Performance Enhancement of Biopolyester Blends by Reactive Compatibilization with Maleic Anhydride-Grafted Poly(butylene succinate-co-adipate)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
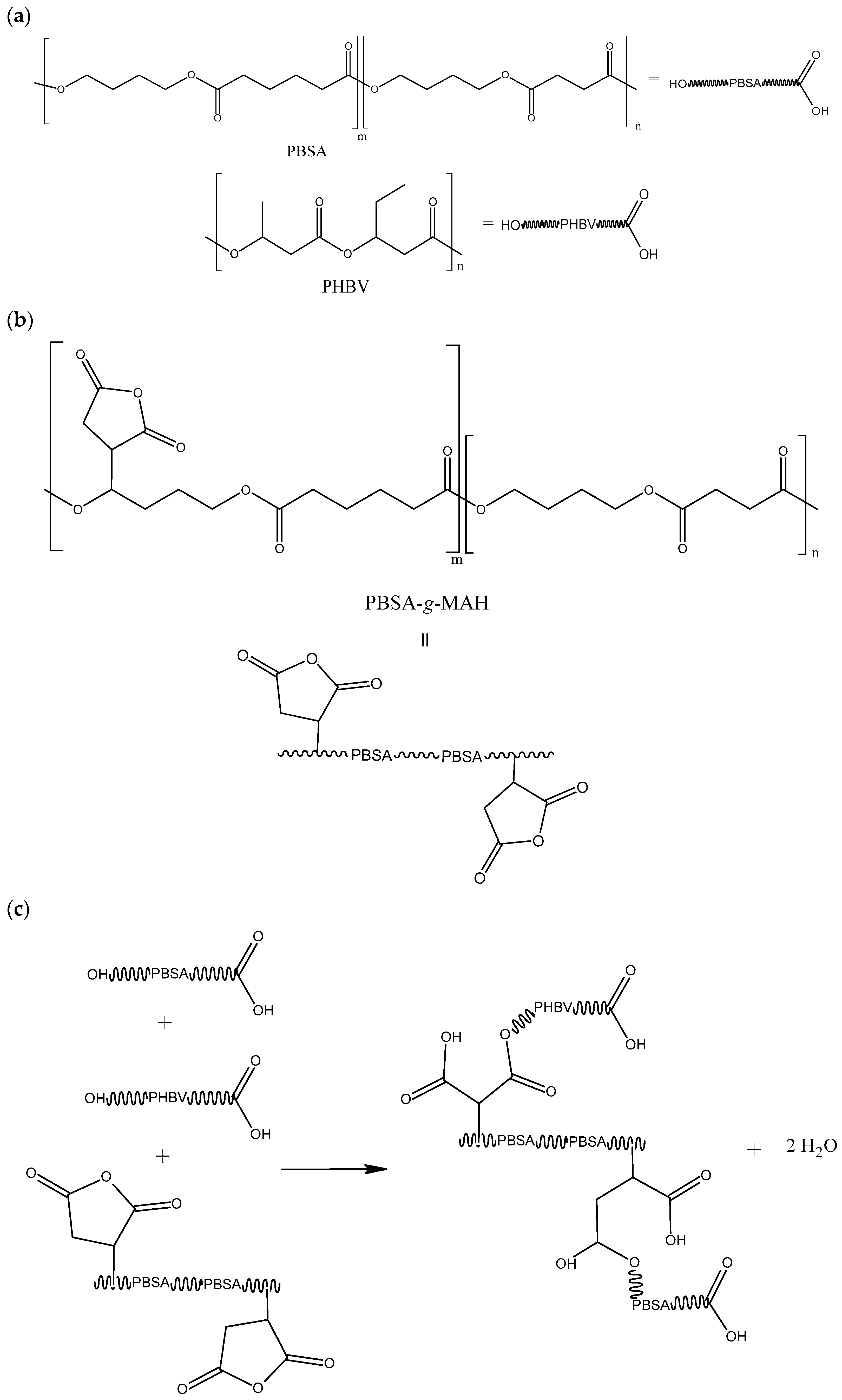
2.2. Grafting Procedure
2.3. Cast-Film Extrusion
2.4. Film Characterization
2.4.1. Morphology
2.4.2. Thermal Analyses
2.4.3. Mechanical Characterization
2.4.4. Permeability Test
3. Results
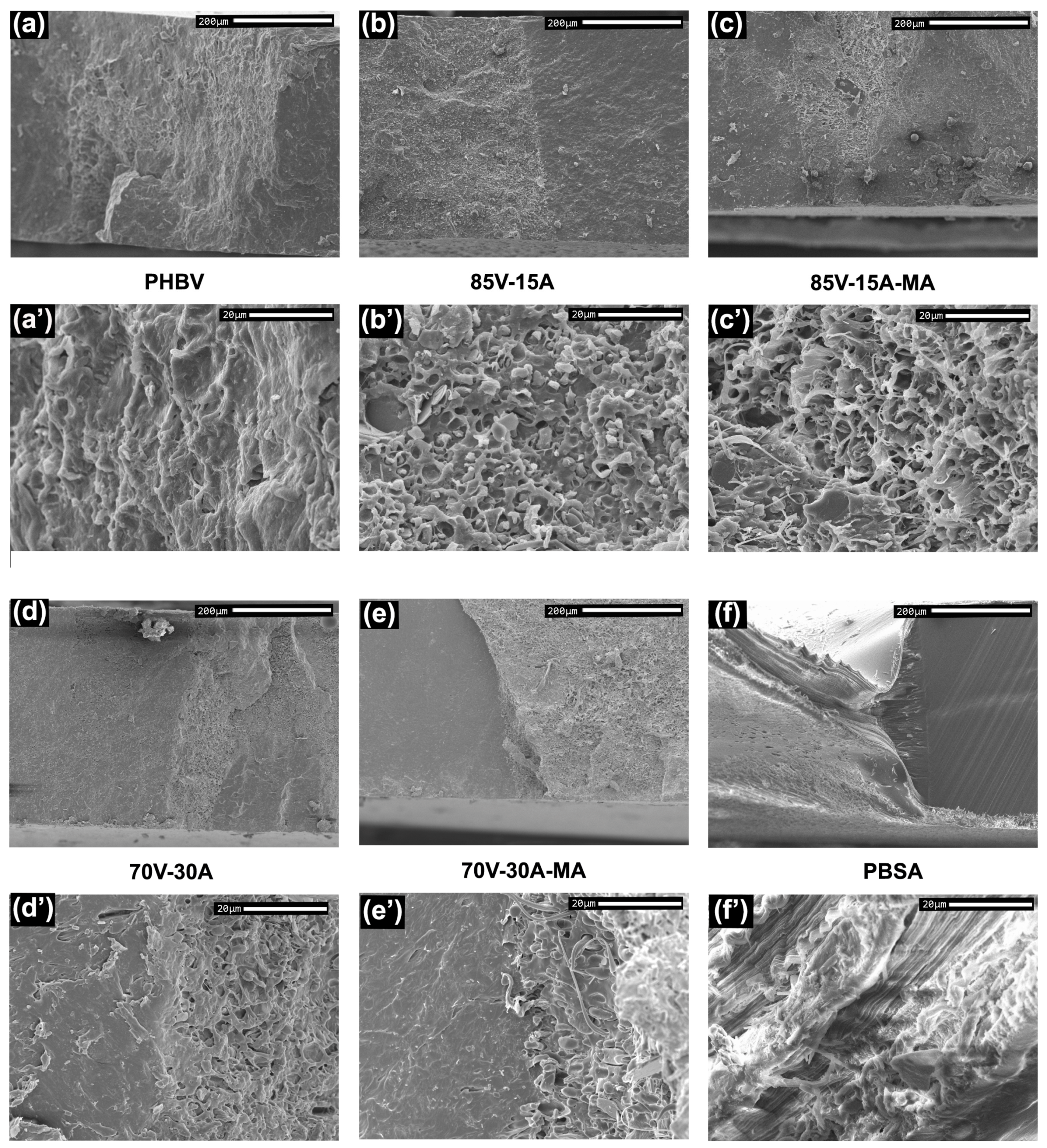
3.1. Morphological Characterization
3.2. Thermal Characterization
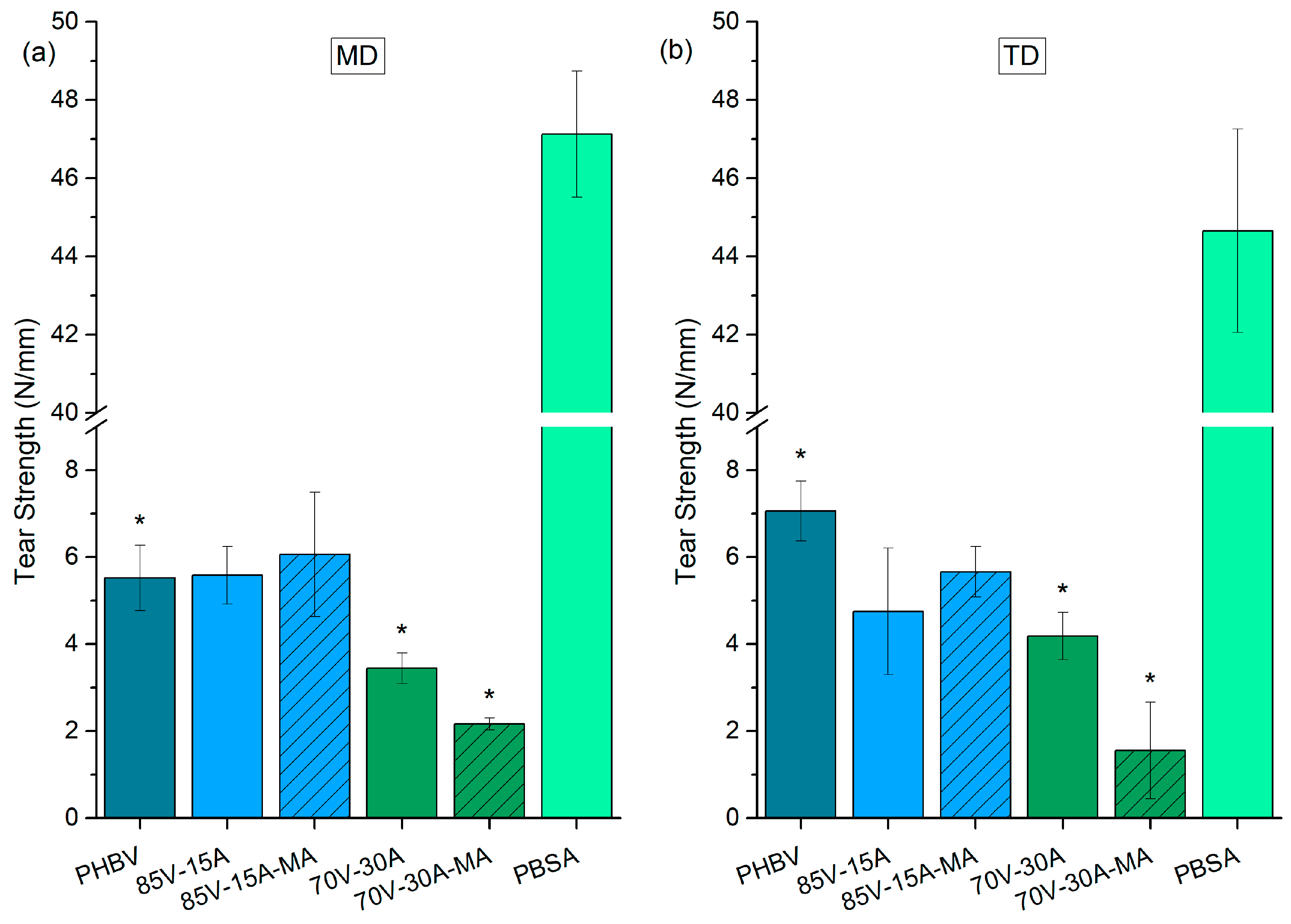
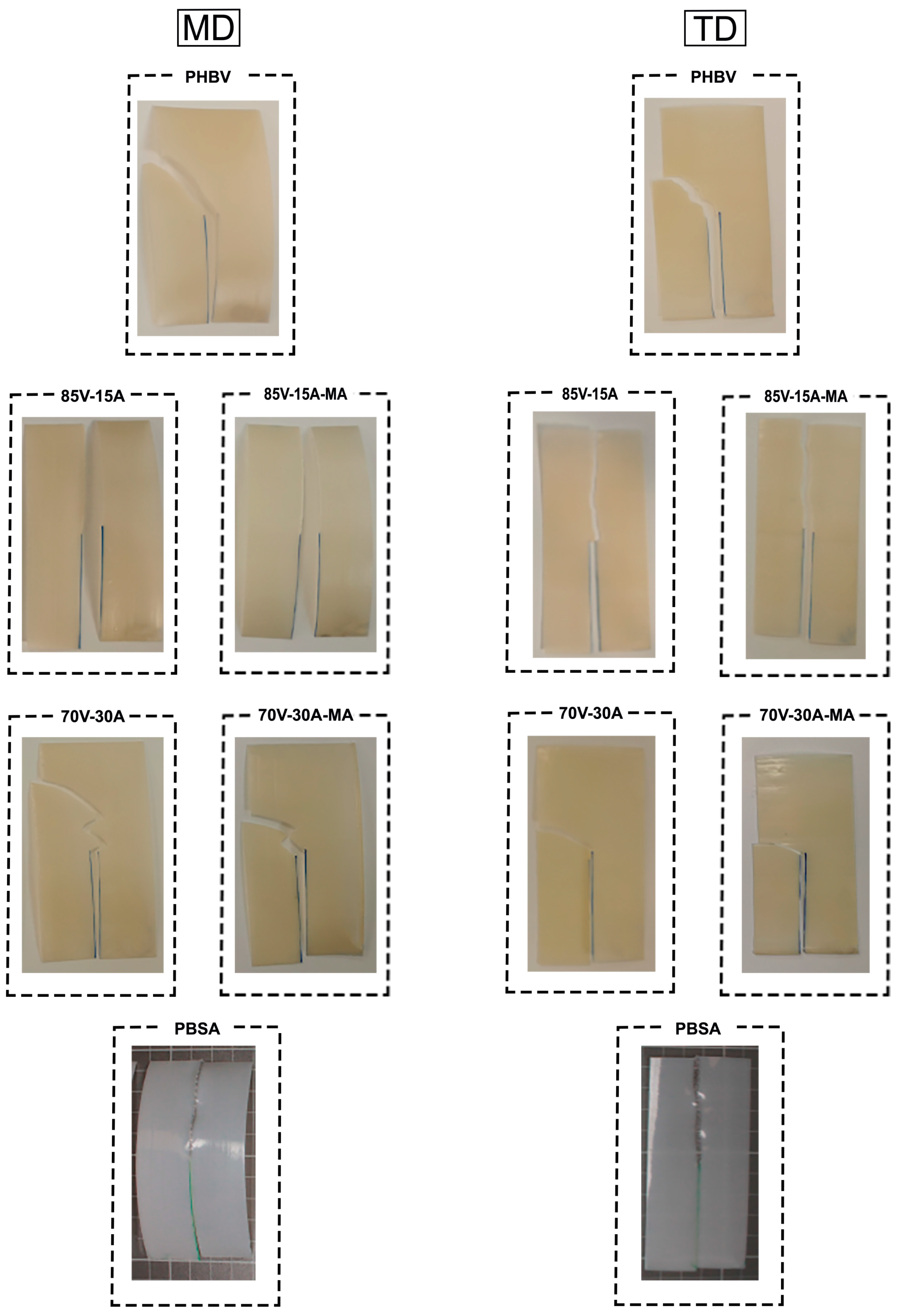
3.3. Mechanical Characterization
3.4. Barrier Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodríguez-Cendal, A.I.; Gómez-Seoane, I.; de Toro-Santos, F.J.; Fuentes-Boquete, I.M.; Señarís-Rodríguez, J.; Díaz-Prado, S.M. Biomedical Applications of the Biopolymer Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) (PHBV): Drug Encapsulation and Scaffold Fabrication. Int. J. Mol. Sci. 2023, 24, 11674. [Google Scholar] [CrossRef]
- Khanna, S.; Srivastava, A.K. Recent Advances in Microbial Polyhydroxyalkanoates. Process Biochem. 2005, 40, 607–619. [Google Scholar] [CrossRef]
- Laycock, B.; Halley, P.; Pratt, S.; Werker, A.; Lant, P. The Chemomechanical Properties of Microbial Polyhydroxyalkanoates. Prog. Polym. Sci. 2014, 39, 397–442. [Google Scholar] [CrossRef]
- Policastro, G.; Panico, A.; Fabbricino, M. Improving Biological Production of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) (PHBV) Co-Polymer: A Critical Review. Rev. Environ. Sci. Biotechnol. 2021, 20, 479–513. [Google Scholar] [CrossRef]
- Rivera-Briso, A.L.; Serrano-Aroca, Á. Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate): Enhancement Strategies for Advanced Applications. Polymers 2018, 10, 732. [Google Scholar] [CrossRef] [PubMed]
- Kiruthika, A.V. PHBV Based Blends and Composites. In Biodegradable Polymers, Blends and Composites; Woodhead Publishing: Sawston, UK, 2021; pp. 283–308. ISBN 9780128237915. [Google Scholar]
- Le Delliou, B.; Vitrac, O.; Castro, M.; Bruzaud, S.; Domenek, S. Characterization of a New Bio-Based and Biodegradable Blends of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) and Poly(Butylene-Co-Succinate-Co-Adipate). J. Appl. Polym. Sci. 2022, 139, 52124. [Google Scholar] [CrossRef]
- Ma, P.; Hristova-Bogaerds, D.G.; Lemstra, P.J.; Zhang, Y.; Wang, S. Toughening of PHBV/PBS and PHB/PBS Blends via In Situ Compatibilization Using Dicumyl Peroxide as a Free-Radical Grafting Initiator. Macromol. Mater. Eng. 2012, 297, 402–410. [Google Scholar] [CrossRef]
- Samaniego-Aguilar, K.; Sánchez-Safont, E.; Arrillaga, A.; Anakabe, J.; Gamez-Perez, J.; Cabedo, L. In Service Performance of Toughened PHBV/TPU Blends Obtained by Reactive Extrusion for Injected Parts. Polymers 2022, 14, 2337. [Google Scholar] [CrossRef] [PubMed]
- Avella, M.; Martuscelli, E.; Raimo, M. Review Properties of Blends and Composites Based on Poly(3-Hydroxy)Butyrate (PHB) and Poly(3-Hydroxybutyrate-Hydroxyvalerate) (PHBV) Copolymers. J. Mater. Sci. 2000, 35, 523–545. [Google Scholar] [CrossRef]
- Dong, W.; Ma, P.; Wang, S.; Chen, M.; Cai, X.; Zhang, Y. Effect of Partial Crosslinking on Morphology and Properties of the Poly(β-Hydroxybutyrate)/Poly(d,l-Lactic Acid) Blends. Polym. Degrad. Stab. 2013, 98, 1549–1555. [Google Scholar] [CrossRef]
- Tubio, C.R.; Valle, X.; Carvalho, E.; Moreira, J.; Costa, P.; Correia, D.M.; Lanceros-Mendez, S. Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Blends with Poly(Caprolactone) and Poly(Lactic Acid): A Comparative Study. Polymers 2023, 15, 4566. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Garcia, D.; Ferri, J.M.; Boronat, T.; Lopez-Martinez, J.; Balart, R. Processing and Characterization of Binary Poly(Hydroxybutyrate) (PHB) and Poly(Caprolactone) (PCL) Blends with Improved Impact Properties. Polym. Bull. 2016, 73, 3333–3350. [Google Scholar] [CrossRef]
- Javadi, A.; Kramschuster, A.J.; Pilla, S.; Lee, J.; Gong, S.; Turng, L.S. Processing and Characterization of Microcellular PHBV/PBAT Blends. Polym. Eng. Sci. 2010, 50, 1440–1448. [Google Scholar] [CrossRef]
- Gupta, A.; Lolic, L.; Mekonnen, T.H. Reactive Extrusion of Highly Filled, Compatibilized, and Sustainable PHBV/PBAT—Hemp Residue Biocomposite. Compos. Part A Appl. Sci. Manuf. 2022, 156, 106885. [Google Scholar] [CrossRef]
- Pal, A.K.; Wu, F.; Misra, M.; Mohanty, A.K. Reactive Extrusion of Sustainable PHBV/PBAT-Based Nanocomposite Films with Organically Modified Nanoclay for Packaging Applications: Compression Moulding vs. Cast Film Extrusion. Compos. Part B Eng. 2020, 198, 108141. [Google Scholar] [CrossRef]
- Rodriguez-Uribe, A.; Wang, T.; Pal, A.K.; Wu, F.; Mohanty, A.K.; Misra, M. Injection Moldable Hybrid Sustainable Composites of BioPBS and PHBV Reinforced with Talc and Starch as Potential Alternatives to Single-Use Plastic Packaging. Compos. Part C Open Access 2021, 6, 100201. [Google Scholar] [CrossRef]
- Feijoo, P.; Mohanty, A.K.; Rodriguez-Uribe, A.; Gámez-Pérez, J.; Cabedo, L.; Misra, M. Biodegradable Blends from Bacterial Biopolyester PHBV and Bio-Based PBSA: Study of the Effect of Chain Extender on the Thermal, Mechanical and Morphological Properties. Int. J. Biol. Macromol. 2023, 225, 1291–1305. [Google Scholar] [CrossRef]
- Righetti, M.C.; Cinelli, P.; Aliotta, L.; Bianchi, E.; Tricoli, F.; Seggiani, M.; Lazzeri, A. Immiscible PHB/PBS and PHB/PBSA Blends: Morphology, Phase Composition and Modelling of Elastic Modulus. Polym. Int. 2021, 71, 47–56. [Google Scholar] [CrossRef]
- Aliotta, L.; Vannozzi, A.; Canesi, I.; Cinelli, P.; Coltelli, M.B.; Lazzeri, A. Poly(Lactic Acid) (PLA)/Poly(Butylene Succinate-Co-Adipate) (PBSA) Compatibilized Binary Biobased Blends: Melt Fluidity, Morphological, Thermo-Mechanical and Micromechanical Analysis. Polymers 2021, 13, 218. [Google Scholar] [CrossRef]
- Strangis, G.; Rossi, D.; Cinelli, P.; Seggiani, M. Seawater Biodegradable Poly(Butylene Succinate-Co-Adipate)—Wheat Bran Biocomposites. Materials 2023, 16, 2593. [Google Scholar] [CrossRef] [PubMed]
- Batista Nicolino, M.V.; Oliveira Passos, M.A.; Branciforti, M.C. Study of Miscibility, Crystallization, and Biodegradation of Casting Films of Poly(Butylene Succinate-Co-Adipate) and Poly(ϵ-Caprolactone) Blends. Macromol. Symp. 2019, 383, 1800041. [Google Scholar] [CrossRef]
- Yamazaki, H.; Kamitabira, S.; Maeda, T.; Hotta, A. Controlling the Size of Spherulite and the Degradation of Poly(Butylene Succinate-Co-Adipate) by Solvent and Gel Preparation Temperature. Polym. Degrad. Stab. 2019, 162, 106–111. [Google Scholar] [CrossRef]
- Ojijo, V.; Ray, S.S. Poly(Butylene Succinate) and Poly[(Butylene Succinate)-Co-Adipate] Nanocomposites. In Green Energy and Technology; Avérous, L., Pollet, E., Eds.; Springer: Berlin, Germany, 2012; Volume 50, pp. 165–218. ISBN 9781447141013. [Google Scholar]
- Debuissy, T.; Pollet, E.; Avérous, L. Synthesis and Characterization of Biobased Poly(Butylene Succinate-Ran-Butylene Adipate). Analysis of the Composition-Dependent Physicochemical Properties. Eur. Polym. J. 2017, 87, 84–98. [Google Scholar] [CrossRef]
- Chikh, A.; Benhamida, A.; Kaci, M.; Pillin, I.; Bruzaud, S. Synergistic Effect of Compatibilizer and Sepiolite on the Morphology of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)/Poly(Butylene Succinate) Blends. Polym. Test. 2016, 53, 19–28. [Google Scholar] [CrossRef]
- Peshne, H.; Satapathy, B.K. Comparative Studies of Structural, Thermal, Mechanical, Rheological and Dynamic Mechanical Response of Melt Mixed PHB/Bio-PBS and PHBV/Bio-PBS Blends. J. Polym. Res. 2022, 29, 496. [Google Scholar] [CrossRef]
- Dean, K.M.; Petinakis, E.; Meure, S.; Yu, L.; Chryss, A. Melt Strength and Rheological Properties of Biodegradable Poly(Lactic Aacid) Modified via Alkyl Radical-Based Reactive Extrusion Processes. J. Polym. Environ. 2012, 20, 741–747. [Google Scholar] [CrossRef]
- Dubois, P. Reactive Extrusion (REx): Using Chemistry and Engineering to Solve the Problem of Ocean Plastics. Engineering 2022, 14, 15–18. [Google Scholar] [CrossRef]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Biodegradable Compatibilized Polymer Blends for Packaging Applications: A Literature Review. J. Appl. Polym. Sci. 2018, 135, 45726. [Google Scholar] [CrossRef]
- Pouriman, H.; Lin, R.; Graham, K.; Jayaraman, K. Evaluating the Grafting of Maleic Anhydride with Poly(Lactic Acid) (PLA) and Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) (PHBV) via Batch Compounding. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Ahmad Thirmizir, M.Z.; Mohd Ishak, Z.A.; Mat Taib, R.; Kanapathi Pillai, K.S.K.C.; Salim, M.S.; Hassan, A.; Abu Bakar, M.B. The Effects of Melt Grafted Maleated Polybutylene Succinate on the Properties of Poly(Hydroxybutyrate-Co-Hydroxyhexanoate)/Polybutylene Succinate Blends. J. Vinyl Addit. Technol. 2021, 27, 567–588. [Google Scholar] [CrossRef]
- Rojas-Lema, S.; Arevalo, J.; Gomez-Caturla, J.; Garcia-Garcia, D.; Torres-Giner, S. Peroxide-Induced Synthesis of Maleic Anhydride-Grafted Poly(Butylene Succinate) and Its Compatibilizing Effect on Poly(Butylene Succinate)/Pistachio Shell Flour Composites. Molecules 2021, 26, 5927. [Google Scholar] [CrossRef]
- Kennouche, S.; Le Moigne, N.; Kaci, M.; Quantin, J.C.; Caro-Bretelle, A.S.; Delaite, C.; Lopez-Cuesta, J.M. Morphological Characterization and Thermal Properties of Compatibilized Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) (PHBV)/Poly(Butylene Succinate) (PBS)/Halloysite Ternary Nanocomposites. Eur. Polym. J. 2016, 75, 142–162. [Google Scholar] [CrossRef]
- Kato, S.; Ueda, T.; Aoshima, T.; Kosaka, N.; Nitta, S. BioPBSTM (Polybutylene Succinate). Adv. Polym. Sci. 2023, 293, 269–304. [Google Scholar] [CrossRef]
- Phua, Y.J.; Chow, W.S.; Mohd Ishak, Z.A. Reactive Processing of Maleic Anhydride-Grafted Poly(Butylene Succinate) and the Compatibilizing Effect on Poly(Butylene Succinate) Nanocomposites. Express Polym. Lett. 2013, 7, 340–354. [Google Scholar] [CrossRef]
- Barham, P.J.; Keller, A.; Otun, E.L.; Holmes, P.A. Crystallization and Morphology of a Bacterial Thermoplastic: Poly-3-Hydroxybutyrate. J. Mater. Sci. 1984, 19, 2781–2794. [Google Scholar] [CrossRef]
- Siracusa, V.; Lotti, N.; Munari, A.; Dalla Rosa, M. Poly(Butylene Succinate) and Poly(Butylene Succinate-Co-Adipate) for Food Packaging Applications: Gas Barrier Properties after Stressed Treatments. Polym. Degrad. Stab. 2015, 119, 35–45. [Google Scholar] [CrossRef]
- ISO 6383-1:2015; Plastics—Film and Sheeting—Determination of Tear Resistance. Part 1: Trouser Tear Method. ISO: Geneva, Switzerland, 2015.
- ASTM E96/E96M-14; Standard Test Methods for Water Vapor Transmission of Materials. ASTM: West Conshohocken, PA, USA, 2015.
- ASTM D3985-05; Standard Test Method for Oxygen Gas Transmission Rate Through Plastic Film and Sheeting Using a Coulometric Sensor. ASTM: West Conshohocken, PA, USA, 2010.
- Pesaranhajiabbas, E.; Pal, A.K.; Rodriguez-Uribe, A.; Mohanty, A.K.; Misra, M. Biodegradable Polymer Blends: Studies on Performance Control through Droplet to Co-Continuous Morphology. ACS Appl. Polym. Mater. 2022, 4, 5546–5556. [Google Scholar] [CrossRef]
- Aliotta, L.; Gigante, V.; Geerinck, R.; Coltelli, M.B.; Lazzeri, A. Micromechanical Analysis and Fracture Mechanics of Poly(Lactic Acid) (PLA)/Polycaprolactone (PCL) Binary Blends. Polym. Test. 2023, 121, 107984. [Google Scholar] [CrossRef]
- Bucknall, C.B.; Paul, D.R. Notched Impact Behavior of Polymer Blends: Part 1: New Model for Particle Size Dependence. Polymer 2009, 50, 5539–5548. [Google Scholar] [CrossRef]
- Bucknall, C.B.; Paul, D.R. Notched Impact Behaviour of Polymer Blends: Part 2: Dependence of Critical Particle Size on Rubber Particle Volume Fraction. Polymer 2013, 54, 320–329. [Google Scholar] [CrossRef]
- Samaniego-Aguilar, K.; Sánchez-Safont, E.; Rodríguez, A.; Marín, A.; Candal, M.V.; Cabedo, L.; Gamez-Perez, J. Valorization of Agricultural Waste Lignocellulosic Fibers for Poly(3-Hydroxybutyrate-Co-Valerate)-Based Composites in Short Shelf-Life Applications. Polymer 2023, 15, 4507. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bhattacharya, M.; Mano, J.F. Thermal Analysis of the Multiple Melting Behavior of Poly(Butylene Succinate-Co-Adipate). J. Polym. Sci. Part B Polym. Phys. 2005, 43, 3077–3082. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Li, L. Multiple Melting Behavior of Poly(Butylene Succinate). Eur. Polym. J. 2007, 43, 3163–3170. [Google Scholar] [CrossRef]
- Fenni, S.E.; Wang, J.; Haddaoui, N.; Favis, B.D.; Müller, A.J.; Cavallo, D. Crystallization and Self-Nucleation of PLA, PBS and PCL in Their Immiscible Binary and Ternary Blends. Thermochim. Acta 2019, 677, 117–130. [Google Scholar] [CrossRef]
- Saengbunkoet, S.; Kerddonfag, N.; Puekpoonpoal, N.; Kumsang, P.; Yoksan, R.; Jariyasakoolroj, P. Structural Evolution and Related Physical Properties of Machine Direction Oriented Poly(Butylene Succinate-Co-Adipate) Films. Polymer 2022, 249, 124859. [Google Scholar] [CrossRef]
- Righetti, M.C.; Aliotta, L.; Mallegni, N.; Gazzano, M.; Passaglia, E.; Cinelli, P.; Lazzeri, A. Constrained Amorphous Interphase and Mechanical Properties of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate). Front. Chem. 2019, 7, 489742. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Yu, S.; Zhang, Y.; Zhao, G. Mechanical Properties and Structure of Injection Molded Poly(Hydroxybutyrate-Co-Hydroxyvalerate)/Poly(Butylene Adipate-Co-Terephthalate) (PHBV/PBAT) Blends. J. Appl. Polym. Sci. 2023, 140, e53880. [Google Scholar] [CrossRef]
- Parameswaranpillai, J.; Thomas, S.; Grohens, Y. Polymer Blends: State of the Art, New Challenges, and Opportunities. In Characterization of Polymer Blends: Miscibility, Morphology and Interfaces; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; Volume 9783527331, pp. 1–6. ISBN 9783527645602. [Google Scholar]
- Li, Y.; Zhang, K.; Nie, M.; Wang, Q. Application of Compatibilized Polymer Blends in Packaging. In Compatibilization of Polymer Blends: Micro and Nano Scale Phase Morphologies, Interphase Characterization, and Properties; Elsevier: Amsterdam, The Netherlands, 2019; pp. 539–561. ISBN 9780128160060. [Google Scholar]
- George, S.M.; Puglia, D.; Kenny, J.M.; Causin, V.; Parameswaranpillai, J.; Thomas, S. Morphological and Mechanical Characterization of Nanostructured Thermosets from Epoxy and Styrene-Block-Butadiene-Block-Styrene Triblock Copolymer. Ind. Eng. Chem. Res. 2013, 52, 9121–9129. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Gil, L.; Pascual-Ramírez, L.; Garde-Belza, J.A. Packaging: Food Waste Reduction. In Encyclopedia of Polymer Application; Mishra, M., Ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 1990–2009. [Google Scholar]
- Quiles-Carrillo, L.; Montanes, N.; Lagaron, J.M.; Balart, R.; Torres-Giner, S. In Situ Compatibilization of Biopolymer Ternary Blends by Reactive Extrusion with Low-Functionality Epoxy-Based Styrene–Acrylic Oligomer. J. Polym. Environ. 2019, 27, 84–96. [Google Scholar] [CrossRef]
- Hernández-García, E.; Pacheco-Romeralo, M.; Zomeño, P.; Viscusi, G.; Malvano, F.; Gorrasi, G.; Torres-Giner, S. Development and Characterization of Thermoformed Bilayer Trays of Paper and Renewable Succinic Acid Derived Biopolyester Blends and Their Application to Preserve Fresh Pasta. Materials 2023, 16, 3872. [Google Scholar] [CrossRef]
- Lagarón, J.M. Multifunctional and Nanoreinforced Polymers for Food Packaging. In Multifunctional and Nanoreinforced Polymers for Food Packaging; Woodhead Publishing: Sawston, UK, 2011; pp. 1–28. ISBN 9781845697389. [Google Scholar]
- Sanchez-Garcia, M.D.; Gimenez, E.; Lagaron, J.M. Morphology and Barrier Properties of Solvent Cast Composites of Thermoplastic Biopolymers and Purified Cellulose Fibers. Carbohydr. Polym. 2008, 71, 235–244. [Google Scholar] [CrossRef]
- Hernández-García, E.; Vargas, M.; Torres-Giner, S. Quality and Shelf-Life Stability of Pork Meat Fillets Packaged in Multilayer Polylactide Films. Foods 2022, 11, 426. [Google Scholar] [CrossRef] [PubMed]
- Gabirondo, E.; Melendez-Rodriguez, B.; Arnal, C.; Lagaron, J.M.; Martínez De Ilarduya, A.; Sardon, H.; Torres-Giner, S. Organocatalyzed Closed-Loop Chemical Recycling of Thermo-Compressed Films of Poly(Ethylene Furanoate). Polym. Chem. 2021, 12, 1571–1580. [Google Scholar] [CrossRef]
- Tejada-Oliveros, R.; Fiori, S.; Gomez-Caturla, J.; Lascano, D.; Montanes, N.; Quiles-Carrillo, L.; Garcia-Sanoguera, D. Development and Characterization of Polylactide Blends with Improved Toughness by Reactive Extrusion with Lactic Acid Oligomers. Polymers 2022, 14, 1874. [Google Scholar] [CrossRef] [PubMed]
- Quiles-Carrillo, L.; Montanes, N.; Jorda-Vilaplana, A.; Balart, R.; Torres-Giner, S. A Comparative Study on the Effect of Different Reactive Compatibilizers on Injection-Molded Pieces of Bio-Based High-Density Polyethylene/Polylactide Blends. J. Appl. Polym. Sci. 2019, 136, 47396. [Google Scholar] [CrossRef]
- Teacă, C.A.; Bodîrləu, R.; Spiridon, I. Maleic Anhydride Treatment of Softwood—Effect on Wood Structure and Properties. Proc. Cellul. Chem. Technol. 2014, 48, 863–868. [Google Scholar]
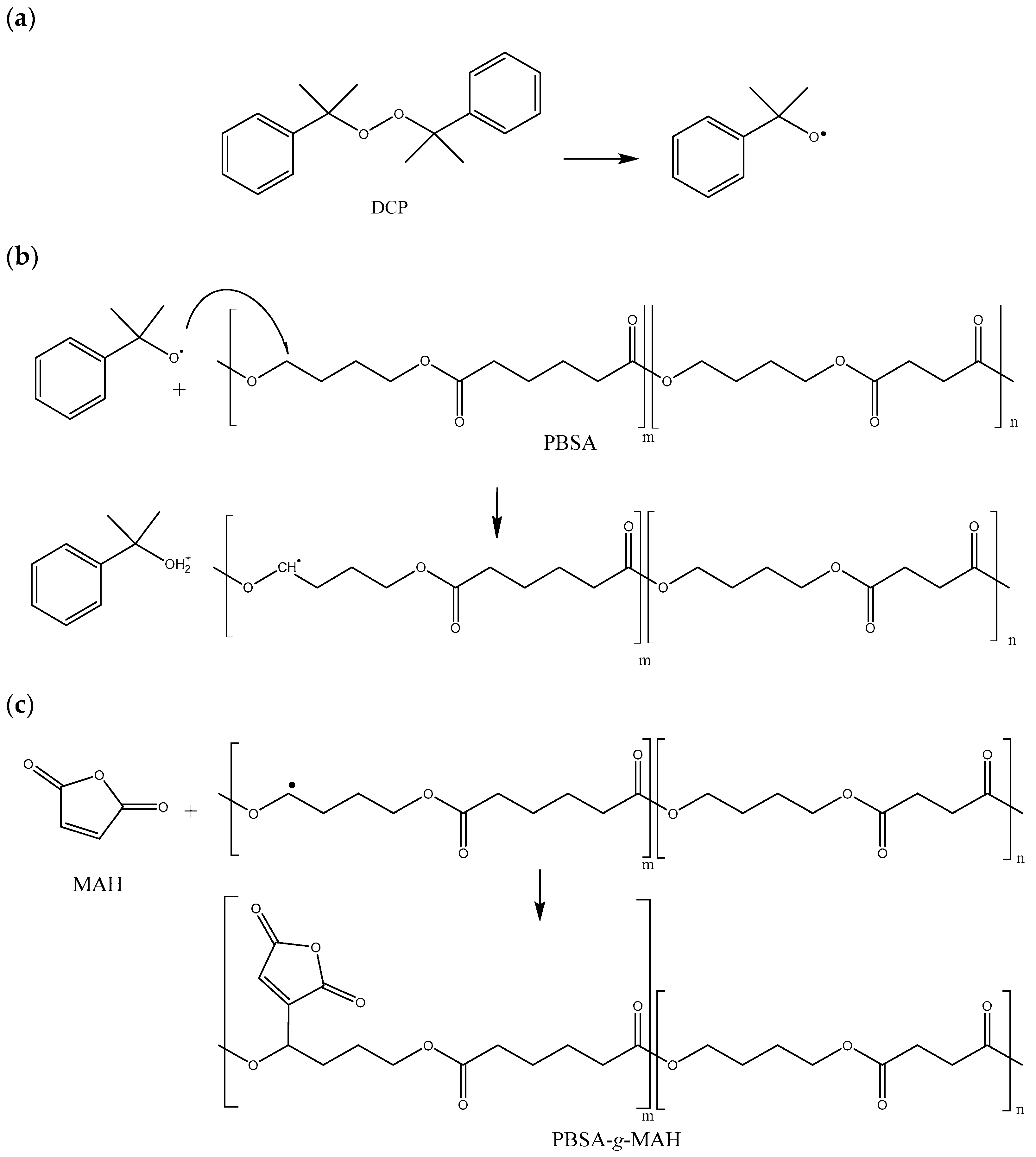
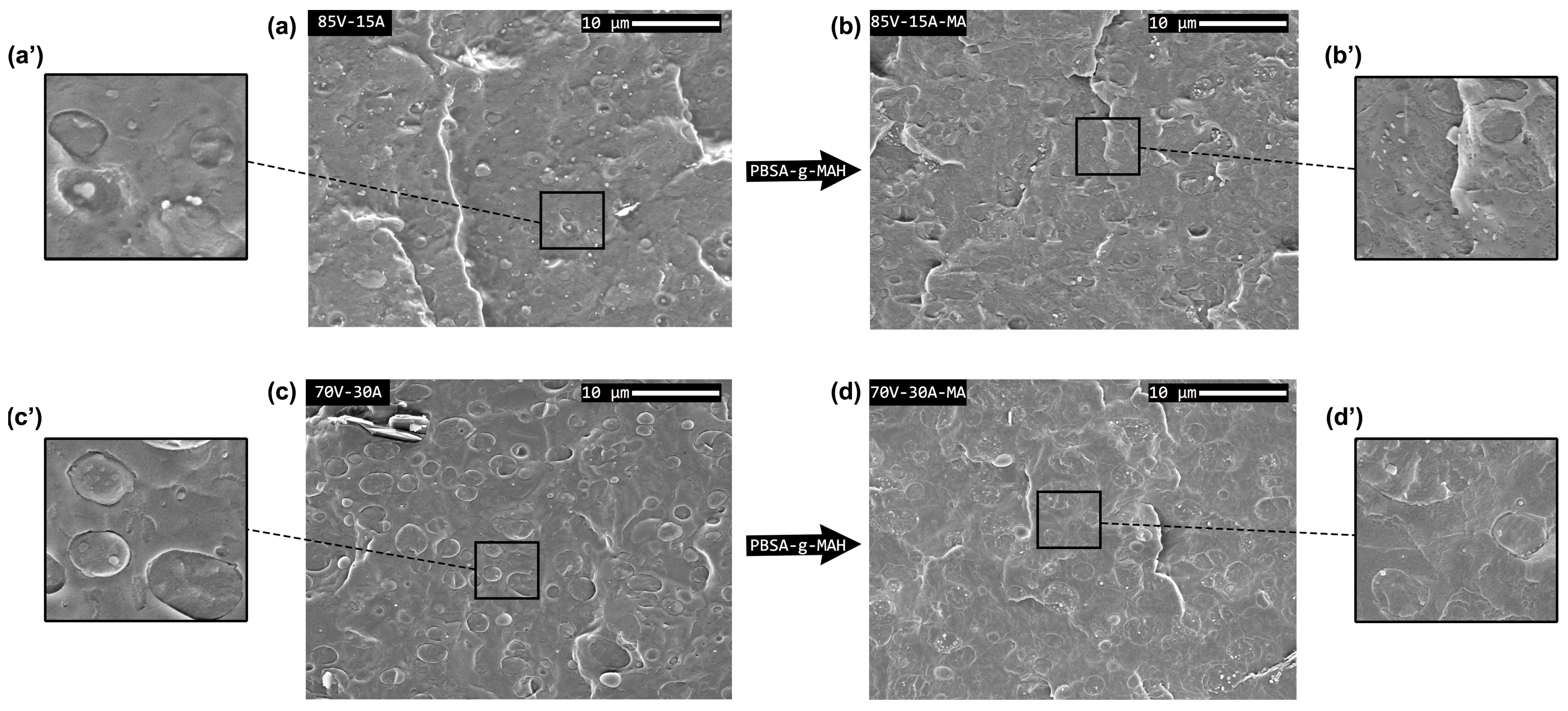
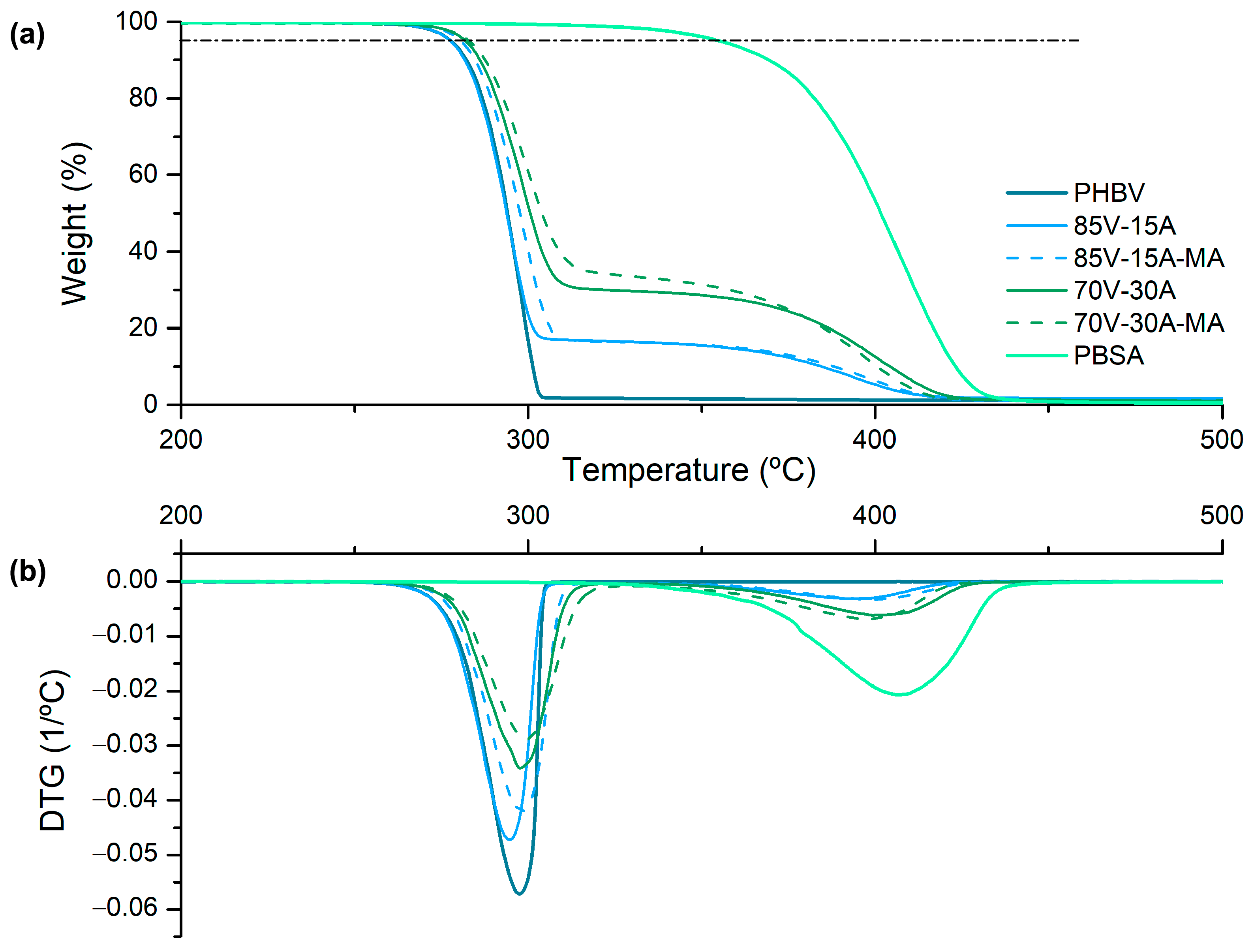
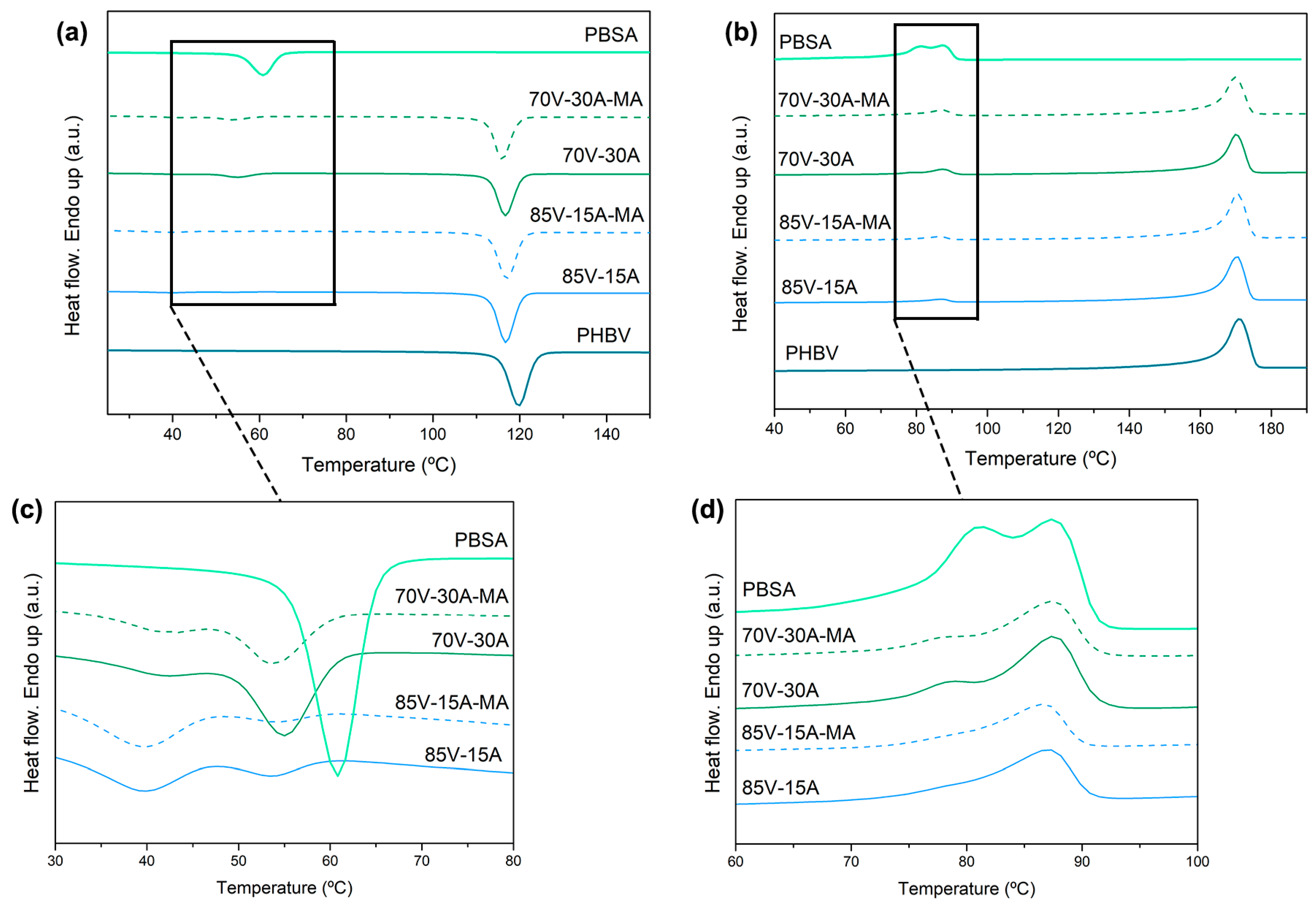


| Sample | PHBV (wt%) | PBSA (wt%) | PBSA-g-MAH (phr) |
|---|---|---|---|
| PHBV | 100 | - | - |
| 85V-15A | 85 | 15 | - |
| 85V-15A-MA | 85 | 15 | 3 |
| 70V-30A | 70 | 30 | - |
| 70V-30A-MA | 70 | 30 | 3 |
| PBSA | - | 100 | - |
| 85V-15A | 85V-15A-MA | 70V-30A | 70V-30A-MA | |
|---|---|---|---|---|
| d10 (µm) | 0.65 | 0.55 | 0.65 | 0.50 |
| d50 (µm) | 1.40 | 1.65 | 2.15 | 1.95 |
| d90 (µm) | 2.45 | 3.70 | >4 | 3.90 |
| Average size (µm) | 1.47 | 1.90 | 2.42 | 2.13 |
| Materials | T5% °C | Td (PHBV) °C | Td (PBSA) °C |
|---|---|---|---|
| PHBV | 279 | 299 | - |
| 85V-15A | 279 | 295 | 393 |
| 85V-15A-MA | 282 | 298 | 398 |
| 70V-30A | 283 | 298 | 399 |
| 70V-30A-MA | 283 | 286 | 399 |
| PBSA | 357 | - | 407 |
| PHBV | PBSA | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First Heating | Cooling | Second Heating | First Heating | Cooling | Second Heating | |||||||||||
| Film | ΔHm1 (J/g) | Tm1 (°C) | ΔHc1 (J/g) | Tc1 (°C) | ΔHm1 (J/g) | Tm1 (°C) | Χc1 (%) | ΔHm2 (J/g) | Tm2 (°C) | ΔHc2 (J/g) | Tc2.1 (°C) | Tc2.2 (°C) | ΔHm2 (J/g) | Tm2.1 (°C) | Tm2.2 (°C) | Χc2 (%) |
| PHBV | 85 | 169 | 90 | 120 | 91 | 171 | 62 | - | - | - | - | - | - | - | - | - |
| 85V-15A | 64 | 168 | 72 | 117 | 74 | 170 | 60 | 6 | 86 | 3 | 39 | 54 | 5 | - | 87 | 27 |
| 85V-15A-MA | 71 | 169 | 72 | 118 | 75 | 170 | 62 | 6 | 86 | 4 | 40 | 54 | 5 | - | 87 | 27 |
| 70V-30A | 62 | 173 | 60 | 117 | 63 | 170 | 62 | 12 | 87 | 5 | 41 | 55 | 11 | 79 | 87 | 26 |
| 70V-30A-MA | 61 | 172 | 59 | 116 | 63 | 169 | 63 | 12 | 87 | 4 | 41 | 53 | 11 | 78 | 87 | 28 |
| PBSA | - | - | - | - | - | - | - | 41 | 89 | 41 | - | 61 | 41 | 83 | 89 | 31 |
| Sample | WPV × 1014 (kg·m/Pa·s·m2) | LP × 1014 (kg·m/Pa·s·m2) | OP × 1019 (m3·m/Pa·s·m2) |
|---|---|---|---|
| PHBV | 0.19 ± 0.04 | 1.03 ± 0.70 | 2.16 ± 0.08 |
| 85V-15A | 1.15 ± 0.22 | 2.29 ± 0.28 | 6.79 ± 0.37 |
| 85V-15A-MA | 0.86 ± 0.15 | 1.97 ± 0.17 | 5.96 ± 0.15 |
| 70V-30A | 1.24 ± 0.21 | 2.69 ± 0.23 | 7.99 ± 0.51 |
| 70V-30A-MA | 1.09 ± 0.23 | 2.38 ± 0.12 | 6.75 ± 0.13 |
| PBSA | 2.79 ± 0.17 | 5.35 ± 0.22 | 79.62 ± 1.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samaniego-Aguilar, K.; Sanchez-Safont, E.; Pisa-Ripoll, I.; Torres-Giner, S.; Flores, Y.; Lagaron, J.M.; Cabedo, L.; Gamez-Perez, J. Performance Enhancement of Biopolyester Blends by Reactive Compatibilization with Maleic Anhydride-Grafted Poly(butylene succinate-co-adipate). Polymers 2024, 16, 2325. https://doi.org/10.3390/polym16162325
Samaniego-Aguilar K, Sanchez-Safont E, Pisa-Ripoll I, Torres-Giner S, Flores Y, Lagaron JM, Cabedo L, Gamez-Perez J. Performance Enhancement of Biopolyester Blends by Reactive Compatibilization with Maleic Anhydride-Grafted Poly(butylene succinate-co-adipate). Polymers. 2024; 16(16):2325. https://doi.org/10.3390/polym16162325
Chicago/Turabian StyleSamaniego-Aguilar, Kerly, Estefania Sanchez-Safont, Ignacio Pisa-Ripoll, Sergio Torres-Giner, Yaiza Flores, Jose M. Lagaron, Luis Cabedo, and Jose Gamez-Perez. 2024. "Performance Enhancement of Biopolyester Blends by Reactive Compatibilization with Maleic Anhydride-Grafted Poly(butylene succinate-co-adipate)" Polymers 16, no. 16: 2325. https://doi.org/10.3390/polym16162325
APA StyleSamaniego-Aguilar, K., Sanchez-Safont, E., Pisa-Ripoll, I., Torres-Giner, S., Flores, Y., Lagaron, J. M., Cabedo, L., & Gamez-Perez, J. (2024). Performance Enhancement of Biopolyester Blends by Reactive Compatibilization with Maleic Anhydride-Grafted Poly(butylene succinate-co-adipate). Polymers, 16(16), 2325. https://doi.org/10.3390/polym16162325












