Crystallization of Poly(ethylene terephthalate): A Review
Abstract
1. Introduction
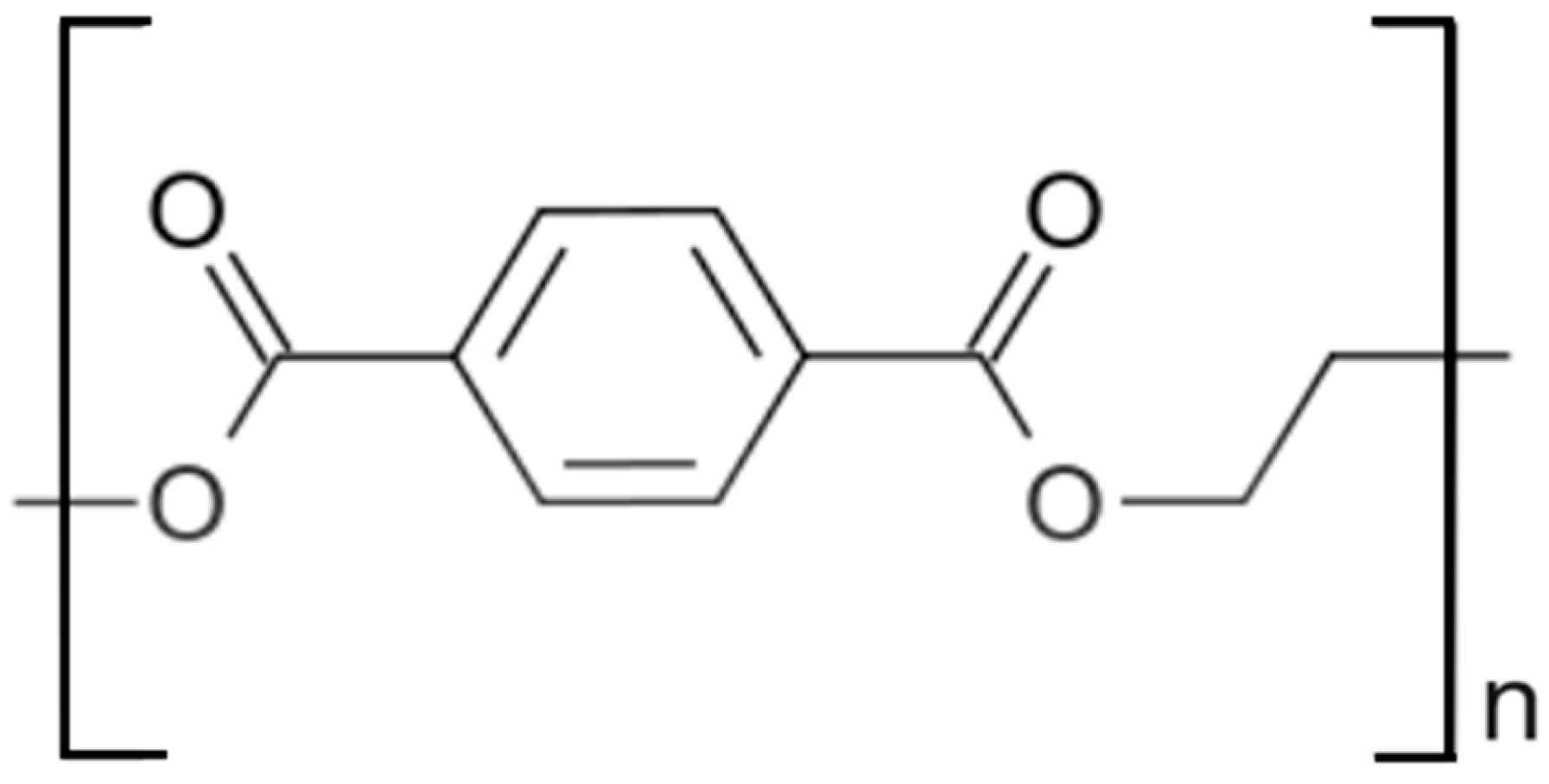
2. Crystal Structure
3. Crystallization Kinetics
3.1. Homogeneous and Heterogeneous Nucleation in PET
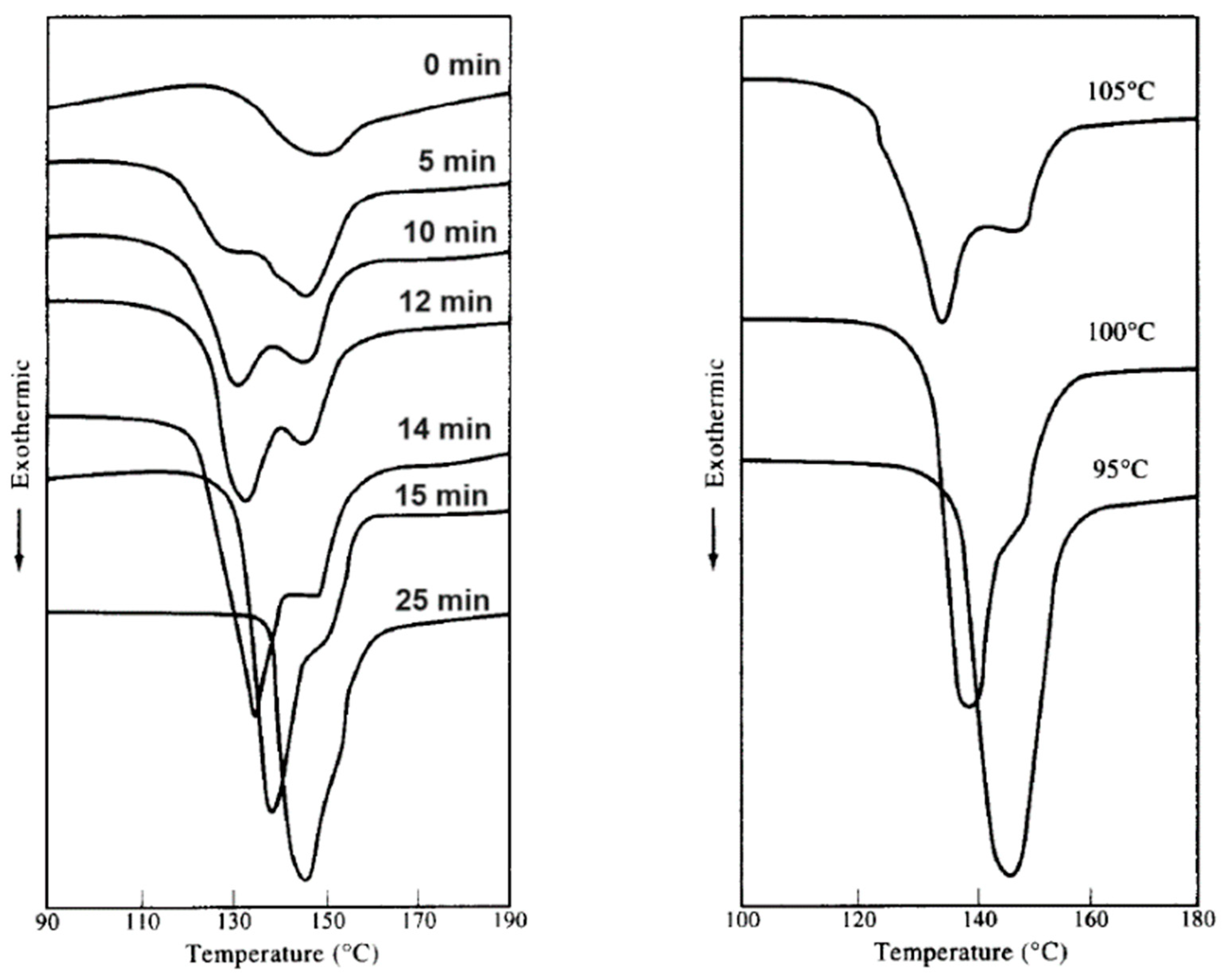
3.2. Crystal Growth
4. Influence of Chain Features
5. Crystallization of PET in Blends and Composites
6. Influence of Liquid and Gases
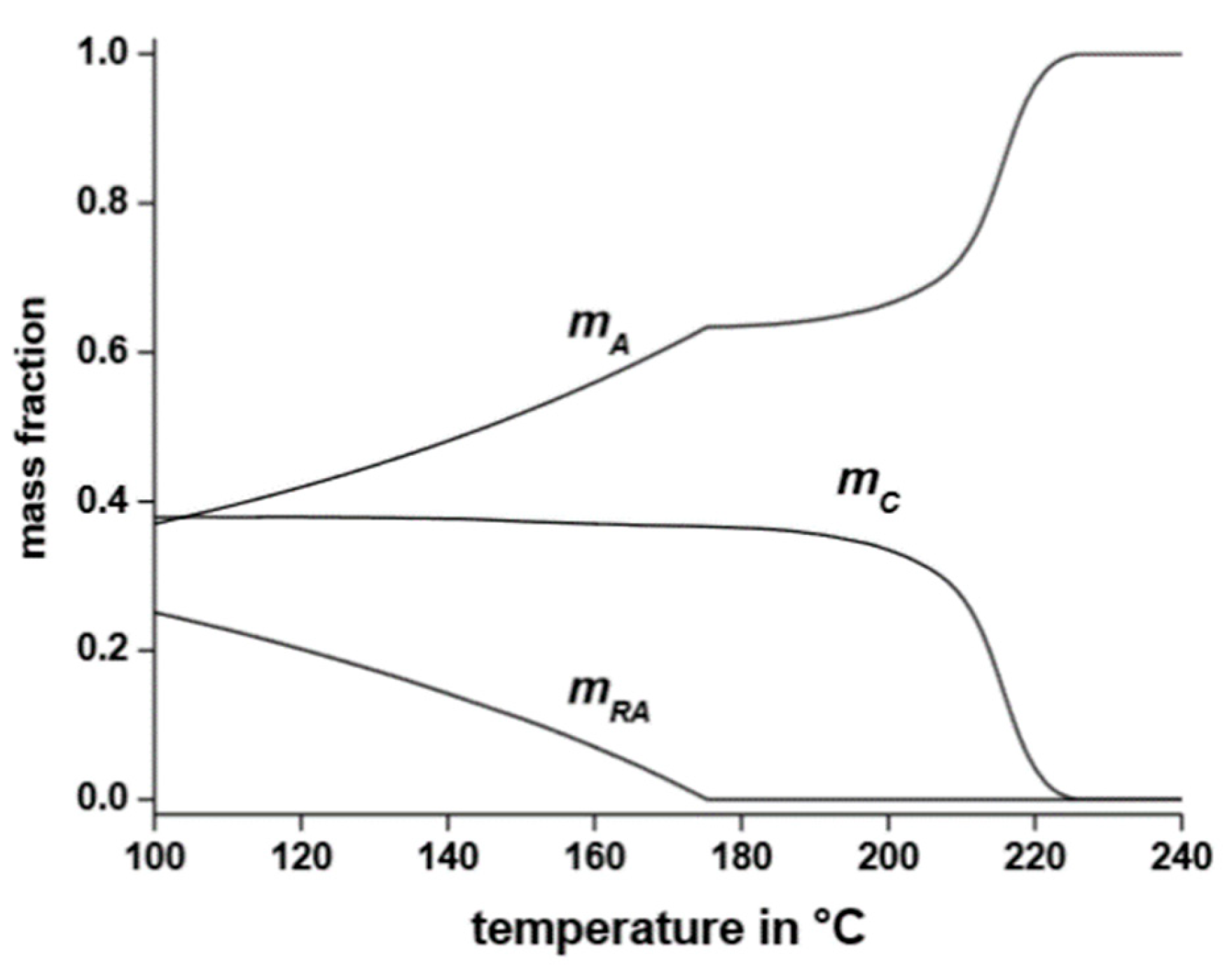
7. Coupling between Crystalline and Amorphous Parts: The Rigid Amorphous Fraction
8. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Available online: https://plasticseurope.org/wp-content/uploads/2022/10/PE-PLASTICS-THE-FACTS_V7-Tue_19-10-1.pdf (accessed on 14 June 2024).
- Jiang, M.; Wang, X.; Xi, W.; Yang, P.; Zhou, H.; Duan, J.; Ratova, M.; Wu, D. Chemical catalytic upgrading of polyethylene terephthalate plastic waste into value-added materials, fuels and chemicals. Sci. Total Environ. 2023, 912, 169342. [Google Scholar] [CrossRef]
- Romero-Hernández, O.; Romero Hernández, S.; Muñoz, D.; Detta-Silveira, E.; Palacios-Brun, A.; Laguna, A. Environmental implications and market analysis of soft drink packaging systems in Mexico. A waste management approach. Int. J. Life Cycle Assess. 2009, 14, 107–113. [Google Scholar] [CrossRef]
- Sevigné-Itoiz, E.; Gasol, C.M.; Rieradevall, J.; Gabarrell, X. Contribution of plastic waste recovery to greenhouse gas (GHG) savings in Spain. Waste Manag. 2015, 46, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Gaur, U.; Lau, F.F.; Wunderlich, B.B.; Wunderlich, B. Heat Capacity and Other Thermodynamic Properties of Linear Macromolecules. VIII. Polyesters and Polyamides. J. Phys. Chem. Ref. Data 1983, 12, 65–89. [Google Scholar] [CrossRef][Green Version]
- Cheng, S.Z.D.; Lim, S.; Judovits, L.H.; Wunderlich, B. Heat capacities of high melting polymers containing phenylene groups. Polymer 1987, 28, 10–22. [Google Scholar] [CrossRef]
- Mehta, A.; Gaur, U.; Wunderlich, B. Equilibrium melting parameters of poly(ethylene terephthalate). J. Polym. Sci. Polym. Phys. Ed. 1978, 16, 289–296. [Google Scholar] [CrossRef]
- Gaonkar, A.A.; Murudkar, V.V.; Deshpande, V.D. Comparison of crystallization kinetics of polyethylene terephthalate (PET) and reorganized PET. Thermochim. Acta 2020, 683, 178472. [Google Scholar] [CrossRef]
- Pilati, F.; Toselli, M.; Messori, M.; Manzoni, C.; Turturro, A.; Gattiglia, E.G. On specific factors affecting the crystallization of PET: The role of carboxyl terminal groups and residual catalysts on the crystallization rate. Polymer 1997, 38, 4469–4476. [Google Scholar] [CrossRef]
- Babaei, M.; Jalilian, M.; Shahbaz, K. Chemical recycling of Polyethylene terephthalate: A mini-review. J. Envir. Chem. Eng. 2024, 12, 112507. [Google Scholar] [CrossRef]
- De Vos, L.; Van de Voorde, B.; Van Daele, L.; Dubruel, P.; Van Vlierberghe, S. Poly (alkylene terephthalate) s: From current developments in synthetic strategies towards applications. Eur. Polym. J. 2021, 161, 110840. [Google Scholar] [CrossRef]
- Cheng, Z.; Liu, Y.; Li, Z.; Song, Y. Condensation polymers: Synthesis, properties, and applications. In E3S Web Conferences; EDP Sciences: Les Ulis, France, 2021; Volume 290, p. 01025. [Google Scholar]
- Lu, W.; Debelak, K.A.; Witt, A.R.; Yang, C.; Collins, W.E.; Lott, C. Structural features of crystallized poly(ethylene terephthalate) polymers. J. Polym. Sci. B Polym. Phys. 2002, 40, 245–254. [Google Scholar] [CrossRef]
- Piorkowska, E. Overview of Biobased Polymers. In Thermal Properties of Bio-Based Polymers; Advances in Polymer Science; Di Lorenzo, M., Androsch, R., Eds.; Springer: Cham, Switzerland, 2019; Volume 283, pp. 1–35. [Google Scholar]
- Benyathiar, P.; Kumar, P.; Carpenter, G.; Brace, J.; Mishra, D.K. Polyethylene terephthalate (PET) bottle-to-bottle recycling for the beverage industry: A Review. Polymers 2022, 14, 2366. [Google Scholar] [CrossRef] [PubMed]
- Al-Salem, S.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef] [PubMed]
- Sheel, A.; Pant, D. Chemical depolymerization of PET bottles via glycolysis. In Recycling of Polyethylene Terephthalate Bottles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 61–84. [Google Scholar]
- Sinha, V.; Patel, M.R.; Patel, J.V. PET waste management by chemical recycling: A review. J. Polym. Environ. 2010, 18, 8–25. [Google Scholar] [CrossRef]
- Das, S.K.; Eshkalak, S.K.; Chinnappan, A.; Ghosh, R.; Jayathilaka, W.; Baskar, C.; Ramakrishna, S. Plastic recycling of polyethylene terephthalate (PET) and polyhydroxybutyrate (PHB)—A comprehensive review. Mater. Circ. Econ. 2021, 3, 9. [Google Scholar] [CrossRef]
- Frounchi, M. Studies on degradation of PET in mechanical recycling. Macromol. Symp. 1999, 144, 465–469. [Google Scholar] [CrossRef]
- Tan, S.; Su, A.; Li, W.; Zhou, E. New insight into melting and crystallization behavior in semicrystalline poly(ethylene terephthalate). J. Polym. Sci. B Polym. Phys. 2000, 38, 53–60. [Google Scholar] [CrossRef]
- Cole, K.C.; Ajji, A.; Pellerin, E. New insights into the development of ordered structure in poly (ethylene terephthalate). 1. Results from external reflection infrared spectroscopy. Macromolecules 2002, 35, 770–784. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, J.; Pan, P. Structural Evolutions of Initially Amorphous Polymers during Near-Tg Stretching: A Minireview of Recent Progresses. Macromol. Chem. Phys. 2022, 223, 2100427. [Google Scholar] [CrossRef]
- Pijpers, T.F.J.; Mathot, V.B.F.; Goderis, B.; Scherrenberg, R.L.; van der Vegte, E.W. High-Speed Calorimetry for the Study of the Kinetics of (De)vitrification, Crystallization, and Melting of Macromolecules. Macromolecules 2002, 35, 3601–3613. [Google Scholar] [CrossRef]
- Androsch, R.; Schick, C.; Rhoades, A.M. Application of Tammann’s two-stage crystal nuclei development method for analysis of the thermal stability of homogeneous crystal nuclei of poly (ethylene terephthalate). Macromolecules 2015, 48, 8082–8089. [Google Scholar] [CrossRef]
- Bai, C.; Spontak, R.J.; Koch, C.C.; Saw, C.K.; Balik, C.M. Structural changes in poly (ethylene terephthalate) induced by mechanical milling. Polymer 2000, 41, 7147–7157. [Google Scholar] [CrossRef]
- Daubeny, R.D.P.; Bunn, C.W.; Brown, C.J. The crystal structure of polyethylene terephthalate, Proc. Royal Soc. London. Series A Math. Phys. Sci. 1954, 226, 531–542. [Google Scholar]
- Sauer, T.H.; Wendorff, J.H.; Zimmermann, H.J. Thermotropic rigid-chain copolymers: X-ray investigations of the structure. J. Polym. Sci. B Polym. Phys. 1987, 25, 2471–2485. [Google Scholar] [CrossRef]
- Asano, T.; Seto, T. Morphological Studies of Cold Drawn Poly(ethylene terephthalate). Polym. J. 1973, 5, 72–85. [Google Scholar] [CrossRef][Green Version]
- Zheng, Y.; Pan, P. Crystallization of biodegradable and biobased polyesters: Polymorphism, cocrystallization, and structure-property relationship. Progr. Polym. Sci. 2020, 109, 101291. [Google Scholar] [CrossRef]
- Bonart, R. Parakristalline Strukturen in Polyäthylenterephthalat (PET). Koll. Z. Z. Polym. 1966, 213, 1–11. [Google Scholar] [CrossRef]
- Prevorsek, D.C. Structure of semi crystalline fibers from interpretation of anelastic effects. J. Polym. Sci. C Polym. Symp. 1971, 32, 343–375. [Google Scholar] [CrossRef]
- Lindner, W.L. Characterization of the crystalline, intermediate and amorphous phase in poly(ethylene terephthalate) fibres by X-ray diffraction. Polymer 1973, 14, 9. [Google Scholar] [CrossRef]
- Napolitano, M.J.; Moet, A. Mechanism of cold drawing in melt-spun poly (ethylene terephthalate) fibers. J. Appl. Polym. Sci. 1987, 34, 1285–1300. [Google Scholar] [CrossRef]
- Fakirov, S.; Evstatiev, M. New routes to polyethylene terephthalate with improved mechanical properties. Polymer 1990, 31, 431–434. [Google Scholar] [CrossRef]
- Auriemma, F.; Corradini, P.; De Rosa, C.; Guerra, G.; Petraccone, V.; Bianchi, R.; Di Dino, G. On the mesomorphic form of poly (ethylene terephthalate). Macromolecules 1992, 25, 2490–2497. [Google Scholar] [CrossRef]
- Nicholson, T.M.; Davies, G.R.; Ward, I.M. Conformations in poly(ethylene terephthalate): A molecular modelling study. Polymer 1994, 35, 4259–4262. [Google Scholar] [CrossRef]
- Asano, T.; Baltá Calleja, F.J.; Flores, A.; Tanigaki, M.; Mina, M.F.; Sawatari, C.; Itagaki, H.; Takahashi, H.; Hatta, I. Crystallization of oriented amorphous poly(ethylene terephthalate) as revealed by X-ray diffraction and microhardness. Polymer 1999, 40, 6475–6484. [Google Scholar] [CrossRef]
- Martins, C.I.; Cakmak, M. Control the strain-induced crystallization of polyethylene terephthalate by temporally varying deformation rates: A mechano-optical study. Polymer 2007, 48, 2109–2123. [Google Scholar] [CrossRef]
- Abou-Kandil, A.I.; Windle, A.H. The development of microstructure in oriented polyethylene terephthalate (PET) during annealing. Polymer 2007, 48, 5069–5079. [Google Scholar] [CrossRef]
- Blundell, D.J.; MacKerron, D.H.; Fuller, W.; Mahendrasingam, A.; Martin, C.; Oldman, R.J.; Rule, R.J.; Riekel, C. Characterization of strain-induced crystallization of poly (ethylene terephthalate) at fast draw rates using synchrotron radiation. Polymer 1996, 37, 3303–3311. [Google Scholar] [CrossRef]
- Ajji, A.; Cole, K.C.; Dumoulin, M.M.; Brisson, J. Amorphous orientation of poly (ethylene terephthalate) by X-ray diffraction in combination with Fourier transform infra-red spectroscopy. Polymer 1995, 36, 4023–4030. [Google Scholar] [CrossRef]
- Mahendrasingam, A.; Blundell, D.J.; Wright, A.K.; Urban, V.; Narayanan, T.; Fuller, W. Observations of structure development during crystallisation of oriented poly (ethylene terephthalate). Polymer 2003, 44, 5915–5925. [Google Scholar] [CrossRef]
- Matthews, R.G.; Ajji, A.; Dumoulin, M.M.; Prud’Homme, R.E. The effects of stress relaxation on the structure and orientation of tensile drawn poly (ethylene terephthalate). Polymer 2000, 41, 7139–7145. [Google Scholar] [CrossRef]
- Forestier, E.; Combeaud, C.; Guigo, N.; Sbirrazzuoli, N.; Billon, N. Understanding of strain-induced crystallization developments scenarios for polyesters: Comparison of poly (ethylene furanoate), PEF, and poly (ethylene terephthalate), PET. Polymer 2020, 203, 122755. [Google Scholar] [CrossRef]
- Kawakami, D.; Hsiao, B.S.; Burger, C.; Ran, S.; Avila-Orta, C.; Sics, I.; Kikutani, T.; Jacob, K.I.; Chu, B. Deformation-induced phase transition and superstructure formation in poly (ethylene terephthalate). Macromolecules 2005, 38, 91–103. [Google Scholar] [CrossRef]
- Kawakami, D.; Ran, S.; Burger, C.; Avila-Orta, C.; Sics, I.; Chu, B.; Hsiao, B.S.; Kikutani, T. Superstructure evolution in poly (ethylene terephthalate) during uniaxial deformation above glass transition temperature. Macromolecules 2006, 39, 2909–2920. [Google Scholar] [CrossRef]
- Wang, Z.G.; Xia, Z.Y.; Yu, Z.Q.; Chen, E.Q.; Sue, H.J.; Han, C.C.; Hsiao, B.S. Lamellar formation and relaxation in simple sheared poly (ethylene terephthalate) by small-angle X-ray scattering. Macromolecules 2006, 39, 2930–2939. [Google Scholar] [CrossRef]
- Keum, J.K.; Kim, J.; Lee, S.M.; Song, H.H.; Son, Y.K.; Choi, J.I.; Im, S.S. Crystallization and Transient Mesophase Structure in Cold-Drawn PET Fibers. Macromolecules 2003, 36, 9873–9878. [Google Scholar] [CrossRef]
- Hobbs, J.; Androsch, R.; Hu, W.; Di Lorenzo, M.L.; Schick, C. Polymer Crystallization Kinetics. In Encyclopedia of Polymer Science and Technology; Wiley: Hoboken, NJ, USA, 2024. [Google Scholar]
- Jabarin, S.A. Optical properties of thermally crystallized poly(ethylene terephthalate). Polym. Eng. Sci. 1982, 22, 815–820. [Google Scholar] [CrossRef]
- Natu, A.A.; Lofgren, E.A.; Jabarin, S.A. Effect of morphology on barrier properties of poly (ethylene terephthalate). Polym. Eng. Sci. 2005, 45, 400–409. [Google Scholar] [CrossRef]
- Masayuki, I.; Kaji, K. Polymer crystallization from the metastable melt: The formation mechanism of spherulites. Polymer 2006, 47, 5544–5554. [Google Scholar]
- Rybnikař, F. Secondary crystallization of polymers. J. Polym. Sci. 1960, 44, 517–522. [Google Scholar] [CrossRef]
- Wunderlich, B. Macromolecular physics. In Crystal Nucleation, Growth, Annealing; Academic: New York, NY, USA, 1976; Volume 2. [Google Scholar]
- Mandelkern, L. Crystallization of Polymers: Kinetics and Mechanisms; Cambridge University Press: Cambridge, UK, 2004; Volume 2. [Google Scholar]
- Goderis, B.; Reynaers, H.; Koch, M.H.J. Primary and secondary crystallization in a homogeneous ethylene-1-octene copolymer: Crystallinity heterogeneity studied by SAXS. Macromolecules 2002, 35, 5840–5853. [Google Scholar] [CrossRef]
- Wunderlich, B. Reversible crystallization and the rigid amorphous phase in semicrystalline macromolecules. Prog. Polym. Sci. 2003, 28, 383–450. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Righetti, M.C. Crystallization-induced formation of rigid amorphous fraction. Polym. Cryst. 2018, 1, e10023. [Google Scholar] [CrossRef]
- Turnbull, D.; Fisher, J.C. Rate of nucleation in condensed systems. J. Chem. Phys. 1949, 17, 71–73. [Google Scholar] [CrossRef]
- Hu, W. Intramolecular crystal nucleation. In Progress in Understanding of Polymer Crystallization, Lecture Notes Physics; Reiter, G., Strobl, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 714. [Google Scholar]
- Piorkowska, E.; Rutledge, G.G. Handbook of Polymer Crystallization; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Androsch, R.; Di Lorenzo, M.L.; Schick, C. Optical Microscopy to Study Crystal Nucleation in Polymers Using a Fast Scanning Chip Calorimeter for Precise Control of the Nucleation Pathway, Macromol. Chem. Phys. 2018, 219, 1700479. [Google Scholar] [CrossRef]
- Tammann, G. Ueber die Abhängigkeit der Zahl der Kerne, welche sich in verschiedenen unterkühlten Flüssigkeiten bilden, von der Temperatur. Z. Phys. Chem. 1898, 25U, 441–479. [Google Scholar] [CrossRef]
- Tammann, G.; Jenckel, E. Die Kristallisationsgeschwindigkeit und die Kernzahl des Glycerins in Abhängigkeit von der Temperatur. Z. Anorg. Allg. Chem. 1930, 193, 76–80. [Google Scholar] [CrossRef]
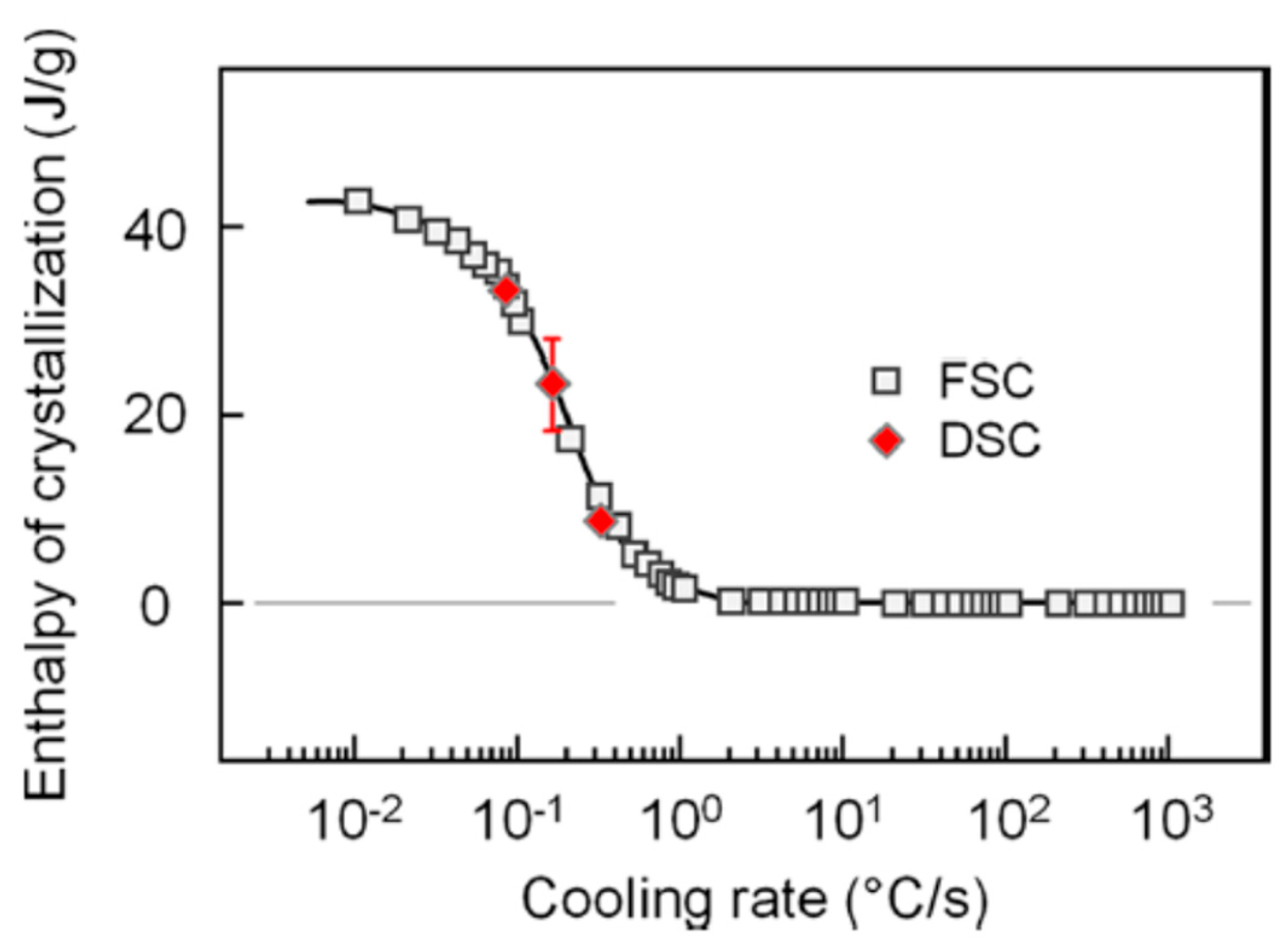
- Toda, A.; Androsch, R.; Schick, C. Insights into polymer crystallization and melting from fast scanning chip calorimetry. Polymer 2016, 91, 239–263. [Google Scholar] [CrossRef]
- Zhuravlev, E.; Schmelzer, J.W.; Androsch, R.; Schick, C. Experimental test of Tammann’s nuclei development approach in crystallization of macromolecules. Int. Polym. Proc. 2016, 31, 628–637. [Google Scholar] [CrossRef]
- Schick, C.; Androsch, R.; Schmelzer, J.W.P. Homogeneous crystal nucleation in polymers. J. Phys. Cond. Matt. 2017, 29, 453002. [Google Scholar] [CrossRef]
- Androsch, R.; Zhuravlev, E.; Schmelzer, J.W.; Schick, C. Relaxation and crystal nucleation in polymer glasses. Eur. Polym. J. 2018, 102, 195–208. [Google Scholar] [CrossRef]
- Androsch, R.; Di Lorenzo, M.L. Kinetics of crystal nucleation of poly (l-lactic acid). Polymer 2013, 54, 6882–6885. [Google Scholar] [CrossRef]
- Androsch, R.; Di Lorenzo, M.L. Crystal nucleation in glassy poly (l-lactic acid). Macromolecules 2013, 46, 6048–6056. [Google Scholar] [CrossRef]
- Androsch, R.; Di Lorenzo, M.L.; Schick, C. Effect of molar mass on enthalpy relaxation and crystal nucleation of poly (l-lactic acid). Eur. Polym. J. 2017, 96, 361–369. [Google Scholar] [CrossRef]
- Illers, K.H. Geordnete Strukturen in “amorphem” Polyäthylenterephthalat. Kolloid. Z. Z. Polym. 1971, 245, 393–398. [Google Scholar] [CrossRef]
- Bove, L.; D’Aniello, C.; Gorrassi, G.; Guadagno, L.; Vittoria, V. Influence of ageing on the cold crystallization of glassy poly (ethyleneterephthalate). Polym. Bull. 1997, 38, 579–585. [Google Scholar] [CrossRef]
- Billon, N. Strain induced crystallization of PET under biaxial conditions. From laboratory tests to injection stretch-blow molding. Polymer 2023, 277, 125953. [Google Scholar] [CrossRef]
- Weissmann, D. PET Use in Blow Molded Rigid Packaging. In Applied Plastics Engineering Handbook; William Andrew Publishing: Norwich, NY, USA, 2024; pp. 809–835. [Google Scholar]
- Tomisawa, R.; Okazaki, M.; Ikaga, T.; Kim, K.; Ohkoshi, Y.; Okada, K.; Kabe, T.; Kanaya, T.; Katsuta, H.; Funatsu, Y. Fiber structure development of poly (ethylene terephthalate-co-isophthalate) copolymer. Polymer 2022, 245, 124708. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Pang, Y.; Zhu, J.; Zheng, W. Dependence of the foaming window of poly (ethylene terephthalate-co-ethylene 2, 5-furandicarboxylate) copolyesters on FDCA content. Polymer 2022, 254, 125101. [Google Scholar] [CrossRef]
- Sorrentino, L.; Di Maio, E.; Iannace, S. Poly (ethylene terephthalate) foams: Correlation between the polymer properties and the foaming process. J. Appl. Polym. Sci. 2010, 116, 27–35. [Google Scholar] [CrossRef]
- Baldwin, D.F.; Shimbo, M.; Suh, N.P. The role of gas dissolution and induced crystallization during microcellular polymer processing: A study of poly (ethylene terephthalate) and carbon dioxide systems. J. Eng. Mater. Technol. 1995, 117, 62–74. [Google Scholar] [CrossRef]
- Longo, A.; Di Maio, E.; Di Lorenzo, M.L. Heterogeneous bubble nucleation by homogeneous crystal nuclei in poly (l-lactic acid) foaming. Macromol. Chem. Phys. 2022, 223, 2100428. [Google Scholar] [CrossRef]
- Jiang, X.L.; Luo, S.J.; Sun, K.; Chen, X.D. Effect of nucleating agents on crystallization kinetics of PET. Expr. Polym. Lett. 2007, 1, 245–251. [Google Scholar] [CrossRef]
- Gonçalves Marques, G.; Couffin, A.; Hajji, P.; Inoubli, R.; Bounor-Legaré, V.; Fulchiron, R. A Review on the Formulation and Rupture Properties of Polyethylene Terephthalate in a Mechanical Recycling Context. Ind. Eng. Chem. Res. 2024, 63, 887–920. [Google Scholar] [CrossRef]
- Tianbin, W.; Yangchuan, K. Preparation of silica–PS composite particles and their application in PET. Eur. Polym. J. 2006, 42, 274–285. [Google Scholar] [CrossRef]
- Antoniadis, G.; Paraskevopoulos, K.M.; Bikiaris, D.; Chrissafis, K. Non-isothermal crystallization kinetic of poly(ethylene terephthalate)/fumed silica (PET/SiO2) prepared by in situ polymerization. Thermochim. Acta 2010, 510, 103–112. [Google Scholar] [CrossRef]
- Wang, Y.M.; Shen, C.Y.; Li, H.M.; Li, Q.; Chen, J. Nonisothermal melt crystallization kinetics of poly (ethylene terephthalate)/clay nanocomposites. J. Appl. Polym. Sci. 2004, 91, 308–314. [Google Scholar] [CrossRef]
- Ke, Y.-C.; Wu, T.-B.; Xia, Y.-F. The nucleation, crystallization and dispersion behavior of PET–monodisperse SiO2 composites. Polymer 2007, 48, 3324–3336. [Google Scholar] [CrossRef]
- Calcagno, C.I.W.; Mariani, C.M.; Teixeira, S.R.; Mauler, R.S. The effect of organic modifier of the clay on morphology and crystallization properties of PET nanocomposites. Polymer 2007, 48, 966–974. [Google Scholar] [CrossRef]
- Yang, F.; Mubarak, C.; Keiegel, R.; Kannan, R.M. Supercritical carbon dioxide (scCO2) dispersion of poly(ethylene terephthalate)/clay nanocomposites: Structural, mechanical, thermal, and barrier properties. J. Appl. Polym. Sci. 2017, 134, 44779. [Google Scholar] [CrossRef]
- Chowreddy, R.R.; Nord-Varhaug, K.; Rapp, F. Recycled poly (ethylene terephthalate)/clay nanocomposites: Rheology, thermal and mechanical properties. J. Polym. Environ. 2019, 27, 37–49. [Google Scholar] [CrossRef]
- Gao, W.; Ma, X.Y.; Liu, Y.; Wang, Z.C.; Zhu, Y.C. Effect of calcium carbonate on PET physical properties and thermal stability. Powder Technol. 2013, 244, 45–51. [Google Scholar] [CrossRef]
- Cayuela, D.; Cot, M.; Algaba, I.; Manich, A.M. Effect of different dispersing agents in the non-isothermal kinetics and thermomechanical behavior of PET/TiO2 composites. J. Macromol. Sci. Part A 2016, 53, 237–244. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, H.; Ke, M.; Xiao, L.; Zuo, D.; Qian, Q.; Chen, Q. Non-isothermal crystallization kinetics of poly(ethylene terephthalate)/mica composites. Polym. Bull. 2014, 71, 2287–2301. [Google Scholar] [CrossRef]
- Miri, F.S.; Ehsani, M.; Khonakdar, H.A.; Kavyani, B. A Comprehensive Study on Physical, Mechanical, and Thermal Properties of Poly(Ethylene Terephthalate) Filled by Micro- and Nanoglass Flakes. J. Vinyl Addit. Technol. 2020, 26, 380–389. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, Y.; Meng, L. UIO-66 as nucleating agent on the crystallization behavior and properties of poly (ethylene terephthalate). Polymers 2021, 13, 2266. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Errico, M.E.; Avella, M. Thermal and morphological characterization of poly (ethylene terephthalate)/calcium carbonate nanocomposites. J. Mat. Sci. 2002, 37, 2351–2358. [Google Scholar] [CrossRef]
- Dekoninck, J.M.; Legras, R.; Mercier, J.P. Nucleation of poly(ethylene terephthalate) by sodium compounds: A unique mechanism. Polymer 1989, 30, 910–913. [Google Scholar] [CrossRef]
- Gilmer, J.W.; Neu, R.P.; Liu, Y.J.; Jen, A.K.-Y. The use of sodium salts as nucleation agents for polyethylene terephthalate with minimal molecular weight reduction. Polym. Eng. Sci. 1995, 35, 1407–1412. [Google Scholar] [CrossRef]
- Legras, R.; Bailly, C.; Daumerie, M.; Dekoninck, J.M.; Mercier, J.P.; Zichy, M.V.; Nield, E. Chemical nucleation, a new concept applied to the mechanism of action of organic acid salts on the crystallization of polyethylene terephthalate and bisphenol-A polycarbonate. Polymer 1984, 25, 835–844. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, S.; Mohamed, M.G.; Kuo, S.-W.; Xin, Z. Crystallization behaviors of poly(ethylene terephthalate) (PET) with monosilane isobutyl-polyhedral oligomeric silsesquioxanes (POSS). J. Mater. Sci. 2020, 55, 14642–14655. [Google Scholar] [CrossRef]
- Ozdemir, E.; Arenas, D.R.; Kelly, N.L.; Hanna, J.V.; van Rijswijk, B.; Degirmenci, V.; McNally, T. Ethylene methyl acrylate copolymer (EMA) assisted dispersion of few-layer graphene nanoplatelets (GNP) in poly(ethylene terephthalate) (PET). Polymer 2020, 205, 122836. [Google Scholar] [CrossRef]
- Xing, L.; Wang, Y.; Wang, S.; Zhang, Y.; Mao, S.; Wang, G.; Liu, J.; Huang, L.; Li, H.; Belfiore, L.A.; et al. Effects of Modified Graphene Oxide on Thermal and Crystallization Properties of PET. Polymers 2018, 10, 613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Li, H.; Gong, X.; Liu, J.; Huang, L.; Wang, W.; Wang, Y.; Zhao, Z.; Belfiore, L.A.; et al. Fluorescent SiO2@Tb3+(PET-TEG)3Phen Hybrids as Nucleating Additive for Enhancement of Crystallinity of PET. Polymers 2020, 12, 568. [Google Scholar] [CrossRef] [PubMed]
- Bouma, K.; Gaymans, R.J. Crystallization of poly (ethylene terephthalate) and poly (butylene terephthalate) modified by diamides. Polym. Eng. Sci. 2001, 41, 466–474. [Google Scholar] [CrossRef]
- Yu, Z.Z.; Ou, Y.C.; Feng, Y.P. Effects of interfacial adhesion on crystallization and mechanical properties of polyethylene terephthalate/polyamide-66 blends. Polym. Mater. Sci. Eng. 1997, 13, 57–61. [Google Scholar]
- Du, X.H.; Wang, Y.Z.; Chen, X.T.; Tang, X.D. Properties of phosphorus-containing thermotropic liquid crystal copolyester/poly (ethylene terephthalate) blends. Polym. Degrad. Stab. 2005, 88, 52–56. [Google Scholar] [CrossRef]
- Tang, S.; Xin, Z. Structural effects of ionomers on the morphology, isothermal crystallization kinetics and melting behaviors of PET/ionomers. Polymer 2009, 50, 1054–1061. [Google Scholar] [CrossRef]
- Lin, C.C. The rate of crystallization of poly (ethylene terephthalate) by differential scanning calorimetry. Polym. Eng. Sci 1983, 23, 113–116. [Google Scholar] [CrossRef]
- Lu, X.F.; Hay, J.N. Isothermal crystallization kinetics and melting behaviour of poly (ethylene terephthalate). Polymer 2001, 42, 9423–9431. [Google Scholar] [CrossRef]
- Lee, J.K.; Lee, K.H.; Jin, B.S. Spherulitic morphologies of poly (ethylene terephthalate), poly (ethylene 2, 6-naphthalate), and their blend. Macromol. Res. 2002, 10, 44–48. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Androsch, R.; Rhoades, A.M.; Righetti, M.C. Analysis of Polymer Crystallization by Calorimetry. In Handbook of Thermal Analysis and Calorimetry; Elsevier Science BV: Amsterdam, The Netherlands, 2018; Volume 6, pp. 253–299. [Google Scholar]
- Jabarin, S.A. Crystallization behavior of poly(ethylene terephthalate). Polym. Eng. Sci. 1989, 29, 1259–1264. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Sajkiewicz, P.; Gradys, A.; La Pietra, P. Optimization of melting conditions for the analysis of crystallization kinetics of poly(3-hydroxybutyrate). e-Polymers 2009, 9, 313–324. [Google Scholar] [CrossRef]
- Miyagi, A.; Wunderlich, B. Annealing during polymerization of crystalline poly(ethylene terephthalate). J. Polym. Sci. Polym. Phys. Ed. 1972, 10, 2085–2092. [Google Scholar] [CrossRef]
- McAlea, K.P.; Schultz, J.M.; Gardner, K.H.; Wignall, G.D. Ester interchange reactions in poly (ethylene terephthalate): Observation using small-angle neutron scattering. Polymer 1986, 27, 1581–1584. [Google Scholar] [CrossRef]
- Kugler, J.; Gilmer, J.W.; Wiswe, D.; Zachmann, H.G.; Hahn, K.; Fischer, E.W. Study of transesterification in poly (ethylene terephthalate) by small-angle neutron scattering. Macromolecules 1987, 20, 1116–1119. [Google Scholar] [CrossRef]
- Liangbin, L.; Rui, H.; Ling, Z.; Shiming, H. A new mechanism in the formation of PET extended-chain crystal. Polymer 2001, 42, 2085–2089. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Androsch, R. Crystallization of Poly[(R)-3-hydroxybutyrate]. In Thermal Properties of Bio-Based Polymers; Advances in Polymer Science; Di Lorenzo, M., Androsch, R., Eds.; Springer: Cham, Switzerland, 2019; Volume 283, pp. 119–142. [Google Scholar]
- Mathot, V.B.F. (Ed.) The crystallization and melting region. In Calorimetry and Thermal Analysis of Polymers; Hanser Publisher: Munich, Germany, 1994; pp. 231–299. [Google Scholar]
- Vilanova, P.C.; Ribas, S.M.; Guzmán, G.M. Isothermal crystallization of poly (ethylene-terephthalate) of low molecular weight by differential scanning calorimetry: 1. Crystallization kinetics. Polymer 1985, 26, 423–428. [Google Scholar] [CrossRef]
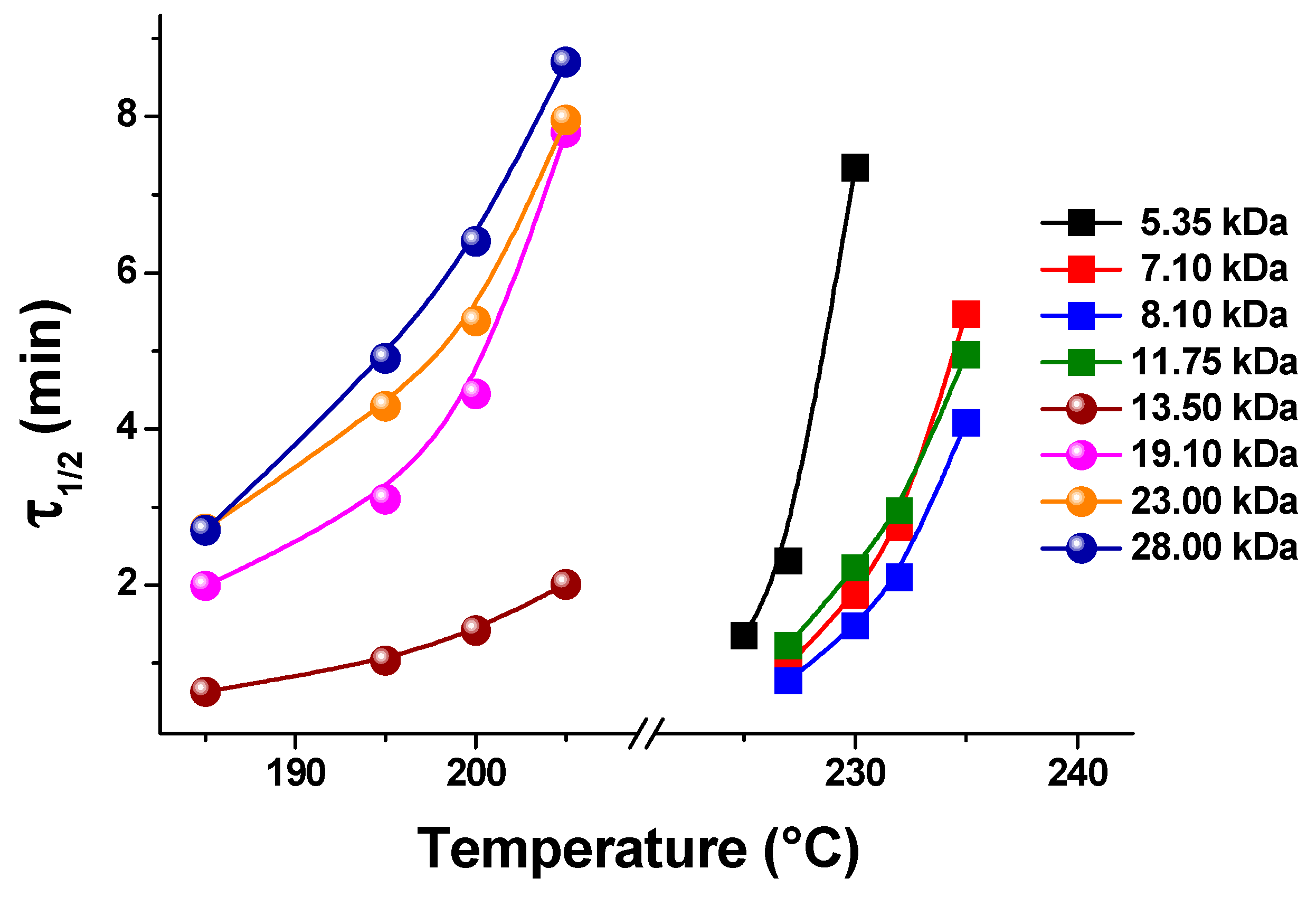
- Zendehzaban, M.; Shamsipur, M. Isothermal crystallization kinetics of poly (ethylene terephthalate) s of different molecular weights. J. Iran. Chem. Soc. 2013, 10, 77–84. [Google Scholar] [CrossRef]
- Godovsky, Y.K.; Slonimsky, G.L.; Garbar, N.M. Effect of molecular weight on the crystallization and morphology of poly (ethylene oxide) fractions. J. Polym. Sci. Part C Polym. Symp. 1972, 38, 1–21. [Google Scholar] [CrossRef]
- Perret, R.; Skoulios, A. Etude de la cristallisation des polymères. VII. Structure lamellaire de quelques poly-ε-caprolactones de différentes masses moléculaires. Makromol. Chem. 1972, 156, 157–172. [Google Scholar] [CrossRef]
- Phillips, P.J.; Tseng, H.T. Influence of pressure on crystallization in poly (ethylene terephthalate). Macromolecules 1989, 22, 1649–1655. [Google Scholar] [CrossRef]
- Fakirov, S.; Seganov, I.; Kurdowa, E. Effect of chain composition of poly(ethylene terephthalate) structure and properties. Makromol. Chem. 1981, 182, 185–197. [Google Scholar] [CrossRef]
- Yu, T.; Bu, H.; Chen, J.; Mei, J.; Hu, J. The effect of units derived from diethylene glycol on crystallization kinetics of poly(ethylene terephthalate). Makromol. Chem. 1986, 187, 2697–2709. [Google Scholar] [CrossRef]
- Patkar, M.; Jabarin, S.A. Effect of diethylene glycol (DEG) on the crystallization behavior of poly (ethylene terephthalate)(PET). J. Appl. Polym. Sci. 1993, 47, 1749–1763. [Google Scholar] [CrossRef]
- Pohl, H.A. The thermal degradation of polyesters. J. Am. Chem. Soc. 1951, 73, 5660–5661. [Google Scholar] [CrossRef]
- Janssen, R.; Ruysschaert, H.; Vroom, R. The determination of the diethylene glycol incorporated in poly(ethylene terephthalate). Makromol. Chem. 1964, 77, 153–158. [Google Scholar] [CrossRef]
- Kirby, J.R.; Baldwin, A.J.; Heidner, R.H. Determination of Diethylene Glycol in Polyethylene Terephthalate. Anal. Chem. 1965, 37, 1306–1309. [Google Scholar] [CrossRef]
- Buxbaum, L.H. Der Abbau von Polyäthylenterephthalat. Angew. Chem. 1968, 80, 225–233. [Google Scholar] [CrossRef]
- Whitehead, B.D. The crystallization and drying of polyethylene terephthalate (PET). Ind. Eng. Chem. Proc. Des. Devel. 1977, 16, 341–346. [Google Scholar] [CrossRef]
- Seganov, I.; Schultz, J.M.; Farikov, S. Effect of diethylene glycol content and annealing temperature on the structure and properties of poly(ethylene terephthalate). J. Appl. Polym. Sci. 1986, 32, 3371–3392. [Google Scholar] [CrossRef]
- Golike, R.C.; Cobbs, W.H., Jr. Crystallization of copolymers of ethylene glycol and diethylene glycol terephthalate. J. Polym. Sci. 1961, 54, 277–285. [Google Scholar] [CrossRef]
- Turi, E.A. Thermal Analysis in Polymer Characterization; Heydin & Son: Philadelphia, PA, USA, 1981. [Google Scholar]
- Di Lorenzo, M.L. Spherulite growth rates in binary polymer blends. Progr. Polym. Sci. 2003, 28, 663–689. [Google Scholar] [CrossRef]
- Farikov, S. Effect of chain composition of PET on small-angle X-ray scattering. Polymer 1980, 21, 373–375. [Google Scholar]
- Lee, J.W.; Lee, S.W.; Lee, B.; Ree, M. Synthesis and non-isothermal crystallization characteristics of poly[(ethylene)-co-(trimethylene terephthalate)]s. Macromol. Chem. Phys. 2001, 202, 3072–3080. [Google Scholar] [CrossRef]
- Kiyotsukuri, T.; Masuda, T.; Tsutsumi, N. Preparation and properties of poly(ethylene terephthalate) copolymers with 2, 2-dialkyl-1, 3-propanediols. Polymer 1994, 35, 1274–1279. [Google Scholar] [CrossRef]
- Kint, D.P.; Martínez de Ilarduya, A.; Sansalvado, A.; Ferrer, J.; Iribarren, J.I.; Muñoz-Guerra, S. Structural characterization and thermal properties of poly (ethylene terephthalate) copolymers containing 2-butyl-2-ethyl-1, 3-propanediol. J. Appl. Polym. Sci. 2002, 86, 1077–1086. [Google Scholar] [CrossRef]
- Chou, R.M.; Chang, C.C.; Yu, T.L.; Tseng, Y.H.; Wu, M.J. Crystallization kinetics of poly (1,4-butylene-co-ethylene terephthalate). Polym. Int. 2001, 50, 213–221. [Google Scholar] [CrossRef]
- Kint, D.P.; Wigström, E.; De Ilarduya, A.M.; Alla, A.; Muñoz-Guerra, S. Poly(ethylene terephthalate) copolyesters derived from (2S, 3S)-2, 3-dimethoxy-1, 4-butanediol. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 3250–3262. [Google Scholar] [CrossRef]
- Yoo, H.Y.; Umemoto, S.; Kikutani, T.; Okui, N. Co-crystallization behaviour and melting-point depression in poly (ethylene terephthalate-co-1, 4-cyclohexylene dimethylene terephthalate) random copolyesters. Polymer 1994, 35, 117–122. [Google Scholar] [CrossRef]
- Yoshie, N.; Inoue, Y.; Yoo, H.Y.; Okui, N. Nuclear magnetic resonance study on isomorphous behaviour in random copolyesters: Poly (ethylene terephthalate-co-1, 4-cyclohexenedimethylene terephthalate). Polymer 1994, 35, 1931–1935. [Google Scholar] [CrossRef]
- Medellín-Rodríguez, F.J.; Phillips, P.J.; Lin, J.S.; Avila-Orta, C.A. Triple melting behavior of poly (ethylene terephthalate-co-1, 4-cyclohexylene dimethylene terephthalate) random copolyesters. J. Polym. Sci. Part B Polym. Phys. 1998, 36, 763–781. [Google Scholar] [CrossRef]
- Sun, Y.M.; Wang, C.S. Preparation and characterization of poly (ethylene-1, 4-cyclohexanedimethylene arylate). Eur. Polym. J. 1999, 35, 1087–1096. [Google Scholar] [CrossRef]
- Kelsey, D.R.; Scardino, B.M.; Grebowicz, J.S.; Chuah, H.H. High impact, amorphous terephthalate copolyesters of rigid 2, 2, 4, 4-tetramethyl-1, 3-cyclobutanediol with flexible diols. Macromolecules 2000, 33, 5810–5818. [Google Scholar] [CrossRef]
- Wu, T.M.; Chang, C.C.; Yu, T.L. Crystallization of poly (ethylene terephthalate–co–isophthalate). J. Polym. Sci. Part B Polymer Physics 2000, 38, 2515–2524. [Google Scholar] [CrossRef]
- Karayannidis, G.P.; Bikiaris, D.N.; Papageorgiou, G.Z.; Pastras, S.V. Synthesis and characterization of poly (ethylene terephthalate-co-isophthalate)s with low content of isophthalate units. J. Appl. Polym. Sci. 2002, 86, 1931–1941. [Google Scholar] [CrossRef]
- Mahendrasingam, A.; Blundell, D.J.; Martin, C.; Urban, V.; Narayanan, T.; Fuller, W. Time resolved WAXS study of the role of mesophase in oriented crystallisation of poly (ethylene terephthalate-co-isophthalate) copolymers. Polymer 2005, 46, 6044–6049. [Google Scholar] [CrossRef]
- Hachiboshi, M.; Fukuda, T.; Kobayashi, S. Studies on solution-grown crystals of polyethylene terephthalate and some copolyesters. I. Hydrolysis of solution-grown crystals and the crystal structure of copolyesters. J. Macromol. Sci. Part B 1969, 3, 525–555. [Google Scholar] [CrossRef]
- Yu, J.; Li, B.; Lee, S.; Ree, M. Relationship between physical properties and chemical structures of poly (ethylene terephthalate-co-ethylene isophthalate). J. Appl. Polym. Sci. 1999, 73, 1191–1195. [Google Scholar] [CrossRef]
- Kint, D.P.; Martínez, A.; Ilarduya, D.; Muñoz-Guerra, S. Poly (ethylene terephthalate) copolymers containing 5-nitroisophthalic units. I. Synthesis and characterization. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 1934–1942. [Google Scholar] [CrossRef]
- Kint, D.P.; De Ilarduya, A.M.; Muñoz-Guerra, S. Poly (ethylene terephthalate) copolymers containing 5-nitroisophthalic units. II. Crystallization studies. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 1553–1564. [Google Scholar] [CrossRef]
- Kint, D.P.; de Ilarduya, A.M.; Muñoz-Guerra, S. Poly (ethylene terephthalate) copolymers containing 5-tert-butyl isophthalic units. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 1994–2004. [Google Scholar] [CrossRef]
- Kint, D.P.; Rudé, E.; Llorens, J.; Muñoz-Guerra, S. Crystallization and properties of poly (ethylene terephthalate) copolymers containing 5-tert-butyl isophthalic units. Polymer 2002, 43, 7529–7537. [Google Scholar] [CrossRef]
- Kint, D.P.; Martínez de Ilarduya, A.; Muñoz-Guerra, S. Sequence analysis of poly (ethylene terephthalate) terpolyesters containing isophthalic and tert-butylisophthalic units by 13C NMR. Macromolecules 2002, 35, 314–317. [Google Scholar] [CrossRef]
- Kint, D.P.; Martínez de Ilarduya, A.; Alla, A.; Muñoz-Guerra, S. Poly (ethylene terephthalate) terpolyesters containing isophthalic and 5-tert-butylisophthalic units. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 124–134. [Google Scholar] [CrossRef]
- Somlai, L.S.; Mathias, L.J.; Schiraldi, D. Investigation of novel PET-5-adamantylisophthalic acid copolymers. Polym. Prepr. (USA) 2000, 41, 56–57. [Google Scholar]
- Lin, Q.; Gariano, N.; Madison, P.H.; Wang, Z.H.; Long, V.K.; Long, T.E.; Armentrout, S. Synthesis and characterization of telechelic and random poly(ethylene terephthalate) (PET) ionomers. Am. Chem. Soc. Polym. Prepr. Div. Polym. Chem. 2002, 43, 496–497. [Google Scholar]
- Kint, D.P.R.; Muñoz-Guerra, S. Modification of the thermal properties and crystallization behaviour of poly (ethylene terephthalate) by copolymerization. Polym. Int. 2003, 52, 321–336. [Google Scholar] [CrossRef]
- Konstantopoulou, M.; Terzopoulou, Z.; Nerantzaki, M.; Tsagkalias, J.; Achilias, D.S.; Bikiaris, D.N.; Exarhopoulos, S.; Papageorgiou, D.G.; Papageorgiou, G.Z. Poly(ethylene furanoate-co-ethylene terephthalate) biobased copolymers: Synthesis, thermal properties and cocrystallization behavior. Eur. Polym. J. 2017, 89, 349–366. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Pang, Y.; Zhu, J.; Zheng, W. Comparing non-isothermal melt and cold crystallization behavior and kinetics of poly (ethylene 2,5-furandicarboxylate-co-ethylene terephthalate) copolyesters. J. Mater. Sci. 2022, 57, 17503–17516. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; Wang, J.; Liu, F.; Jia, Z.; Liu, X.; Zhu, J. 2,5-Furandicarboxylic acid as a sustainable alternative to isophthalic acid for synthesis of amorphous poly(ethylene terephthalate) copolyester with enhanced performance. J. Appl. Polym. Sci. 2019, 136, 47186. [Google Scholar] [CrossRef]
- Kirshanov, K.A.; Gervald, A.Y.; Toms, R.V.; Lobanov, A.N. Obtaining phthalate substituted post-consumer polyethylene terephthalate and its isothermal crystallization. Fine Chem. Techn. 2022, 17, 164–171. [Google Scholar] [CrossRef]
- Descamps, N.; Fernandez, F.; Heijboer, P.; Saint-Loup, R.; Jacquel, N. Isothermal crystallization kinetics of poly (ethylene terephthalate) copolymerized with various amounts of isosorbide. Appl. Sci. 2020, 10, 1046. [Google Scholar] [CrossRef]
- Sun, L.; Huang, L.; Wang, X.; Hu, H.; Guo, J.; Zhu, R.; He, S. Synthesis and structural characterization of sequential structure and crystallization properties for hydrophilic modified polyester. Polymers 2020, 12, 1733. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chu, Y.; Huang, Q.; Jin, X.; Qiu, Z.; Jin, J. Crystallization behavior of copolyesters containing sulfonates. Polymers 2024, 16, 1177. [Google Scholar] [CrossRef] [PubMed]
- Lawton, E.L. Nucleation of crystallization of polyester by catalyst remnants—A review. Polym. Eng. Sci. 1985, 25, 348–354. [Google Scholar] [CrossRef]
- Bier, P.; Binsack, R.; Vernaleken, H.; Rempel, D. Einfluß verzweigter codiole auf das kristallisationsverhalten von aromatischen polyestern. Die Angew. Makromol. Chem. Appl. Macromol. Chem. Phys. 1977, 65, 1–21. [Google Scholar] [CrossRef]
- Weigel, P.; Zimmermann, H. Zum Einfluß von Umesterungs-und Polykondensationskatalysatoren auf das Kristallisationsverhalten von Polyethylenterephthalat. Acta Polym. 1979, 30, 297–301. [Google Scholar] [CrossRef]
- Gümther, B.; Zachmann, H.G. Influence of molar mass and catalysts on the kinetics of crystallization and on the orientation of poly (ethylene terephthalate). Polymer 1983, 24, 1008–1014. [Google Scholar] [CrossRef]
- Jabarin, S.A. Crystallization kinetics of polyethylene terephthalate. I. Isothermal crystallization from the melt. J. Appl. Polym. Sci. 1987, 34, 85–96. [Google Scholar] [CrossRef]
- Jabarin, S.A. Crystallization kinetics of polyethylene terephthalate. II. Dynamic crystallization of PET. J. Appl. Polym. Sci. 1987, 34, 97–102. [Google Scholar] [CrossRef]
- Asano, T.; Dzeick-Pickuth, A.; Zachmann, H.G. Influence of catalysts on the rate of crystallization and on the crystal distortions in poly (ethylene terephthalate). J. Mat. Sci. 1989, 24, 1967–1973. [Google Scholar] [CrossRef]
- Di Fiore, C.; Leone, B.; De Rosa, C.; Guerra, G.; Petraccone, V.; Di Dino, G.; Bianchi, R.; Vosa, R. Influence of the antimony catalyst remnants on the melt crystallization of PET. J. Appl. Polym. Sci. 1993, 48, 1997–2001. [Google Scholar] [CrossRef]
- Flores, I.; Demarteau, J.; Müller, A.J.; Etxeberria, A.; Irusta, L.; Bergman, F.; Koning, C.; Sardon, H. Screening of different organocatalysts for the sustainable synthesis of PET. Eur. Polym. J. 2018, 104, 170–176. [Google Scholar] [CrossRef]
- Shaikh, I.R. Organocatalysis: Key trends in green synthetic chemistry, challenges, scope towards heterogenization, and importance from research and industrial point of view. J. Catal. 2014, 2014, 402860. [Google Scholar] [CrossRef]
- Longo, A.; Dal Poggetto, G.; Malinconico, M.; Laurienzo, P.; Di Maio, E.; Di Lorenzo, M.L. Enhancement of crystallization kinetics of poly (l-lactic acid) by grafting with optically pure branches. Polymer 2021, 227, 123852. [Google Scholar] [CrossRef]
- McKee, M.G.; Unal, S.; Wilkes, G.L.; Long, T.E. Branched polyesters: Recent advances in synthesis and performance. Progr. Polym. Sci. 2005, 30, 507–539. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, Y.; Wang, K.; Xia, Y.; Gao, H.; Luo, K.; Cao, Z.; Qi, J. Effect of the trifunctional chain extender on intrinsic viscosity, crystallization behavior, and mechanical properties of poly (ethylene terephthalate). ACS Omega 2020, 5, 19247–19254. [Google Scholar] [CrossRef] [PubMed]
- Dolatshah, S.; Ahmadi, S.; Ershad-Langroudi, A.; Jashni, H. Rheological/thermal properties of poly(ethylene terephthalate) modified by chain extenders of pyromellitic dianhydride and pentaerythritol. J. Appl. Polym. Sci. 2021, 138, e49917. [Google Scholar] [CrossRef]
- Cusano, I.; Campagnolo, L.; Aurilia, M.; Costanzo, S.; Grizzuti, N. Rheology of recycled PET. Materials 2023, 16, 3358. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Karayannidis, G.P. Thermomechanical analysis of chain-extended PET and PBT. J. Appl. Polym. Sci. 1996, 60, 55–61. [Google Scholar] [CrossRef]
- Hanley, T.; Sutton, D.; Heeley, E.; Moad, G.; Knott, R. A small-angle X-ray scattering study of the effect of chain architecture on the shear-induced crystallization of branched and linear poly(ethylene terephthalate). J. Appl. Cryst. 2007, 40, s599–s604. [Google Scholar] [CrossRef]
- Jayakannan, M.; Ramakrishnan, S. Synthesis and thermal analysis of branched and “kinked” poly (ethylene terephthalate). J. Polym. Sci. Part A Polym. Chem. 1998, 36, 309–317. [Google Scholar] [CrossRef]
- Jayakannan, M.; Ramakrishnan, S. Effect of branching on the crystallization kinetics of poly (ethylene terephthalate). J. Appl. Polym Sci. 1999, 74, 59–66. [Google Scholar] [CrossRef]
- Incarnato, L.; Scarfato, P.; Di Maio, L.; Acierno, D. Structure and rheology of recycled PET modified by reactive extrusion. Polymer 2000, 41, 6825–6831. [Google Scholar] [CrossRef]
- Awaja, F.; Pavel, D. Recycling of PET. Eur. Polym. J. 2005, 41, 1453–1477. [Google Scholar] [CrossRef]
- Rosu, R.F.; Shanks, R.A.; Bhattacharya, S.N. Synthesis and Characterisation of Branched Poly (ethylene terephthalate). Polym. Int. 1997, 42, 267–275. [Google Scholar] [CrossRef]
- Rosu, R.F.; Shanks, R.A.; Bhattacharya, S.N. Shear rheology and thermal properties of linear and branched poly (ethylene terephthalate) blends. Polymer 1999, 40, 5891–5898. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Karayannidis, G.P. Synthesis and characterisation of branched and partially crosslinked poly (ethylene terephthalate). Polym. Int. 2003, 52, 1230–1239. [Google Scholar] [CrossRef]
- Li, X.; Zeng, Z.; Chen, Y.; Xu, Y. Determination of phthalate acid esters plasticizers inplastic by ultrasonic solvent extraction combined with solid-phase microextraction using calix[4]arene fiber. Talanta 2004, 63, 1013–1019. [Google Scholar] [CrossRef]
- Chen, S.; Xie, S.; Guang, S.; Bao, J.; Zhang, X.; Chen, W. Crystallization and thermal behaviors of poly (ethylene terephthalate)/bisphenols complexes through melt post-polycondensation. Polymers 2020, 12, 3053. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Frigione, M. Compatibilization criteria and procedures for binary blends: A review. J. Polym. Eng. 1997, 17, 429–460. [Google Scholar] [CrossRef]
- Stewart, M.E.; Cox, A.J.; Naylor, D.M. Reactive processing of poly (ethylene 2, 6-naphthalene dicarboxylate)/poly (ethylene terephthalate) blends. Polymer 1993, 34, 4060–4067. [Google Scholar] [CrossRef]
- Bang, H.J.; Lee, J.K.; Lee, K.H. Phase behavior and structure development in extruded poly (ethylene terephthalate)/poly (ethylene-2, 6-naphthalate) blend. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 2625–2633. [Google Scholar] [CrossRef]
- Patcheak, T.D.; Jabarin, S.A. Structure and morphology of PET/PEN blends. Polymer 2001, 42, 8975–8985. [Google Scholar] [CrossRef]
- Kampert, W.G.; Sauer, B.B. Temperature modulated DSC studies of melting and recrystallization in poly(ethylene-2,6-naphthalene dicarboxylate) (PEN) and blends with poly(ethylene terephthalate) (PET). Polymer 2001, 42, 8703–8714. [Google Scholar] [CrossRef]
- Denchev, Z.; Duchesne, A.; Stamm, M.; Fakirov, S. Sequence length determination in poly (ethylene terephthalate)–bisphenol-A polycarbonate random copolymers by application of selective degradation. J. Appl. Polym. Sci. 1998, 68, 429–440. [Google Scholar] [CrossRef]
- Montaudo, G.; Puglisi, C.; Samperi, F. Mechanism of exchange in PBT/PC and PET/PC blends. Composition of the copolymer formed in the melt mixing process. Macromolecules 1998, 31, 650–661. [Google Scholar] [CrossRef]
- Pilati, F.; Marianucci, E.; Berti, C. Study of the reactions occurring during melt mixing of poly (ethylene terephthalate) and polycarbonate. J. Appl. Polym. Sci. 1985, 30, 1267–1275. [Google Scholar] [CrossRef]
- Schneggenburger, L.A.; Osenar, P.; Economy, J. Direct evidence for sequence ordering of random semicrystalline copolyesters during high-temperature annealing. Macromolecules 1997, 30, 3754–3758. [Google Scholar] [CrossRef]
- Acar, I.; Durmuş, A.; Özgümüş, S. Nonisothermal crystallization kinetics and morphology of poly(ethylene terephthalate) modified with poly(lactic acid). J. Appl. Polym. Sci. 2007, 106, 4180–4191. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Botta, L.; Morreale, M.; Scaffaro, R. Effect of small amounts of poly (lactic acid) on the recycling of poly (ethylene terephthalate) bottles. Polym. Degrad. Stab. 2012, 97, 21–24. [Google Scholar] [CrossRef]
- Guclu, M.; Alkan Göksu, Y.; Özdemir, B.; Ghanbari, A.; Nofar, M. Thermal stabilization of recycled PET through chain extension and blending with PBT. J. Polym. Envir 2022, 30, 719–727. [Google Scholar] [CrossRef]
- Himmelsbach, A.; Brütting, C.; Akdevelioglu, Y.; Albuquerque, R.Q.; Nofar, M.; Ruckdäschel, H. Non-isothermal crystallization kinetic of recycled PET and its blends with PBT modified with epoxy-based multifunctional chain extender. J. Appl. Polym. Sci. 2024, 141, e55357. [Google Scholar] [CrossRef]
- Ma, G.Q.; Yang, H.; Li, L.; Sun, Z.B.; Miao, X.R.; Bian, F.G.; Xu, J.Z.; Zhong, G.J.; Gao, X.Q.; Li, Z.M. Structure of polyamide 6/poly (ethylene terephthalate) blends under high cooling rate and shear stress and their moisture-sensitive properties. Polymer 2020, 203, 122817. [Google Scholar] [CrossRef]
- Costa, A.R.D.M.; Henrique, M.A.; Luna, C.B.B.; Carvalho, L.H.D.; Almeida, Y.M.B.D. Influence of a multifunctional epoxy additive on the performance of polyamide 6 and PET post-consumed blends during processing. Sustainability 2022, 14, 16658. [Google Scholar] [CrossRef]
- Moghanlou, S.; Khamseh, M.; Razavi Aghjeh, M.; Pourabbas, B. Influence of chain extension and blending on crystallinity and morphological behavior of recycled-PET/ethylene vinyl acetate blends. J. Polym. Environ. 2020, 28, 1526–1533. [Google Scholar] [CrossRef]
- Dobrzyńska-Mizera, M.; Dutkiewicz, M.; Sterzyński, T.; Di Lorenzo, M.L. Polypropylene-based composites containing sorbitol-based nucleating agent and siloxane-silsesquioxane resin. J. Appl. Polym. Sci. 2016, 133, 43476. [Google Scholar] [CrossRef]
- Dobrzyńska-Mizera, M.; Knitter, M.; Woźniak-Braszak, A.; Baranowski, M.; Sterzyński, T.; Di Lorenzo, M.L. Poly (l-lactic acid)/pine wood bio-based composites. Materials 2020, 13, 3776. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Rhoades, A.M.; Androsch, R. Polymer Crystallization. In Thermal Analysis of Polymeric Materials; Pielichowski, K., Pielichowska, K., Eds.; Wiley: Hoboken, NJ, USA, 2022; pp. 263–302. [Google Scholar]
- Gauthier, C.; Chailan, J.-F.; Chauchard, J. Utilisation de l’analyse viscoélastique dynamique à l’étude de la cristallisation isotherme du poly(téréphtalate d’ethylène) amorphe. Application à des composites unidirectionnels avec fibres de verre. Makromol. Chem. 1992, 193, 1001–1009. [Google Scholar] [CrossRef]
- Lin, G.; Li, D.; Liu, M.; Zhang, X.; Zheng, Y. Rheological behavior, mechanical properties, and nonisothermal crystallization behavior of poly (ethylene terephthalate)/modified carbon fiber composites. High Perf. Polym. 2019, 31, 733–740. [Google Scholar] [CrossRef]
- Owen, M.M.; Achukwu, E.O.; Shuib, S.B.; Ahmad, Z.R.; Abdullah, A.H.; Ishiaku, U.S. Effects of high-temperature optimization and resin coating treatment on the mechanical, thermal, and morphological properties of natural kenaf fiber-filled engineering plastic composites. Polym. Compos. 2023, 44, 2512–2529. [Google Scholar] [CrossRef]
- Yadav, Y.K.; Dixit, G.; Dixit, S. Natural fiber reinforced rPET/polyester composites: A review on development, mechanical performance, and sustainable management. Polym.-Plast. Technol. Mater. 2023, 62, 1823–1843. [Google Scholar] [CrossRef]
- Kostenko, M.; Stetsyshyn, Y.; Harhay, K.; Melnyk, Y.; Donchak, V.; Gubriy, Z.; Kracalik, M. Impact of the functionalized clay nanofillers on the properties of the recycled polyethylene terephthalate nanocomposites. J. Appl. Polym. Sci. 2024, 141, e55543. [Google Scholar] [CrossRef]
- Han, Z.; Wang, Y.; Wang, J.; Wang, S.; Zhuang, H.; Liu, J.; Huang, L.; Wang, Y.; Wang, W.; Belfiore, L.A.; et al. Preparation of hybrid nanoparticle nucleating agents and their effects on the crystallization behavior of poly(ethylene terephthalate). Materials 2018, 11, 587. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Zhuo, W.; Zhou, J.; Huang, R.; Jiang, G. Crystallization behavior and enhanced toughness of poly(ethylene terephthalate) composite with noncovalent modified graphene functionalized by pyrene-terminated molecules: A comparative study. J. Mater. Sci. 2017, 52, 10567–10580. [Google Scholar] [CrossRef]
- Zhao, L.; Yin, Y.; Xiao, W.; Li, H.; Feng, H.; Wang, D.; Qu, C. Rapid crystallization and fluorescence of poly (ethylene terephthalate) using graphene quantum dots as nucleating agents. Polymers 2023, 15, 3506. [Google Scholar] [CrossRef] [PubMed]
- Heeley, E.L.; Hughes, D.J.; Crabb, E.M.; Bowen, J.; Bikondoa, O.; Mayoral, B.; Leung, S.; McNally, T. The formation of a nanohybrid shish-kebab (NHSK) structure in melt-processed composites of poly(ethylene terephthalate) (PET) and multi-walled carbon nanotubes (MWCNTs). Polymer 2017, 117, 208–219. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, D.; Wu, M.; Ruan, S.; Castro, J.M.; Lee, L.J.; Chen, F. Impacts of carbonaceous particulates on extrudate semicrystalline polyethylene terephthalate foams: Nonisothermal crystallization, rheology, and infrared attenuation studies. Ind. Eng. Chem. Res. 2020, 59, 15586–15597. [Google Scholar] [CrossRef]
- Cruz-Delgado, V.J.; Ávila-Orta, C.A.; Espinoza-Martínez, A.B.; Mata-Padilla, J.M.; Solis-Rosales, S.G.; Jalbout, A.F.; Medellín-Rodríguez, F.J.; Hsiao, B.S. Carbon nanotube surface-induced crystallization of polyethylene terephthalate (PET). Polymer 2014, 55, 642–650. [Google Scholar] [CrossRef]
- Qiao, L.; Yan, X.; Tan, H.; Dong, S.; Ju, G.; Shen, H.; Ren, Z. Mechanical properties, melting and crystallization behaviors, and morphology of carbon nanotubes/continuous carbon fiber reinforced polyethylene terephthalate composites. Polymers 2022, 14, 2892. [Google Scholar] [CrossRef]
- Sirin, H.; Turan, D.; Ozkoc, G.; Gurdag, S. POSS reinforced PET based composite fibers: “Effect of POSS type and loading level”. Compos. Part B Eng. 2013, 53, 395–403. [Google Scholar] [CrossRef]
- Lee, A.S.; Jeon, H.; Choi, S.-S.; Park, J.; Hwang, S.Y.; Jegal, J.; Oh, D.X.; Kim, B.C.; Hwang, S.S. Crystallization derivation of amine functionalized T12 polyhedral oligomeric silsesquioxane-conjugated poly(ethylene terephthalate). Compos. Sci. Technol. 2017, 146, 42–48. [Google Scholar] [CrossRef]
- Riba-Moliner, M.; Mijas, G.; Roig, D.; Cayuela, D. Obtaining of a PET/SiC hybrid multifilament: Non-isothermal crystallization studies. J. Text. Inst. 2021, 112, 1779–1787. [Google Scholar] [CrossRef]
- Samieifakhr, M.; Shojaei, A. Improved crystallization behavior and enhanced impact strength of melt processed poly (ethylene terephthalate)/UiO-66 nanocomposites. Polymer 2024, 290, 126593. [Google Scholar] [CrossRef]
- Otero, P.; Saha, S.K.; Moane, S.; Barron, J.; Clancy, G.; Murray, P. Improved method for rapid detection of phthalates in bottled water by gas chromatography-mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 997, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, Z.-W.; Lin, Q.-B.; Hu, C.-Y. Study of the Migration of Stabilizer and Plasticizer from Polyethylene Terephthalate into Food Simulants. J. Chromatogr. Sci. 2016, 54, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Zekriardehani, S.; Joshi, A.S.; Jabarin, S.A.; Gidley, D.W.; Coleman, M.R. Effect of Dimethyl Terephthalate and Dimethyl Isophthalate on the Free Volume and Barrier Properties of Poly(ethylene terephthalate) (PET): Amorphous PET. Macromolecules 2018, 51, 456–467. [Google Scholar] [CrossRef]
- Salazar-Beltrán, D.; Hinojosa-Reyes, L.; Palomino-Cabello, C.; Turnes-Palomino, G.; Hernández-Ramírez, A.; Guzmán-Mar, J.L. Determination of phthalate acid esters plasticizers in polyethylene terephthalate bottles and its correlation with some physicochemical properties. Polym. Test. 2018, 68, 87–94. [Google Scholar] [CrossRef]
- Rahman, M.; Brazel, C. The plasticizer market: An assessment of traditional plasticizers and research trends to meet new challenges. Progr. Polym. Sci. 2004, 29, 1223–1248. [Google Scholar] [CrossRef]
- Bodaghi, A. An overview on the recent developments in reactive plasticizers in polymers. Polym. Adv. Technol. 2020, 31, 355–367. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly (lactic acid) crystallization. Progr. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Gowd, E.B.; Ramesh, C. Effect of poly(ethylene glycol) on the solid-state polymerization of poly(ethylene terephthalate). Polym. Int. 2006, 55, 340–345. [Google Scholar] [CrossRef]
- Piccarolo, S.; Poulose, A.M.; Luzio, A. Influence of Plasticizers Suggests Role of Topology in Polymer Solidification at High Cooling Rates. J. Appl. Polym. Sci. 2012, 125, 4233–4242. [Google Scholar] [CrossRef]
- Akato, K.M.; Nguyen, N.A.; Bonnesen, P.V.; Harper, D.P.; Naskar, A.K. Recycling waste polyester via modification with a renewable fatty acid for enhanced processability. ACS Omega 2018, 3, 10709–10715. [Google Scholar] [CrossRef]
- Zimmerman, H.; Kim, N.T. Investigations on thermal and hydrolytic degradation of poly(ethylene terephthalate). Polym. Eng. Sci. 1980, 20, 680–683. [Google Scholar] [CrossRef]
- Allen, G.; McAinsh, J.; Jeffs, G. Relaxation phenomena in some aromatic polymers: Effect of water content on the low temperature relaxation. Polymer 1971, 12, 85–100. [Google Scholar] [CrossRef]
- Dargent, E.; Denis, G.; Caron, C.; Saiter, J.; Grenet, J. Effect of water molecules on crystallization during unixial drawing of poly (ethylene terephthalate) films. J. Appl. Polym. Sci. 2000, 77, 1056–1066. [Google Scholar] [CrossRef]
- Langevin, D.; Grenet, J.; Saiter, J. Moisture sorption in pet influence on the thermokinetic parameters. Eur. Polym. J. 1994, 30, 339–345. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, Q.; Ye, K.; Zhang, Q.; Chen, W.; Meng, L.; Chen, X.; Wang, D.; Li, L. The effect of water absorption on stretch-induced crystallization of poly (ethylene terephthalate): An in-situ synchrotron radiation wide angle X-ray scattering study. Polymer 2019, 162, 91–99. [Google Scholar] [CrossRef]
- Jabarin, S.A. Crystallization kinetics of poly (ethylene terephthalate). III. Effect of moisture on the crystallization behavior of PET from the glassy state. J. Appl. Polym. Sci. 1987, 34, 103–108. [Google Scholar] [CrossRef]
- Toda, T.; Yoshida, H.; Fukunishi, K. Structure and molecular motion changes in poly (ethylene terephthalate) induced by annealing under dry and wet conditions. Polymer 1995, 36, 699–706. [Google Scholar] [CrossRef]
- Goto, S.; Kitagawa, T.; Miyatake, M.; Mori, T.; Kamijo, T. Polarizing Film, Optical Film Laminate Comprising Polarizing Film, and Stretched Laminate for Manufacturing the Same. U.S. Patent No. US-8320042-B2, 8 March 2012. [Google Scholar]
- Mizoguchi, K.; Hirose, T.; Naito, Y.; Kamiya, Y. CO2-induced crystallization of poly (ethylene terephthalate). Polymer 1987, 28, 1298–1302. [Google Scholar] [CrossRef]
- Lambert, S.M.; Paulaitis, M.E. Crystallization of poly (ethylene terephthalate) induced by carbon dioxide sorption at elevated pressures. J. Supercrit Fluids 1991, 4, 15–23. [Google Scholar] [CrossRef]
- Zhong, Z.; Zheng, S.; Mi, Y. High-pressure DSC study of thermal transitions of a poly(ethylene terephthalate)/carbon dioxide system. Polymer 1999, 40, 3829–3834. [Google Scholar] [CrossRef]
- Mensitieri, G.; Del Nobile, M.A.; Apicella, A.; Guerra, G.; Al Ghatta, H. Low temperature melting behavior of CO2 crystallized modified PETs. Polym. Eng. Sci. 1995, 35, 506–512. [Google Scholar] [CrossRef]
- Takada, M.; Ohshima, M. Effect of CO2 on crystallization kinetics of poly (ethylene terephthalate). Polym. Eng, Sci. 2003, 43, 479–489. [Google Scholar] [CrossRef]
- Shieh, Y.T.; Lin, Y.S. Glass transition and cold crystallization in carbon dioxide treated poly (ethylene terephthalate). J. Appl. Polym. Sci. 2009, 113, 3345–3353. [Google Scholar] [CrossRef]
- Baseri, S.; Karimi, M.; Morshed, M. Study of structural changes and mesomorphic transitions of oriented poly (ethylene therephthalate) fibers in supercritical CO2. Eur. Polym. J. 2012, 48, 811–820. [Google Scholar] [CrossRef]
- Aizawa, T. Effect of crystallinity on Young’s modulus of porous materials composed of polyethylene terephthalate fibers in the presence of carbon dioxide. Polymers 2022, 14, 3724. [Google Scholar] [CrossRef]
- Liang, M.T.; Wang, C.M. Production of engineering plastics foams by supercritical CO2. Ind. Eng. Chem. Res. 2000, 39, 4622–4626. [Google Scholar] [CrossRef]
- Yao, S.; Chen, Y.; Ling, Y.; Hu, D.; Xi, Z.; Zhao, L. Analysis of bubble growth in supercritical CO2 extrusion foaming polyethylene terephthalate process based on dynamic flow simulation. Polymers 2021, 13, 2799. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y. Preparation of renewable porous carbons for CO2 capture—A review. Fuel Proc. Techn. 2022, 236, 107437. [Google Scholar] [CrossRef]
- Chen, Y.; Yao, S.; Ling, Y.; Zhong, W.; Hu, D.; Zhao, L. Microcellular PETs with high expansion ratio produced by supercritical CO2 molding compression foaming process and their mechanical properties. Adv. Eng. Mater. 2022, 24, 2101124. [Google Scholar] [CrossRef]
- Longo, A.; Di Maio, E.; Du, F.; Androsch, R.; Di Lorenzo, M.L. CO2-diffusion controlled mesophase formation in poly (l-lactic acid) and its effect on cold-crystallization. Polymer 2023, 285, 126380. [Google Scholar] [CrossRef]
- Zia, Q.; Mileva, D.; Androsch, R. Rigid amorphous fraction in isotactic polypropylene. Macromolecules 2008, 41, 8095–8102. [Google Scholar] [CrossRef]
- Lin, J.; Shenogin, S.; Nazarenko, S. Oxygen solubility and specific volume of rigid amorphous fraction in semicrystalline poly (ethylene terephthalate). Polymer 2002, 43, 4733–4743. [Google Scholar] [CrossRef]
- Badia, J.D.; Strömberg, E.; Karlsson, S.; Ribes-Greus, A. The role of crystalline, mobile amorphous and rigid amorphous fractions in the performance of recycled poly (ethylene terephthalate)(PET). Polym. Degrad. Stab. 2012, 97, 98–107. [Google Scholar] [CrossRef]
- Righetti, M.C.; Tombari, E.; Angiuli, M.; Di Lorenzo, M.L. Enthalpy-based determination of crystalline, mobile amorphous and rigid amorphous fractions in semicrystalline polymers: Poly(ethylene terephthalate). Thermochim. Acta 2007, 462, 15–24. [Google Scholar] [CrossRef]
- Righetti, M.C.; Di Lorenzo, M.L. Vitrification and devitrification of the rigid amorphous fraction in poly(ethylene terephthalate). e-Polymers 2009, 9, 053. [Google Scholar] [CrossRef]
- Chen, H.; Cebe, P. Vitrification and devitrification of rigid amorphous fraction of PET during quasi-isothermal cooling and heating. Macromolecules 2009, 42, 288–292. [Google Scholar] [CrossRef]
- Pingping, Z.; Dezhu, M. Double cold crystallization peaks of poly(ethylene terephthalate)—1. Samples isothermally crystallized at low temperature. Eur. Polym. J. 1997, 33, 1817–1818. [Google Scholar]
- Pingping, Z.; Dezhu, M. Study on the double cold crystallization peaks of poly(ethylene terephthalate) (PET): 2. Samples isothermally crystallized at high temperature. Eur. Polym. J. 1999, 35, 739–742. [Google Scholar] [CrossRef]
- Pyda, M.; Nowak-Pyda, E.; Heeg, J.; Huth, H.; Minakov, A.A.; Di Lorenzo, M.L.; Schick, C.; Wunderlich, B. Melting and crystallization of poly(butylene terephthalate) by temperature-modulated and superfast calorimetry. J. Polym. Sci. Polym. Phys. 2006, 44, 1364–1377. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Gazzano, M.; Righetti, M.C. The role of the rigid amorphous fraction on cold crystallization of poly(3-hydroxybutyrate). Macromolecules 2012, 45, 5684–5691. [Google Scholar] [CrossRef]
- Androsch, R.; Wunderlich, B. The link between rigid amorphous fraction and crystal perfection in cold-crystallized poly(ethylene terephthalate). Polymer 2005, 46, 12556–125661. [Google Scholar] [CrossRef]
- Verma, R.; Marand, H.; Hsiao, B. Morphological changes during secondary crystallization and subsequent melting in poly (ether ether ketone) as studied by real time small angle X-ray scattering. Macromolecules 1996, 29, 7767–7775. [Google Scholar] [CrossRef]
- Chen, Z.; Hay, J.N.; Jenkins, M.J. The effect of secondary crystallization on crystallization kinetics–Polyethylene terephthalate revisited. Eur. Polym. J. 2016, 81, 216–223. [Google Scholar] [CrossRef]
- Marand, H.; Alizadeh, A.; Farmer, R.; Desai, R.; Velikov, V. Influence of Structural and Topological Constraints on the Crystallization and Melting Behavior of Polymers. 2. Poly(arylene ether ether ketone). Macromolecules 2000, 33, 3392–3403. [Google Scholar] [CrossRef]
- Lotz, B. Fold Surfaces of Polymer Crystals Are Nucleation Sites: The Link between Polymer Decoration, Secondary Crystallization, and the Rigid Amorphous Fraction (RAF). Macromolecules 2023, 56, 4135–4152. [Google Scholar] [CrossRef]
- Hay, J.N. Secondary crystallization kinetics. Polym. Cryst. 2018, 1, e10007. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, W.; Chen, Y.; Zhang, H.; Ye, B.; Yu, W.; Li, L. Structural evolutions of the amorphous fraction of polyethylene terephthalate during the secondary crystallization. Polymer 2022, 253, 124987. [Google Scholar] [CrossRef]
- Holdsworth, P.J.; Turner-Jones, A. The melting behaviour of heat crystallized poly (ethylene terephthalate). Polymer 1971, 12, 195–208. [Google Scholar] [CrossRef]
- Alfonso, G.C.; Pedemonte, E.; Ponzetti, L. Mechanism of densification and crystal perfection of poly (ethylene terephthalate). Polymer 1979, 20, 104–112. [Google Scholar] [CrossRef]
- Groeninckx, G.; Reynaers, H.; Berghmans, H.; Smets, G. Morphology and melting behavior of semicrystalline poly(ethylene terephthalate). I. Isothermally crystallized PET. J. Polym. Sci. Polym. Phys. Ed. 1980, 18, 1311–1324. [Google Scholar] [CrossRef]
- Stribeck, N. SAXS data analysis of a lamellar two-phase system. Layer statistics and compansion. Colloid Polym. Sci. 1993, 271, 1007–1023. [Google Scholar] [CrossRef]
- Imai, M.; Kaji, K.; Kanaya, T.; Sakai, Y. Ordering process in the induction period of crystallization of poly (ethylene terephthalate). Phys. Rev. B 1995, 52, 12696. [Google Scholar] [CrossRef] [PubMed]
- Medellin-Rodriguez, F.J.; Phillips, P.J.; Lin, J.S. Melting behavior of high-temperature polymers. Macromolecules 1996, 29, 7491–7501. [Google Scholar] [CrossRef]
- Wang, Z.G.; Hsiao, B.S.; Sauer, B.B.; Kampert, W.G. The nature of secondary crystallization in poly (ethylene terephthalate). Polymer 1999, 40, 4615–4627. [Google Scholar] [CrossRef]
- Ivanov, D.A.; Amalou, Z.; Magonov, S.N. Real-time evolution of the lamellar organization of poly(ethylene terephthalate) during crystallization from the melt: High-temperature atomic force microscopy study. Macromolecules 2001, 34, 8944–8952. [Google Scholar] [CrossRef]
- Lee, B.; Shin, T.J.; Lee, S.W.; Yoon, J.; Kim, J.; Youn, H.S.; Ree, M. Time-resolved X-ray scattering and calorimetric studies on the crystallization behaviors of poly (ethylene terephthalate)(PET) and its copolymers containing isophthalate units. Polymer 2003, 44, 2509–2518. [Google Scholar] [CrossRef]
- Schick, C.; Dobbertin, J.; Potter, M.; Dehne, H.; Hensel, A.; Wurm, A.; Ghoneim, A.M.; Weyer, S. Separation of components of different molecular mobility by calorimetry, dynamic mechanical and dielectric spectroscopy. J. Therm. Anal. 1997, 49, 499–511. [Google Scholar] [CrossRef]
- Righetti, M.C.; Di Lorenzo, M.L.; Tombari, E.; Angiuli, M. The low-temperature endotherm in poly (ethylene terephthalate): Partial melting and rigid amorphous fraction mobilization. J. Phys. Chem. B 2008, 112, 4233–4241. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, M.L.; Righetti, M.C.; Cocca, M.; Wunderlich, B. Coupling between crystal melting and rigid amorphous fraction mobilization in poly (ethylene terephthalate). Macromolecules 2010, 43, 7689–7694. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Righetti, M.C.; Wunderlich, B. Influence of crystal polymorphism on the three-phase structure and on the thermal properties of isotactic poly (1-butene). Macromolecules 2009, 42, 9312–9320. [Google Scholar] [CrossRef]
- Wang, W.; Fenni, S.E.; Ma, Z.; Righetti, M.C.; Cangialosi, D.; Di Lorenzo, M.L.; Cavallo, D. Glass transition and aging of the rigid amorphous fraction in polymorphic poly (butene-1). Polymer 2021, 226, 123830. [Google Scholar] [CrossRef]
- Monnier, X.; Cavallo, D.; Righetti, M.C.; Di Lorenzo, M.L.; Marina, S.; Martin, J.; Cangialosi, D. Physical aging and glass transition of the rigid amorphous fraction in poly(l-lactic acid). Macromolecules 2020, 53, 8741–8750. [Google Scholar] [CrossRef]
- Righetti, M.C.; Tombari, E. Crystalline, mobile amorphous and rigid amorphous fractions in poly(l-lactic acid) by TMDSC. Thermochim. Acta 2011, 522, 118–127. [Google Scholar] [CrossRef]
- Righetti, M.C.; Tombari, E.; Di Lorenzo, M.L. The role of the crystallization temperature on the nanophase structure evolution of poly[(R)-3-hydroxybutyrate]. J. Phys. Chem. B 2013, 117, 12303–12311. [Google Scholar] [CrossRef]
- Righetti, M.C.; Laus, M.; Di Lorenzo, M.L. Temperature dependence of the rigid amorphous fraction in poly(ethylene terephthalate). Eur. Polym. J. 2014, 58, 60–68. [Google Scholar] [CrossRef]
- Righetti, M.C.; Laus, M.; Di Lorenzo, M.L. Rigid amorphous fraction and melting behavior of poly (ethylene terephthalate). Colloid Polym. Sci. 2014, 292, 1365–1374. [Google Scholar] [CrossRef]
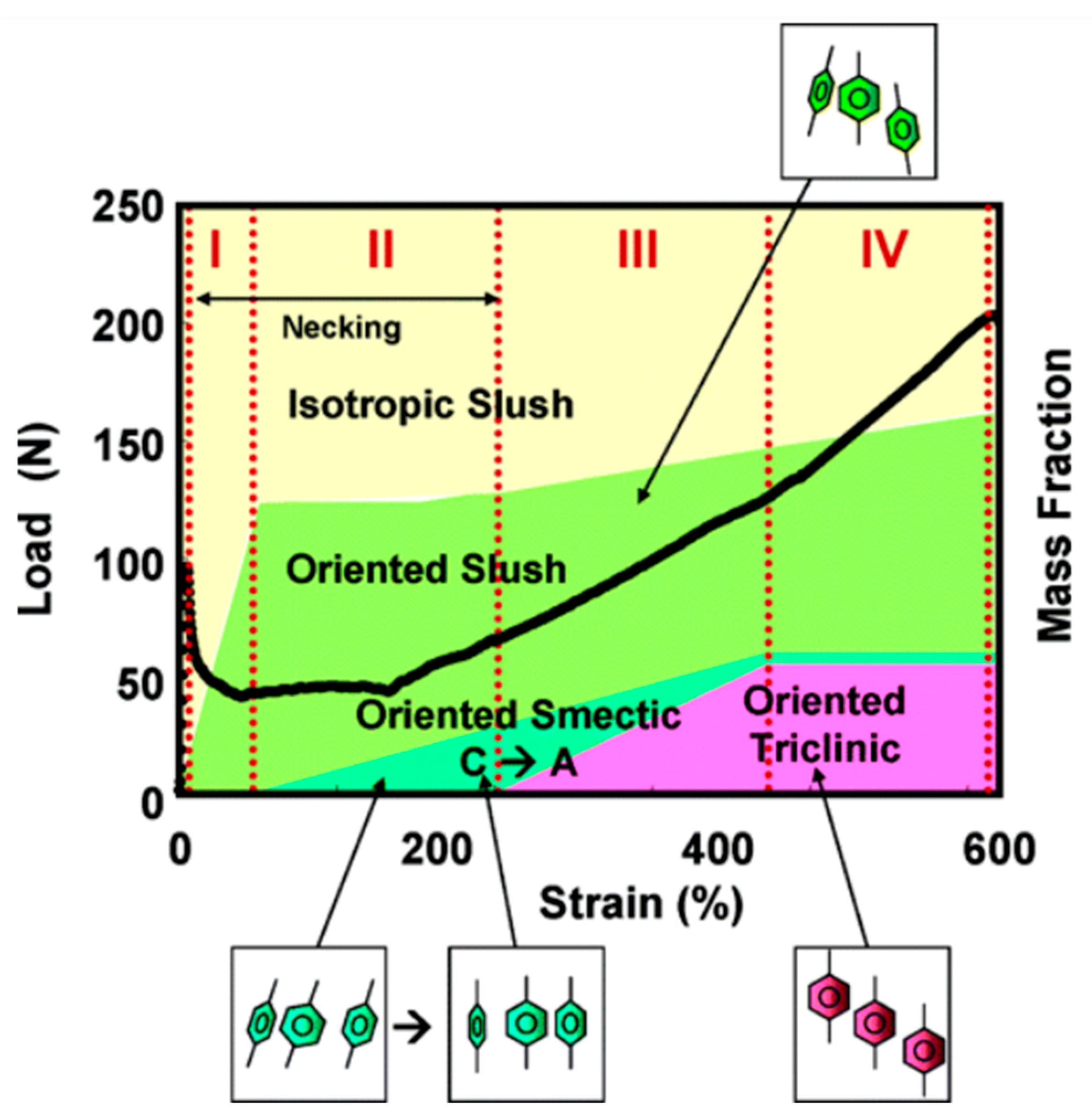
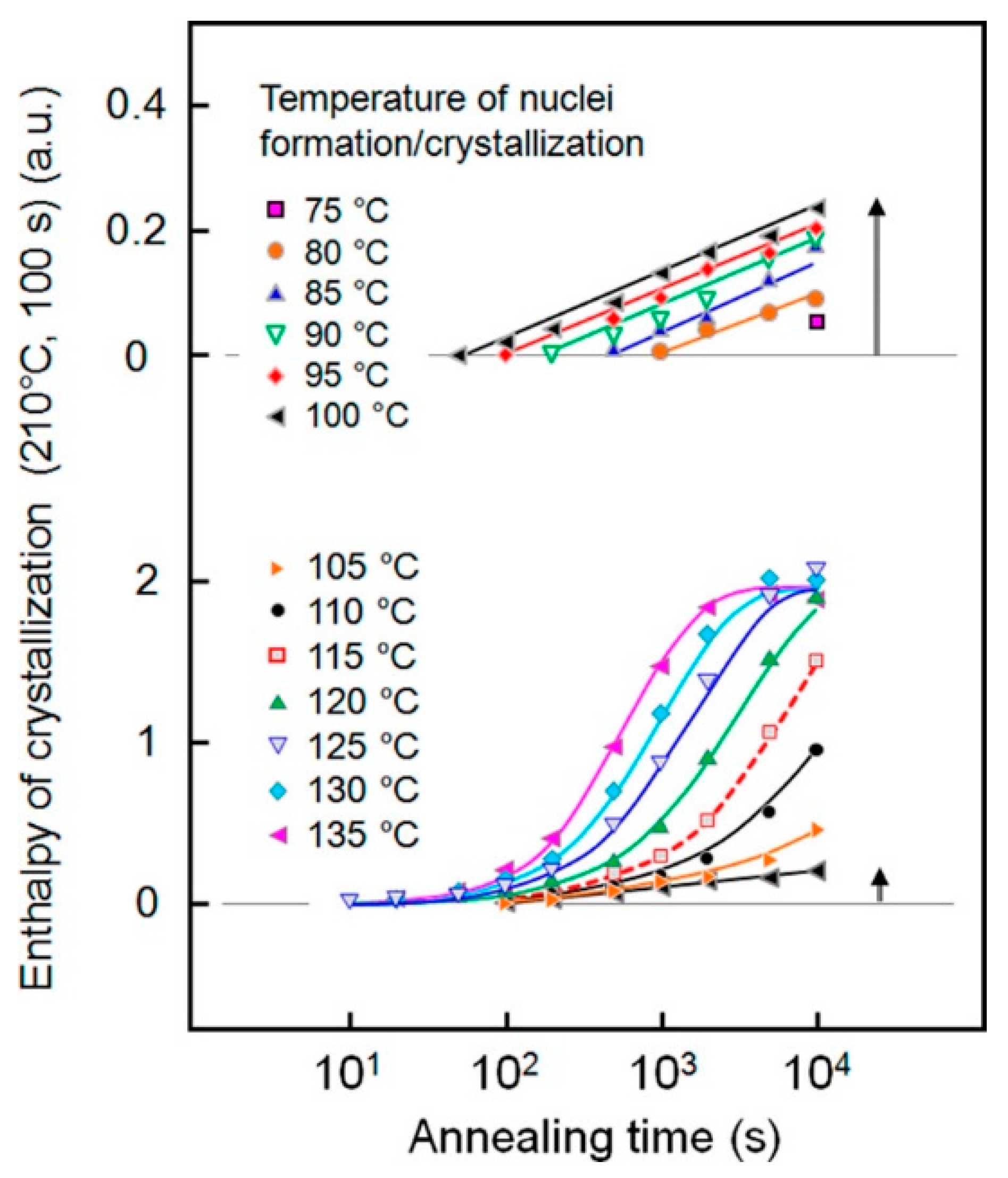
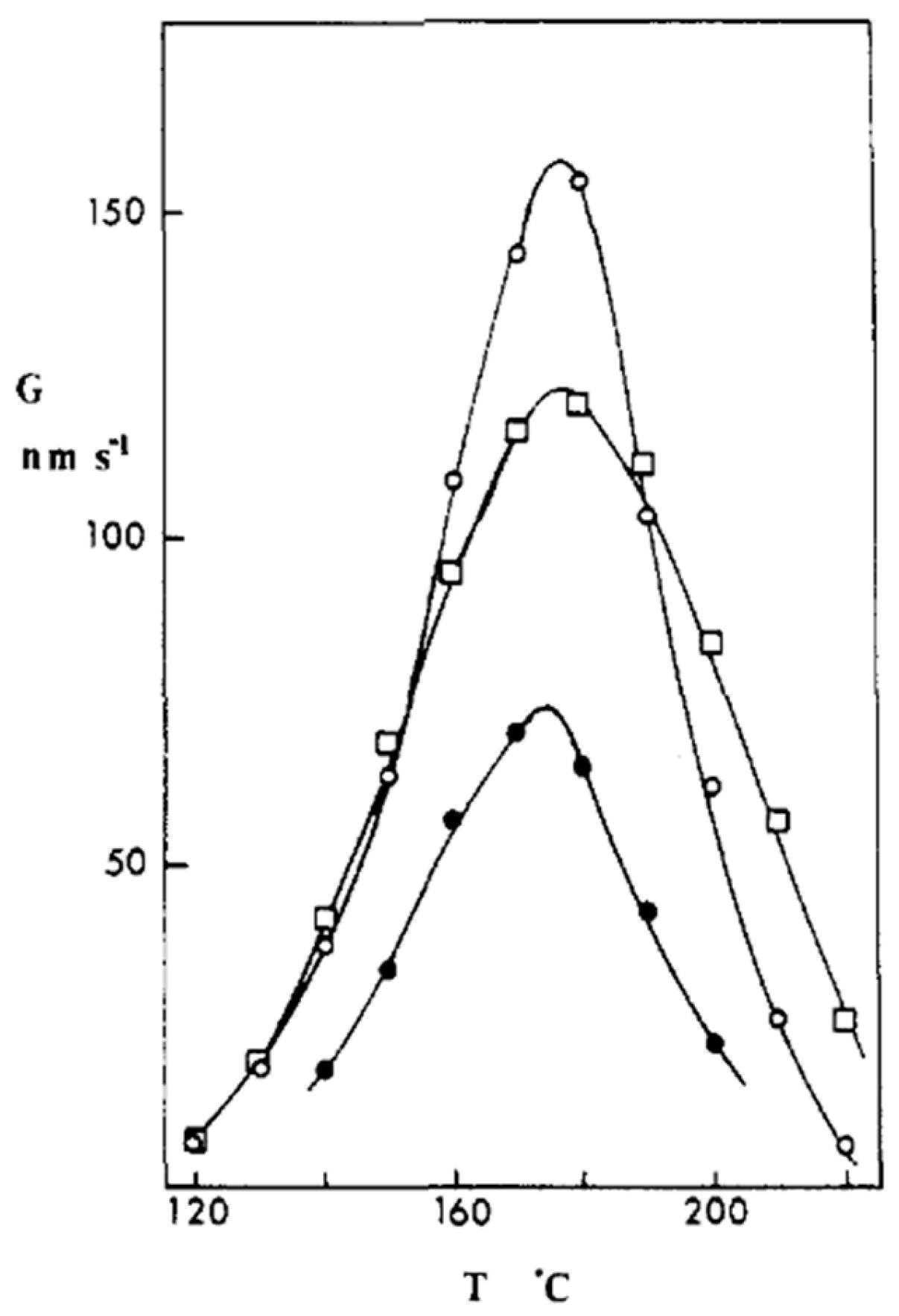
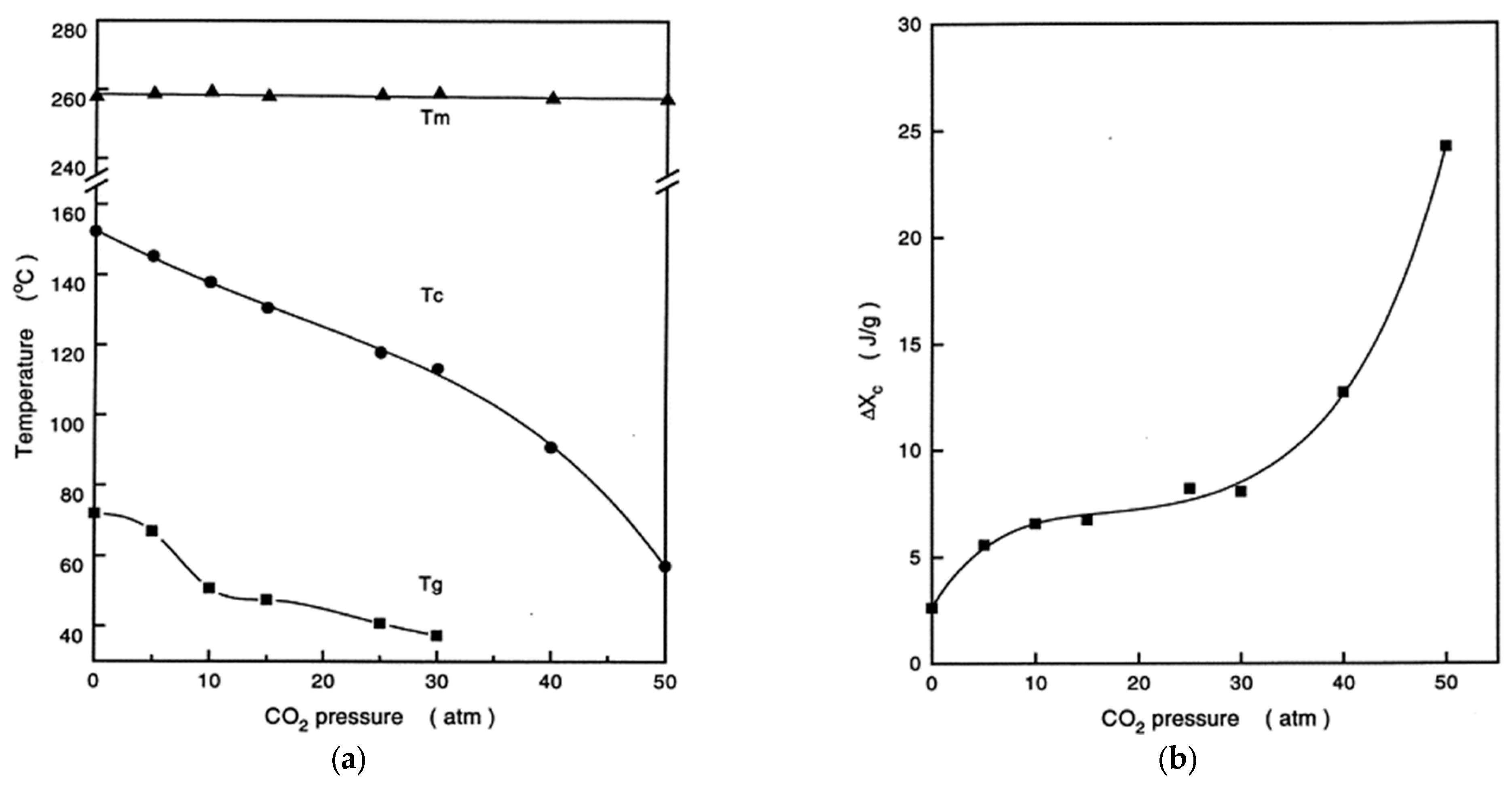

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Lorenzo, M.L. Crystallization of Poly(ethylene terephthalate): A Review. Polymers 2024, 16, 1975. https://doi.org/10.3390/polym16141975
Di Lorenzo ML. Crystallization of Poly(ethylene terephthalate): A Review. Polymers. 2024; 16(14):1975. https://doi.org/10.3390/polym16141975
Chicago/Turabian StyleDi Lorenzo, Maria Laura. 2024. "Crystallization of Poly(ethylene terephthalate): A Review" Polymers 16, no. 14: 1975. https://doi.org/10.3390/polym16141975
APA StyleDi Lorenzo, M. L. (2024). Crystallization of Poly(ethylene terephthalate): A Review. Polymers, 16(14), 1975. https://doi.org/10.3390/polym16141975