Preparation and Properties of Multi-Responsive Liquid Crystalline Poly(urethane-acrylate)s and Its Composite Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
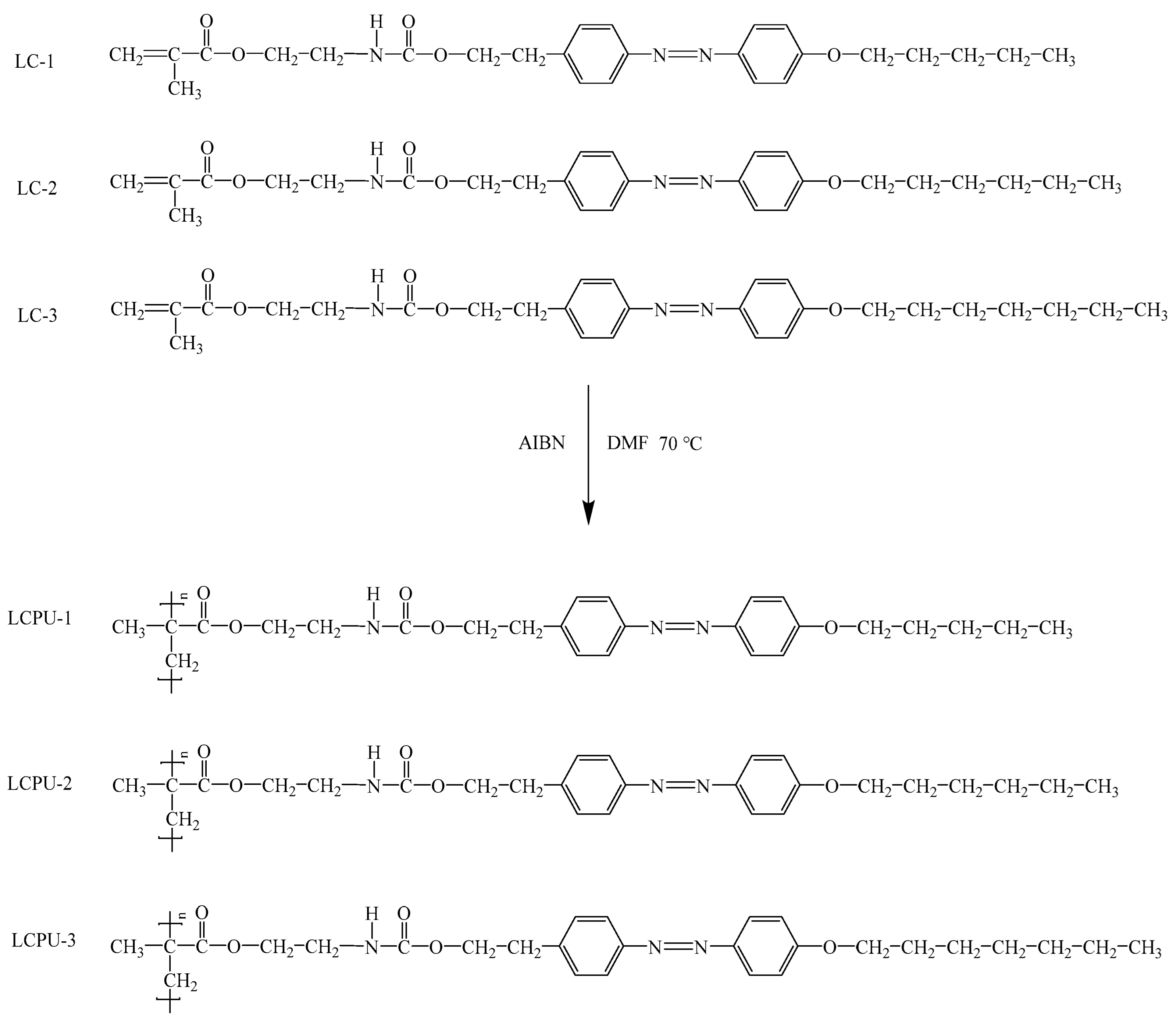
2.2. Synthesis of Poly(urethane-acrylate)s Containing Azophenyl Groups in Side Chains
2.3. Synthesis of Shape Memory Polyurethane (SMPU)
2.4. Preparation of SMPU Fiber Membranes
2.5. Preparation of Composite Fiber Membranes
2.6. Characterization
3. Results and Discussion
3.1. Structural Analysis of Poly(urethane-acrylate)s
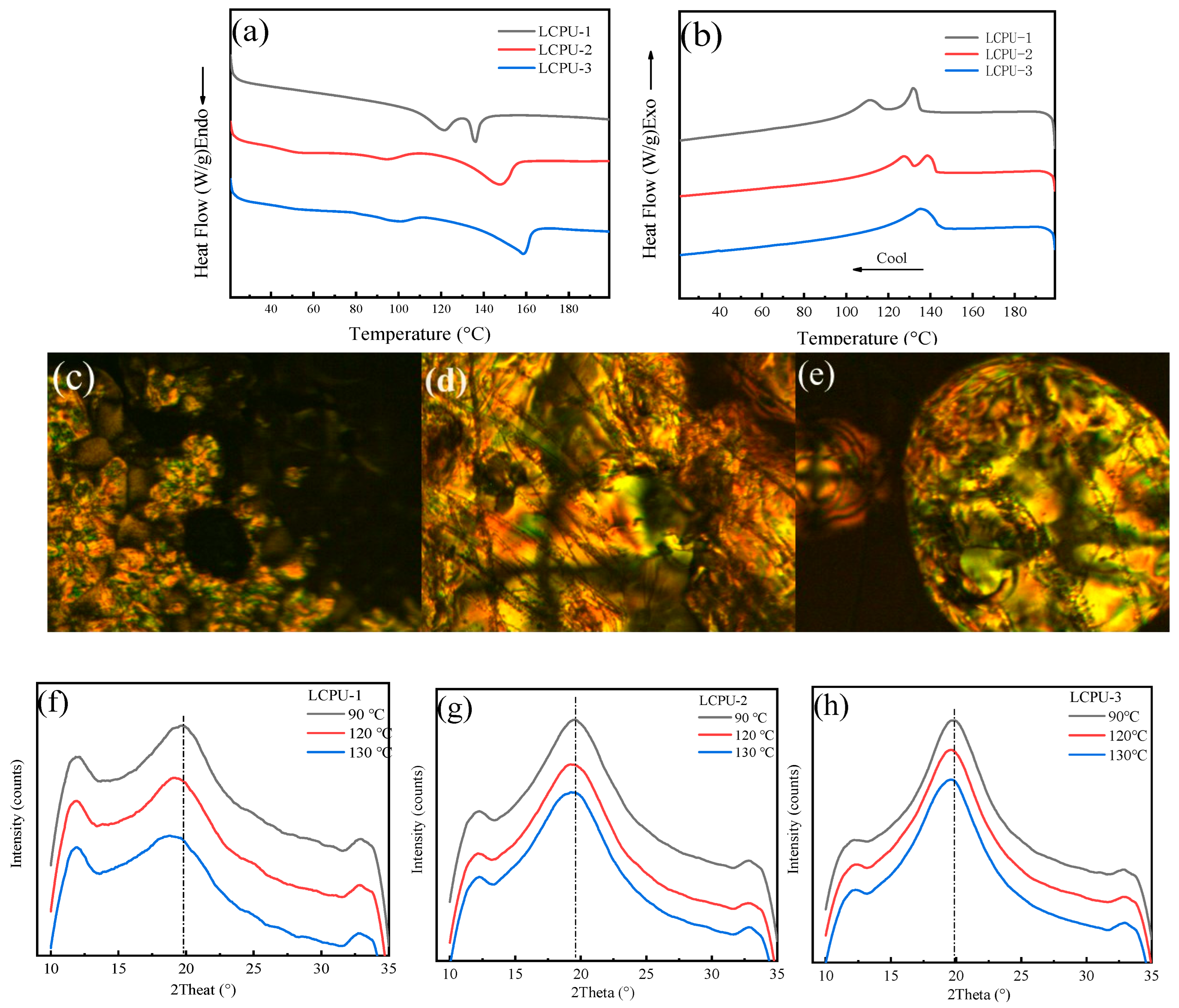
3.2. Liquid Crystal Properties of Poly(urethane-acrylate)s
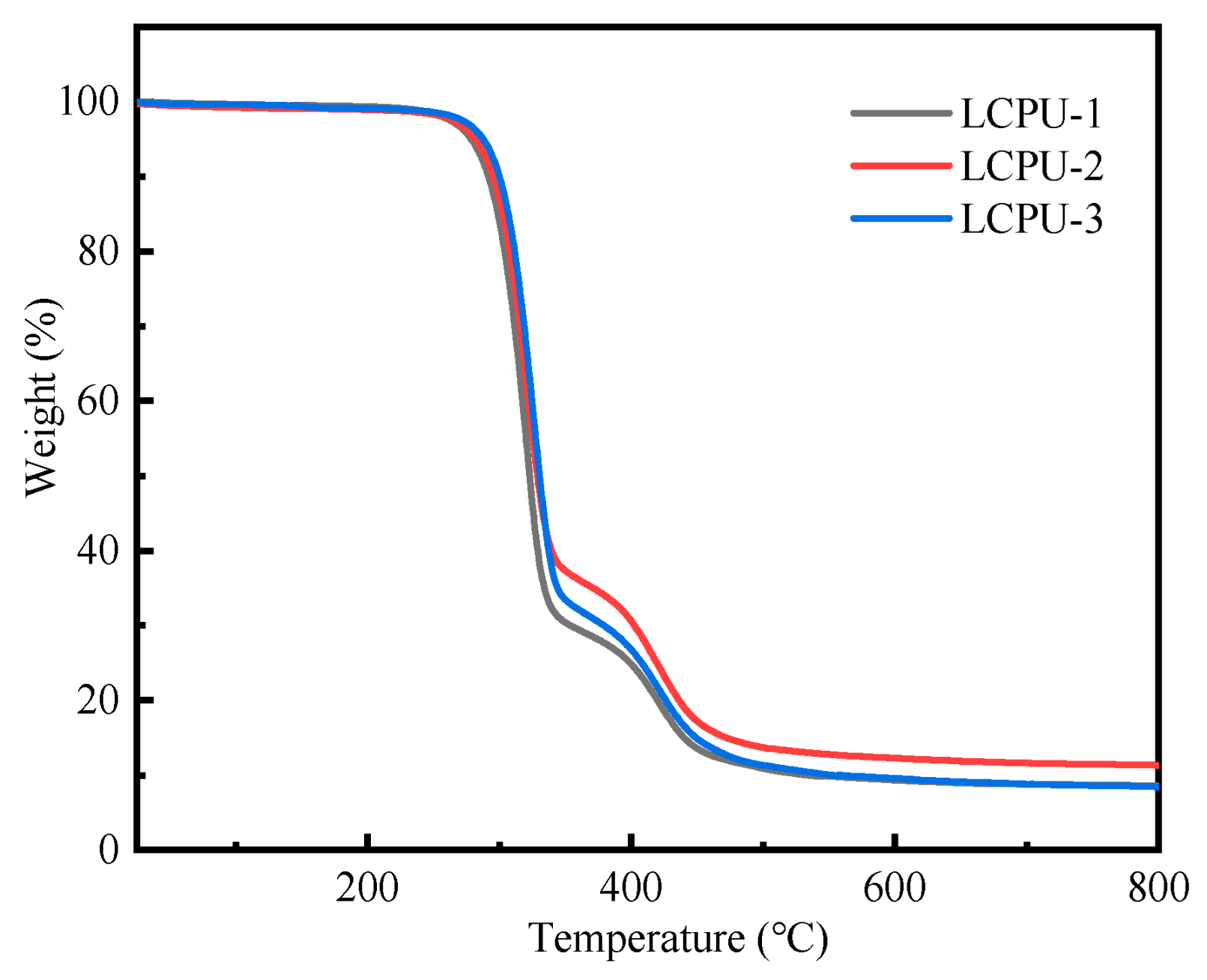
3.3. Thermal Stability of Poly(urethane-acrylate)s
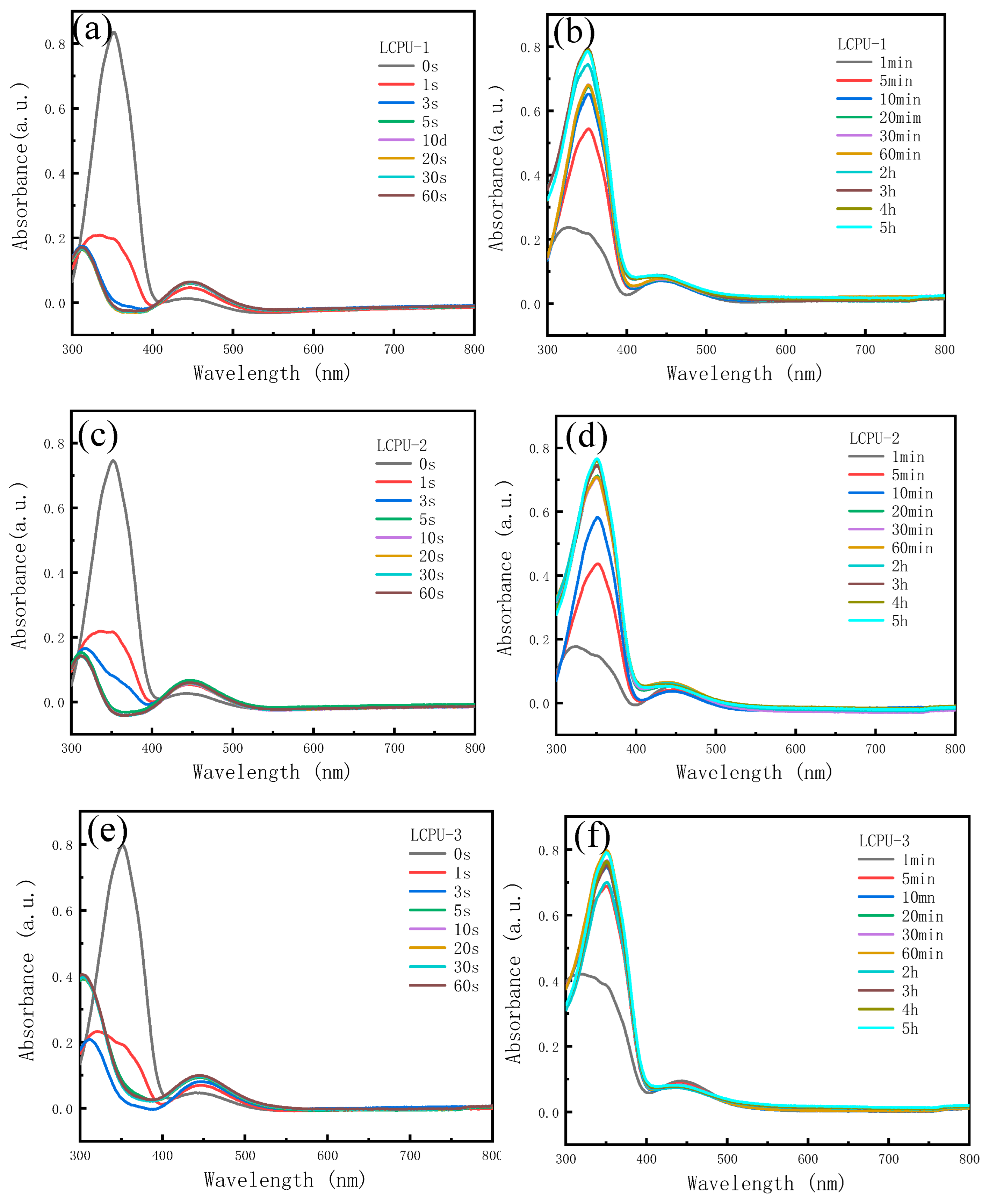
3.4. The Photoresponse Performances of Poly(urethane-acrylate)s
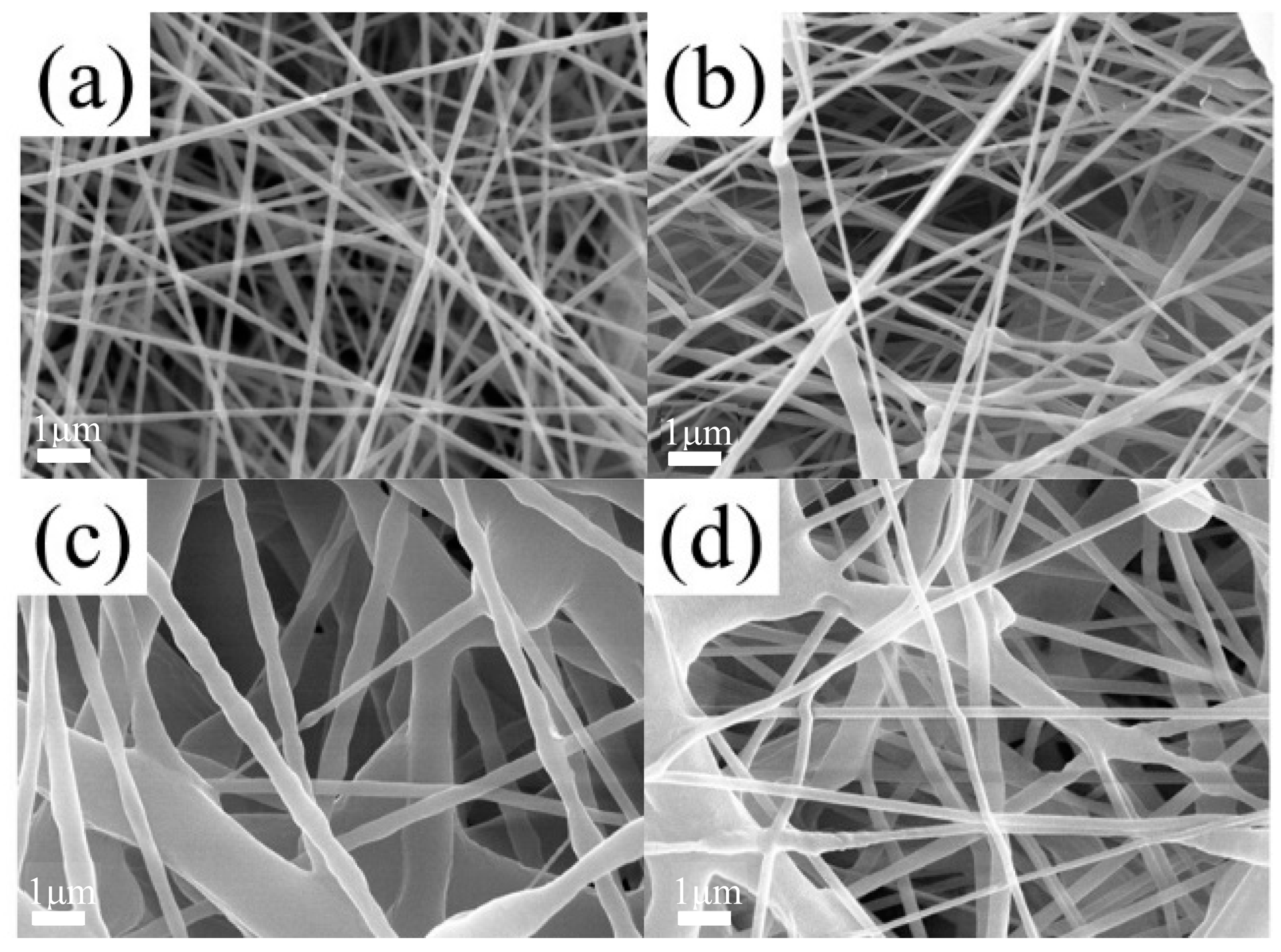
3.5. Microstructure of SMPU Fiber Membranes
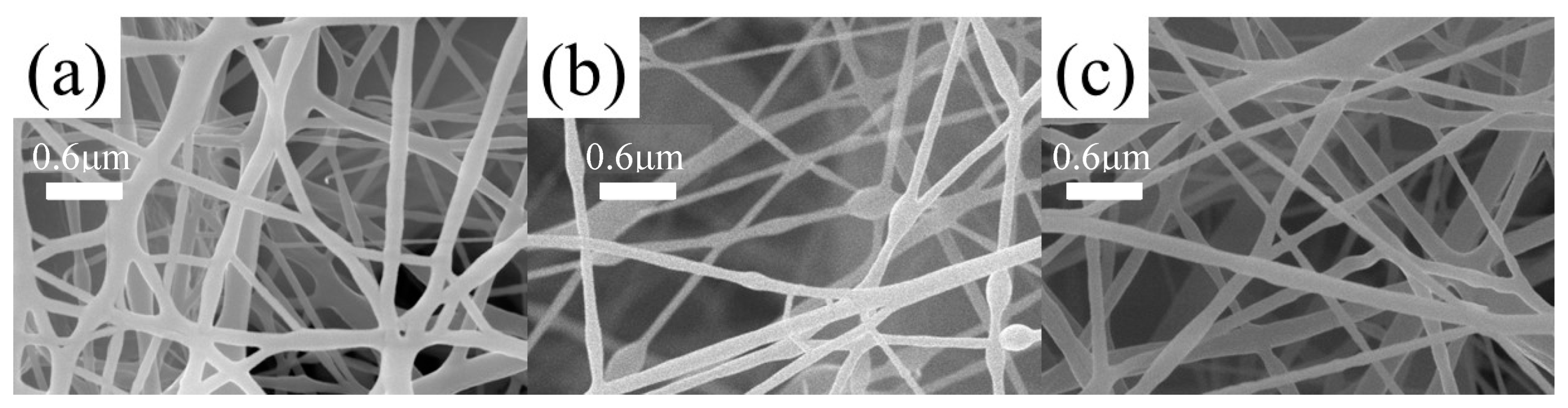
3.6. Microstructure of Composite Fiber Membranes

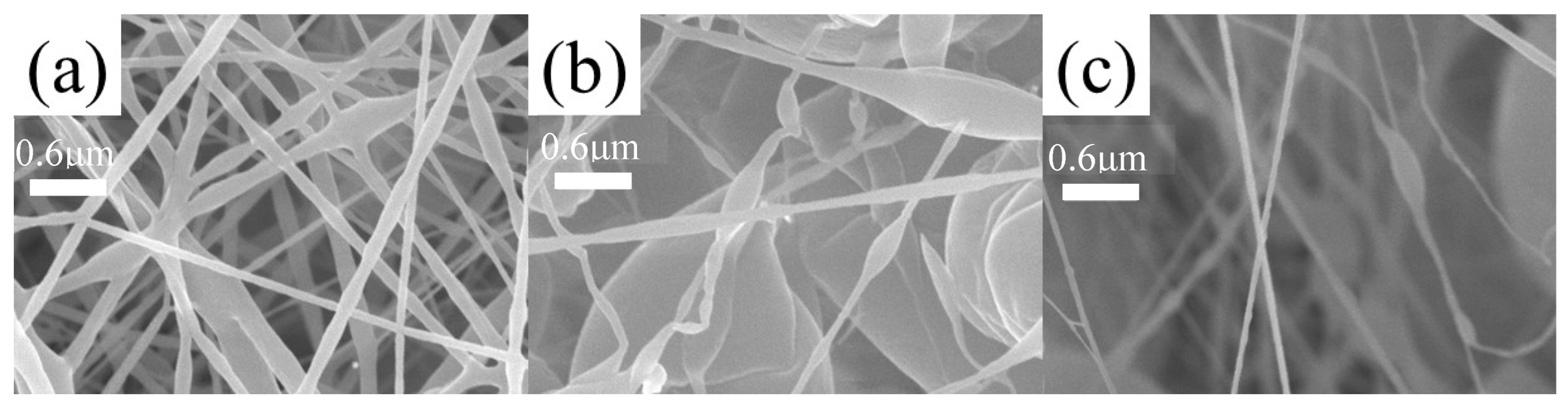
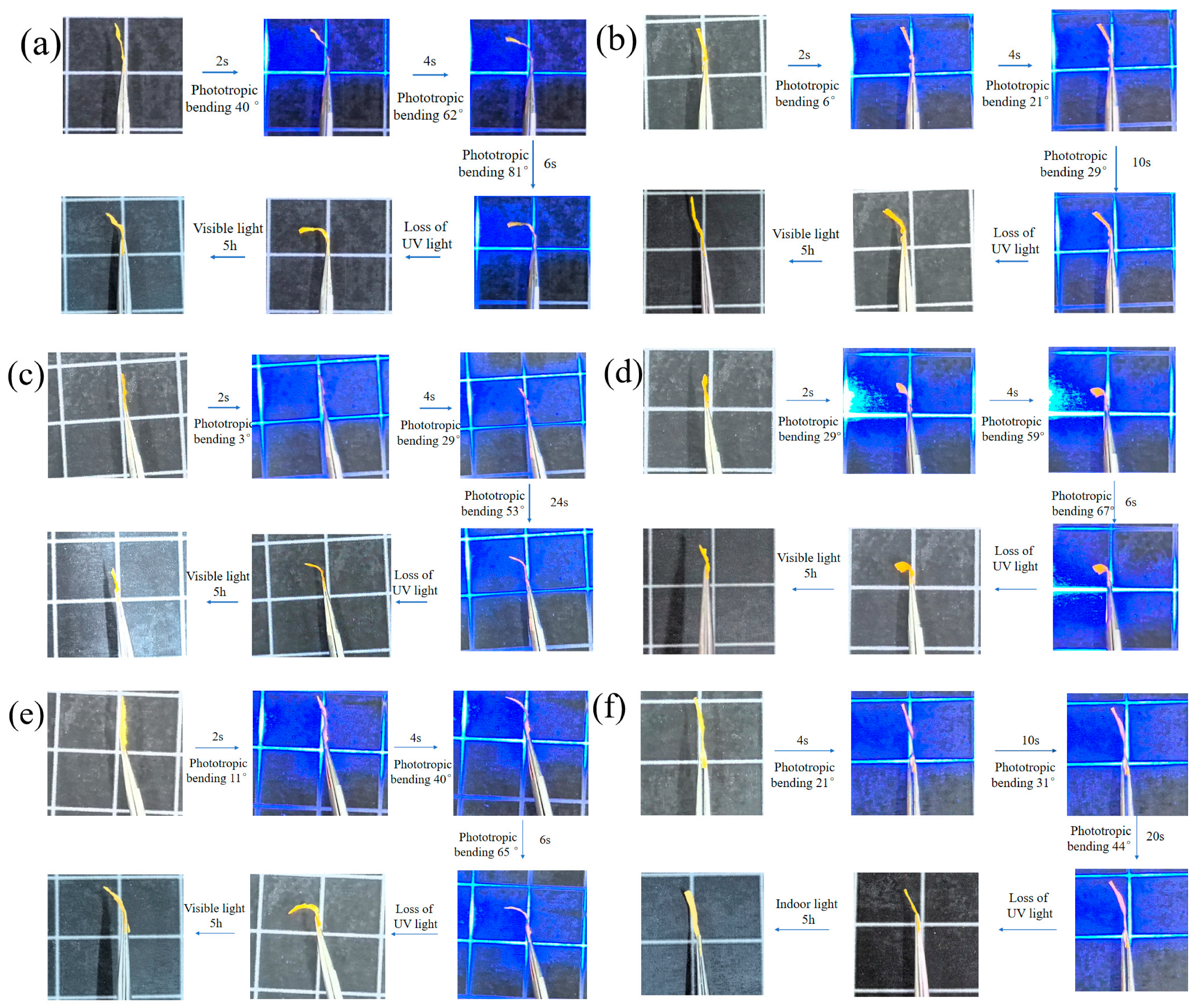
3.7. Photoresponse Behavior of Composite Fiber Membranes
3.8. Thermal Response of Composite Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Pang, X.L.; Lv, J.A.; Zhu, C.Y.; Qi, L.; Yu, Y.L. Photodeformable azobenzene-containing liquid crystal polymers and soft actuators. Adv. Mater. 2019, 31, 1904224. [Google Scholar] [CrossRef] [PubMed]
- Pantuso, E.; De, F.G.; Nicoletta, F.P. Light-responsive polymer membranes. Adv. Opt. Mater. 2019, 7, 1900252. [Google Scholar] [CrossRef]
- Stoychev, G.; Kirillova, A.; Lonov, L. Light-responsive shape-changing polymers. Adv. Opt. Mater. 2019, 7, 1900067. [Google Scholar] [CrossRef]
- Toru, U.; Tomiki, I. Photomobile polymer materials with complex 3D deformation, continuous motions, self-regulation, and enhanced processability. Adv. Opt. Mater. 2019, 7, 1900380. [Google Scholar]
- Tiptipakorn, S.; Angkanawarangkana, C.; Rimdusit, S.; Hemvichian, K.; Lertsarawut, P. Investigation of multiple shape memory behaviors, thermal and physical properties of benzoxazine blended with diamino polysiloxane. Polymers 2023, 15, 3814. [Google Scholar] [CrossRef]
- Yu, Q.; Aguila, B.; Gao, J.; Xu, P.X.; Chen, Q.Z.; Yan, J.; Xing, D.; Chen, Y.; Cheng, P.; Zhang, Z.J.; et al. Photomechanical organic crystals as smart materials for advanced applications. Chem. Eur. J. 2019, 25, 5611–5622. [Google Scholar] [CrossRef]
- Dong, L.L.; Zhao, Y. Photothermally driven liquid crystal polymer actuators. Mater. Chem. Front. 2018, 2, 1932–1943. [Google Scholar] [CrossRef]
- Song, C.J.; Zhang, Y.H.; Bao, J.Y.; Wang, Z.Z.; Zhang, L.Y.; Sun, J.; Lan, R.C.; Yu, Z.; Zhu, S.Q.; Yang, H. Light-responsive programmable shape-memory soft actuator based on liquid crystalline polymer/polyurethane network. Adv. Funct. Mater. 2023, 33, 2213771. [Google Scholar] [CrossRef]
- Morooka, T.; Ohsedo, Y.; Kato, R.; Miyamoto, N. Structure-regulated tough elastomers of liquid crystalline inorganic nanosheet/polyurethane nanocomposites. Adv. Mater. 2021, 2, 1035–1042. [Google Scholar] [CrossRef]
- Ujiie, S.; Watanabe, T.; Shimada, G.; Tomitaka, S.; Nata, M. Liquid-crystalline behavior of reactive main-chain polyurethanes. Mol. Cryst. Liq. Cryst. 2017, 647, 223–227. [Google Scholar] [CrossRef]
- Sultan, M.; Atta, S.; Bhatti, H.N.; Islam, A.J.T.; Bibi, I.; Gull, N. Synthesis, Characterization, and application studies of polyurethane acrylate thermoset coatings: Effect of hard segment. Polym.-Plast. Technol. 2017, 56, 1608–1618. [Google Scholar] [CrossRef]
- Shang, Y.Y.; Wang, J.X.; Ikeda, T.; Jiang, L. Bio-inspired liquid crystal actuator materials. J. Mater. Chem. C 2017, 7, 3413–3428. [Google Scholar] [CrossRef]
- Ge, F.J.; Yang, R.; Tong, X.; Camerel, F.; Zhao, Y. A multifunctional dye-doped liquid crystal polymer actuator: Light-guided transportation, turning in locomotion, and autonomous motion. Angew. Chem. Int. Edit. 2018, 57, 11758–11763. [Google Scholar] [CrossRef]
- Huang, S.; Tao, Y.; Huang, Y.L. Photodeformable composite materials based on liquid crystal polymers. Prog. Chem. 2022, 34, 2012–2023. [Google Scholar]
- Liu, Y.Y.; Zhao, J.; Zhao, L.Y.; Li, W.W.; Zhang, H.; Yu, X.; Zhang, Z. High performance shape memory epoxy/carbon nanotube nanocomposites. ACS Appl. Mater. Interfaces 2016, 8, 311–320. [Google Scholar] [CrossRef]
- Fiorito, F.; Sauchelli, M.; Arroyo, D.; Pesenti, M.; Imperadori, M.; Masera, G.; Ranzi, G. Shape morphing solar shadings: A review. Renew. Sust. Energy Rev. 2016, 55, 863–884. [Google Scholar] [CrossRef]
- Meng, Q.H.; Hu, J.H.; Zhu, Y.; Lu, J.; Liu, B. Biological evaluations of a smart shape memory fabric. Text. Res. J. 2009, 79, 1522–1533. [Google Scholar] [CrossRef]
- Rodriguez, E.D. Linear/network poly(epsilon-caprolactone)blends exhibiting shape memory assisted self-healing(SMASH). ACS Appl. Mater. Interfaces 2011, 3, 152–161. [Google Scholar] [CrossRef]
- Gautam, D.; Park, S.Y. Liquid crystalline elastomer actuators with dynamic covalent bonding: Synthesis, alignment, reprogrammability, and self-healing. Curr. Opin. Solid State Mater. Sci. 2023, 27, 101076. [Google Scholar]
- Lu, H.F.; Wang, M.; Chen, X.M.; Lin, B.P.; Yang, H. Interpenetrating liquid-crystal polyurethane/polyacrylate elastomer with ultrastrong mechanical property. J. Am. Chem. Soc. 2019, 141, 14364–14369. [Google Scholar] [CrossRef]
- Annapooranan, R.; Wang, Y.; Cai, S.Q. Highly durable and tough liquid crystal elastomers. ACS Appl. Mater. Interfaces 2022, 14, 2006–2014. [Google Scholar] [CrossRef]
- Shen, W.B.; Liu, J.S.; Du, B.; Zhuo, H.T.; Chen, S.J. Thermal- and light-responsive programmable shape-memory behavior of liquid crystalline polyurethanes with pendant photosensitive groups. J. Mater. Chem. A 2021, 9, 15087–15094. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, B.; Huang, S.; Cai, F.; Wang, G.J.; Yu, H.F. UV-vis-NIR light-induced bending of shape-memory polyurethane composites doped with azobenzene and upconversion nanoparticles. Polymer 2019, 178, 121644. [Google Scholar] [CrossRef]
- Karim, M.R.; Sheikh, M.R.K.; Yahya, R.; Salleh, N.M.; Azzahari, A.; Hassan, A.; Sarih, N.M. Thermal, optical and electrochemical study of side chain liquid crystalline polymers bearing azo-benzothiazole chromophore in the mesogen. J. Polym. Res. 2013, 20, 259. [Google Scholar] [CrossRef]
- Martinez-Felipe, A.; Lu, Z.B.; Henderson, P.A.; Picken, S.J.; Norder, B.; Imrie, C.T.; Ribes-Greus, A. Synthesis and characterisation of side chain liquid crystal copolymers containing sulfonic acid groups. Polymer 2012, 53, 2604–2612. [Google Scholar] [CrossRef]
- Yuan, Y.J.; Li, J.; He, L.F.; Liu, Y.M.; Zhang, H.L. Preparation and properties of side chain liquid crystalline polymers with aggregation-induced emission enhancement characteristics. J. Mater. Chem. C 2018, 6, 7119–7127. [Google Scholar] [CrossRef]
- Wen, Z.B.; Zhang, T.H.; Hui, Y.; Wang, W.L.; Yang, K.K.; Zhou, Q.; Wang, Y.Z. Elaborate fabrication of well-defined side-chain liquid crystalline polyurethane networks with triple-shape memory capacity. J. Mater. Chem. A 2015, 3, 13435–13444. [Google Scholar] [CrossRef]
- Cui, Z.S.; Yue, X.L.; Wang, Y.C.; Zhang, Y.J.; Ren, Z.H.; Guan, Z.H. A light-responsive poly(urethane-urea) actuator with room temperature self-healing performance. Chem. Eng. J. 2024, 479, 147538. [Google Scholar] [CrossRef]
- Lei, Y.; Yuan, Y.; Zhao, S.W.; Yuan, A.Q.; Zhou, S.Y.; Xiao, Y.; Lei, J.X.; Jiang, L. Catalyst-free, highly sensitive and adjustable photo-responsive azobenzene liquid crystal elastomers based on dynamic multiple hydrogen bond. Polymer 2023, 269, 12537. [Google Scholar] [CrossRef]
- Wen, Z.B.; Liu, D.; Li, X.Y.; Zhu, C.H.; Shao, R.F.; Visvanathan, R.; Clark, N.A.; Yang, K.K.; Wang, Y.Z. Fabrication of liquid crystalline polyurethane networks with a pendant azobenzene group to access thermal/photoresponsive shape-memory effects. ACS Appl. Mater. Interfaces 2017, 9, 24947–24954. [Google Scholar] [CrossRef]
- Yu, L.J.; Peng, R.G.; Wang, Y.Z.; Lie, Y.E.; Yang, Y.K. Liquid crystalline polyurethane elastomers containing mesogenic azobenzene pendant groups for photomechanical actuators. Adv. Mater. Res. 2012, 531, 580–583. [Google Scholar] [CrossRef]


| LCPU-1:SMPU | LCPU-2:SMPU | LCPU-3:SMPU |
|---|---|---|
| 1:2 | 1:2 | 1:2 |
| 3:4 | 3:4 | 3:4 |
| 1:1 | 1:1 | 1:1 |
| Mass Ratio | Tensile Deformation Rate (%) | Recovery Rate (%) |
|---|---|---|
| LCPU-1:SMPU = 1:2 | 36.0 | 99.6 |
| LCPU-1:SMPU = 3:4 | 62.5 | 94.1 |
| LCPU-1:SMPU = 1:1 | 20.0 | 88.0 |
| Sample | Photoisomerization | Photoinduced Bending | Shape Recovery Rate (%) | Ref. | |||
|---|---|---|---|---|---|---|---|
| λ (nm) | t (s) | λ (nm) | t (s) | Angle (°) | |||
| LCPU-1/SMPU | 365 | 5 | 365 | 6 | 81.0 | 99.6 | This work |
| MLCPU | 365 | 8 | 365 | 5 | 7.0 | 85.1 | [28] |
| HAzo-LCEs | 365 | 10 | 365 | 10 | 48.0 | - | [29] |
| SCLCPU(AZO) | 450 | 30 | - | - | 95.3 | [30] | |
| LCPUE | 365 | 285 | 365 | 300 | 13.0 | - | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Wang, Z.; Gao, L.; Yang, H.; Fang, S. Preparation and Properties of Multi-Responsive Liquid Crystalline Poly(urethane-acrylate)s and Its Composite Membranes. Polymers 2024, 16, 1854. https://doi.org/10.3390/polym16131854
Zhou L, Wang Z, Gao L, Yang H, Fang S. Preparation and Properties of Multi-Responsive Liquid Crystalline Poly(urethane-acrylate)s and Its Composite Membranes. Polymers. 2024; 16(13):1854. https://doi.org/10.3390/polym16131854
Chicago/Turabian StyleZhou, Liming, Ziwen Wang, Lijun Gao, Hongcheng Yang, and Shaoming Fang. 2024. "Preparation and Properties of Multi-Responsive Liquid Crystalline Poly(urethane-acrylate)s and Its Composite Membranes" Polymers 16, no. 13: 1854. https://doi.org/10.3390/polym16131854
APA StyleZhou, L., Wang, Z., Gao, L., Yang, H., & Fang, S. (2024). Preparation and Properties of Multi-Responsive Liquid Crystalline Poly(urethane-acrylate)s and Its Composite Membranes. Polymers, 16(13), 1854. https://doi.org/10.3390/polym16131854







