects of citrate esters on properties of poly(lactic acid). J. Polym. Eng. 2015, 36, 371–380. [Google Scholar] [CrossRef]
- Stefaniak, K.; Masek, A. Green copolymers based on poly(lactic acid)—Short review. Materials 2021, 14, 5254. [Google Scholar] [CrossRef] [PubMed]
- Li, S.M. Hydrolytic degradation characteristics of aliphatic polyesters derived from lactic and glycolic acids. J. Biomed. Mater. Res. 1999, 48, 342–353. [Google Scholar] [CrossRef]
- Azevedo, H.; Reis, R.L. Understanding the Enzymatic Degradation of Biodegradable Polymers and Strategies to Control Their Degradation Rate; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar] [CrossRef]
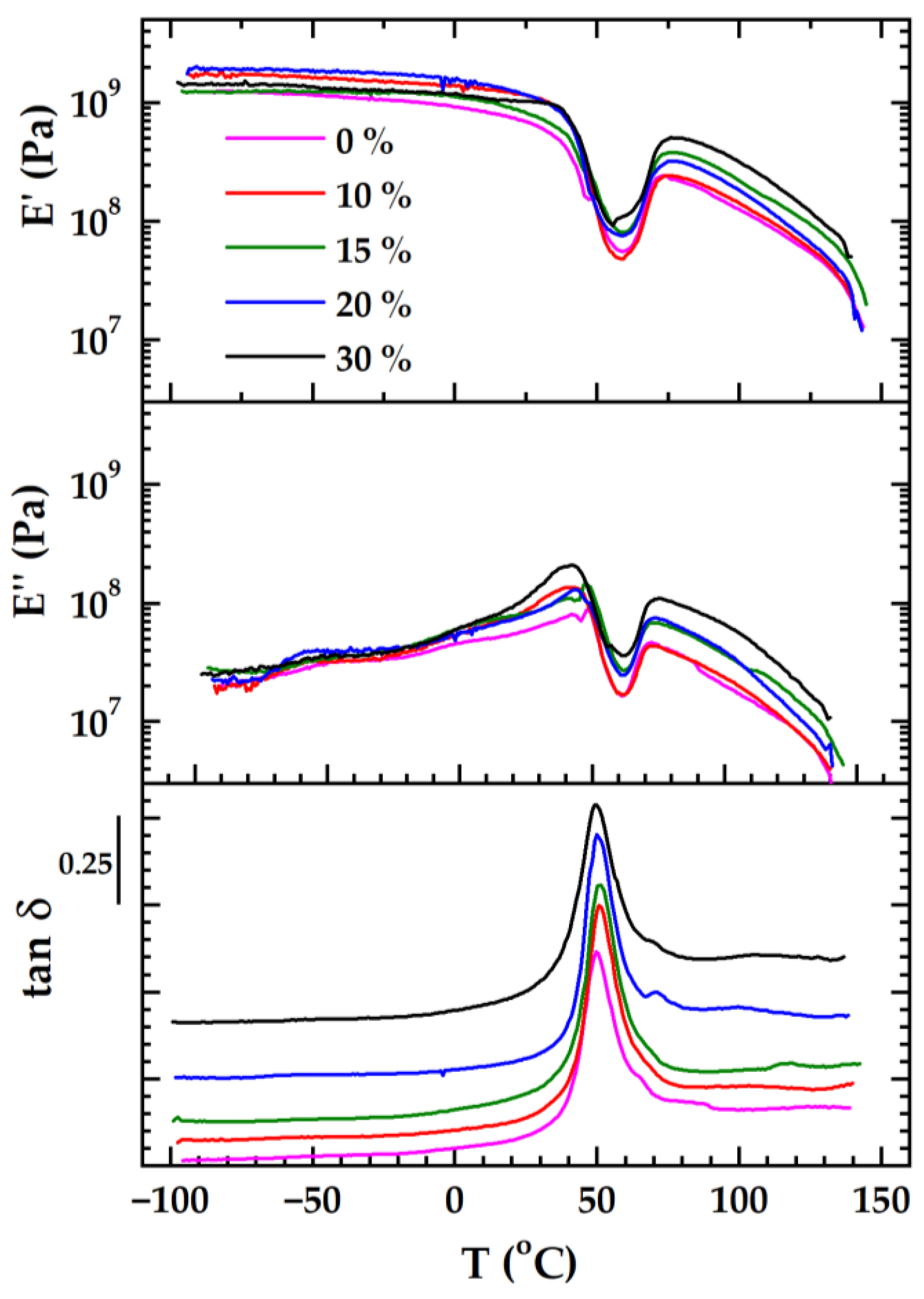
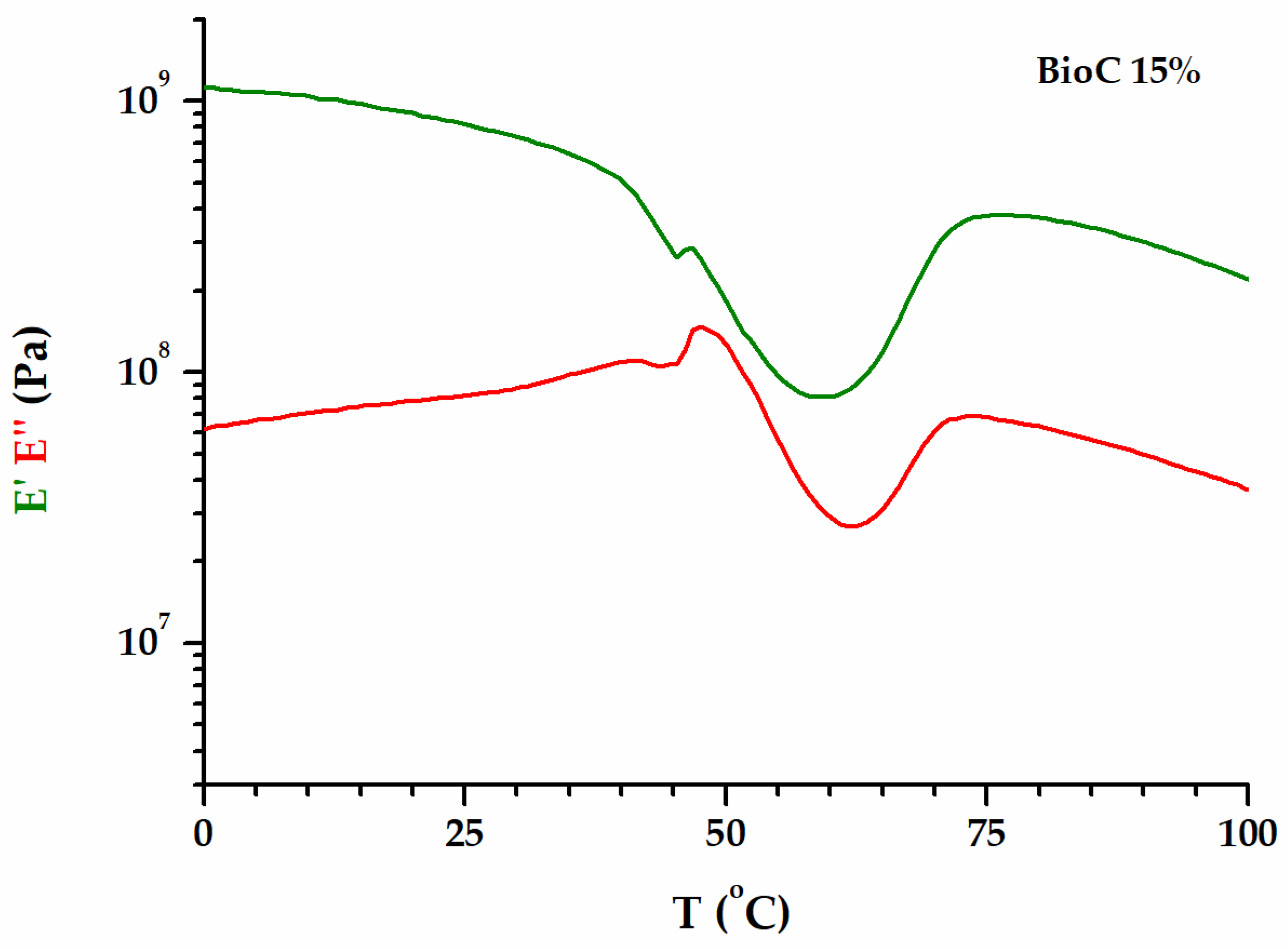


| PLA/P(3HB-co-4HB)/Biochar (Mass Ratio) | E′ [MPa] (Glassy Region at 0 °C) | Tg [°C] 1 (by E′onset) | T 2 [°C] | E′ 2 [MPa] | T [°C] 3 | E′ [MPa] |
|---|---|---|---|---|---|---|
| 100/0 | 925 | 36.1 | 59.0 | 55.5 | 75.6 | 230 |
| 90/10 | 1400 | 39.2 | 58.5 | 48.1 | 76.6 | 241 |
| 85/15 | 1120 | 37.3 | 60.0 | 81.3 | 77.6 | 379 |
| 80/20 | 1200 | 41.6 | 59.3 | 76.0 | 78.3 | 318 |
| 70/30 | 1180 | 41.4 | 56.0 | 99.0 | 79.2 | 501 |
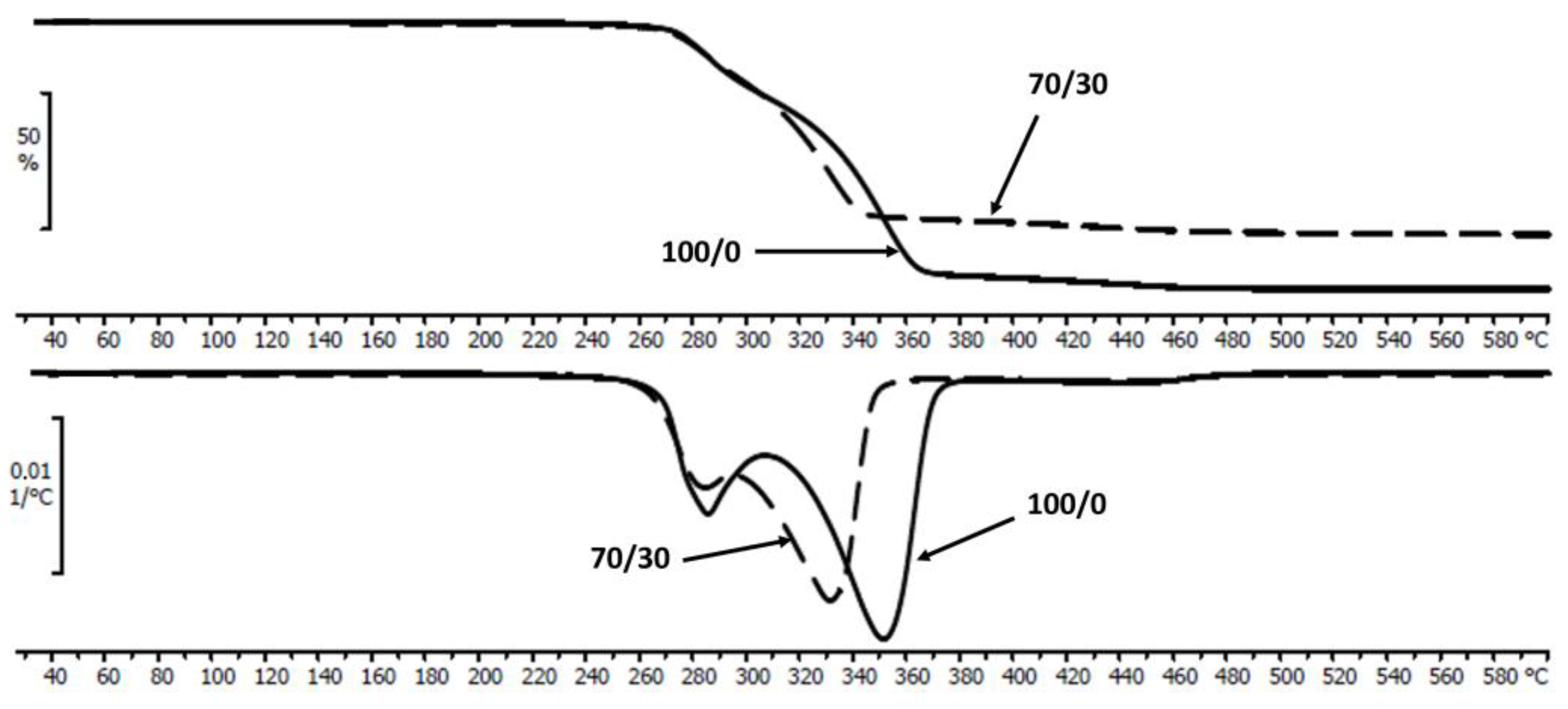
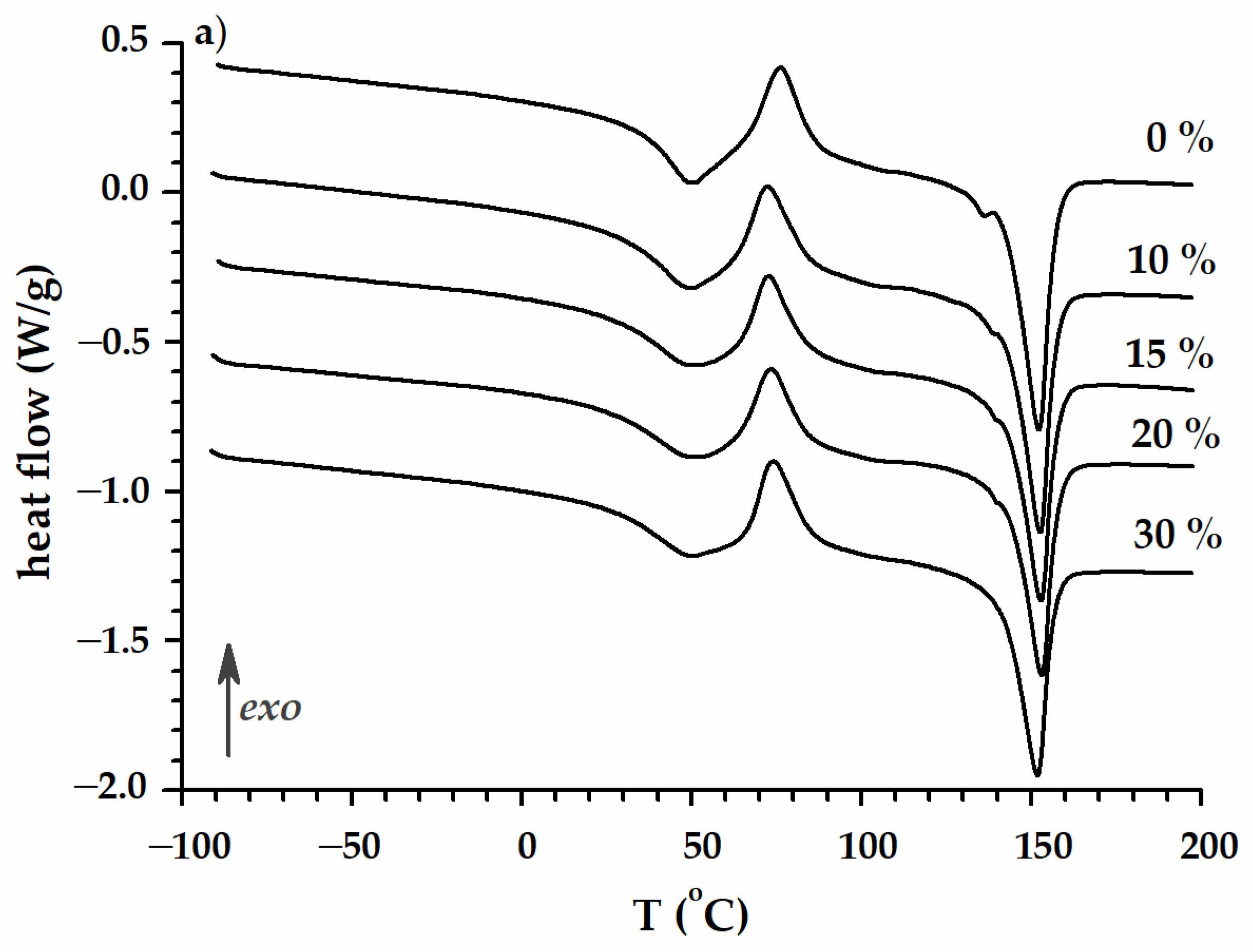
| PLA/P(3HB-co-4HB)/Biochar (Mass Ratio) | Time [Days] | Tmax [°C] | R600 [%] | Tcc [°C] | ∆Hcc [J/g ] | Tm [°C] | ∆Hm [J/g ] | TgPHA [°C] | TgPLA [°C] |
|---|---|---|---|---|---|---|---|---|---|
| Abiotic degradation | |||||||||
| 100/0 | 0 | 283.8/351.1 | 1.8 | 83.6 | 20.4 | 157.1 | 29.1 | −5.9 | 35.9 |
| 7 | 300.2/352.5 | 1.2 | ---- | ---- | 141.8 | 41.3 | ---- | 13.8 | |
| 14 | 303.7/350.0 | 1.0 | ---- | ----- | 135.3 | 57.0 | ---- | 14.7 | |
| 21 | 301.7/344.5 | 1.6 | ---- | ----- | 124.9 | 61.7 | ---- | 14.9 | |
| 70 | 286.5 | 1.7 | ---- | ----- | 128.3 | 57.3 | −21.3 | ---- | |
| 90/10 | 0 | 286.3/341.6 | 9.5 | 86.0 | 20.1 | 160.5 | 27.0 | −5.9 | 41.4 |
| 7 | 295.5/341.8 | 8.6 | ---- | ---- | 146.4 | 37.7 | ---- | 20.2 | |
| 14 | 305.2/344.8 | 10.3 | ---- | ---- | 133.2 | 48.2 | ---- | 17.0 | |
| 21 | 302.6/349.0 | 13.1 | ---- | ----- | 127.2 | 51.7 | ---- | 15.5 | |
| 70 | 289.1 | 30.2 | ---- | ----- | 132.7 | 43.8 | −17.7 | ---- | |
| 85/15 | 0 | 285.9/338.8 | 13.6 | 85.7 | 22.1 | 158.8 | 23.7 | −4.6 | 39.5 |
| 7 | 302.9/342.7 | 13.5 | ---- | ---- | 144.7 | 33.6 | ---- | 19.1 | |
| 14 | 305.9/349.1 | 15.3 | ---- | ---- | 133.6 | 43.3 | ---- | 17.7 | |
| 21 | 303.3/351.2/ | 17.5 | ---- | ---- | 123.7 | 46.8 | ---- | 19.1 | |
| 70 | 290.0 | 40.4 | ---- | ---- | 126.6 | 37.7 | −16.2 | ---- | |
| 80/20 | 0 | 283.7/336.9 | 15.7 | 88.6 | 19.5 | 157.7 | 21.0 | −5.7 | 39.6 |
| 7 | 298.6/339.0 | 15.7 | ---- | ---- | 144.7 | 35.6 | ---- | 22.7 | |
| 14 | 306.3/347.7 | 18.0 | ---- | ---- | 135.8 | 44.7 | ---- | 18.5 | |
| 21 | 303.7/347.1 | 21.4 | ---- | ---- | 124.6 | 49.0 | ---- | 18.9 | |
| 70 | 290.0 | 44.9 | ---- | ---- | 123.4 | 50.3 | −16.8 | ---- | |
| 70/30 | 0 | 283.9/331.6 | 21.3 | 89.7 | 19.9 | 159.2 | 22.6 | −5.5 | 41.4 |
| 7 | 302.1/340.2 | 21.2 | ---- | ---- | 143.7 | 39.3 | ---- | 23.3 | |
| 14 | 306.2/341.9 | 24.2 | ---- | ---- | 135.5 | 44.9 | ---- | 20.4 | |
| 21 | 303.5/345.6 | 27.3 | ---- | ---- | 130.3 | 43.9 | ---- | 21.9 | |
| 70 | 289.9 | 52.4 | ---- | ---- | 72.1/ 118.5 | 60.0 | −16.2 | ---- | |
| Degradation in compost (respirometer) | |||||||||
| 100/0 | 21 | 285.4/327.7 | 2.6 | ---- | ----- | 148.9 | 54.3 | ----- | 29.6 |
| 90/10 | 21 | 273.6/317.6 | 12.3 | ---- | ----- | 144.1 | 47.6 | ----- | 28.1 |
| 85/15 | 21 | 290.3/336.4 | 14.0 | ---- | ----- | 145.8 | 49.8 | ----- | 29.8 |
| 80/20 | 21 | 272.2/305.3 | 21.2 | ---- | ------ | 150.1 | 40.1 | ----- | 32.0 |
| 70/30 | 21 | 293.4/325.6 | 23.2 | ---- | ----- | 145.9 | 45.4 | ----- | 32.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musioł, M.; Rydz, J.; Janeczek, H.; Andrzejewski, J.; Cristea, M.; Musioł, K.; Kampik, M.; Kowalczuk, M. (Bio)degradable Biochar Composites of PLA/P(3HB-co-4HB) Commercial Blend for Sustainable Future—Study on Degradation and Electrostatic Properties. Polymers 2024, 16, 2331. https://doi.org/10.3390/polym16162331
Musioł M, Rydz J, Janeczek H, Andrzejewski J, Cristea M, Musioł K, Kampik M, Kowalczuk M. (Bio)degradable Biochar Composites of PLA/P(3HB-co-4HB) Commercial Blend for Sustainable Future—Study on Degradation and Electrostatic Properties. Polymers. 2024; 16(16):2331. https://doi.org/10.3390/polym16162331
Chicago/Turabian StyleMusioł, Marta, Joanna Rydz, Henryk Janeczek, Jacek Andrzejewski, Mariana Cristea, Krzysztof Musioł, Marian Kampik, and Marek Kowalczuk. 2024. "(Bio)degradable Biochar Composites of PLA/P(3HB-co-4HB) Commercial Blend for Sustainable Future—Study on Degradation and Electrostatic Properties" Polymers 16, no. 16: 2331. https://doi.org/10.3390/polym16162331
APA StyleMusioł, M., Rydz, J., Janeczek, H., Andrzejewski, J., Cristea, M., Musioł, K., Kampik, M., & Kowalczuk, M. (2024). (Bio)degradable Biochar Composites of PLA/P(3HB-co-4HB) Commercial Blend for Sustainable Future—Study on Degradation and Electrostatic Properties. Polymers, 16(16), 2331. https://doi.org/10.3390/polym16162331











