Enhanced Marine Biodegradation of Polycaprolactone through Incorporation of Mucus Bubble Powder from Violet Sea Snail as Protein Fillers
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of PCL/Mucus Bubble Powder (PCL/Bubble) Sheets
2.1.1. Preparation of Dried Mucus Bubbles from the VSS
2.1.2. Preparation of Solvent-Cast Film-Shaped PCL Specimens
2.1.3. Preparation of PCL/Bubble Composite Powder via Freeze Grinding
2.1.4. Preparation of PCL/Bubble Composite Sheets by Heat Pressing
2.2. Characterization
2.2.1. Differential Scanning Calorimetry (DSC)
2.2.2. Thermal Gravimetric Analysis (TGA)
2.2.3. Tensile Testing
2.2.4. Wide-Angle X-ray Scattering (WAXS)
2.2.5. Biodegradability Testing
Biochemical Oxygen Demand (BOD)
Weight Loss in Extracted Seawater
2.2.6. Scanning Electron Microscope (SEM)
2.2.7. Contact Angle Measurement
3. Results and Discussion
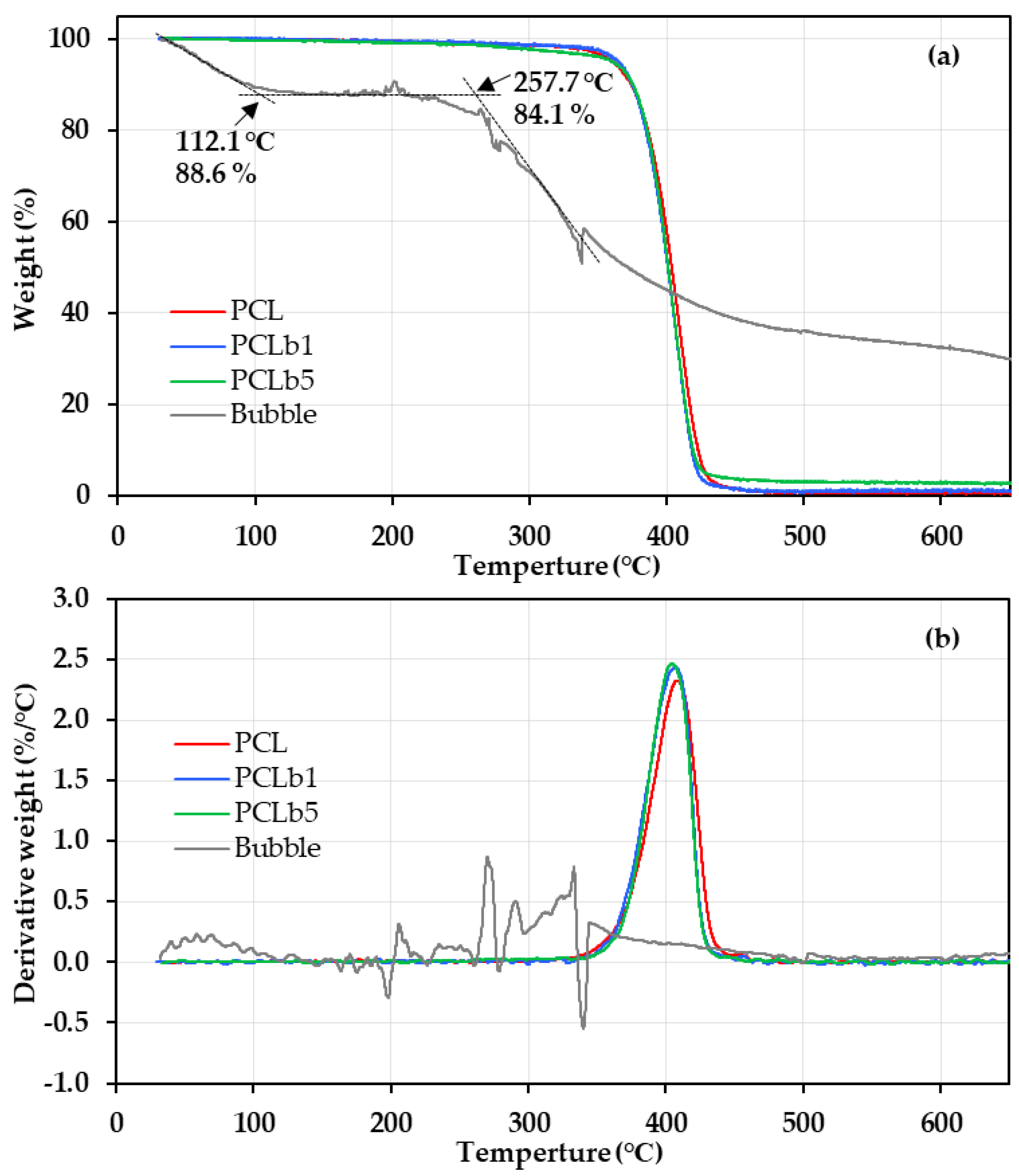
3.1. Thermal Properties of PCL/Bubble Composite Sheets and Powder
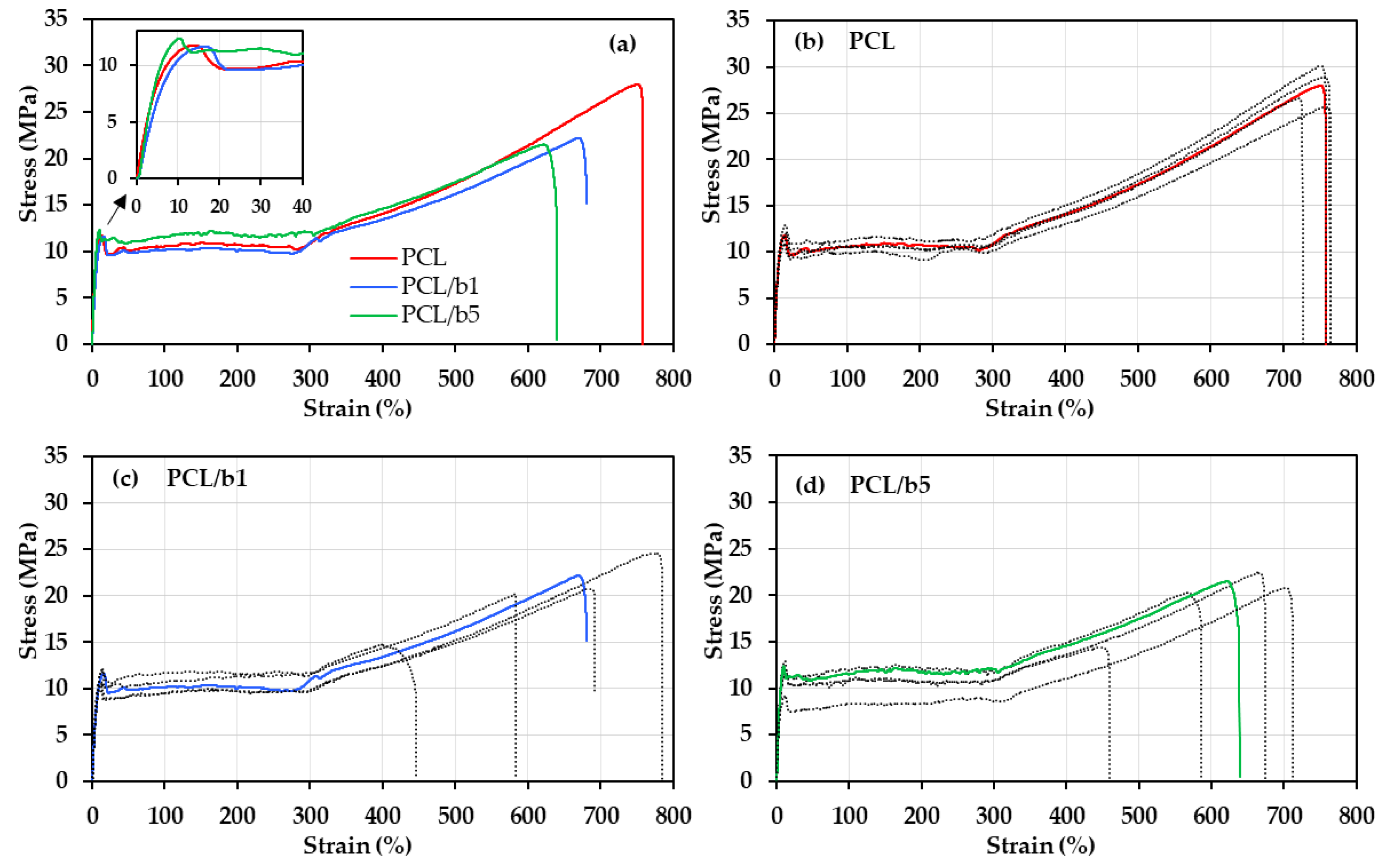
3.2. Mechanical Properties of PCL/Bubble Composite Sheets
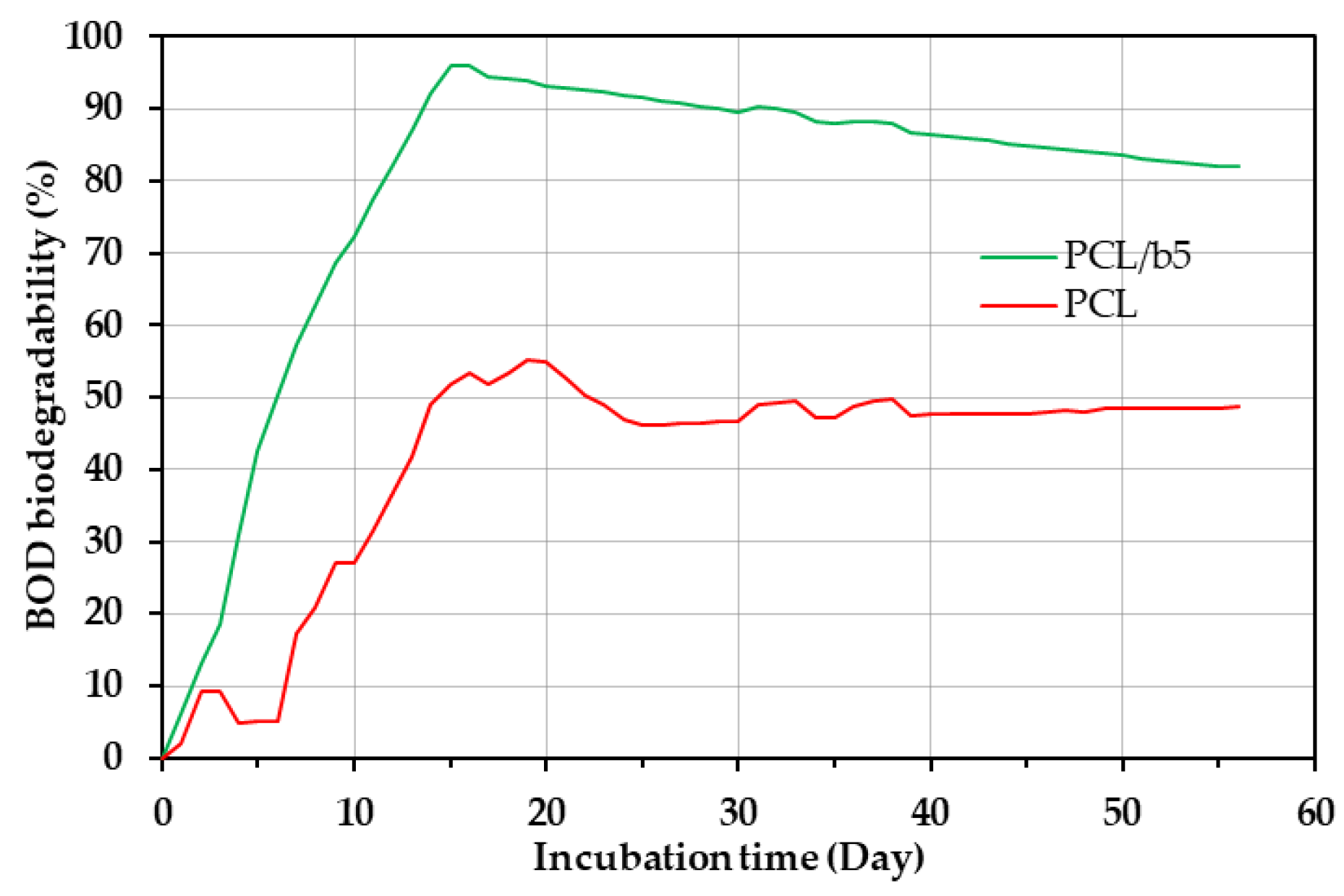
3.3. Degradability of PCL/Bubble Composites in Marine Environments
3.4. Weight Loss of PCL/Bubble Composites
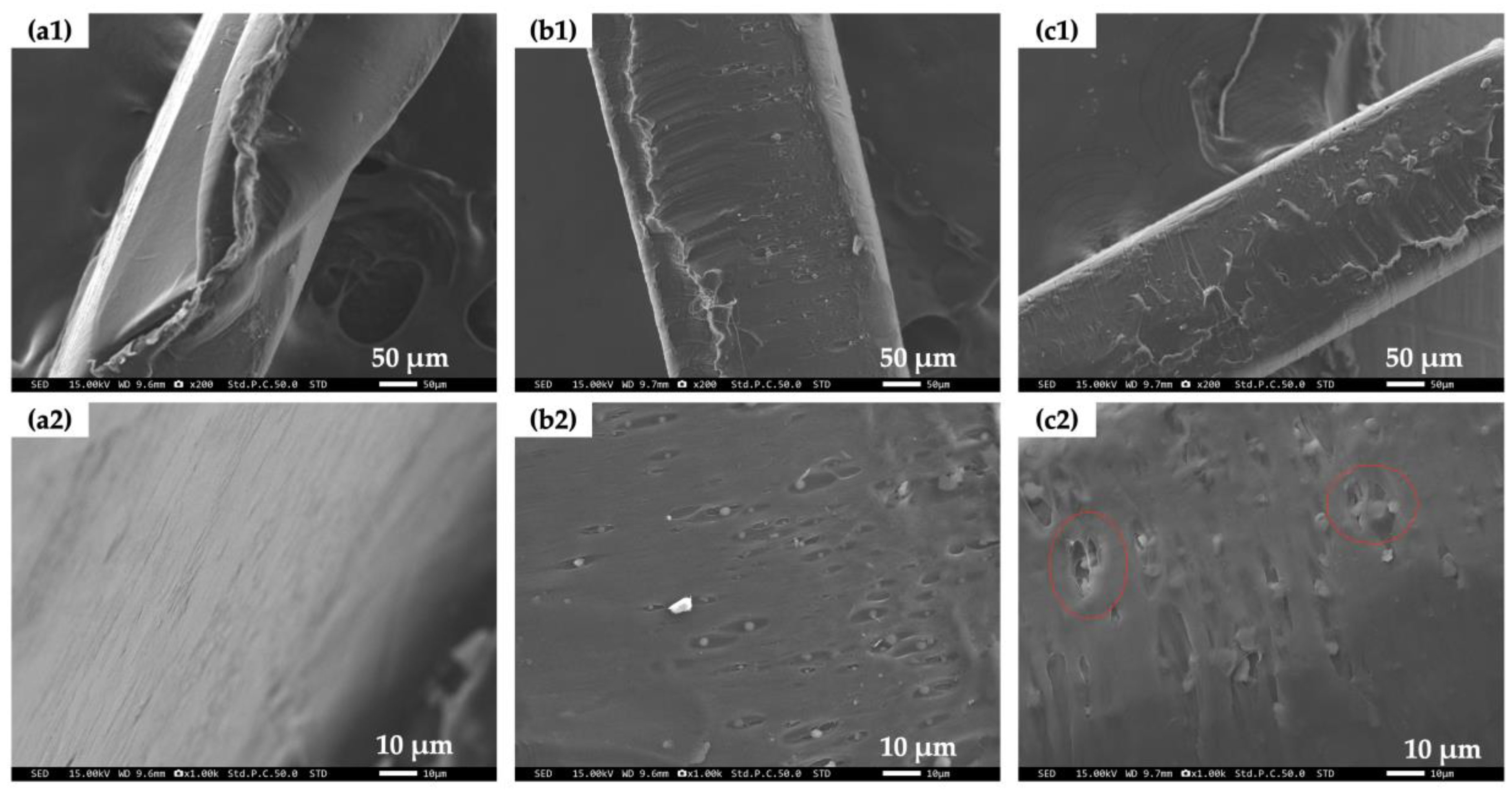
3.5. Surface Structure of PCL/Bubble Composite Sheets before and after the BOD Test
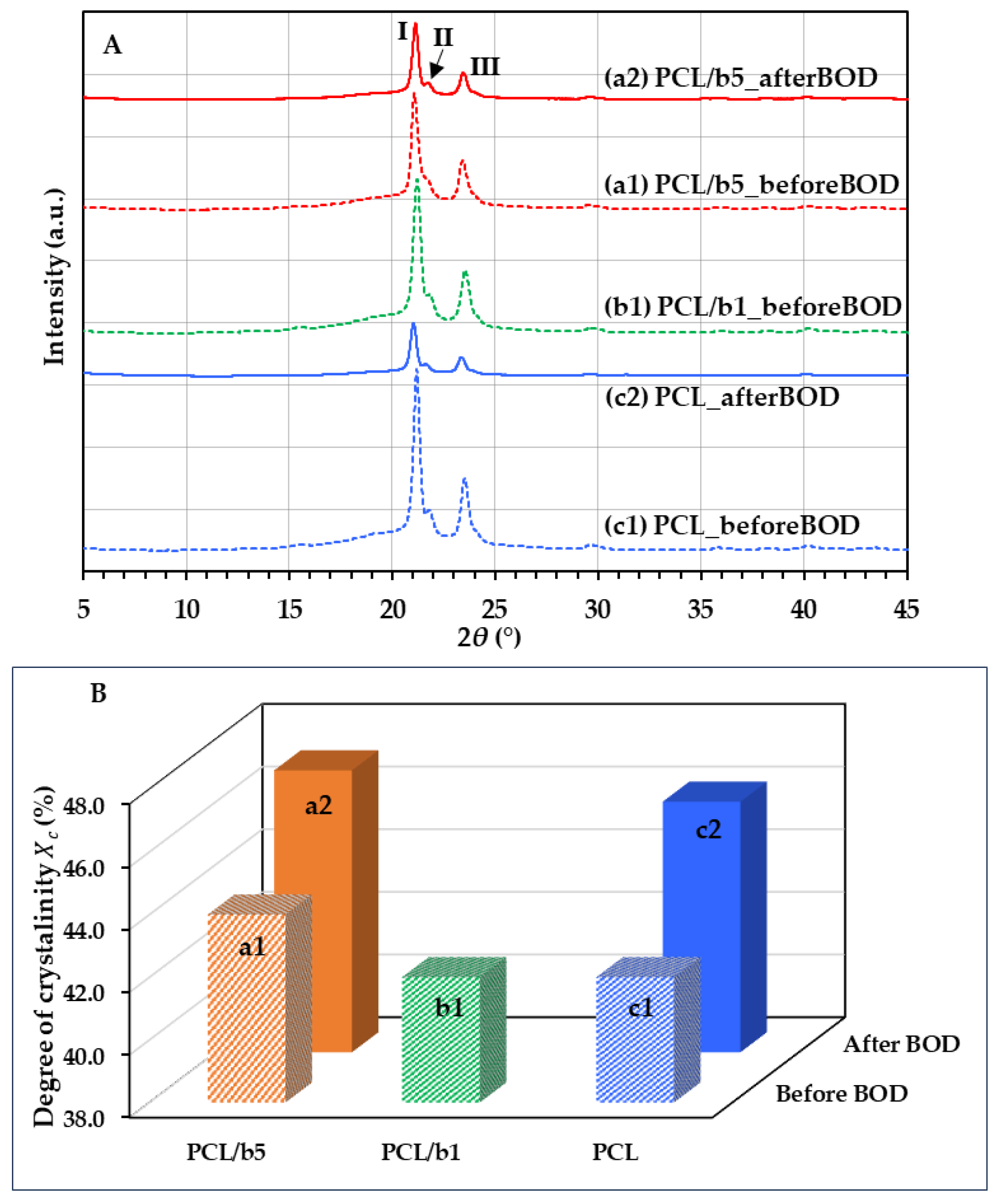
3.6. Crystal Structure of PCL/Bubble Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, G.; Huang, D.; Ji, J.; Völker, C.; Wurm, F.R. Seawater-Degradable Polymers—Fighting the Marine Plastic Pollution. Adv. Sci. 2021, 8, 2001121. [Google Scholar] [CrossRef] [PubMed]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic Waste Inputs from Land into the Ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- da Costa, J.P.; Santos, P.S.M.; Duarte, A.C.; Rocha-Santos, T. (Nano)Plastics in the Environment—Sources, Fates and Effects. Sci. Total Environ. 2016, 566–567, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Wang, G.-X.; Huang, D.; Ren, Z.-L.; Wang, X.-W.; Wang, P.-L.; Zhen, Z.-C.; Zhang, W.; Ji, J.-H. Comparison of PCL Degradation in Different Aquatic Environments: Effects of Bacteria and Inorganic Salts. Polym. Degrad. Stab. 2018, 150, 133–139. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Zuhri, M.Y.M.; Norrrahim, M.N.F.; Misenan, M.S.M.; Jenol, M.A.; Samsudin, S.A.; Nurazzi, N.M.; Asyraf, M.R.M.; Supian, A.B.M.; Bangar, S.P.; et al. Natural Fiber-Reinforced Polycaprolactone Green and Hybrid Biocomposites for Various Advanced Applications. Polymers 2022, 14, 182. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimifar, M.; Taherimehr, M. Evaluation of In-Vitro Drug Release of Polyvinylcyclohexane Carbonate as a CO2-Derived Degradable Polymer Blended with PLA and PCL as Drug Carriers. J. Drug Deliv. Sci. Technol. 2021, 63, 102491. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Shalaby, E.S.; Abd-Al-Aleem, A.H.; Abu-Saied, M.A.; Youssef, A.M. Synthesis of Environmentally Benign Antimicrobial Dressing Nanofibers Based on Polycaprolactone Blended with Gold Nanoparticles and Spearmint Oil Nanoemulsion. J. Mater. Res. Technol. 2021, 15, 3447–3460. [Google Scholar] [CrossRef]
- Herrero-Herrero, M.; Alberdi-Torres, S.; González-Fernández, M.L.; Vilariño-Feltrer, G.; Rodríguez-Hernández, J.C.; Vallés-Lluch, A.; Villar-Suárez, V. Influence of Chemistry and Fiber Diameter of Electrospun PLA, PCL and Their Blend Membranes, Intended as Cell Supports, on Their Biological Behavior. Polym. Test. 2021, 103, 107364. [Google Scholar] [CrossRef]
- Doganci, M.D. Effects of Star-Shaped PCL Having Different Numbers of Arms on the Mechanical, Morphological, and Thermal Properties of PLA/PCL Blends. J. Polym. Res. 2021, 28, 11. [Google Scholar] [CrossRef]
- Ouled Ltaief, A.; Ghorbel, N.; Benhamou, K.; Arous, M.; Kaddami, H.; Kallel, A. Impact of Cellulose Nanocrystals Reinforcement on Molecular Dynamics and Dielectric Properties of PCL-based Polyurethane. Polym. Compos. 2021, 42, 2737–2750. [Google Scholar] [CrossRef]
- dos Santos Filho, E.A.; Siqueira, D.D.; Araújo, E.M.; Luna, C.B.B.; de Medeiros, E.P. The Impact of the Macaíba Components Addition on the Biodegradation Acceleration of Poly (Ɛ-Caprolactone) (PCL). J. Polym. Environ. 2022, 30, 443–460. [Google Scholar] [CrossRef]
- Reis, R.S.; Souza, D.D.H.S.; Marques, M.D.F.V.; da Luz, F.S.; Monteiro, S.N. Novel Bionanocomposite of Polycaprolactone Reinforced with Steam-Exploded Microfibrillated Cellulose Modified with ZnO. J. Mater. Res. Technol. 2021, 13, 1324–1335. [Google Scholar] [CrossRef]
- AlMaadeed, M.A.; Kahraman, R.; Noorunnisa Khanam, P.; Madi, N. Date Palm Wood Flour/Glass Fibre Reinforced Hybrid Composites of Recycled Polypropylene: Mechanical and Thermal Properties. Mater. Des. 2012, 42, 289–294. [Google Scholar] [CrossRef]
- Herzele, S.; Veigel, S.; Liebner, F.; Zimmermann, T.; Gindl-Altmutter, W. Reinforcement of Polycaprolactone with Microfibrillated Lignocellulose. Ind. Crops Prod. 2016, 93, 302–308. [Google Scholar] [CrossRef]
- Hejna, A.; Sulyman, M.; Przybysz, M.; Saeb, M.R.; Klein, M.; Formela, K. On the Correlation of Lignocellulosic Filler Composition with the Performance Properties of Poly(ε-Caprolactone) Based Biocomposites. Waste Biomass Valor 2020, 11, 1467–1479. [Google Scholar] [CrossRef]
- Beu, A.G. Evolution of Janthina and Recluzia (Mollusca: Gastropoda: Epitoniidae). Rec. Aust. Mus. 2017, 69, 119–222. [Google Scholar] [CrossRef]
- JIS K 6251; Rubber, Vulcanized or Thermoplastic Determination of Tensile Stress-Strain Properties. Japanese Standards Association: Tokyo, Japan, 2023.
- Ushida, K.; Sato, R.; Momma, T.; Tanaka, S.; Kaneko, T.; Morishita, H. Jellyfish Mucin (Qniumucin) Extracted with a Modified Protocol Indicated Its Existence as a Constituent of the Extracellular Matrix. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2022, 1866, 130189. [Google Scholar] [CrossRef] [PubMed]
- Masuda, A.; Baba, T.; Dohmae, N.; Yamamura, M.; Wada, H.; Ushida, K. Mucin (Qniumucin), a Glycoprotein from Jellyfish, and Determination of Its Main Chain Structure. J. Nat. Prod. 2007, 70, 1089–1092. [Google Scholar] [CrossRef]
- Svärd, A.; Brännvall, E.; Edlund, U. Modified and Thermoplastic Rapeseed Straw Xylan: A Renewable Additive in PCL Biocomposites. Ind. Crops Prod. 2018, 119, 73–82. [Google Scholar] [CrossRef]
- Hejna, A.; Formela, K.; Saeb, M.R. Processing, Mechanical and Thermal Behavior Assessments of Polycaprolactone/Agricultural Wastes Biocomposites. Ind. Crops Prod. 2015, 76, 725–733. [Google Scholar] [CrossRef]
- Jayathilaka, L.P.I.; Ariyadasa, T.U.; Egodage, S.M. Development of Biodegradable Natural Rubber Latex Composites by Employing Corn Derivative Bio-fillers. J. Appl. Polym. Sci 2020, 137, 49205. [Google Scholar] [CrossRef]
- Jeon, H.; Kim, M.-S.; Park, S.B.; Kim, S.; Lee, M.; Park, S.-A.; Hwang, S.Y.; Koo, J.M.; Oh, D.X.; Park, J. Improved Mechanical Properties of Biodegradable Polycaprolactone Nanocomposites Prepared Using Cellulose Nanocrystals. Cellulose 2023, 30, 11561–11574. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of Solid Surfaces to Wetting by Water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Wenzel, R.N. Surface Roughness and Contact Angle. J. Phys. Chem. 1949, 53, 1466–1467. [Google Scholar] [CrossRef]
- Cheng, B.K.; Naccarato, B.; Kim, K.J.; Kumar, A. Theoretical Consideration of Contact Angle Hysteresis Using Surface-Energy-Minimization Methods. Int. J. Heat Mass Transf. 2016, 102, 154–161. [Google Scholar] [CrossRef]
- Ponjavic, M.; Nikolic, M.S.; Nikodinovic-Runic, J.; Jeremic, S.; Stevanovic, S.; Djonlagic, J. Degradation Behaviour of PCL/PEO/PCL and PCL/PEO Block Copolymers under Controlled Hydrolytic, Enzymatic and Composting Conditions. Polym. Test. 2017, 57, 67–77. [Google Scholar] [CrossRef]
- Yang, K.-C. A Self-Reinforcing Biodegradable Implant Made of Poly (3-Caprolactone)/Calcium Phosphate Ceramic Composite for Craniomaxillofacial Fracture Fixation. J. Cranio-Maxillofac. Surg. 2016, 44, 1333–1341. [Google Scholar]
- Sung, H.-J.; Meredith, C.; Johnson, C.; Galis, Z.S. The Effect of Scaffold Degradation Rate on Three-Dimensional Cell Growth and Angiogenesis. Biomaterials 2004, 25, 5735–5742. [Google Scholar] [CrossRef]



| Sample | Tc (°C) | Tm (°C) | ||
|---|---|---|---|---|
| Powder | PCL | 31.7 | 54.7 | |
| PCL/bubble composite | PCL/b1 | 30.5 | 56.0 | |
| PCL/b5 | 31.7 | 55.4 | ||
| Bubble | - | 108.9 | ||
| Sheet | PCL | 31.8 | 55.8 | |
| PCL/bubble composite | PCL/b1 | 29.5 | 54.0 | |
| PCL/b5 | 29.3 | 57.1 | ||
| Sample | Tonset (°C) | T5% (°C) | T10% (°C) | Tinflep (°C) | ||
|---|---|---|---|---|---|---|
| Powder | PCL | 381.5 | 360.3 | 374.1 | 408.8 | |
| PCL/bubble composite | PCL/b1 | 378.6 | 364.5 | 375.1 | 406.0 | |
| PCL/b5 | 381.2 | 360.2 | 375.2 | 404.5 | ||
| Bubble | 257.7 | - | - | 276.1/292.6 | ||
| Sample | Breaking Strength (MPa) | Yield Strength (MPa) | Breaking Strain (%) | Toughness (MJ/m3) | Young’s Modulus (MPa) |
|---|---|---|---|---|---|
| PCL | 27.5 | 11.2 | 757.2 | 117.0 | 210.2 |
| PCL/b1 | 20.9 | 11.3 | 648.8 | 85.5 | 199.8 |
| PCL/b5 | 20.1 | 12.5 | 626.6 | 85.5 | 238.0 |
| Sample (a) | Weight Loss (%) | Average Weight Loss (%) | |
|---|---|---|---|
| PCL/b5 | 1 | 34.5 | 34.1 |
| 2 | 36.8 | ||
| 3 | 30.9 | ||
| PCL | 1 | 34.6 | 33.4 |
| 2 | 25.6 | ||
| 3 | 40.1 | ||
| Sample | Peak I | Peak II (Shoulder Peak) | Peak III | ||||
|---|---|---|---|---|---|---|---|
| 2θ (°) | d1 (Å) | 2θ (°) | d2 (Å) | 2θ (°) | d3 (Å) | ||
| PCL/b5 | After BOD | 21.14 | 4.20 | 21.65 | 4.10 | 23.45 | 3.79 |
| Before BOD | 21.08 | 4.21 | 21.73 | 4.09 | 23.42 | 3.80 | |
| PCL/b1 | Before BOD | 21.21 | 4.19 | 21.81 | 4.07 | 23.55 | 3.78 |
| PCL | After BOD | 21.03 | 4.22 | 21.67 | 4.10 | 23.35 | 3.81 |
| Before BOD | 21.20 | 4.19 | 21.83 | 4.07 | 23.54 | 3.78 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, K.; Teramoto, S.; Gong, J.; Kobayashi, Y.; Ito, H. Enhanced Marine Biodegradation of Polycaprolactone through Incorporation of Mucus Bubble Powder from Violet Sea Snail as Protein Fillers. Polymers 2024, 16, 1830. https://doi.org/10.3390/polym16131830
Yoshida K, Teramoto S, Gong J, Kobayashi Y, Ito H. Enhanced Marine Biodegradation of Polycaprolactone through Incorporation of Mucus Bubble Powder from Violet Sea Snail as Protein Fillers. Polymers. 2024; 16(13):1830. https://doi.org/10.3390/polym16131830
Chicago/Turabian StyleYoshida, Koh, Sayaka Teramoto, Jin Gong, Yutaka Kobayashi, and Hiroshi Ito. 2024. "Enhanced Marine Biodegradation of Polycaprolactone through Incorporation of Mucus Bubble Powder from Violet Sea Snail as Protein Fillers" Polymers 16, no. 13: 1830. https://doi.org/10.3390/polym16131830
APA StyleYoshida, K., Teramoto, S., Gong, J., Kobayashi, Y., & Ito, H. (2024). Enhanced Marine Biodegradation of Polycaprolactone through Incorporation of Mucus Bubble Powder from Violet Sea Snail as Protein Fillers. Polymers, 16(13), 1830. https://doi.org/10.3390/polym16131830







