Biological Evaluation of Thermosensitive Hydrogels of Chitosan/Hydrolyzed Collagen/β-GP in an In Vitro Model of Induced Cardiac Ischemia
Abstract
1. Introduction
2. Methods
2.1. Natural Biocomposite Hydrogel Development
2.2. Hydrogels’ Physicochemical Characterization
2.2.1. Scanning Electron Microscopy (SEM)
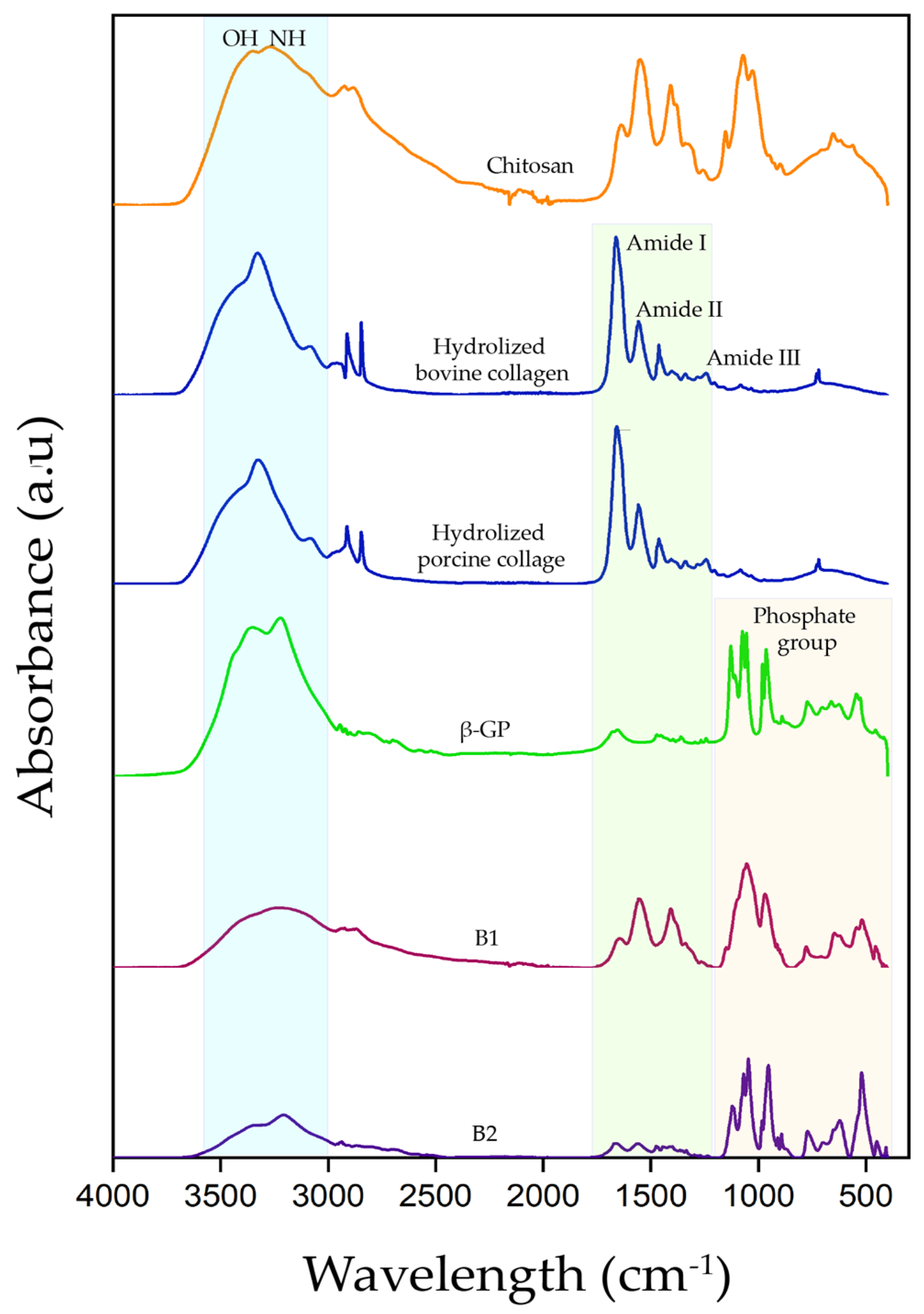
2.2.2. Fourier Transform Infrared Spectroscopy with an Attenuated Total Reflectance Modulus (ATR)
2.2.3. Mass Loss Assay
2.3. Hydrogel Biocompatibility
2.3.1. In Vitro Model: RL-14 Human Fetal Ventricular Cardiomyocytes
2.3.2. Hydrogel Sterilization
2.3.3. Hydrogel Cytotoxicity
2.3.4. Cell Viability
2.3.5. Hydrogel Hemolytic Capacity
2.4. Biological Evaluation of the Hydrogel in an In Vitro Model of Induced Ischemia
2.4.1. Isolation and Culture of Primary Cardiomyocytes
2.4.2. Cell Encapsulation
2.4.3. Ischemia Model: Glucose Decrease and Hypoxia Induction
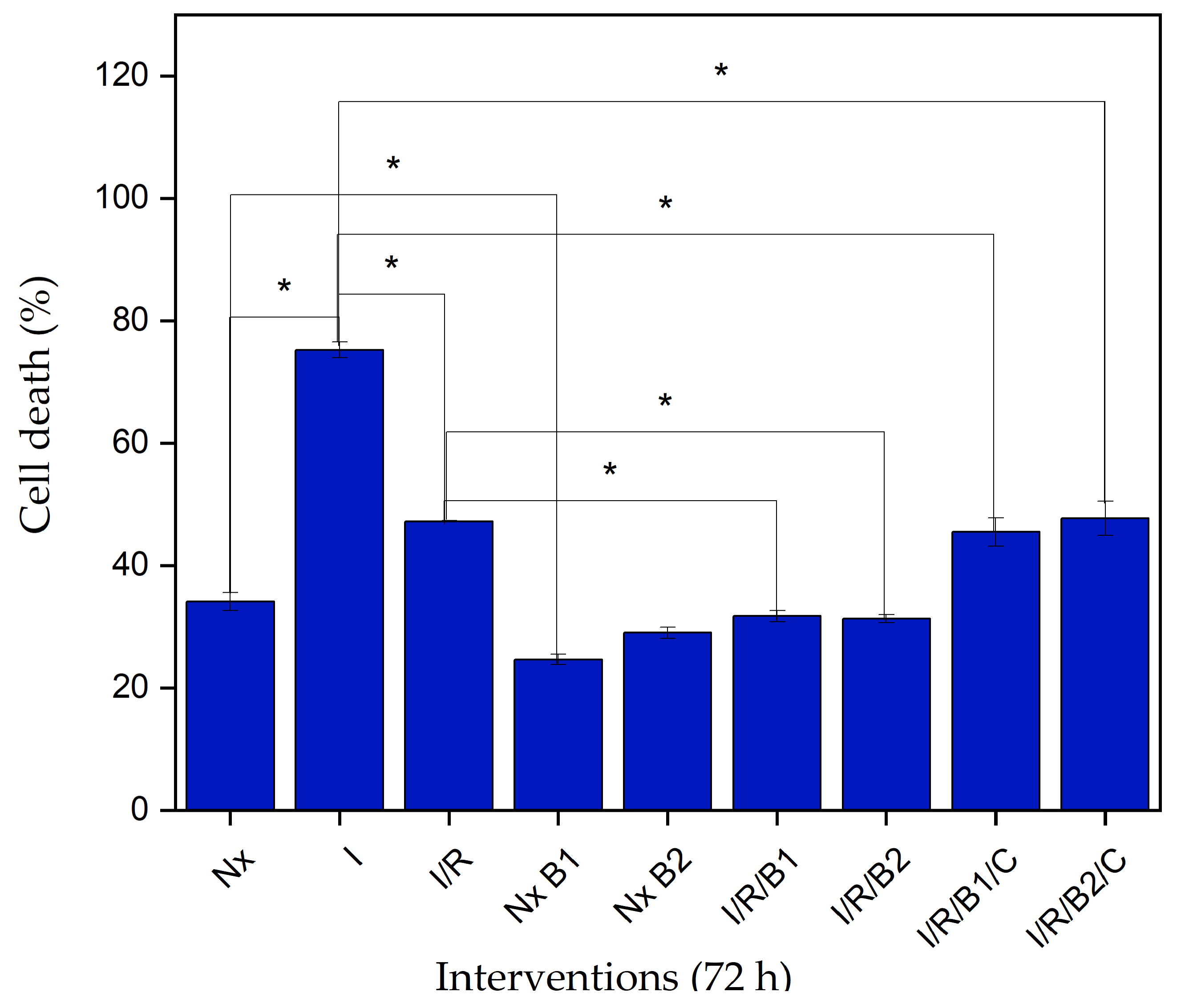
2.4.4. Lactate Dehydrogenase (LDH) Cytotoxicity
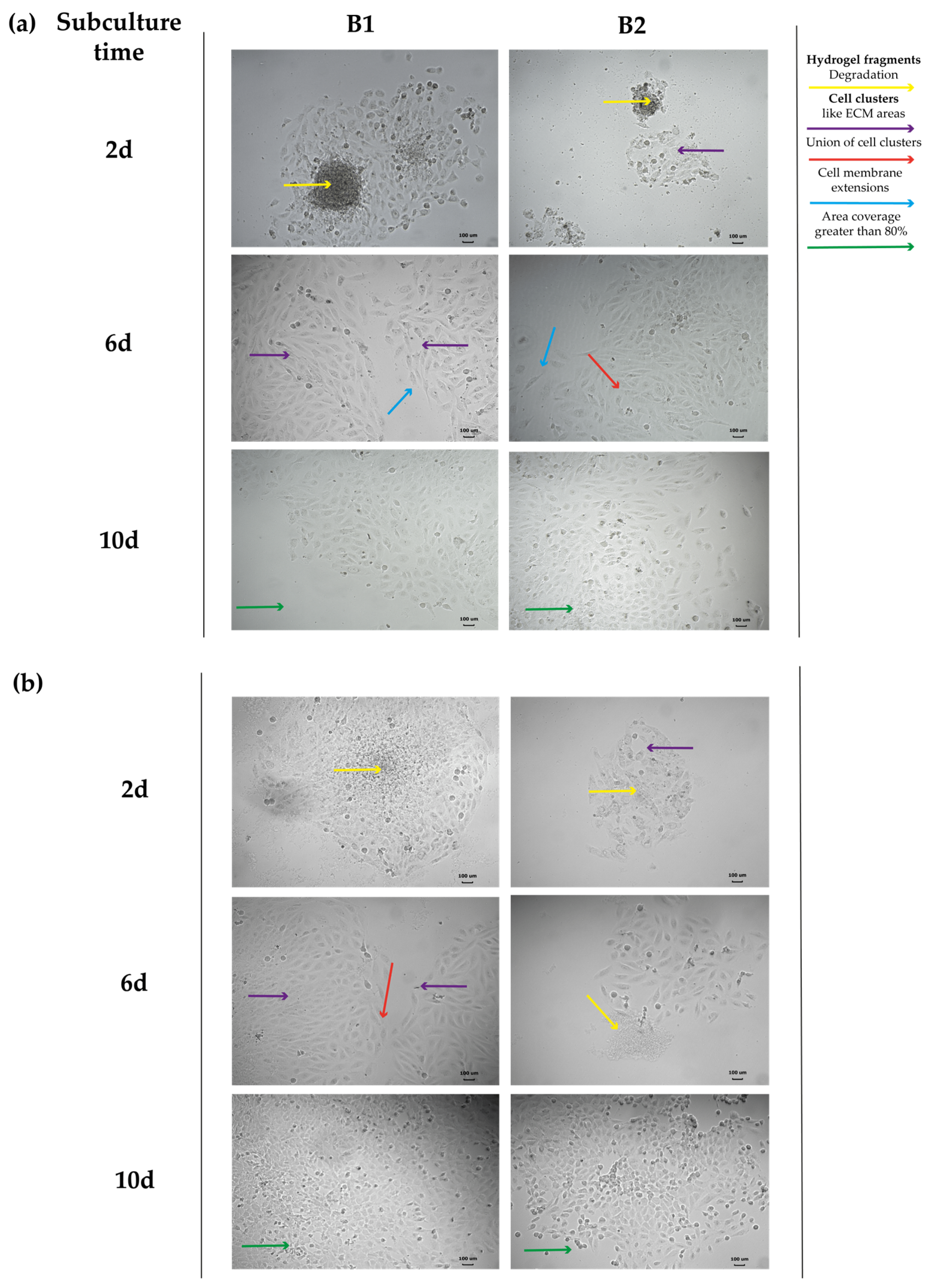
2.4.5. Evaluation of the Cell Structural Changes
2.4.6. Intracellular Calcium Measurements
2.5. Statistical Analysis
3. Results and Discussion
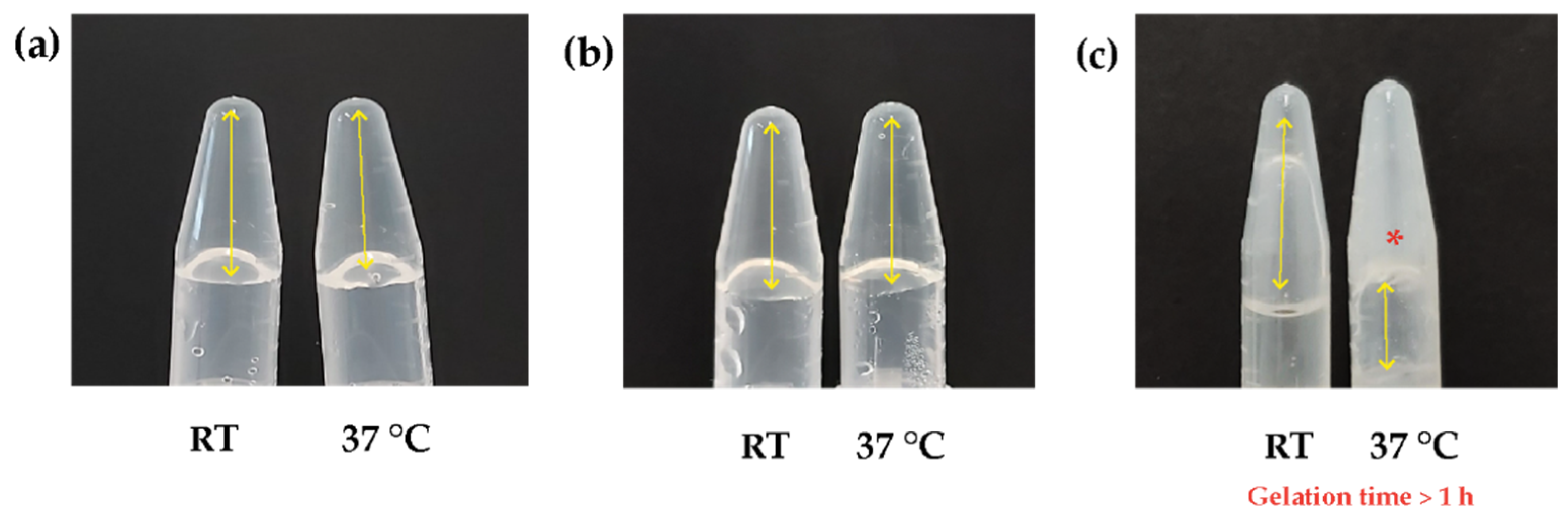
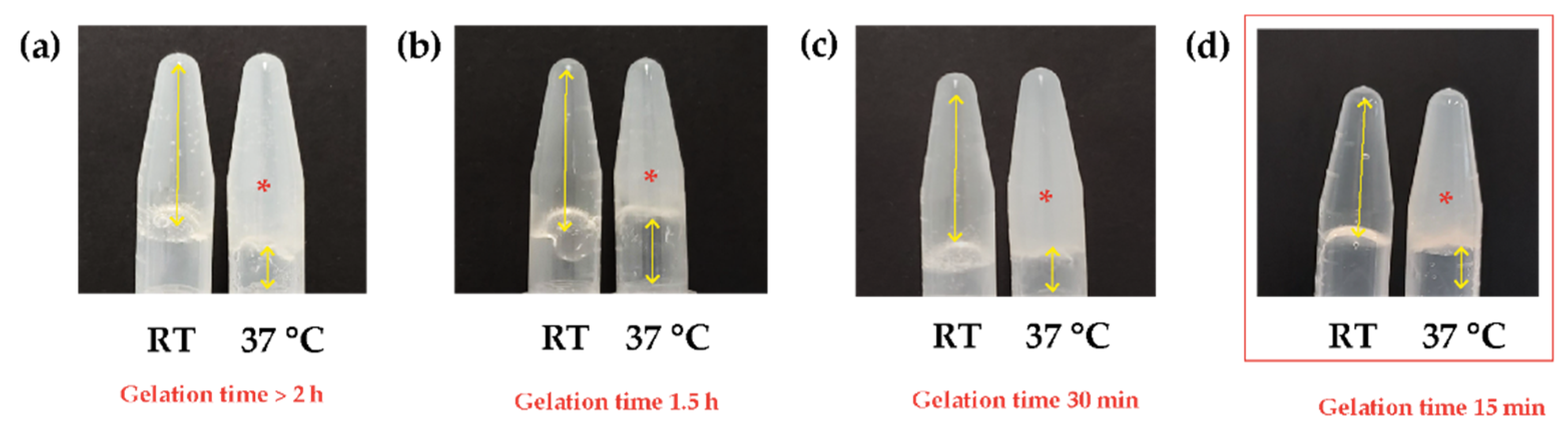
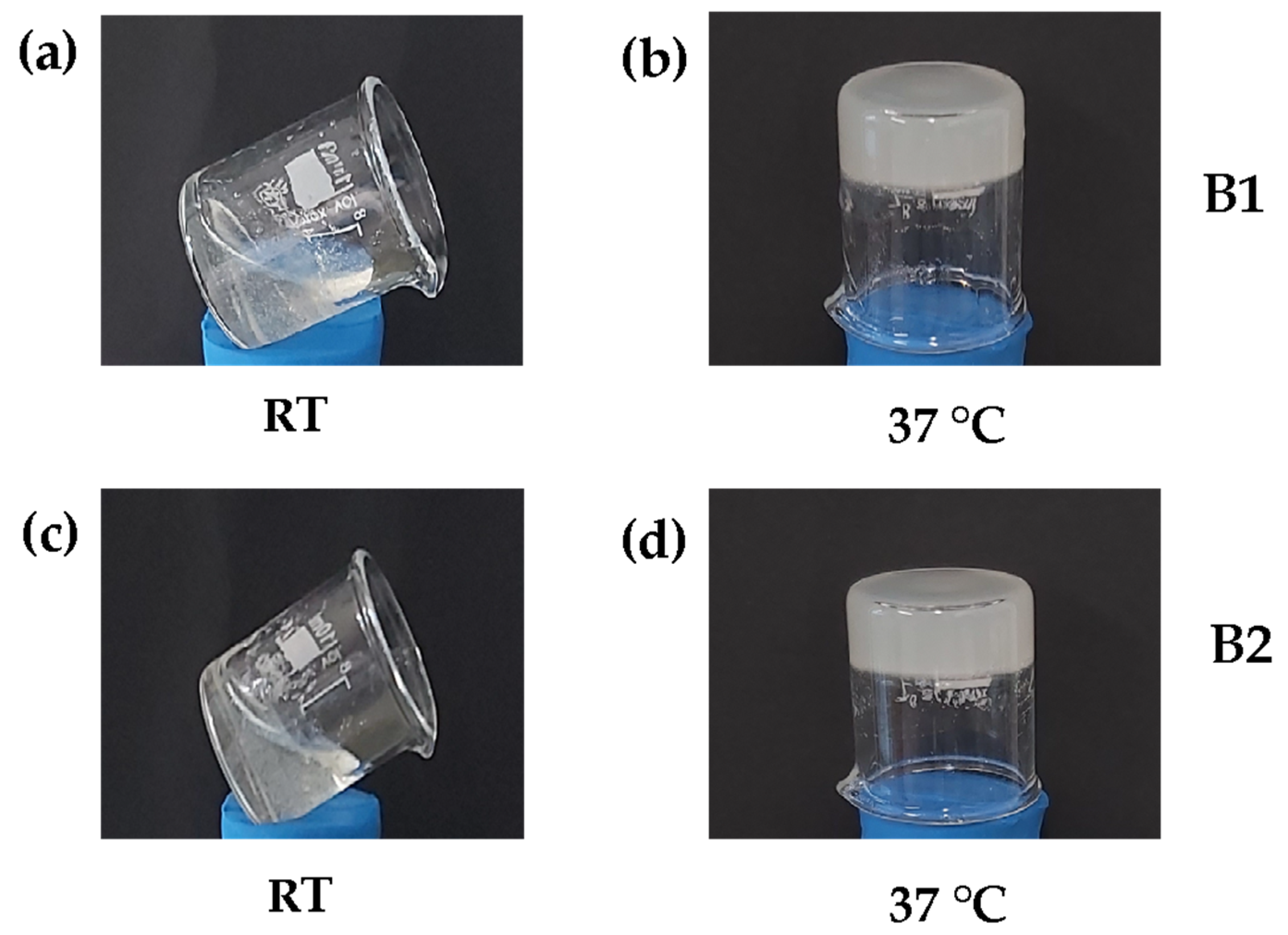
3.1. Natural Biocomposite Hydrogel Development: Determination of the Volumetric Ratios of Chitosan, β-Glycerolphosphate, and Hydrolyzed Collagen
3.2. Physicochemical Characterization of Hydrogels
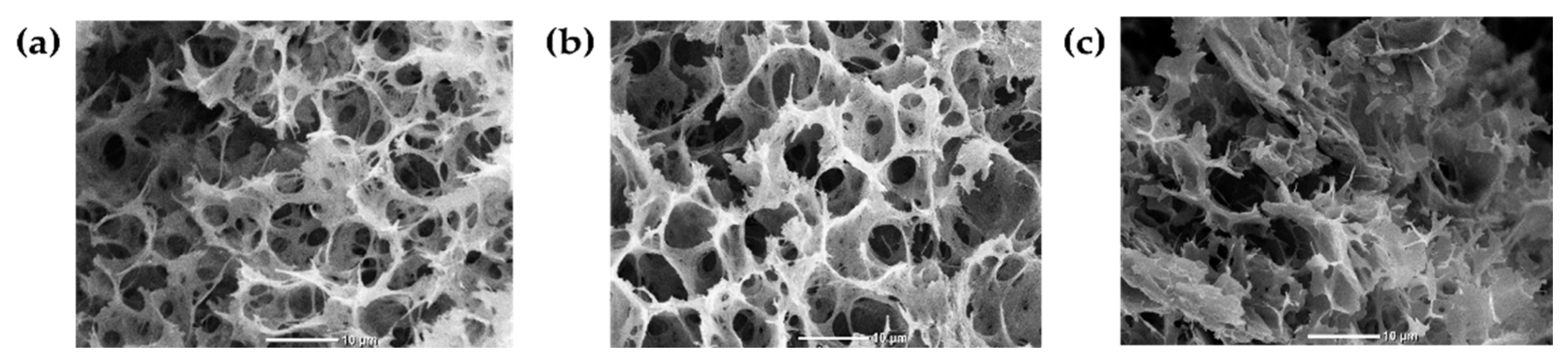
3.2.1. Scanning Electron Microscopy (SEM)
3.2.2. Fourier Transform Infrared Spectroscopy with Attenuated Total Reflectance Modulus (ATR)
3.2.3. Mass Loss Assay
3.3. Hydrogel Biocompatibility
3.3.1. Hydrogel Cytotoxicity
3.3.2. Cell Viability
3.3.3. Hydrogel Hemolytic Capacity
3.4. Biological Evaluation of Hydrogels in an In Vitro Model of Induced Ischemia
3.4.1. Hypoxia Model and Cell Characteristics by Ischemia
3.4.2. Lactate Dehydrogenase (LDH) Cytotoxicity
3.4.3. Evaluation of Cell Structural Changes
Connexin 43
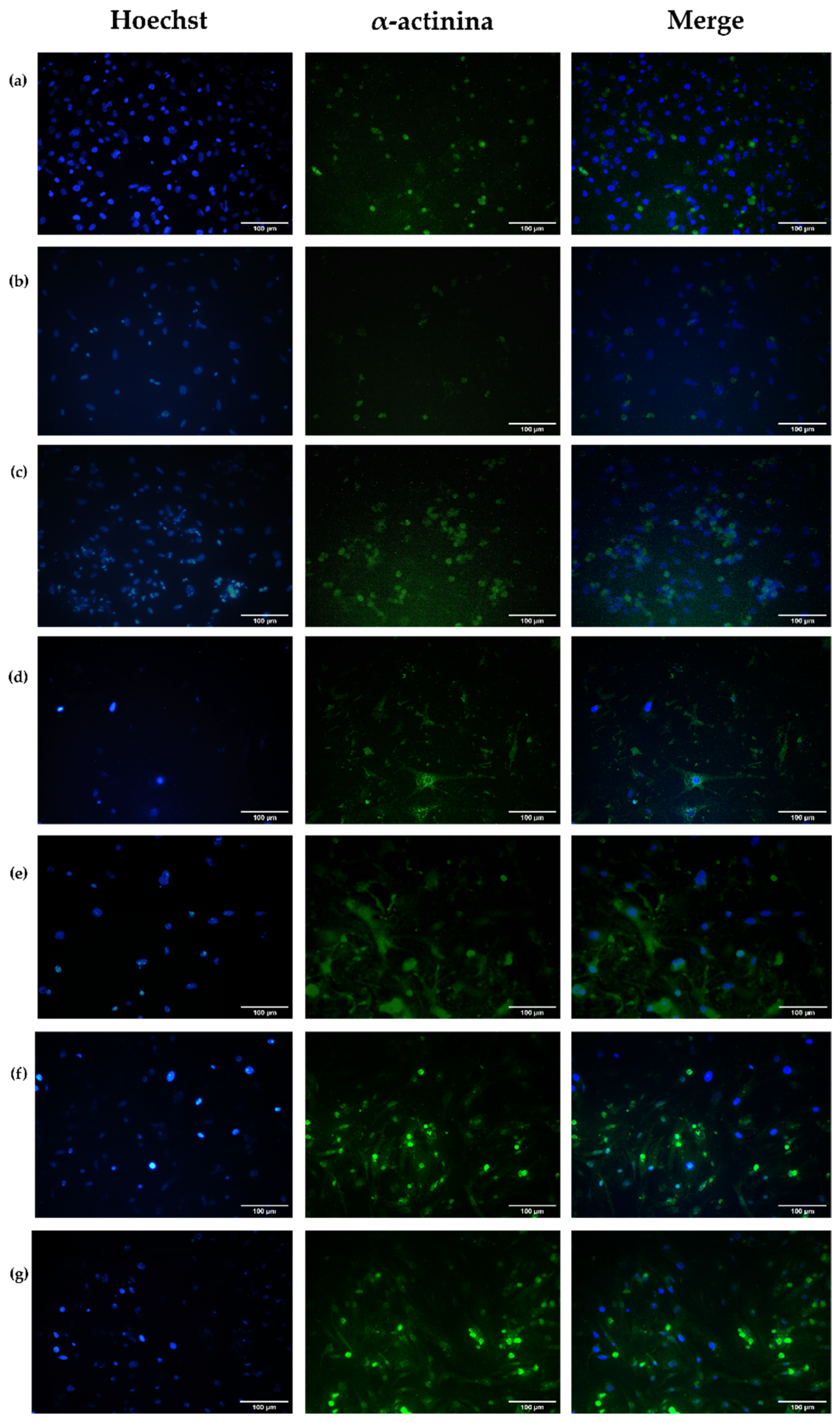
Alpha-Actinin
3.4.4. Intracellular Calcium Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuraitis, D.; Giordano, C.; Suuronen, E.J.; Ruel, M. Cell Therapy to Regenerate the Ischemic Heart; Woodhead Publishing Limited, University of Ottawa Heart Institute: Ottawa, ON, Canada, 2014; Volume 1. [Google Scholar] [CrossRef]
- Kikuchi, K.; Poss, K.D. Cardiac Regenerative Capacity and Mechanisms Kazu. Ann. Rev. Cell Dev. Biol. 2012, 28, 719–741. [Google Scholar] [CrossRef] [PubMed]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, M.F.; Hanif, M.; Ranjha, N.M. Methods of synthesis of hydrogels…A review. Saudi Pharm. J. 2016, 24, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Klouda, L.; Mikos, A.G. Thermoresponsive hydrogels in biomedical applications—A review. Eur. J. Pharm. Biopharm. 2011, 68, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Peña, B.; Laughter, M.; Jett, S.; Rowland, T.J.; Taylor, M.R.G.; Mestroni, L.; Park, D. Injectable Hydrogels for Cardiac Tissue Engineering. Macromol. Biosci. 2018, 18, e1800079. [Google Scholar] [CrossRef] [PubMed]
- Segers, V.F.; Lee, R.T. Biomaterials to Enhance Stem Cell Function in the Heart. Circ. Res. 2011, 109, 910–922. [Google Scholar] [CrossRef] [PubMed]
- Dang, Q.; Liu, K.; Zhang, Z.; Liu, C.; Liu, X.; Xin, Y.; Cheng, X.; Xu, T.; Cha, D.; Fan, B. Fabrication and evaluation of thermosensitive chitosan/collagen/α, β-glycerophosphate hydrogels for tissue regeneration. Carbohydr. Polym. 2017, 167, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Reist, M.; Chenite, A.; Felt-Baeyens, O.; Mayer, J.; Gurny, R. Pseudo-thermosetting chitosan hydrogels for biomedical application. Int. J. Pharm. 2005, 288, 197–206. [Google Scholar] [CrossRef]
- ISO 10993-5; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- ISO 10993-4; Biological Evaluation of Medical Devices—Part 4: Selection of Tests for Interactions with Blood. International Organization for Standardization: Geneva, Switzerland, 2017; Volume 2002, p. 13.
- Vidyasekar, P.; Shyamsunder, P.; Santhakumar, R.; Arun, R.; Verma, R.S. A simplified protocol for the isolation and culture of cardiomyocytes and progenitor cells from neonatal mouse ventricles. Eur. J. Cell Biol. 2015, 94, 444–452. [Google Scholar] [CrossRef]
- Hafez, P.; Chowdhury, S.R.; Jose, S.; Law, J.X.; Ruszymah, B.H.I.; Ramzisham, A.R.M.; Ng, M.H. Development of an In Vitro Cardiac Ischemic Model Using Primary Human Cardiomyocytes. Cardiovasc. Eng. Technol. 2018, 9, 529–538. [Google Scholar] [CrossRef]
- Smith, S.M.; Wunder, M.B.; Norris, D.A.; Shellman, Y.G. A Simple Protocol for Using a LDH-Based Cytotoxicity Assay to Assess the Effects of Death and Growth Inhibition at the Same Time. PLoS ONE 2011, 6, e26908. [Google Scholar] [CrossRef] [PubMed]
- Panyamao, P.; Ruksiriwanich, W.; Sirisa-Ard, P.; Charumanee, S. Injectable Thermosensitive Chitosan/Pullulan-Based Hydrogels with Improved Mechanical Properties and Swelling Capacity. Polymers 2020, 12, 2514. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Li, M.; Wang, X.; Liang, J.; Wang, X.; Wu, S.; Long, M.; Hu, C. An injectable chitosan/dextran/β -glycerophosphate hydrogel as cell delivery carrier for therapy of myocardial infarction. Carbohydr. Polym. 2020, 229, 115516. [Google Scholar] [CrossRef] [PubMed]
- Dang, Q.F.; Yan, J.Q.; Lin, H.; Chen, X.G.; Liu, C.S.; Ji, Q.X.; Li, J.J. Design and evaluation of a highly porous thermosensitive hydrogel with low gelation temperature as a 3D culture system for Penaeus chinensis lymphoid cells. Carbohydr. Polym. 2012, 88, 361–368. [Google Scholar] [CrossRef]
- Wang, R.; Wang, M.; He, S.; Sun, G.; Sun, X. Targeting Calcium Homeostasis in Myocardial Ischemia/Reperfusion Injury: An Overview of Regulatory Mechanisms and Therapeutic Reagents. Front. Pharmacol. 2020, 11, 872. [Google Scholar] [CrossRef]
- Vu, T.D.; Kofidis, T. Biomaterials and cells for cardiac tissue engineering. In Cardiac Regeneration and Repair; Woodhead Publishing: Cambridge, UK, 2014. [Google Scholar] [CrossRef]
- Gorgieva, S.; Kokol, V. Collagen- vs. Gelatine-Based Biomaterials and Their Biocompatibility: Review and Perspectives. In Biomaterials Applications for Nanomedicine; Pignatello, R., Ed.; IntechOpen: Ljubljana, Slovenia, 2011; pp. 17–52. [Google Scholar] [CrossRef]
- Ahmadi, R.; de Bruijn, J.D. Biocompatibility and gelation of chitosan–glycerol phosphate hydrogels. J. Biomed. Mater. Res. Part A 2008, 86, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Langenbach, F.; Handschel, J. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem. Cell Res. Ther. 2013, 4, 1. [Google Scholar] [CrossRef]
- Hernández Fernández, R.A. Kinasas y fosfatasas: El yin y el yan de la vida. Rev. Habanera Cienc. Medicas 2012, 11, 15–24. [Google Scholar]
- Sapio, L.; Naviglio, S. Inorganic phosphate in the development and treatment of cancer: A Janus Bifrons? World J. Clin. Oncol. 2015, 6, 198–201. [Google Scholar] [CrossRef]
- Lai, J.Y. The Role of Bloom Index of Gelatin on the Interaction with Retinal Pigment Epithelial Cells. Int. J. Mol. Sci. 2009, 10, 3442–3456. [Google Scholar] [CrossRef]
- Wu, Z.; Korntner, S.H.; Olijve, J.; Mullen, A.M.; Zeugolis, D.I. The Influence of Bloom Index, Endotoxin Levels and Polyethylene Glycol Succinimidyl Glutarate Crosslinking on the Physicochemical and Biological Properties of Gelatin Biomaterials. Biomolecules 2021, 11, 1003. [Google Scholar] [CrossRef] [PubMed]
- Kraehenbuehl, T.P.; Zammaretti, P.; Van der Vlies, A.J.; Schoenmakers, R.G.; Lutolf, M.P.; Jaconi, M.E.; Hubbell, J.A. Three-dimensional extracellular matrix-directed cardioprogenitor differentiation: Systematic modulation of a synthetic cell-responsive PEG-hydrogel. Biomaterials 2008, 29, 2757–2766. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Jiang, L.J.; Cao, P.P.; Li, J.B.; Chen, X.G. Glycerophosphate-based chitosan thermosensitive hydrogels and their biomedical applications. Carbohydr. Polym. 2015, 117, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shi, J.; Wang, Y.; Yin, Y.; Wang, L.; Liu, J.; Liu, Z.; Duan, C.; Zhu, P.; Wang, C. Promotion of cardiac differentiation of brown adipose derived stem cells by chitosan hydrogel for repair after myocardial infarction. Biomaterials 2014, 35, 3986–3998. [Google Scholar] [CrossRef] [PubMed]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures—A comparison of different. Arch. Med. Sci. 2016, 14, 910–919. [Google Scholar] [PubMed]
- Madl, C.M.; Heilshorn, S.C. Engineering Hydrogel Microenvironments to Recapitulate the Stem Cell Niche. Annu. Rev. Biomed. Eng. 2018, 20, 21–47. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lakes, R. Biomaterials. An Introduction, 3rd ed.; Springer: New York, NY, USA, 2007; Chapter 12; p. 332. [Google Scholar] [CrossRef]
- Rushton-Castro, B. ASTM Hemolysis (Direct Contact and Extract Methods) GLP Report; Nelson Laboratories, LLC: Salt Lake City, UT, USA, 2019; pp. 1–6. [Google Scholar]
- Rapido, F. The potential adverse effects of haemolysis. Blood Transfus. 2017, 15, 218–221. [Google Scholar] [CrossRef]
- Sreejit, P.; Kumar, S.; Verma, R.S. An improved protocol for primary culture of cardiomyocyte from neonatal mice. Vitr. Cell. Dev. Biol. Anim. 2008, 44, 45–50. [Google Scholar] [CrossRef]
- Buja, L.M.; Entman, M.L. Modes of Myocardial Cell Injury and Cell Death in Ischemic Heart Disease. Circulation 1998, 98, 1355–1357. [Google Scholar] [CrossRef]
- Lindsey, M.L.; Bolli, R.; Canty, J.M., Jr.; Du, X.-J.; Frangogiannis, N.G.; Frantz, S.; Gourdie, R.G.; Holmes, J.W.; Jones, S.P.; Kloner, R.A.; et al. Guidelines for experimental models of myocardial ischemia and infarction. Am. J. Physiol. Heart Circ. Physiol. 2018, 314, H812–H838. [Google Scholar] [CrossRef]
- Schorl, C.; Sedivy, J.M. Analysis of cell cycle phases and progression in cultured mammalian cells. Methods 2007, 41, 143–150. [Google Scholar] [CrossRef]
- Wei, H.; Wang, C.; Guo, R.; Takahashi, K.; Naruse, K. Development of a model of ischemic heart disease using cardiomyocytes differentiated from human induced pluripotent stem cells. Biochem. Biophys. Res. Commun. 2019, 520, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.S.L.; Hanner, F.; Peti-Peterdi, J.A. Intercellular Junctions. In Seldin and Geibisch’s the Kidney; Academic Press: Cambridge, MA, USA, 2013; Volume 1, pp. 347–368. [Google Scholar] [CrossRef]
- Boengler, K.; Dodoni, G.; Rodriguezsinovas, A.; Cabestrero, A.; Ruizmeana, M.; Gres, P.; Konietzka, I.; Lopeziglesias, C.; Garciadorado, D.; Dilisa, F.; et al. Connexin 43 in cardiomyocyte mitochondria and its increase by ischemic preconditioning. Cardiovasc. Res. 2005, 67, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Schuiz, R.; Gorge, P.M.; Gorbe, A.; Ferdinandy, P.; Lampe, P.D.; Leybaert, L. Connexin 43 is an emerging therapeutic target in ischemia/ reperfusion injury, cardioprotection and neuroprotection. Pharmacol. Ther. 2015, 153, 90–106. [Google Scholar] [CrossRef]
- Ramaccini, D.; Montoya-Uribe, V.; Aan, F.J.; Modesti, L.; Potes, Y.; Wieckowski, M.R.; Krga, I.; Glibetić, M.; Pinton, P.; Giorgi, C.; et al. Mitochondrial Function and Dysfunction in Dilated Cardiomyopathy. Front. Cell Dev. Biol. 2021, 8, 624216. [Google Scholar] [CrossRef] [PubMed]
- Derks, W.; Bergmann, O. Polyploidy in Cardiomyocytes: Roadblock to Heart Regeneration? Circ. Res. 2020, 126, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Axelsen, L.N.; Stahlhut, M.; Mohammed, S.; Larsen, B.D.; Nielsen, M.S.; Holstein-Rathlou, N.-H.; Andersen, S.; Jensen, O.N.; Hennan, J.K.; Kjølbye, A.L. Identification of ischemia-regulated phosphorylation sites in connexin43: A possible target for the antiarrhythmic peptide analogue rotigaptide (ZP123). J. Mol. Cell. Cardiol. 2006, 40, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Kells-Andrews, R.M.; Margraf, R.A.; Fisher, C.G.; Falk, M.M. Connexin 43 K63-polyubiquitylation on lysines 264 and 303 regulates gap junction internalization. J. Cell Sci. 2018, 131, jcs204321. [Google Scholar] [CrossRef] [PubMed]
- Solan, J.L.; Lampe, P.D. Connexin 43 Phosphorylation—Structural Changes and biological effects. Biochem. J. 2009, 419, 261–272. [Google Scholar] [CrossRef]
- Danon, A.; Zeevi-Levin, N.; Pinkovich, D.Y.; Michaeli, T.; Berkovich, A.; Flugelman, M.; Eldar, Y.C.; Rosen, M.R.; Binah, O. Hypoxia causes connexin 43 internalization in neonatal rat ventricular myocytes. Gen. Physiol. Biophys. 2010, 29, 222–233. [Google Scholar] [CrossRef]
- Chen, T.; Vunjak-Novakovic, G. In Vitro Models of Ischemia-Reperfusion Injury. Regen. Eng. Transl. Med. 2019, 4, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Imbeault, S.; Gauvin, L.G.; Toeg, H.D.; Pettit, A.; Sorbara, C.D.; Migahed, L.; DesRoches, R.; Menzies, A.S.; Nishii, K.; Paul, D.L.; et al. The extracellular matrix controls gap junction protein expression and function in postnatal hippocampal neural progenitor cells. BMC Neurosci. 2009, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Prados, J.; Melguizo, C.; Marchal, J.A.; Velez, C.; Alvarez, L.; Aránega, A. Estudio Inmunohistoquimico de los Cambios en la Expresion de la alfa-Actinina durante el Desarrollo Cardiaco de Pollo. Rev. Chil. Anatomía 1998, 16, 255–261. [Google Scholar] [CrossRef]
- Ribeiro, E.D.A.; Pinotsis, N.; Ghisleni, A.; Salmazo, A.; Konarev, P.V.; Kostan, J.; Sjöblom, B.; Schreiner, C.; Polyansky, A.A.; Gkougkoulia, E.A.; et al. The Structure and Regulation of Human Muscle α-Actinin. Cell 2014, 159, 1447–1460. [Google Scholar] [CrossRef]
- Van Eyk, J.E.; Powers, F.; Law, W.; Larue, C.; Hodges, R.S.; Solaro, R.J. Breakdown and Release of Myofilament Proteins During Ischemia and Ischemia/Reperfusion in Rat Hearts Identification of Degradation Products and Effects on the pCa-Force Relation. Circ. Res. 1998, 82, 261–271. [Google Scholar] [CrossRef]
- Brady, T.; Siegel, J.; Albers, R.; Price. Calcium Chapter 24. In Basic Neurochemistry; Elsevier: Amsterdam, The Netherlands, 2012; p. xxi. [Google Scholar] [CrossRef]
- Bannwarth, M.; Corrêa, I.R.; Sztretye, M.; Pouvreau, S.; Fellay, C.; Aebischer, A.; Royer, L.; Ríos, E.; Johnsson, K. Indo-1 Derivatives for Local Calcium Sensing. ACS Chem. Biol. 2009, 4, 179–190. [Google Scholar] [CrossRef]



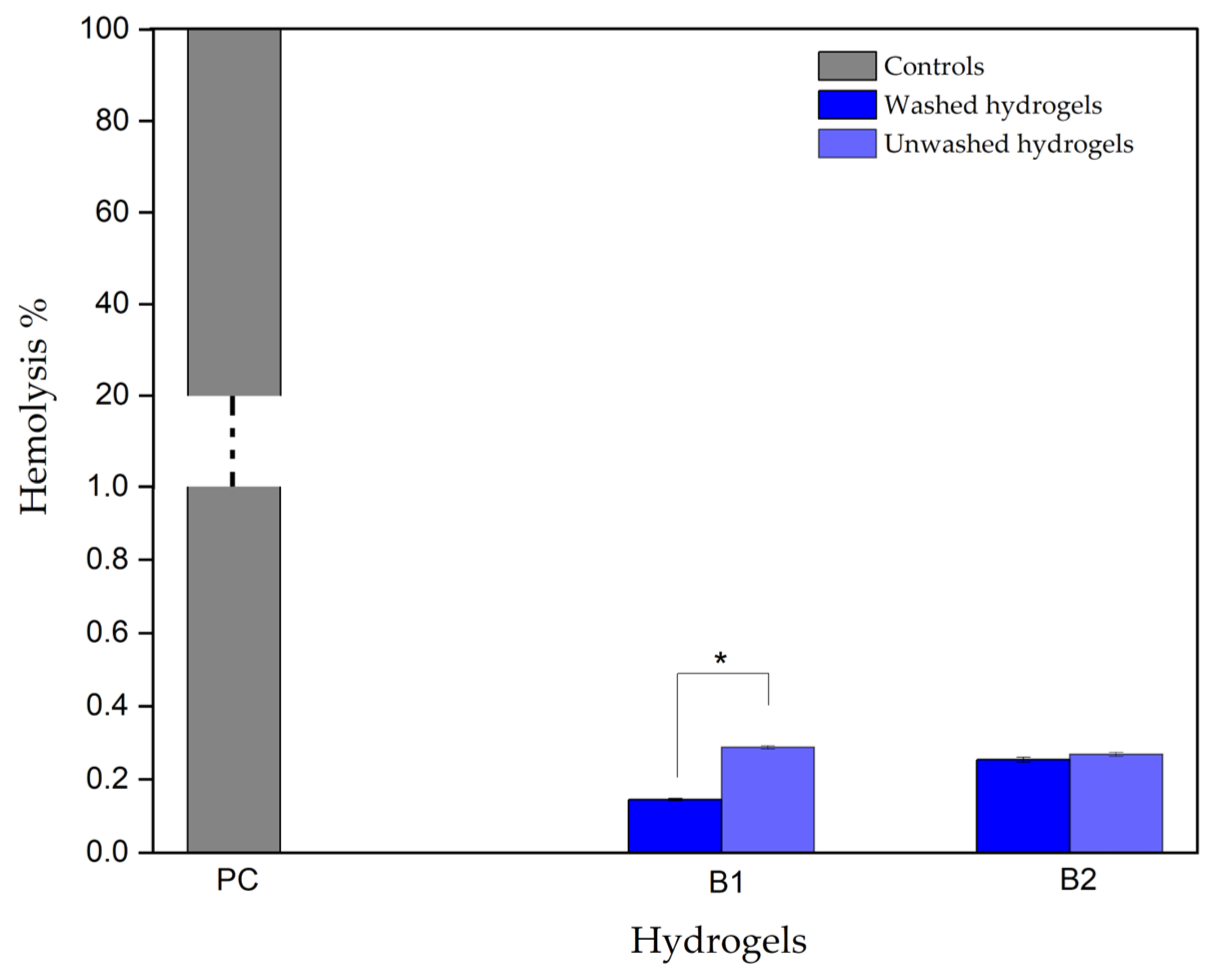




| Hemolytic Index | Hemolytic Degree |
|---|---|
| 0–2 | Non-hemolytic |
| 2–5 | Slightly hemolytic |
| >5 | Hemolytic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marín, L.O.; Montoya, Y.; Bustamante, J. Biological Evaluation of Thermosensitive Hydrogels of Chitosan/Hydrolyzed Collagen/β-GP in an In Vitro Model of Induced Cardiac Ischemia. Polymers 2024, 16, 2206. https://doi.org/10.3390/polym16152206
Marín LO, Montoya Y, Bustamante J. Biological Evaluation of Thermosensitive Hydrogels of Chitosan/Hydrolyzed Collagen/β-GP in an In Vitro Model of Induced Cardiac Ischemia. Polymers. 2024; 16(15):2206. https://doi.org/10.3390/polym16152206
Chicago/Turabian StyleMarín, Lina Orozco, Yuliet Montoya, and John Bustamante. 2024. "Biological Evaluation of Thermosensitive Hydrogels of Chitosan/Hydrolyzed Collagen/β-GP in an In Vitro Model of Induced Cardiac Ischemia" Polymers 16, no. 15: 2206. https://doi.org/10.3390/polym16152206
APA StyleMarín, L. O., Montoya, Y., & Bustamante, J. (2024). Biological Evaluation of Thermosensitive Hydrogels of Chitosan/Hydrolyzed Collagen/β-GP in an In Vitro Model of Induced Cardiac Ischemia. Polymers, 16(15), 2206. https://doi.org/10.3390/polym16152206







