Preparation of Functionalized Ethylene–Vinyl-Alcohol Nanofibrous Membrane Filter for Rapid and Cyclic Removing of Organic Dye from Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Apparatus
2.2. Fabrication of SiW12@EVOH NFM
2.3. Preparation of SiW12@EVOH-CCA NFM
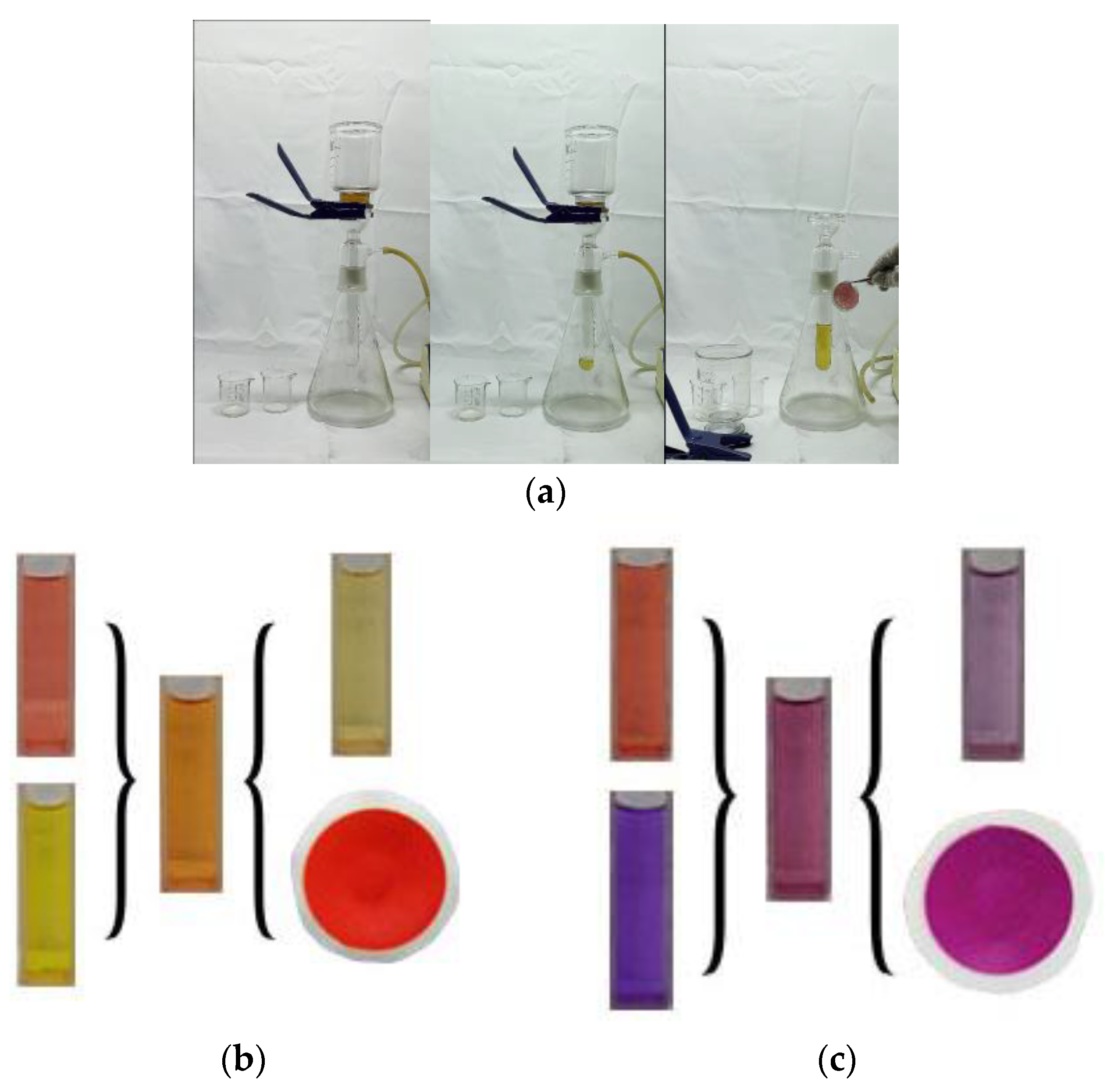
2.4. Selective Adsorption of CR by SiW12@EVOH-CCA NFM
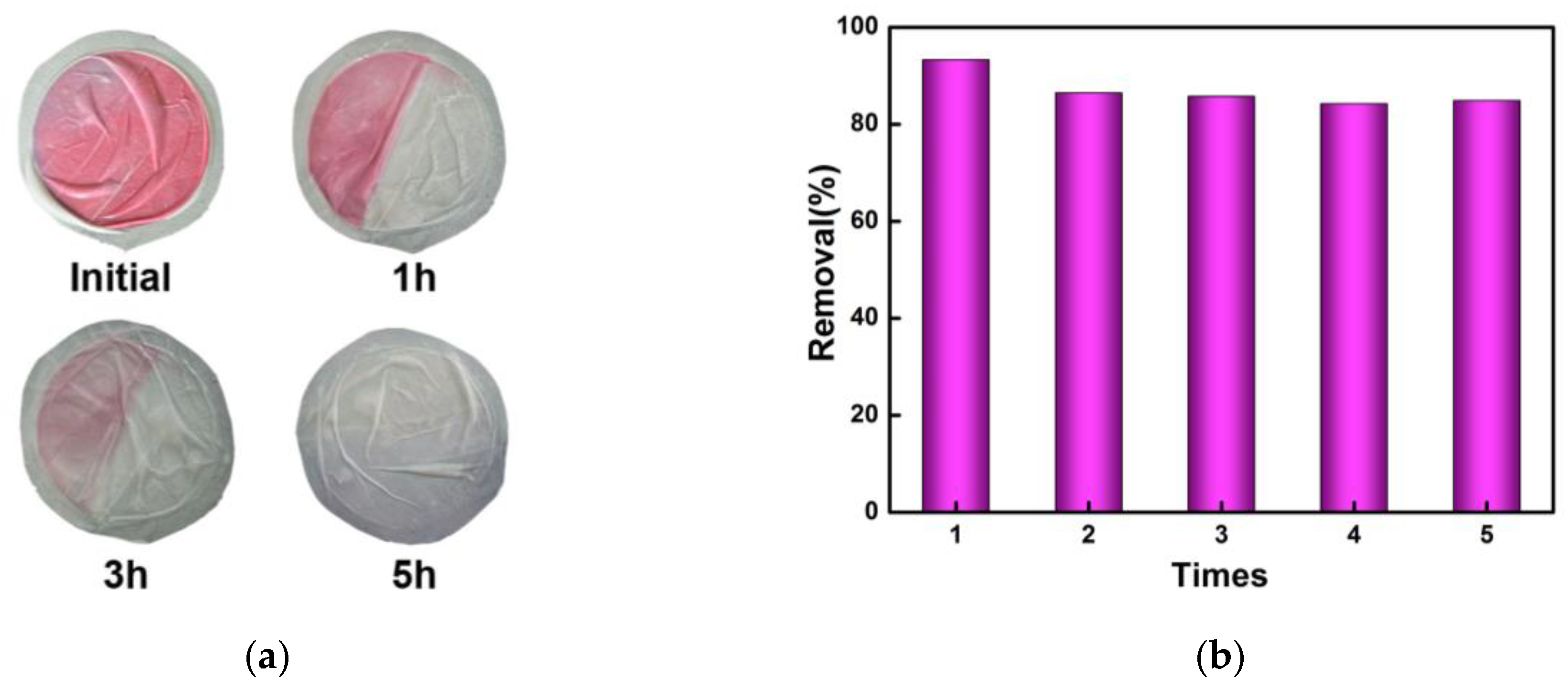
2.5. Regeneration and Reuse of SiW12@EVOH-CCA NFM
3. Results and Discussion
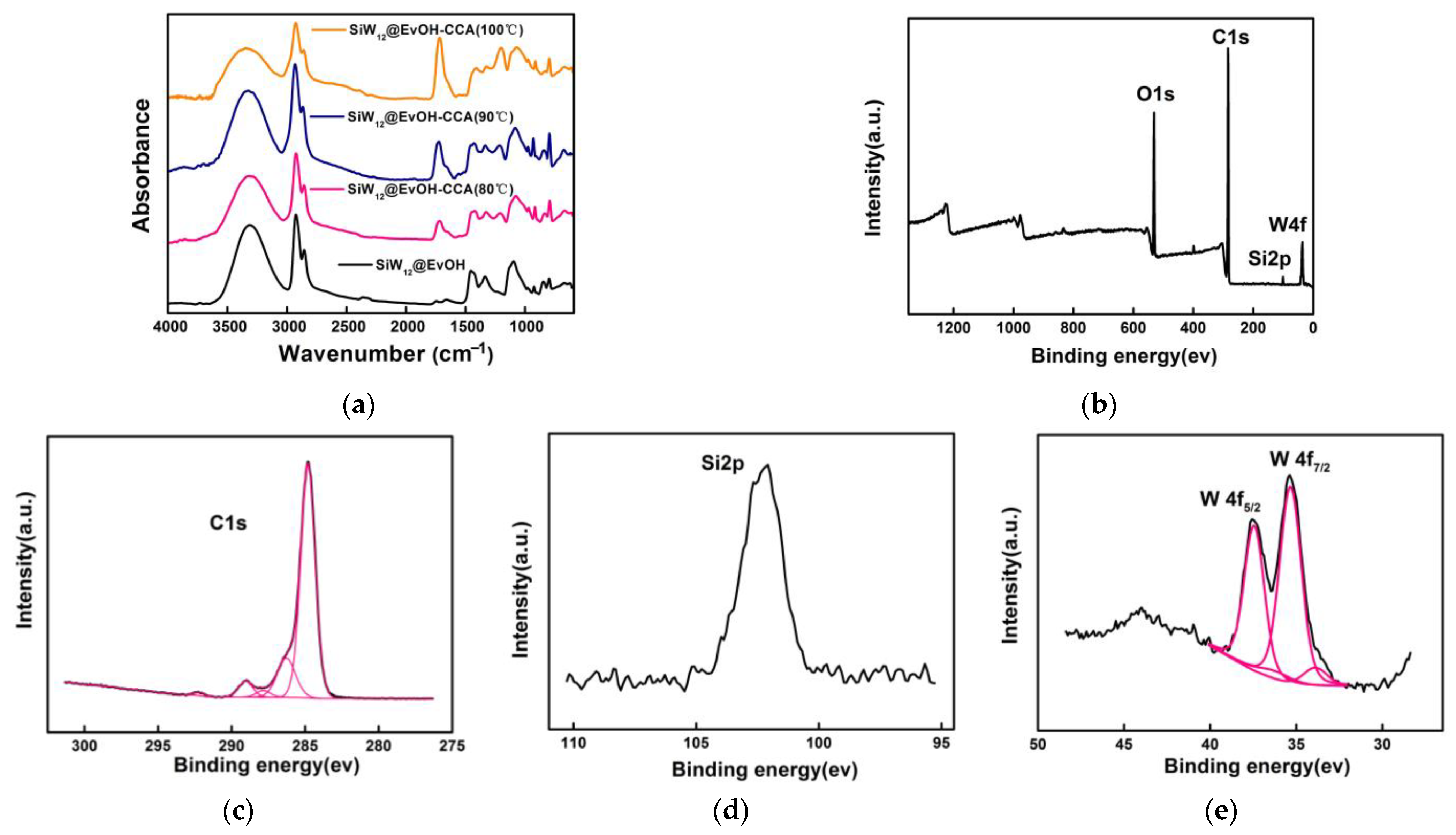
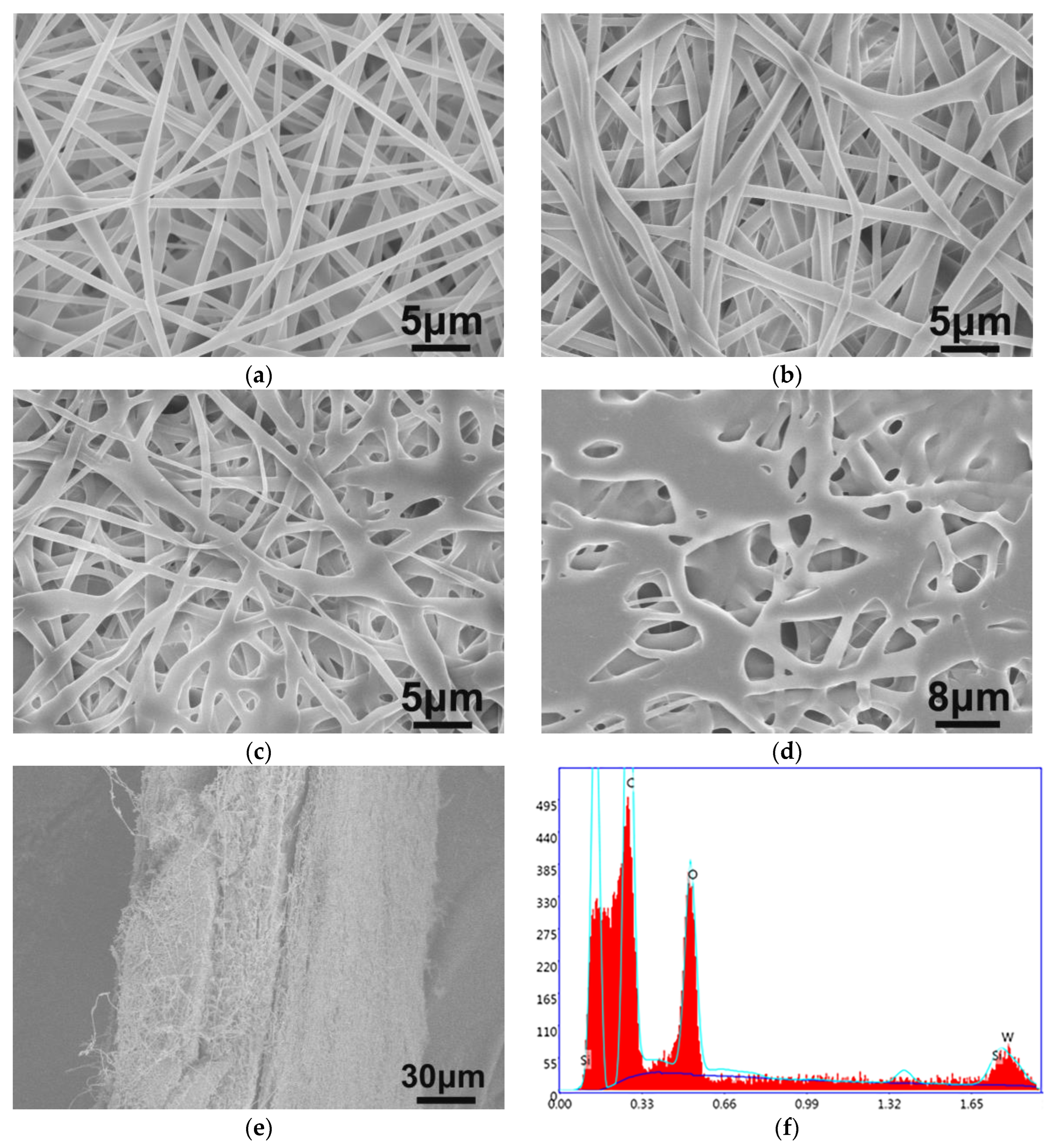
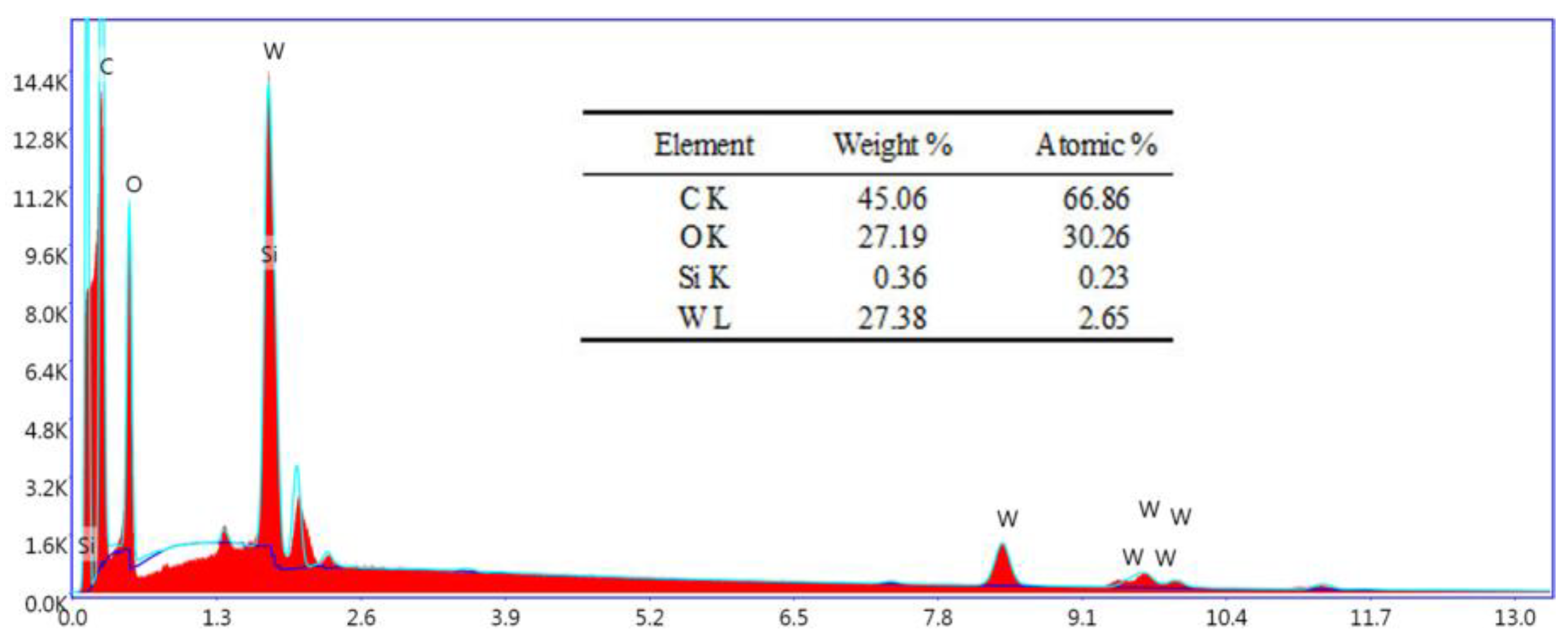
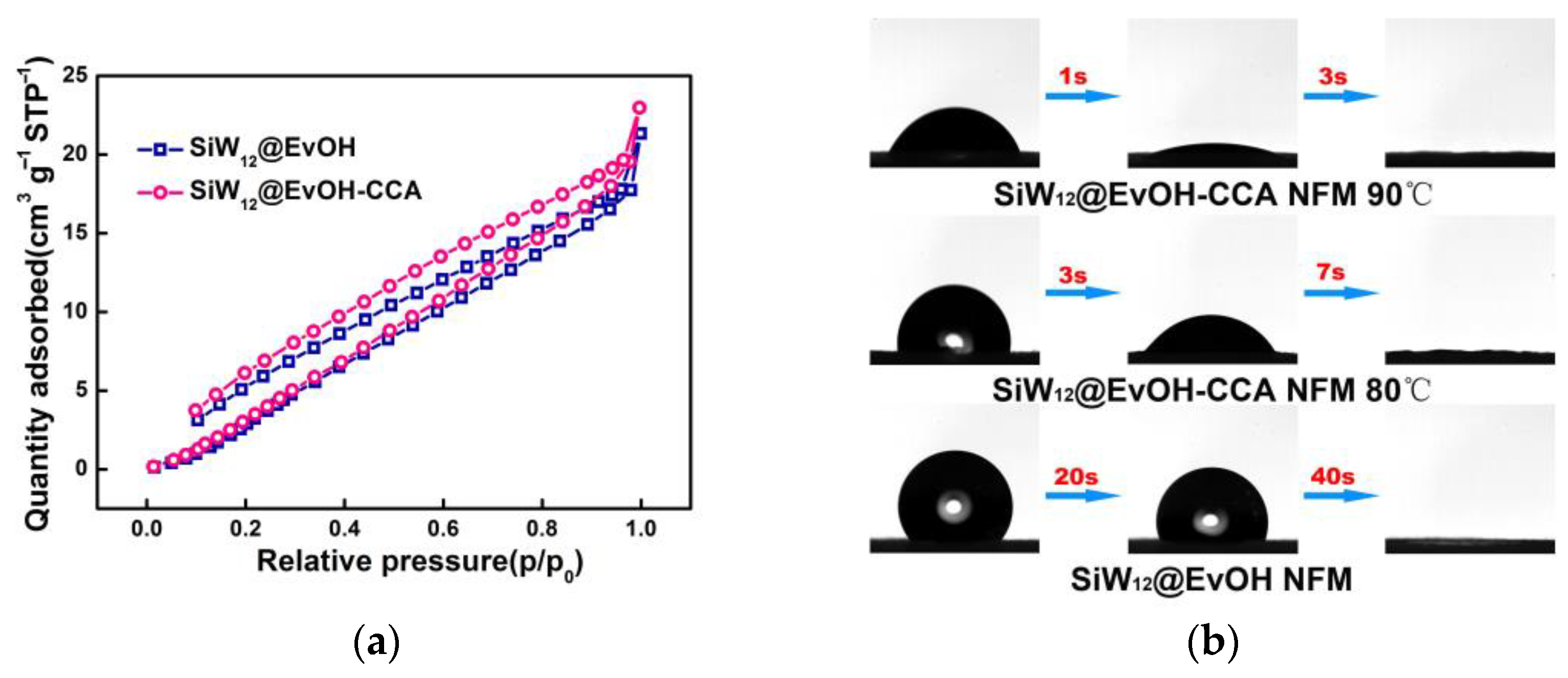
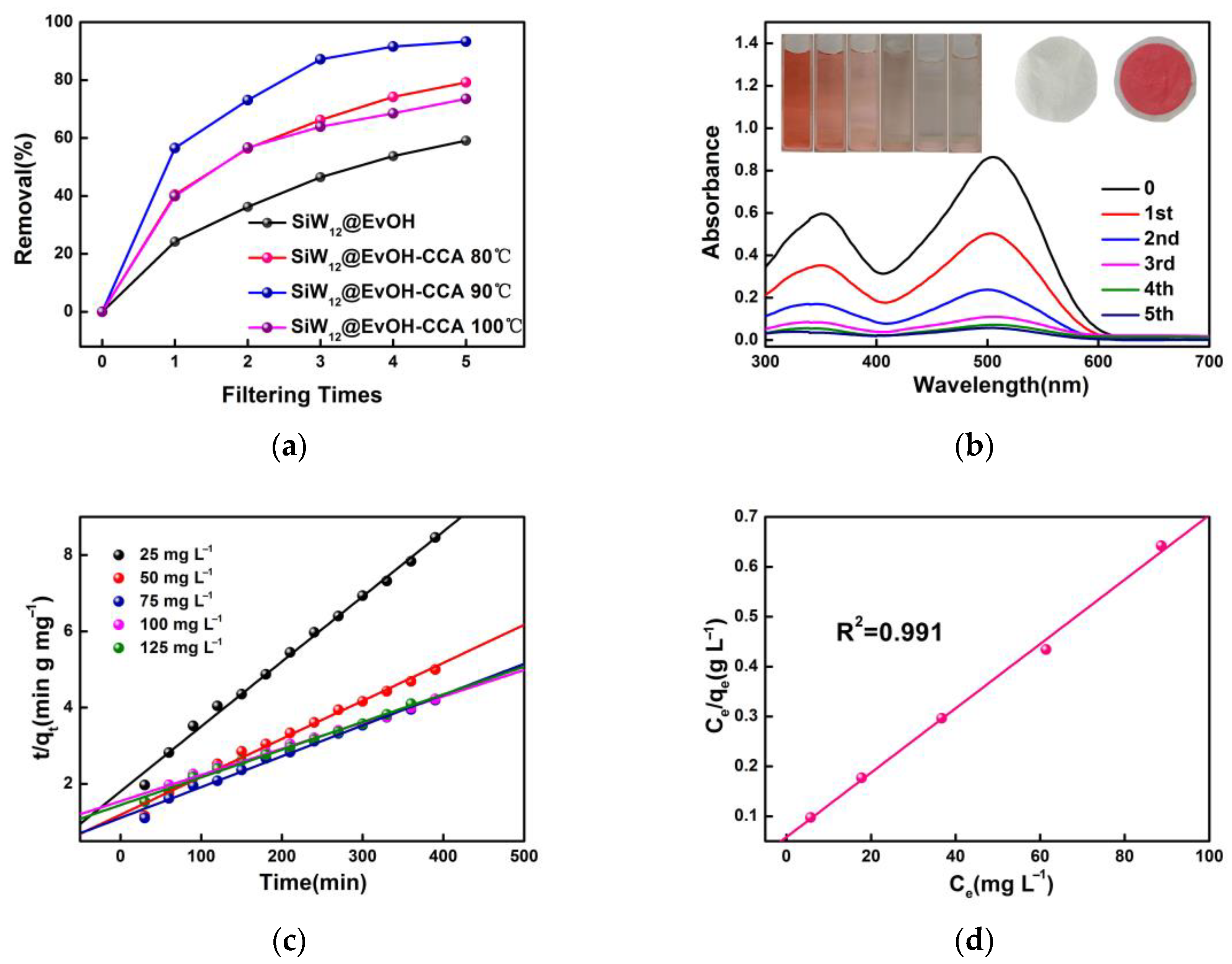
3.1. Characterization of SiW12@EVOH-CCA NFM
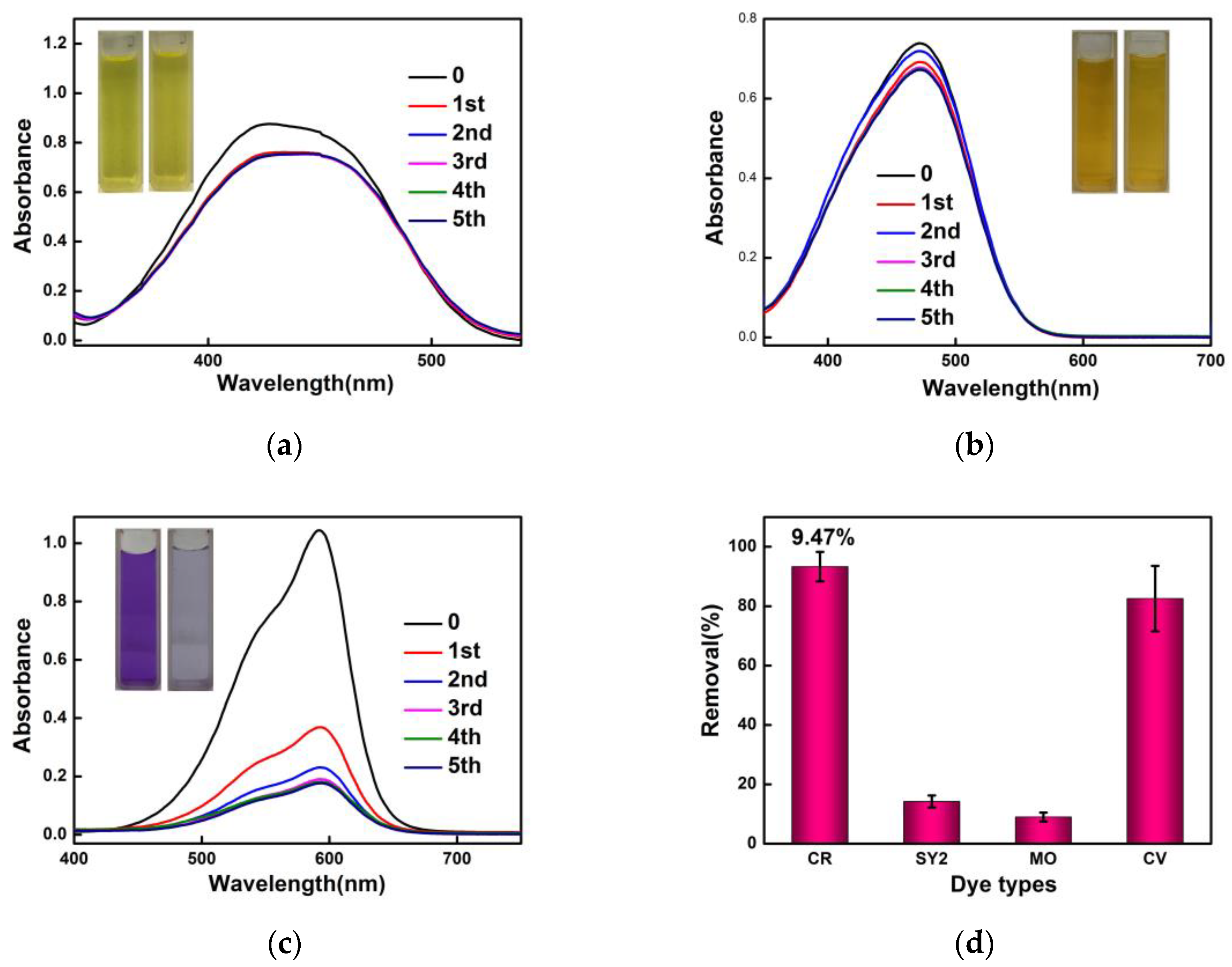
3.2. Selective Adsorption of Dyes by SiW12@EVOH-CCA NFM
3.3. Recycling Process of SiW12@EVOH-CCA NFM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Long, J.; Xie, Z.; Xue, S.; Shi, W.; Liu, Y. Highly stable and permeable graphene oxide membrane modified by carbohydrazide for efficient dyes separation. Sep. Purif. Technol. 2022, 298, 121586. [Google Scholar] [CrossRef]
- Ma, H.; Kong, A.; Ji, Y.; He, B.; Song, Y.; Li, J. Ultrahigh adsorption capacities for anionic and cationic dyes from wastewater using only chitosan. J. Clean. Prod. 2019, 214, 89–94. [Google Scholar] [CrossRef]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Hoijang, S.; Wangkarn, S.; Ieamviteevanich, P.; Pinitsoontorn, S.; Ananta, S.; Randall Lee, T.; Srisombat, L. Silica-coated magnesium ferrite nanoadsorbent for selective removal of methylene blue. Colloids Surf. A 2020, 606, 125483. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, Y.; Lin, T.; Li, Y.; Zhou, R.; Peng, Y. Adsorption performance and mechanism of methylene blue by H3PO4− modified corn stalks. J. Environ. Chem. Eng. 2019, 7, 103398. [Google Scholar] [CrossRef]
- Pan, J.; Zhou, L.; Chen, H.; Liu, X.; Hong, C.; Chen, D.; Pan, B. Mechanistically understanding adsorption of methyl orange, indigo carmine, and methylene blue onto ionic/nonionic polystyrene adsorbents. J. Hazard. Mater. 2021, 418, 126300. [Google Scholar] [CrossRef]
- Santhosh, A.S.; Sandeep, S.; Kumara Swamy, N. Green synthesis of nano silver from euphorbia geniculata leaf extract: Investigations on catalytic degradation of methyl orange dye and optical sensing of Hg2+. Surf. Interfaces 2019, 14, 50–54. [Google Scholar] [CrossRef]
- Nath, J.; Bag, S.; Bera, D.; Ray, L. Biotreatment of malachite green from aqueous solution and simulated textile effluent by growing cells (batch mode) and activated sludge system. Groundw. Sustain. Dev. 2019, 8, 172–178. [Google Scholar] [CrossRef]
- Handojo, L.; Pramudita, D.; Mangindaan, D.; Indarto, A. Application of Nanoparticles in Environmental Cleanup: Production, Potential Risks and Solutions. In Emerging Eco-Friendly Green Technologies for Wastewater Treatment. Microorganisms for Sustainability; Bharagava, R., Ed.; Springer: Singapore, 2020; Volume 18, pp. 45–76. [Google Scholar]
- Ramutshatsha-Makhwedzha, D.; Nomngongo, P.N. Application of Ultrafiltration Membrane Technology for Removal of Dyes from Wastewater. In Membrane Based Methods for Dye Containing Wastewater. Sustainable Textiles: Production, Processing, Manufacturing & Chemistry; Muthu, S.S., Khadir, A., Eds.; Springer: Singapore, 2022; pp. 37–47. [Google Scholar]
- Yan, W.; Han, L.J.; Jia, H.L.; Shen, K.; Wang, T.; Zheng, H.G. Three Highly Stable Cobalt MOFs Based on “Y”-Shaped Carboxylic Acid: Synthesis and Absorption of Anionic Dyes. Inorg. Chem. 2016, 55, 8816–8821. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, Y.; Nie, W.; Song, L.; Chen, P. Highly efficient methylene blue dyes removal from aqueous systems by chitosan coated magnetic mesoporous silica nanoparticles. J. Porous Mat. 2015, 22, 1383–1392. [Google Scholar] [CrossRef]
- Viana, M.M.; do Amparo, S.Z.S.; Lima, M.C.F.S.; Lopes, R.C.F.G.; Vasconcelos, C.K.B.; Caliman, V.; Silva, G.G. Microwave-assisted synthesis of polyacrylamide-aminated graphene oxide hybrid hydrogel with improved adsorption properties. J. Environ. Chem. Eng. 2020, 8, 104415. [Google Scholar] [CrossRef]
- Li, K.; Yan, J.; Zhou, Y.; Li, B.; Li, X. β-cyclodextrin and magnetic graphene oxide modified porous composite hydrogel as a superabsorbent for adsorption cationic dyes: Adsorption performance, adsorption mechanism and hydrogel column process investigates. J. Mol. Liq. 2021, 335, 116291. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, G.; Liu, J.; Wang, T.; Wang, X.; Liu, B.; Liu, Y.; Huo, Q.; Chu, Z. Synthesis of hierarchical hollow sodium titanate microspheres and their application for selective removal of organic dyes. J. Colloid Interface Sci. 2018, 528, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, D.; Xiang, C.; Zhang, F.; Liu, L.; Zhou, X.; Zhang, H. Facile Synthesis of Boron Organic Polymers for Efficient Removal and Separation of Methylene Blue, Rhodamine B, and Rhodamine 6G. ACS Sustain. Chem. Eng. 2018, 6, 16777–16787. [Google Scholar] [CrossRef]
- Molavi, H.; Shojaei, A.; Pourghaderi, A. Rapid and tunable selective adsorption of dyes using thermally oxidized nanodiamond. J. Colloid Interface Sci. 2018, 524, 52–64. [Google Scholar] [CrossRef]
- Li, K.; Li, X.; Li, B. Investigation the adsorption behavior of functional carbon-based composites for efficient removing anions/cations in single and multicomponent systems. Sep. Purif. Technol. 2022, 289, 120737. [Google Scholar] [CrossRef]
- Akbari, Z.; Ghiaci, M.; Nezampour, F. Encapsulation of Vanadium Phosphorus Oxide into TiO2 Matrix for Selective Adsorption of Methylene Blue from Aqueous Solution. J. Chem. Eng. Data 2018, 63, 3923–3932. [Google Scholar] [CrossRef]
- Kim, C.; Hwang, J.Y.; Ku, K.S.; Angupillai, S.; Son, Y.A. A renovation of non-aqueous Al3+ sensor to aqueous media sensor by simple recyclable immobilize electrospun nano-fibers and its uses for live sample analysis. Sens. Actuators B Chem. 2016, 228, 259–269. [Google Scholar] [CrossRef]
- Ge, J.; Zong, D.; Jin, Q.; Yu, J.; Ding, B. Biomimetic and Superwettable Nanofibrous Skins for Highly Efficient Separation of Oil-in-Water Emulsions. Adv. Funct. Mater. 2018, 28, 1705051. [Google Scholar] [CrossRef]
- Rocha, J.M.; Sousa, R.P.C.L.; Fangueiro, R.; Ferreira, D.P. The Potential of Electrospun Membranes in the Treatment of Textile Wastewater: A Review. Polymers 2024, 16, 801. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, X.; Wu, F.; Ji, Y. High adsorption capability and selectivity of ZnO nanoparticles for dye removal. Colloids Surf. A 2016, 509, 474–483. [Google Scholar] [CrossRef]
- Fu, Q.; Wang, X.; Si, Y.; Liu, L.; Yu, J.; Ding, B. Scalable Fabrication of Electrospun Nanofibrous Membranes Functionalized with Citric Acid for High-Performance Protein Adsorption. ACS Appl. Mater. Interface 2016, 8, 11819–11829. [Google Scholar] [CrossRef]
- Li, T.T.; Liu, L.; Gao, M.L.; Han, Z.B. A highly stable nanofibrous Eu-MOF membrane as a convenient fluorescent test paper for rapid and cyclic detection of nitrobenzene. Chem. Commun. 2019, 55, 4941–4944. [Google Scholar] [CrossRef]
- Makhtar, S.N.N.M.; Yusof, N.; Fajrina, N.; Hairom, N.H.H.; Aziz, F.; Salleh, W.N.W. V2O5/CdS as nanocomposite catalyst for Congo red dye photocatalytic degradation under visible light. Mater. Today Proc. 2024, 96, 69–72. [Google Scholar] [CrossRef]
- Rianjanu, A.; Mustamin, A.S.P.; Melati, E.K.A.; Aflaha, R.; Khamidy, N.I.; Utami, M.; Khairurrijal, K.; Triyana, K.; Abdi, F.F.; Wasisto, H.S.; et al. Photocatalytic degradation of aqueous Congo red dye pollutants by rare-earth metal oxide (CeO2) nanorods. Colloids Surf. A 2024, 682, 132919. [Google Scholar] [CrossRef]
- Gadore, V.; Singh, A.K.; Mishra, S.R.; Ahmaruzzaman, M. RSM approach for process optimization of the photodegradation of congo red by a novel NiCo2S4/chitosan photocatalyst. Sci. Rep. 2024, 14, 1118. [Google Scholar] [CrossRef] [PubMed]
- Sui, C.; Li, C.; Guo, X.; Cheng, T.; Gao, Y.; Zhou, G.; Gong, J.; Du, J. Facile synthesis of silver nanoparticles-modified PVA/H4SiW12O40 nanofibers-based electrospinning to enhance photocatalytic activity. Appl. Surf. Sci. 2012, 258, 7105–7111. [Google Scholar] [CrossRef]
- Jang, K.S.; Eom, Y.S.; Choi, K.S.; Bae, H.C. Synchronous curable deoxidizing capability of epoxy–anhydride adhesive: Deoxidation quantification via spectroscopic analysis. J. Appl. Polym. Sci. 2018, 135, 46639. [Google Scholar] [CrossRef]
- Fang, D.; He, F.; Xie, J.; Xue, L. Calibration of binding energy positions with C1s for XPS results. J. Wuhan Univ. Technol. Mater Sci. Ed. 2020, 35, 711–718. [Google Scholar] [CrossRef]
- Feng, Z.; Shao, Z.; Yao, J.; Huang, Y.; Chen, X. Protein adsorption and separation with chitosan-based amphoteric membranes. Polymer 2009, 50, 1257–1263. [Google Scholar] [CrossRef]
- Angin, D. Utilization of activated carbon produced from fruit juice industry solid waste for the adsorption of Yellow 18 from aqueous solutions. Bioresour. Technol. 2014, 168, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wen, Y.; Wang, L.; He, J.; Al-Deyab, S.S.; El-Newehy, M.; Yu, J.; Ding, B. Simultaneous visual detection and removal of lead(ii) ions with pyromellitic dianhydride-grafted cellulose nanofibrous membranes. J. Mater. Chem. A 2015, 3, 18180–18189. [Google Scholar] [CrossRef]
- Troupis, A.; Gkika, E.; Triantis, T.; Hiskia, A.; Papaconstantinou, E. Photocatalytic reductive destruction of azo dyes by polyoxometallates: Naphthol blue black. J. Photochem. Photobiol. A 2007, 188, 272–278. [Google Scholar] [CrossRef]
- Troupis, A.; Triantis, T.M.; Gkika, E.; Hiskia, A.; Papaconstantinou, E. Photocatalytic reductive–oxidative degradation of Acid Orange 7 by polyoxometalates. Appl. Catal. B Environ. Energy 2009, 86, 98–107. [Google Scholar] [CrossRef]
- Li, T.T.; Zhang, Z.M.; Yang, Z.Y. Encapsulation of phosphotungstic acid into UiO-66-(OH)2 for synergistic and rapid photocatalytic degradation of organic pollutants. Mater. Lett. 2023, 348, 134699. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, J.; Li, T.; Wang, X.; Li, M.; Li, T.; Zhang, Z. Preparation of Functionalized Ethylene–Vinyl-Alcohol Nanofibrous Membrane Filter for Rapid and Cyclic Removing of Organic Dye from Aqueous Solution. Polymers 2024, 16, 2328. https://doi.org/10.3390/polym16162328
Ding J, Li T, Wang X, Li M, Li T, Zhang Z. Preparation of Functionalized Ethylene–Vinyl-Alcohol Nanofibrous Membrane Filter for Rapid and Cyclic Removing of Organic Dye from Aqueous Solution. Polymers. 2024; 16(16):2328. https://doi.org/10.3390/polym16162328
Chicago/Turabian StyleDing, Jiachen, Tingting Li, Xiangyi Wang, Mengyang Li, Tianyu Li, and Zhiming Zhang. 2024. "Preparation of Functionalized Ethylene–Vinyl-Alcohol Nanofibrous Membrane Filter for Rapid and Cyclic Removing of Organic Dye from Aqueous Solution" Polymers 16, no. 16: 2328. https://doi.org/10.3390/polym16162328
APA StyleDing, J., Li, T., Wang, X., Li, M., Li, T., & Zhang, Z. (2024). Preparation of Functionalized Ethylene–Vinyl-Alcohol Nanofibrous Membrane Filter for Rapid and Cyclic Removing of Organic Dye from Aqueous Solution. Polymers, 16(16), 2328. https://doi.org/10.3390/polym16162328





