Poly(2-isopropenyl-2-oxazoline) as a Versatile Functional Polymer for Biomedical Applications
Abstract
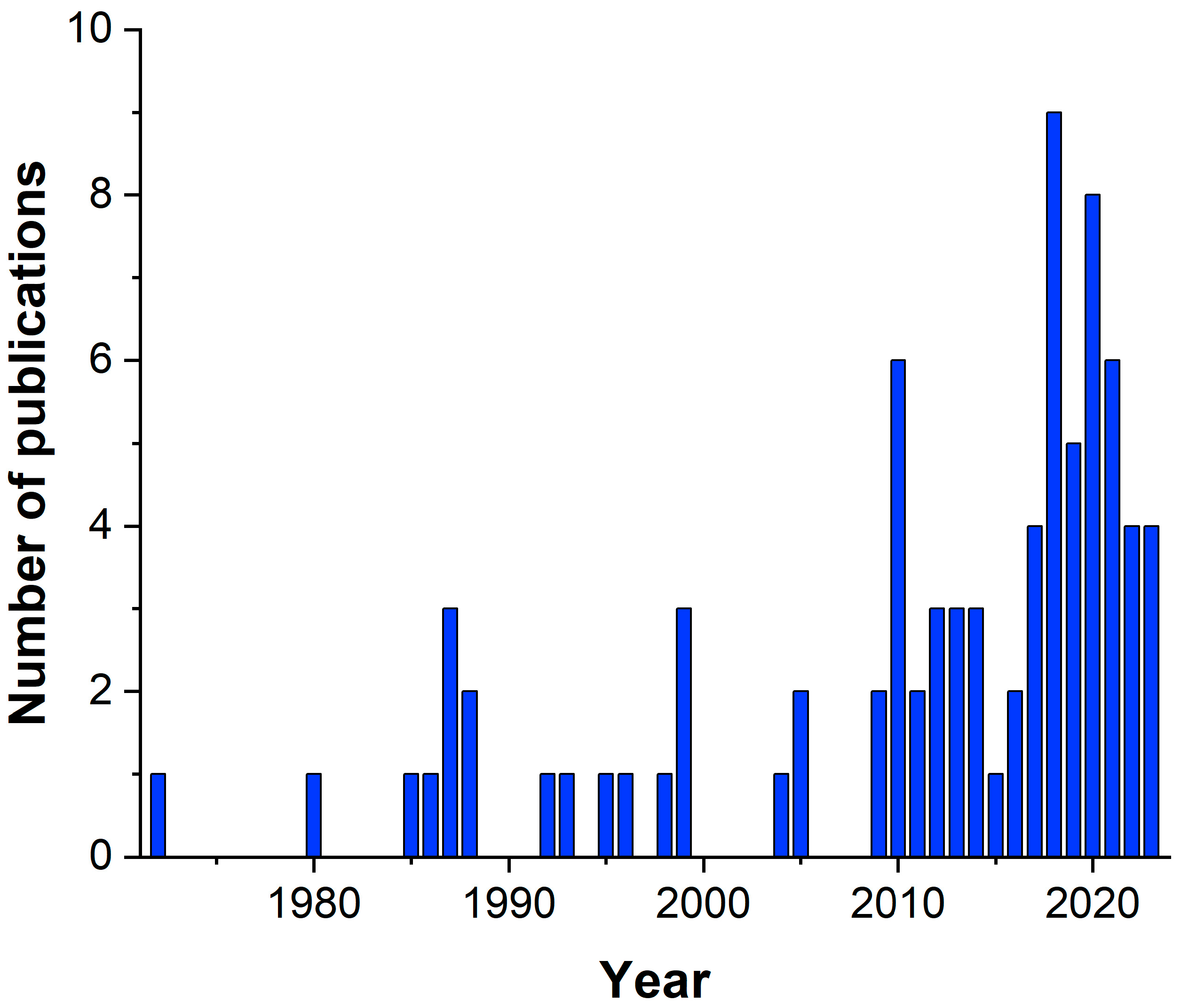
1. Introduction
| Year | Milestone | Authors | Ref. |
|---|---|---|---|
| 1884 | First synthesis of 2-oxazoline cycle from allylurea | Andreash | [22] |
| 1966 | Cationic polymerization of 2-methyl-2-oxazoline | Kagiya, Litt, Tomalia, Seeliger | [23,24,25,26] |
| 1972 | Free-radical polymerization of IPOx | Kagyia | [27] |
| Hydrogels based on PIPOx | Kagiya | [28] | |
| Addition reactions of PIPOx with carboxylic acids | Kagiya | [28] | |
| 1980 | Anionic polymerization of IPOx | Tomalia | [29] |
| 1993 | Compatibilization of polymer blends using PIPOx | Baker | [30] |
| 1996 | Selective addition reactions of PIPOx with thiols and carboxylic acids | Nishikubo | [32] |
| 2009 | Polymer brushes based on PIPOx | Jordan | [33] |
| 2010 | Controlled radical polymerization of IPOx | Schubert | [34] |
| 2013 | Group-transfer polymerization of IPOx | Rieger | [35] |
| 2016 | In vitro cytotoxicity studies of PIPOx | Kronek | [31] |
| 2018 | Plasma-polymerized PIPOx coatings | Zanini | [36] |
| Thermosensitive polymers based on PIPOx | Hoogenboom | [37] | |
| 2020 | ATRP of PIPOx | Raus | [38] |
| Drug delivery systems based on PIPOx | Hoogenboom | [39] | |
| 2021 | Segmented networks based on PIPOx | Basko | [40] |
| In vitro hydrolytic stability of PIPOx | Hoogenboom | [41] | |
| 2023 | Hybrid metal nanoparticles based on PIPOx | Mosnáček | [42] |
| PIPOx polymers for gene delivery | Hoogenboom | [43] |
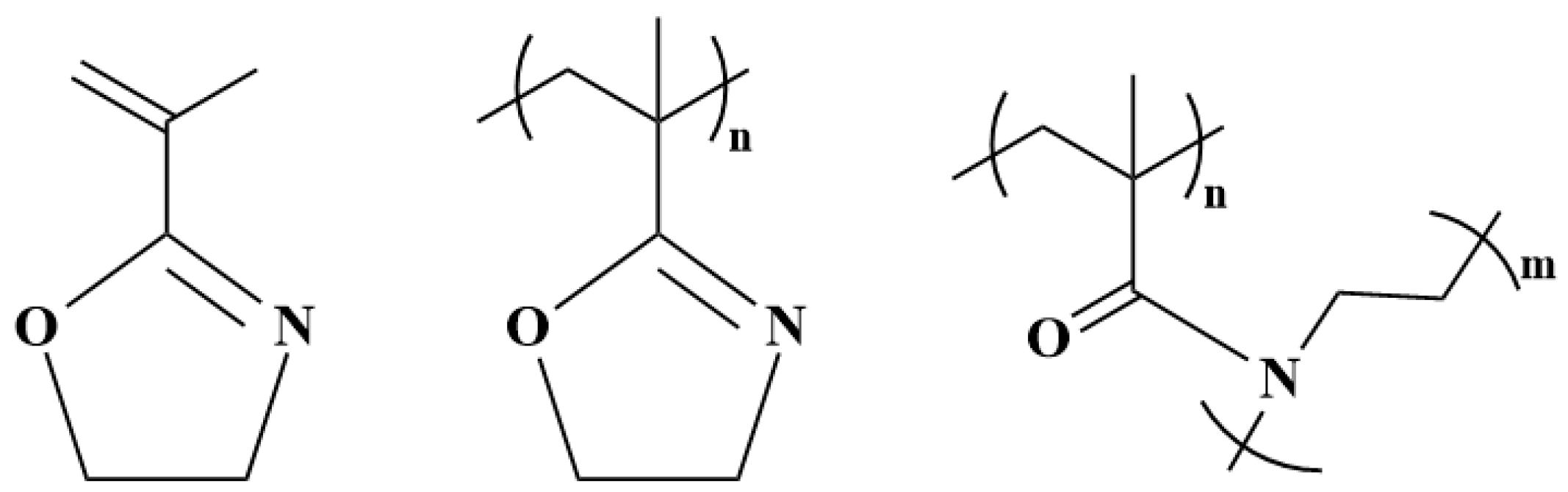
2. Synthesis of 2-Isopropenyl-2-Oxazoline
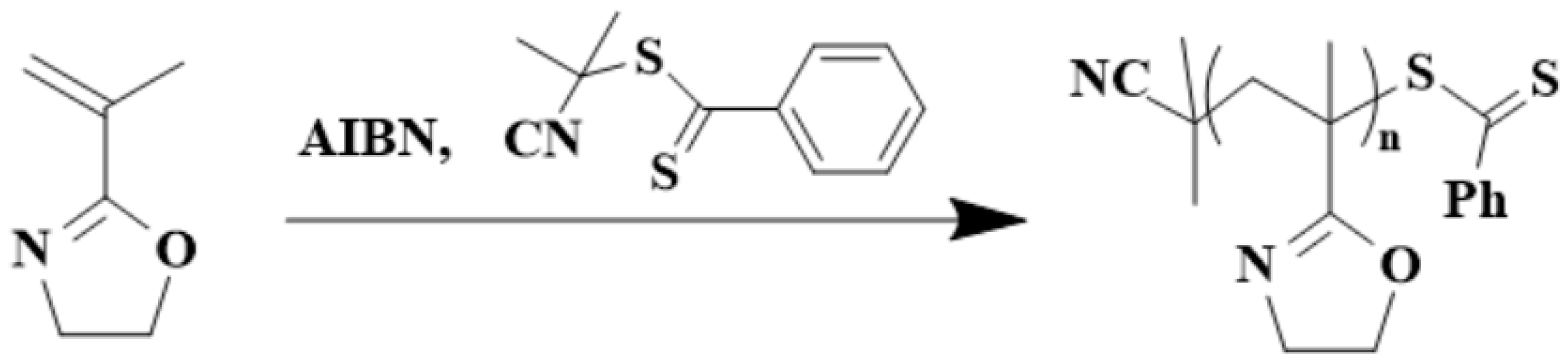
3. Polymerization Methods of 2-Isopropenyl-2-Oxazoline
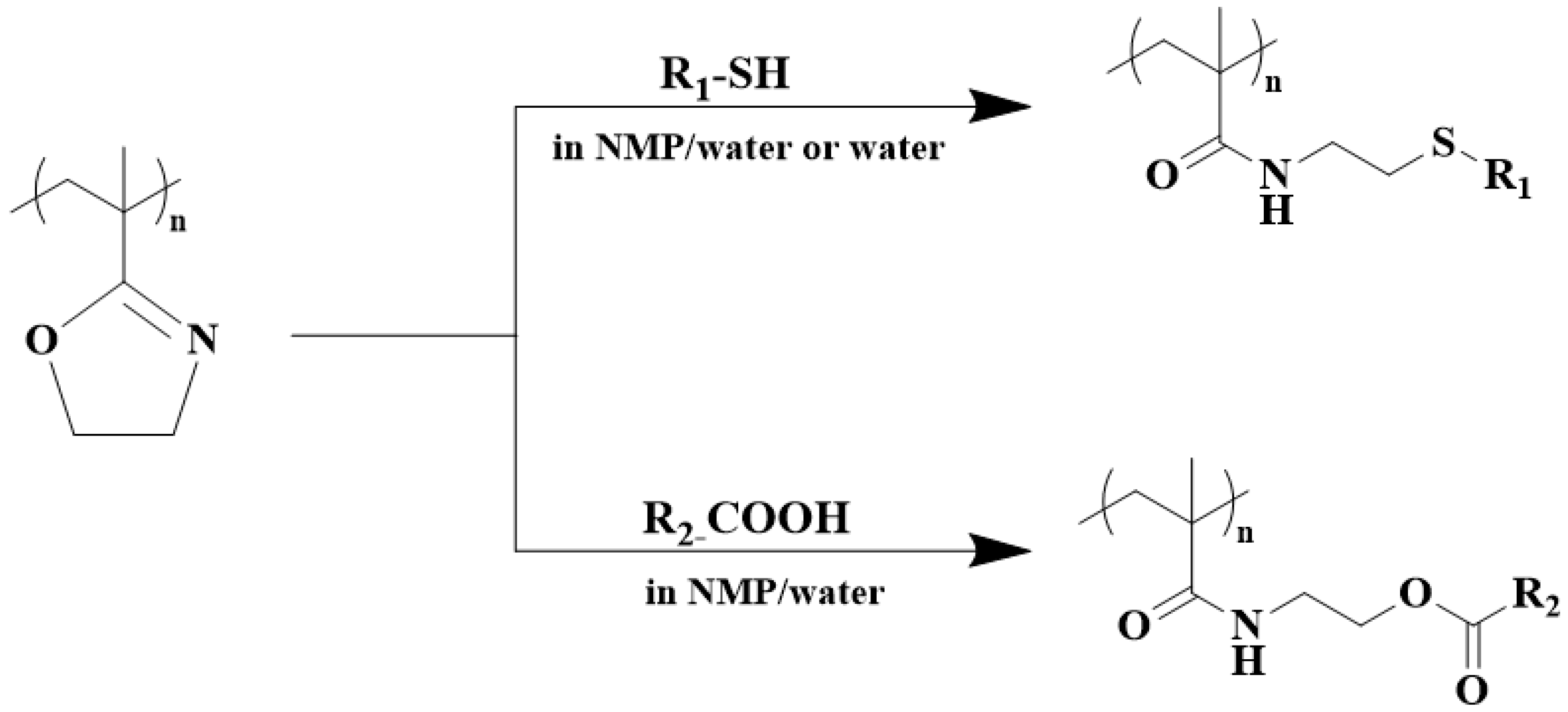
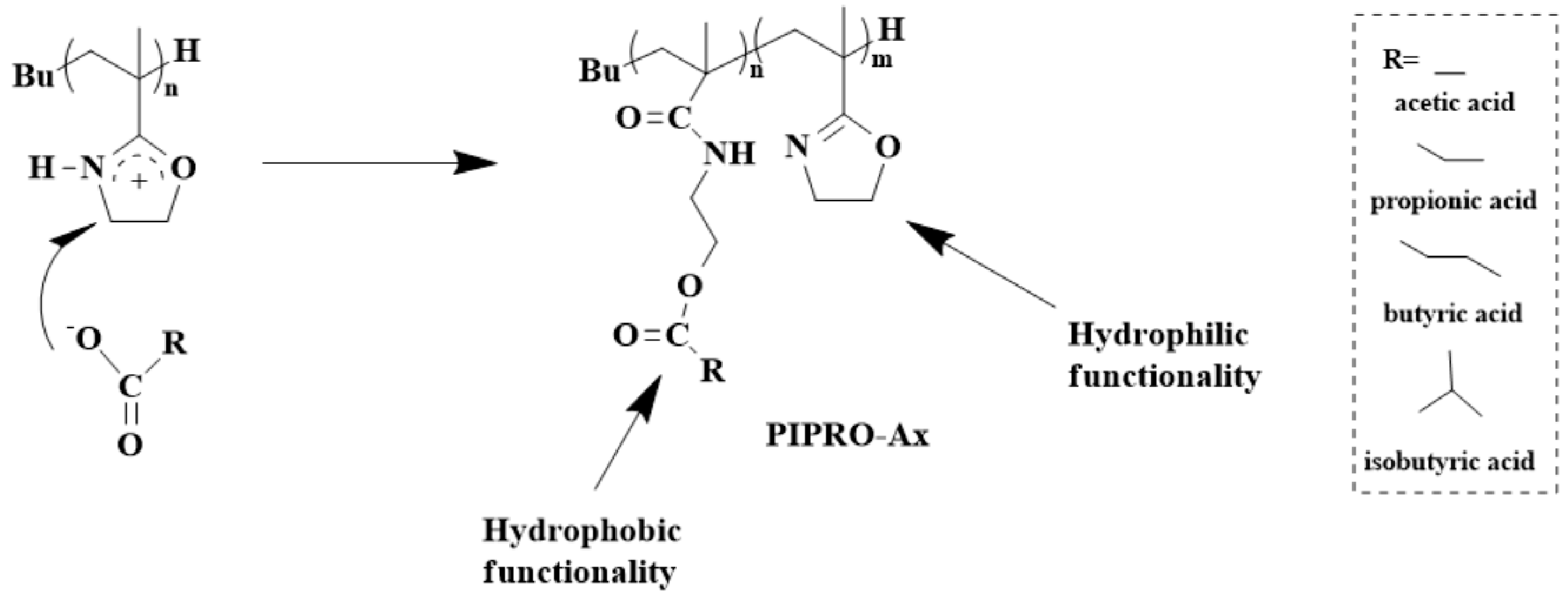
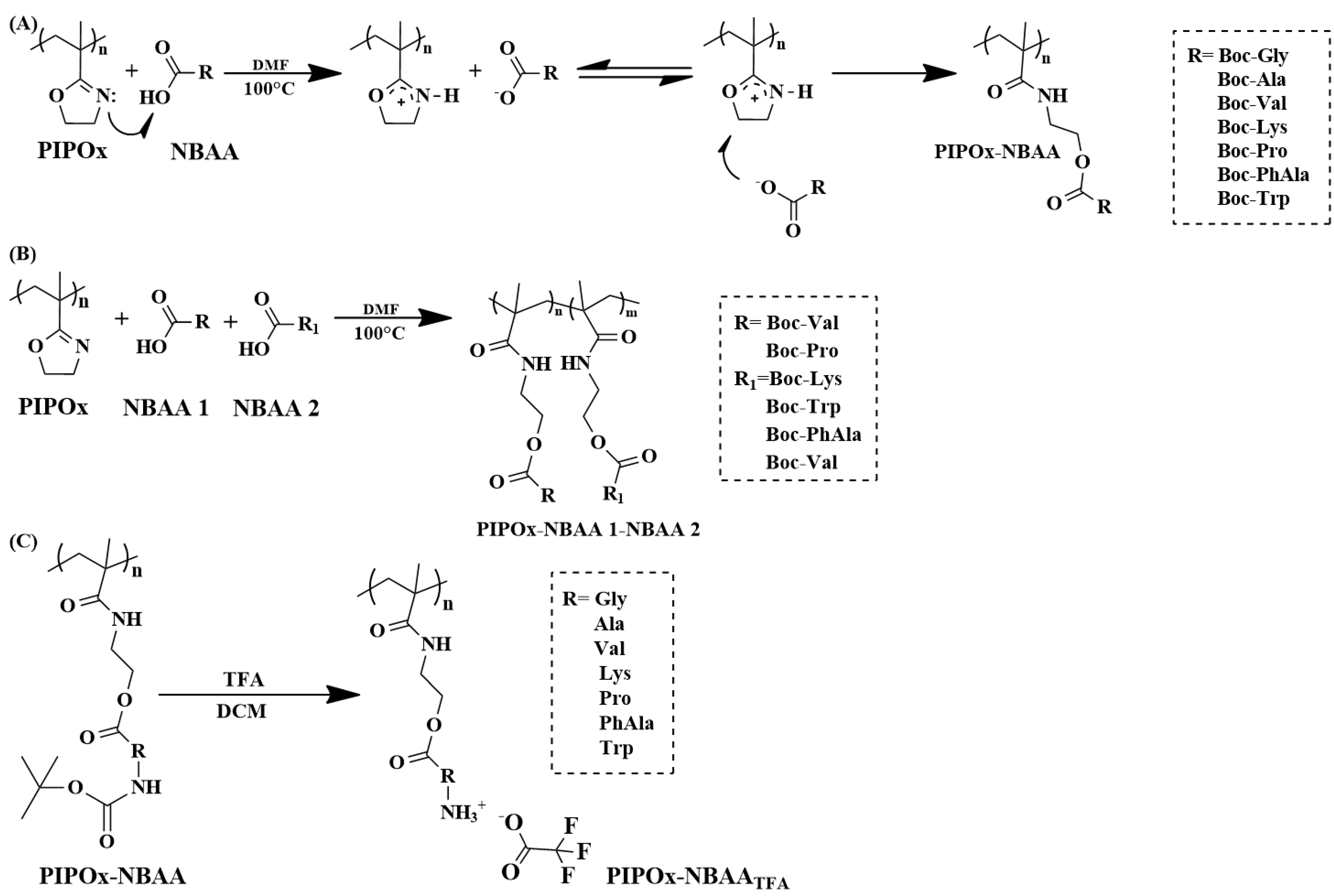
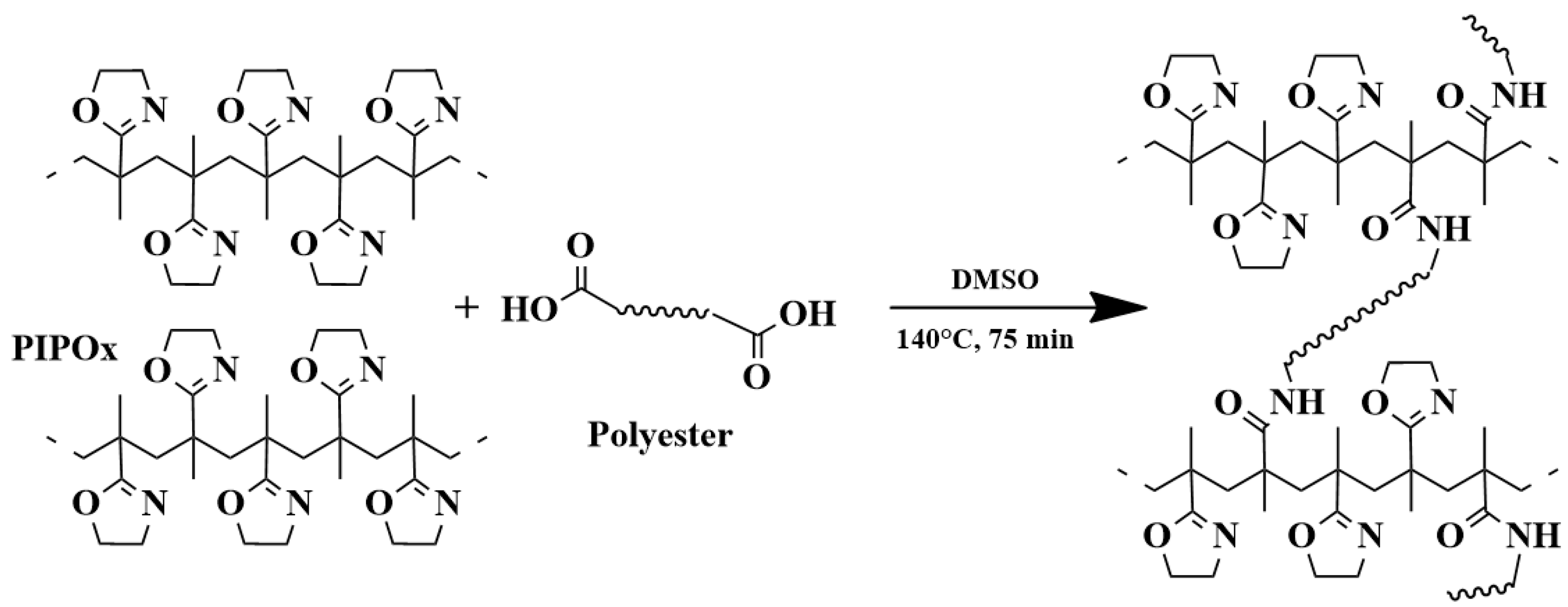
4. Post-Polymerization Modification of Poly(2-isopropenyl-2-oxazoline)
5. Poly(2-isopropenyl-2-oxazoline) in Biomedical Applications
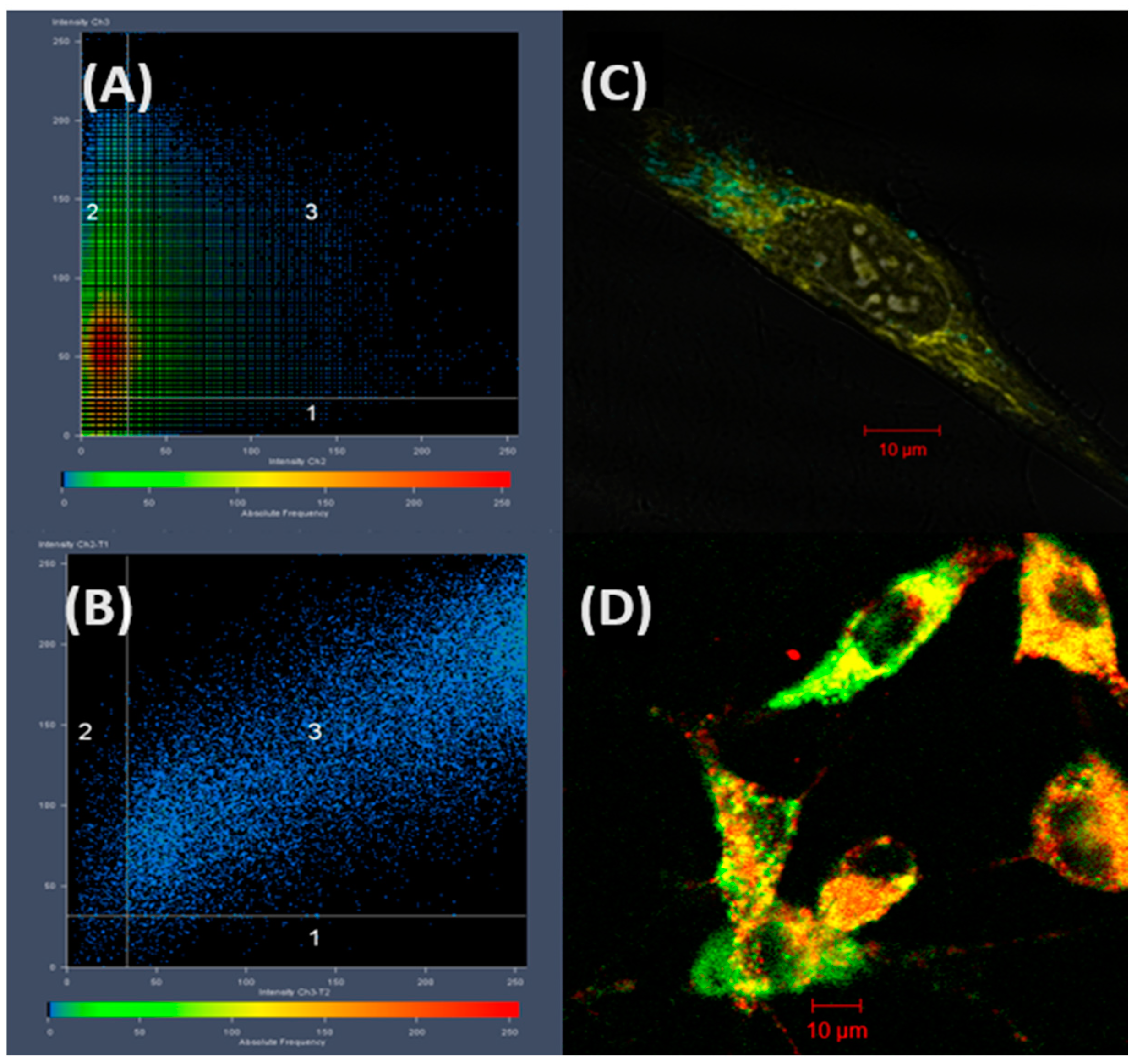
5.1. Biocompatibility and Immunocompatibility of Poly(2-isopropenyl-2-oxazoline)
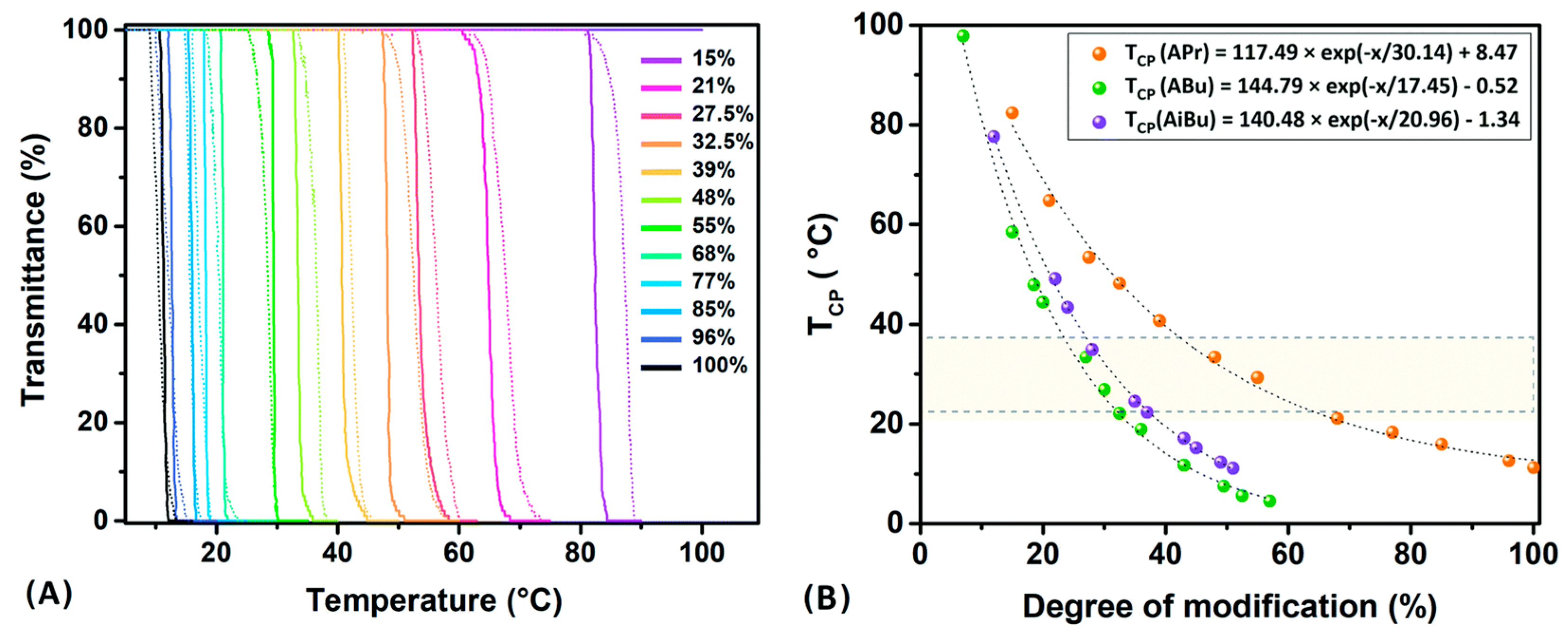
5.2. Thermosensitive Polymers
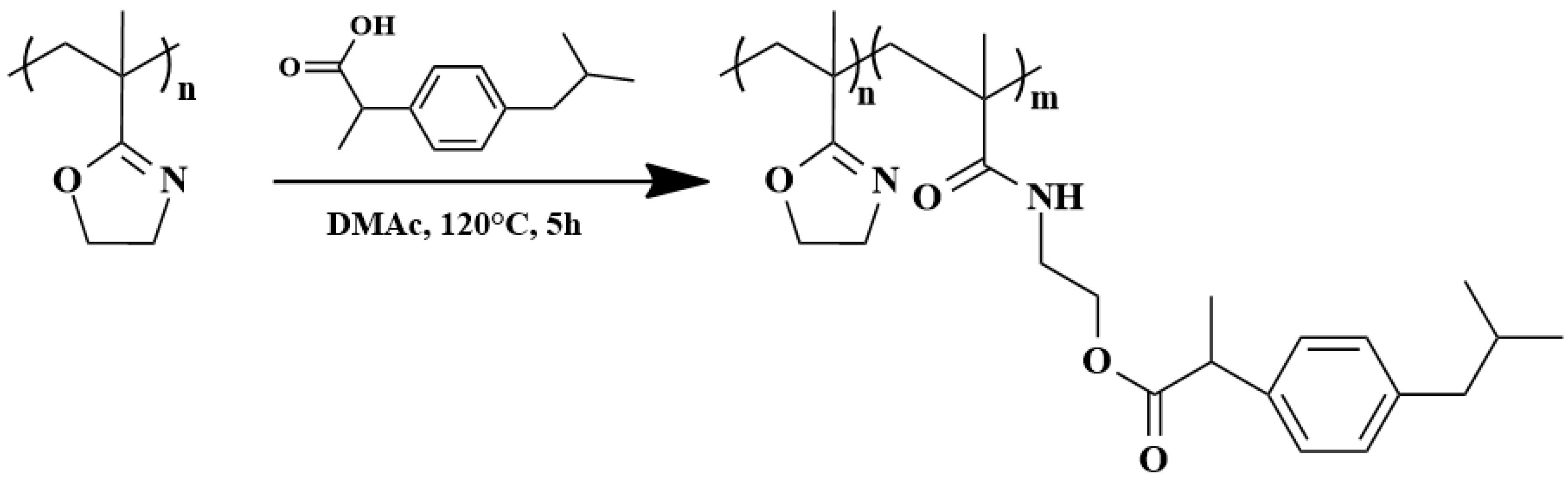
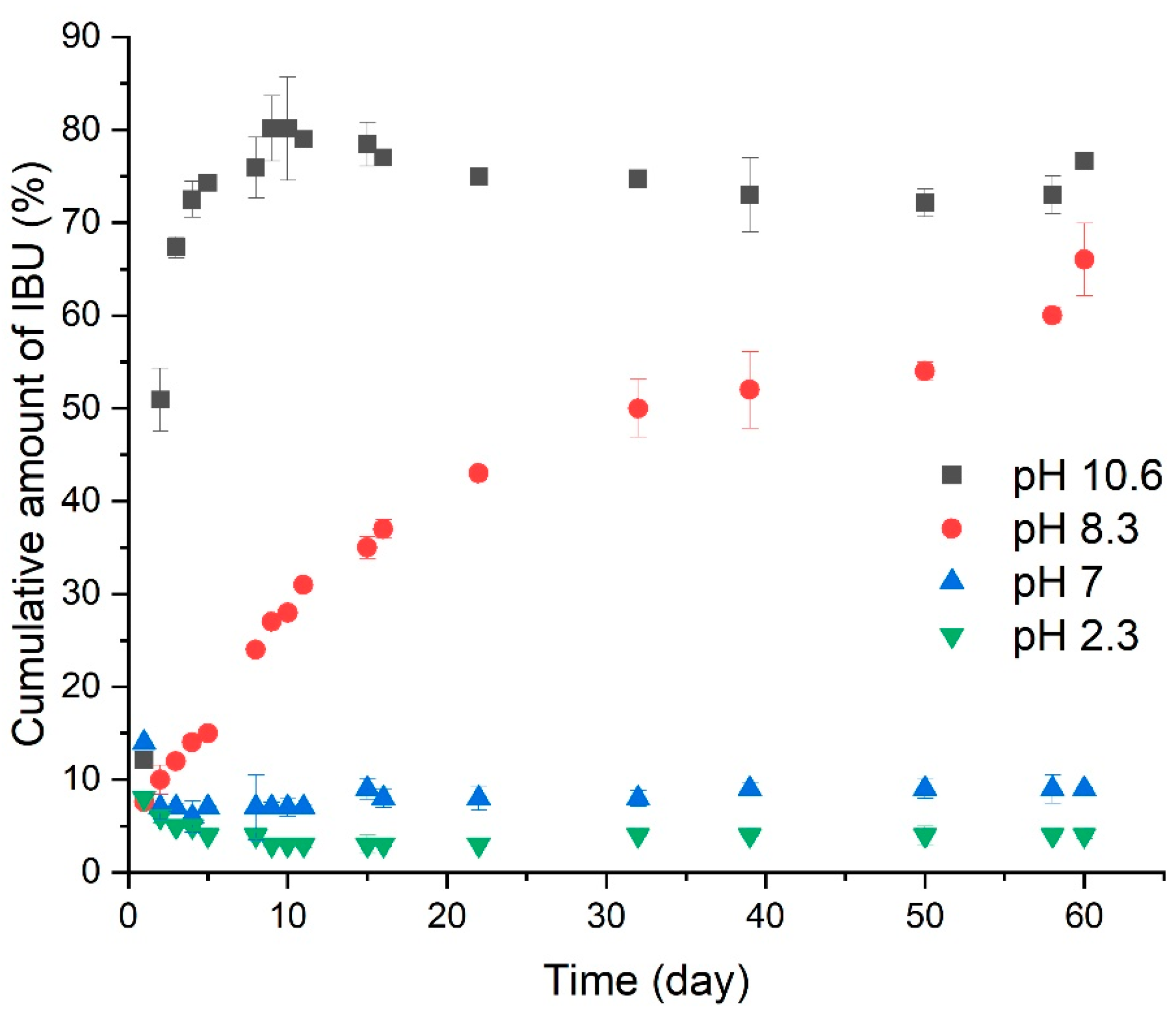
5.3. Drug Conjugates
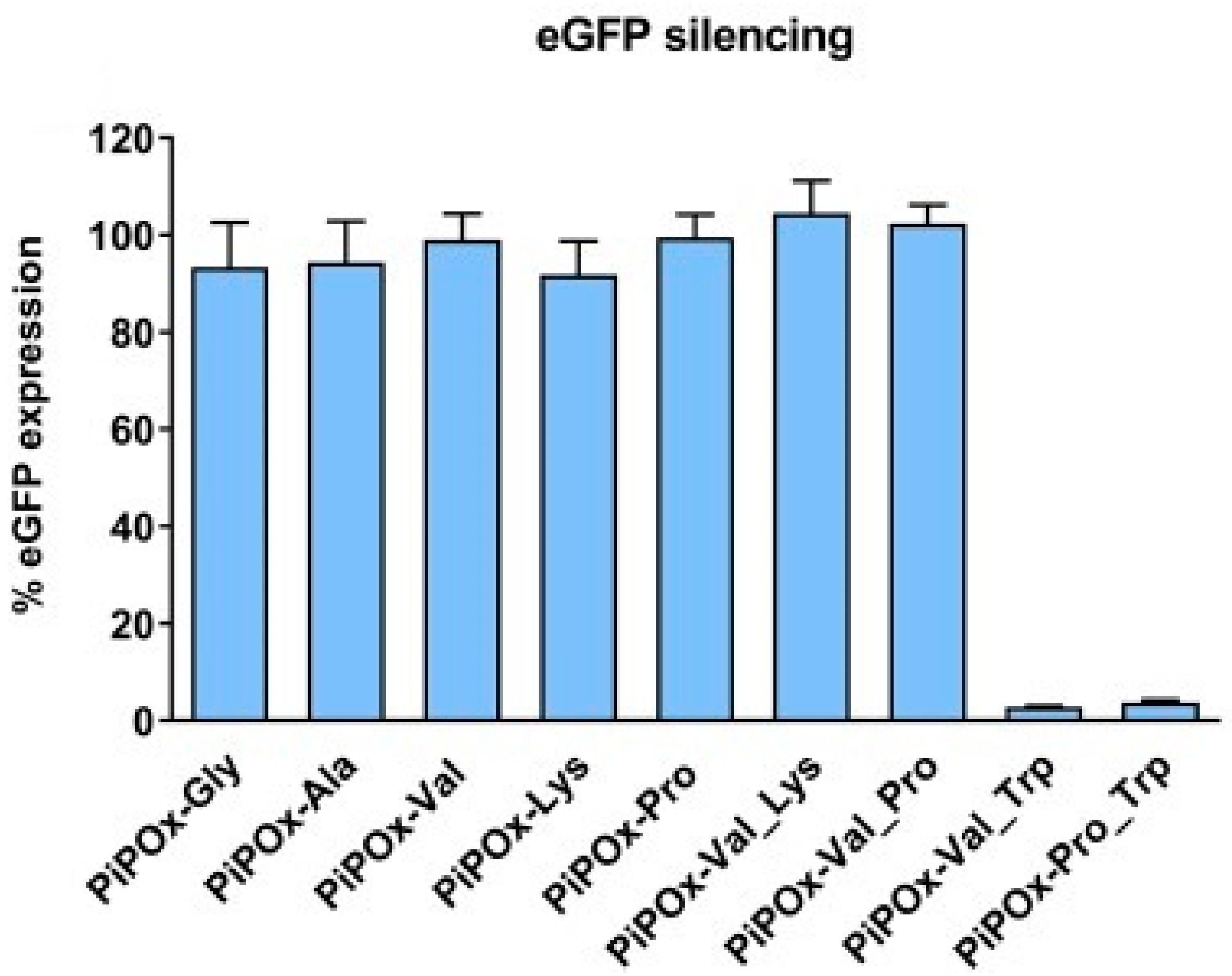
5.4. Cationic Polymers
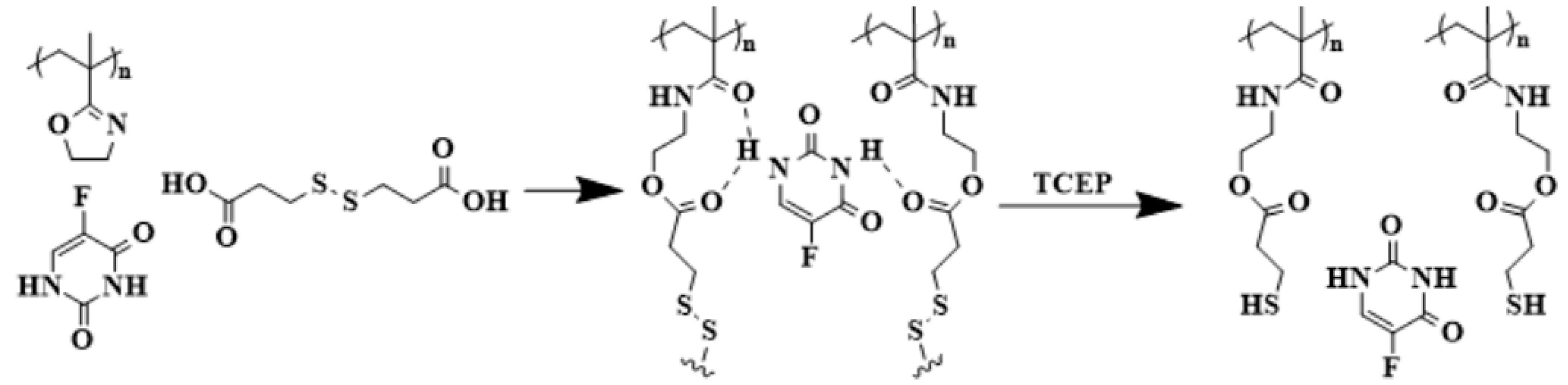
5.5. Hydrogels
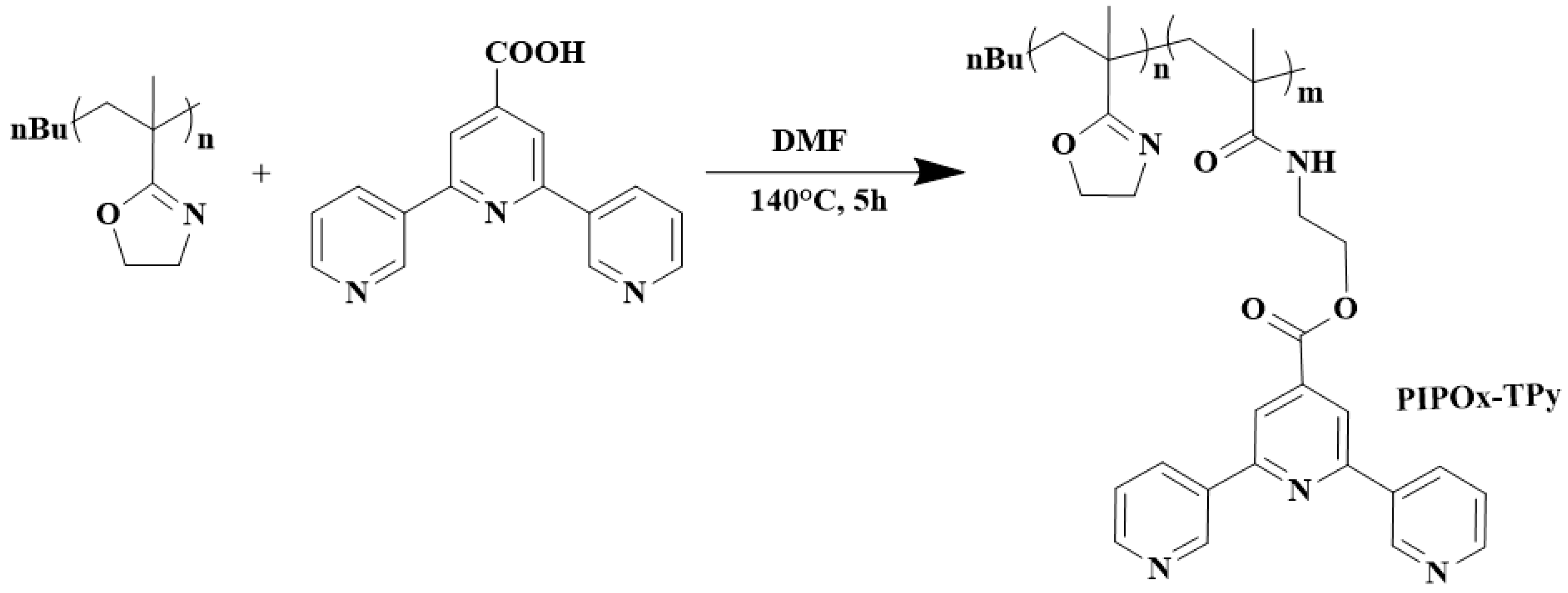
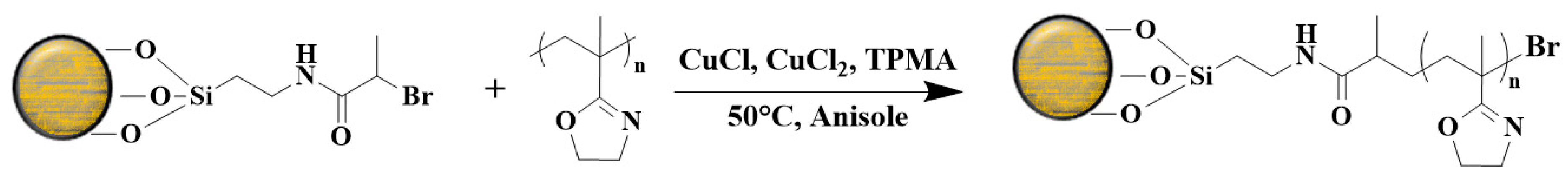
5.6. Surface, Molecular, and Bottle-Brush Polymers
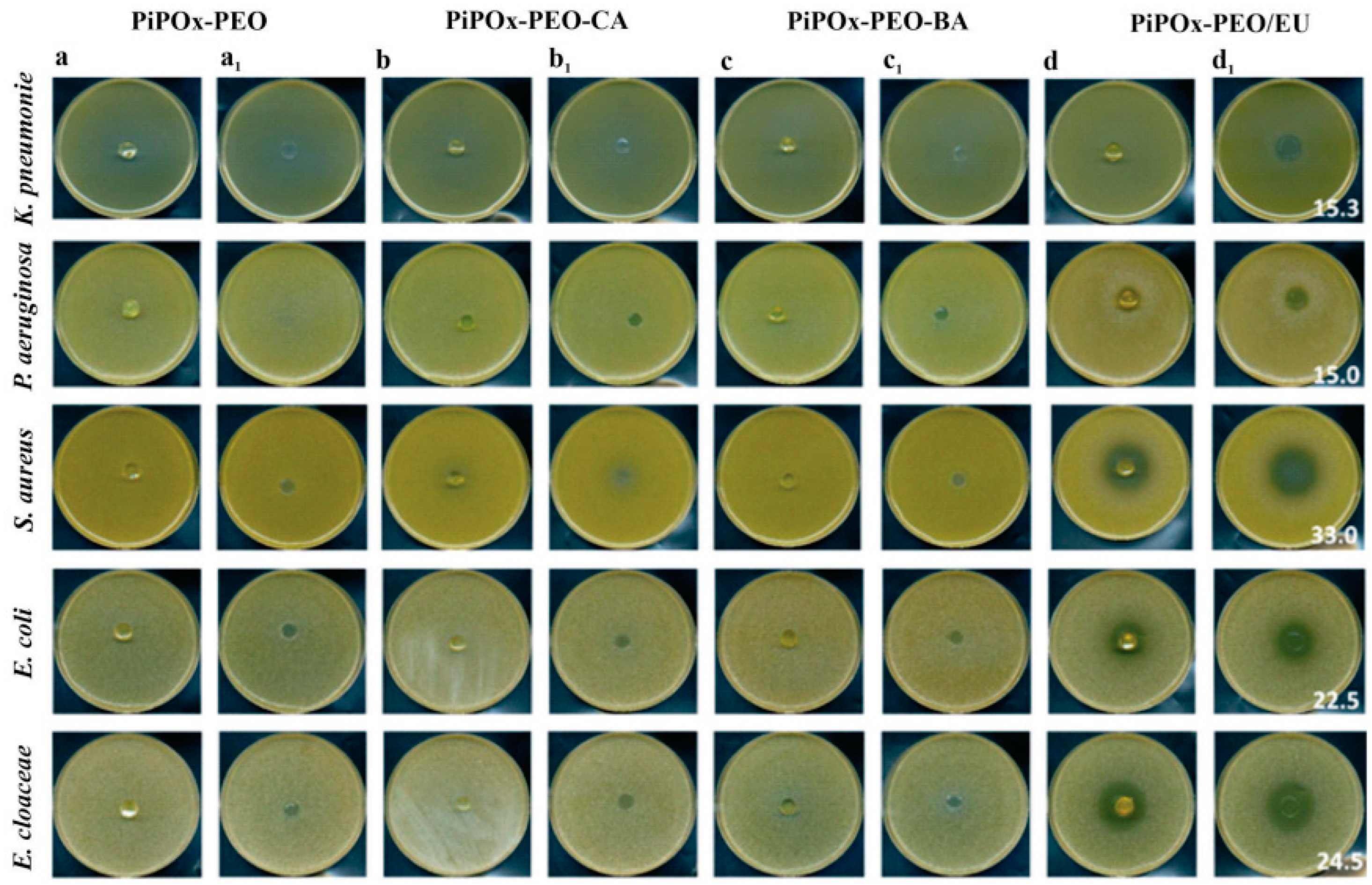
5.7. Polymer Coatings
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- EL-Ghoul, Y.; Alminderej, F.M.; Alsubaie, F.M.; Alrasheed, R.; Almousa, N.H. Recent Advances in Functional Polymer Materials for Energy, Water, and Biomedical Applications: A Review. Polymers 2021, 13, 4327. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.Y.; Hsiue, G.H. Functional biomedical polymers for corneal regenerative medicine. React. Funct. Polym. 2007, 67, 1284–1291. [Google Scholar] [CrossRef]
- Dong, R.; Zhou, Y.; Huang, X.; Zhu, X.; Lu, Y.; Shen, J. Functional Supramolecular Polymers for Biomedical Applications. Adv. Mater. 2015, 27, 498–526. [Google Scholar] [CrossRef] [PubMed]
- Jagur-Grodzinski, J. Biomedical application of functional polymers. React. Funct. Polym. 1999, 39, 99–138. [Google Scholar] [CrossRef]
- Pattanashetti, N.A.; Heggannavar, G.B.; Kariduraganavar, M.Y. Smart Biopolymers and Their Biomedical Applications. Proc. Manufact. 2017, 12, 263–279. [Google Scholar] [CrossRef]
- Biswas, M.C.; Jony, B.; Nandy, P.K.; Chowdhury, R.A.; Halder, S.; Kumar, D.; Ramakrishna, S.; Hassan, M.; Ahsan, A.; Hoque, E.; et al. Recent Advancement of Biopolymers and Their Potential Biomedical Applications. J. Polym. Environ. 2022, 30, 51–74. [Google Scholar] [CrossRef]
- Babu, R.P.; O’Connor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 8. [Google Scholar] [CrossRef]
- Kopeček, J. Smart and genetically engineered biomaterials and drug delivery systems. Eur. J. Pharm. Sci. 2003, 20, 1–16. [Google Scholar] [CrossRef]
- Kopeček, J.; Kopečková, P. HPMA copolymers: Origins, early developments, present, and future. Adv. Drug Deliv. Rev. 2010, 62, 122–149. [Google Scholar] [CrossRef]
- Lanzalaco, S.; Armelin, E. Poly(N-isopropylacrylamide) and Copolymers: A Review on Recent Progresses in Biomedical Applications. Gels 2017, 3, 36. [Google Scholar] [CrossRef]
- Bobde, Y.; Biswas, S.; Ghosh, B. Current trends in the development of HPMA-based block copolymeric nanoparticles for their application in drug delivery. Eur. Polym. J. 2020, 139, 110018. [Google Scholar] [CrossRef]
- Pasut, G.; Veronese, F.M. Polymer–drug conjugation, recent achievements and general strategies. Prog. Polym. Sci. 2007, 32, 933–961. [Google Scholar] [CrossRef]
- Pasut, G.; Veroneso, F.M. State of the art in PEGylation: The great versatility achieved after forty years of research. J. Control. Release 2012, 161, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Kurakula, M.; Kotesware Rao, G.S.N. Pharmaceutical assessment of polyvinylpyrrolidone (PVP): As excipient from conventional to controlled delivery systems with a spotlight on COVID-19 inhibition. J. Drug Deliv. Sci. Technol. 2020, 60, 102046. [Google Scholar] [CrossRef] [PubMed]
- Teasdale, I.; Brüggemann, O. Polyphosphazenes: Multifunctional, Biodegradable Vehicles for Drug and Gene Delivery. Polymers 2013, 5, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Ogueri, K.S.; Ogueri, K.S.; Allcock, H.R.; Laurencin, C.T. Polyphosphazene polymers: The next generation of biomaterials for regenerative engineering and therapeutic drug delivery. J. Vac. Sci. Technol. B 2020, 38, 030801. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.; Ke, P.C.; Davis, T.P.; Kempe, K. Poly(2-oxazoline)-based micro- and nanoparticles: A review. Eur. Polym. J. 2017, 88, 486–515. [Google Scholar] [CrossRef]
- Mahand, S.N.; Aliakbarzadeh, S.; Moghaddam, A.; Moghaddam, A.S.; Kruppke, B.; Nasrollahzadeh, M.; Khonakdar, H.A. Polyoxazoline: A review article from polymerization to smart behaviors and biomedical applications. Eur. Polym. J. 2022, 178, 111484. [Google Scholar] [CrossRef]
- Hoogenboom, R. The future of poly(2-oxazoline)s. Eur. Polym. J. 2022, 179, 111521. [Google Scholar] [CrossRef]
- Lusina, A.; Nazim, T.; Cegłowski, M. Poly(2-oxazoline)s as Stimuli-Responsive Materials for Biomedical Applications: Recent Developments of Polish Scientists. Polymers 2022, 19, 4176. [Google Scholar] [CrossRef]
- Jana, S.; Hoogenboom, R. Poly(2-oxazoline)s: A comprehensive overview of polymer structures and their physical properties—An update. Polym. Int. 2022, 71, 935–949. [Google Scholar] [CrossRef]
- Andreasch, R. Zur Kenntniss des Allylharnstoffs. Monatshefte Für Chem. 1884, 5, 33–46. [Google Scholar] [CrossRef]
- Kagiya, T.; Narisawa, S.; Maeda, T.; Fukui, K. Ring-opening polymerization of 2-substituted 2-oxazolines. J. Polym. Sci. Polym. Lett. Ed. 1966, B4, 441. [Google Scholar] [CrossRef]
- Seeliger, W.; Thier, W. Über die Polymerisation der Δ2-Oxazoline. Angew. Chem. 1966, 78, 613. [Google Scholar] [CrossRef]
- Bassiri, T.G.; Levi, A.J.; Litt, M.H. Polymerization of cyclic imino ethers. I. Oxazolines. J. Polym. Sci. Part B Polym. Lett. 1967, 5, 871–879. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Sheetz, D.P. Homopolymerization of 2-alkyl- and 2-aryl-2-oxazolines. J. Polym. Sci. Part A1 Polym. Chem. 1966, 4, 2253. [Google Scholar] [CrossRef]
- Kagiya, T.; Matsuda, T.; Zushi, K. Radical Copolymerization of 2-lsopropenyl-2-oxazoline with Styrene in the Presence of Lewis Acids. J. Macromol. Sci. Part A Chem. 1972, 6, 1349–1372. [Google Scholar] [CrossRef]
- Kagiya, T.; Matsuda, T. Selective Polymerization of 2-Isopropenyl-2-oxazoline and Cross-linking Reaction of the Polymers. Polym. J. 1972, 3, 307–314. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Thill, B.P.; Fazio, M.J. Ionic Oligomerization and Polymerization of 2-Alkenyl-2-Oxazolines. Polym. J. 1980, 12, 661–675. [Google Scholar] [CrossRef]
- Liu, N.C.; Xie, H.Q.; Baker, W.E. Comparison of the effectiveness of different basic functional groups for the reactive compatibilization of polymer blends. Polymer 1993, 34, 4680–4687. [Google Scholar] [CrossRef]
- Kroneková, Z.; Mikulec, M.; Petrenčíková, N.; Paulovičová, E.; Paulovičová, L.; Jančinová, V.; Nosáľ, R.; Reddy, P.S.; Shimoga, G.D.; Chorvát, D., Jr.; et al. Ex Vivo and In Vitro Studies on the Cytotoxicity and Immunomodulative Properties of Poly(2-isopropenyl-2-oxazoline) as a New Type of Biomedical Polymer. Macromol. Biosci. 2016, 16, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Nishikubo, T.; Kameyama, A.; Tokai, H. Synthesis of Polymers in Aqueous Solutions. Selective Addition Reaction of Poly(2-isopropenyl-2-oxazoline) with Thiols and Carboxylic Acids in Aqueous Solutions. Polym. J. 1996, 28, 134–138. [Google Scholar] [CrossRef]
- Zhang, N.; Huber, S.; Schulz, A.; Luxenhofer, R.; Jordan, R. Cylindrical Molecular Brushes of Poly(2-Oxazoline)s from 2-Isopropenyl-2-Oxazoline. Macromolecules 2009, 42, 2215–2221. [Google Scholar] [CrossRef]
- Weber, C.; Remzi Becer, C.; Guenther, W.; Hoogenboom, R.; Schubert, U.S. Dual Responsive Methacrylic Acid and O1igo(2-Ethyl-2-Oxazoline) Containing Graft Copolymers. Macromolecules 2010, 43, 160–167. [Google Scholar] [CrossRef]
- Zhang, N.; Salzinger, S.; Soller, B.S.; Rieger, B. Rare Earth Metal-Mediated Group-Transfer Polymerization: From Defined Polymer Microstructures to High-Precision Nano-Scaled Objects. J. Am. Chem. Soc. 2013, 135, 8810–8813. [Google Scholar] [CrossRef] [PubMed]
- Zanini, S.; Zoia, L.; Pergola, D.R.; Riccardi, C. Pulsed plasma-polymerized 2-isopropenyl-2-oxazoline coatings: Chemical characterization and reactivity studies. Surf. Coat. Technol. 2018, 334, 173–181. [Google Scholar] [CrossRef]
- Jerca, F.A.; Jerca, V.V.; Anghelache, A.M.; Vuluga, D.M.; Hoogenboom, R. Poly(2-Isopropenyl-2-Oxazoline) as a Versatile Platform towards Thermoresponsive Copolymers. Polym. Chem. 2018, 9, 3473–3478. [Google Scholar] [CrossRef]
- Raus, V.; Hološ, A.; Kronek, J.; Mosnáček, J. Well-Defined Linear and Grafted Poly(2-Isopropenyl-2-Oxazoline)s Prepared via Copper-Mediated Reversible-Deactivation Radical Polymerization Methods. Macromolecules 2020, 53, 2077–2087. [Google Scholar] [CrossRef]
- Cegłowski, M.; Jerca, V.V.; Jerca, F.A.; Hoogenboom, R. Reduction-Responsive Molecularly Imprinted Poly(2-isopropenyl-2-oxazoline) for Controlled Release of Anticancer Agents. Pharmaceutics 2020, 12, 506. [Google Scholar] [CrossRef]
- Kopka, B.; Kost, B.; Pawlak, A.; Tomaszewska, A.; Krupa, A.; Basko, M. Covalent segmented polymer networks composed of poly(2-isopropenyl-2-oxazoline) and selected aliphatic polyesters: Designing biocompatible amphiphilic materials containing degradable blocks. Soft Matter 2023, 19, 6987–6999. [Google Scholar] [CrossRef]
- Jerca, F.A.; Jerca, V.V.; Hoogenboom, R. In Vitro Assessment of the Hydrolytic Stability of Poly(2-Isopropenyl-2-Oxazoline). Biomacromolecules 2021, 22, 5020–5032. [Google Scholar] [CrossRef] [PubMed]
- Ilčíková, M.; Mrlík, M.; Cvek, M.; Bondarev, D.; Kroneková, Z.; Kronek, J.; Kasák, P.; Mosnáček, J. Atom Transfer Radical Polymerization of 2-Isopropenyl-2-Oxazoline in Solution and from the Surface of Carbonyl Iron Particles toward Fabrication of a Cytocompatible Magneto-Responsive Hybrid Filler. Macromolecules 2023, 56, 3904–3912. [Google Scholar] [CrossRef]
- Jerca, F.A.; Muntean, C.; Remaut, K.; Jerca, V.V.; Raemdonck, K.; Hoogenboom, R. Cationic amino-acid functionalized polymethacrylamide vectors for siRNA transfection based on modification of poly(2-isopropenyl-2-oxazoline). J. Control. Release 2023, 364, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Luxenhofer, R.; Jordan, R. Thermoresponsive Poly(2-Oxazoline) Molecular Brushes by Living Ionic Polymerization: Modulation of the Cloud Point by Random and Block Copolymer Pendant Chains. Macromol. Chem. Phys. 2012, 213, 1963. [Google Scholar] [CrossRef]
- Paulovičová, E.; Kroneková, Z.; Paulovičová, L.; Majerčíková, M.; Kronek, J. Cell-Mediated Immunoreactivity of Poly(2-isopropenyl-2-oxazoline) as Promising Formulation for Immunomodulation. Materials 2021, 14, 1371. [Google Scholar] [CrossRef] [PubMed]
- Kopka, B.; Kost, B.; Basko, M. Poly(2-isopropenyl-2-oxazoline) as a reactive polymer for materials development. Polym. Chem. 2022, 13, 4736. [Google Scholar] [CrossRef]
- Witte, H.; Seeliger, W. Simple Synthesis of 2-Substituted 2-Oxazolines and 5,6-Dihydro-4H-1,3-oxazines. Angew. Chem. Int. Ed. Engl. 1972, 11, 287–288. [Google Scholar] [CrossRef]
- Seeliger, W.; Aufderhaar, E.; Diepers, W.; Feinauer, R.; Nehring, R.; Thier, W.; Hellmann, H. Recent syntheses and reactions of cyclic imidic esters. Angew. Chem. Int. Ed. 1966, 5, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Frump, J.A. Oxazolines. Their preparation, reactions, and applications. Chem. Rev. 1971, 71, 483–505. [Google Scholar] [CrossRef]
- Kempe, K.; Lobert, M.; Hoogenboom, R.; Schubert, U.S. Screening the synthesis of 2-substituted-2-oxazolines. J. Comb. Chem. 2009, 11, 274–280. [Google Scholar] [CrossRef]
- Elliott, M.C.; Kruiswijk, E. Asymmetric hetero-Diels–Alder reactions of alkenyldihydrooxazoles. Synthesis of oxazolo [3, 2-c] pyrimidines and related compounds. J. Chem. Soc. Perkin Trans. 1 1999, 21, 3157. [Google Scholar] [CrossRef]
- Karpoormath, R.; Sayyad, N. Synthesis of Heterocyclic Compounds from Carboxamide and Carboxamide Derivatives with Haloalkanols. Patent WO/2022018651A2, 27 January 2022. [Google Scholar]
- Ude, W. Process for the Preparation of 2-Alkenyl-2-Oxazolines. German Patent DE19853532691A1, 26 March 1987. [Google Scholar]
- Culbertson, B.M. Cyclic imino ethers in step-growth polymerizations. Prog. Polym. Sci. 2002, 27, 579. [Google Scholar] [CrossRef]
- Miyamoto, M.; Sano, Y.; Kimura, Y.; Saegusa, T. “Spontaneous” Vinyl Polymerization of 2-Vinyl-2-oxazolines. Macromolecules 1985, 18, 1641–1648. [Google Scholar] [CrossRef]
- Rossegger, E.; Schenk, V.; Wiesbrock, F. Design Strategies for Functionalized Poly(2-oxazoline)s and Derived Materials. Polymers 2013, 5, 956–1011. [Google Scholar] [CrossRef]
- Aoi, K.; Okada, M. Polymerization of oxazolines. Prog. Polym. Sci. 1996, 21, 151–208. [Google Scholar] [CrossRef]
- Miyamoto, M.; Sano, Y.; Saegusa, T. Reactivity of Cyclic Imino Ether Salts Having Vinyl Group V. Spontaneous and Base-Catalyzed Polymerization of N-Protio Salts of 2-Alkenyl-2-oxazolines and 2-Alkenyl-5,6-dihydro-4H-1,3-oxazines. Polym. J. 1987, 19, 557–566. [Google Scholar] [CrossRef][Green Version]
- Miyamoto, M.; Lange, P.; Kanetaka, S.; Saegusa, T. Nucleophilic vinyl polymerization of 2-alkenyl-2-oxazolines activated by trimethylsilyl trifluoromethanesulfonate. Polym. Bull. 1995, 34, 249–256. [Google Scholar] [CrossRef]
- Hutter, N.A.; Reitinger, A.; Zhang, N.; Steenackers, M.; Williams, O.A.; Garrido, J.A.; Jordan, R. Microstructured poly(2-oxazoline) bottle-brush brushes on nanocrystalline diamond. Phys. Chem. Chem. Phys. 2010, 12, 4360–4366. [Google Scholar] [CrossRef] [PubMed]
- Kopka, B.; Kost, B.; Rajkowska, K.; Pawlak, A.; Kunicka-Styczynska, A.; Biela, T.; Basko, M. A simple strategy for efficient preparation of networks based on poly(2-isopropenyl-2- oxazoline), poly(ethylene oxide), and selected biologically active compounds: Novel hydrogels with antibacterial properties. Soft Matter 2021, 17, 10683–10695. [Google Scholar] [CrossRef]
- He, J.; Zhang, Y.; Chen, E.Y.X. Synthesis of Pyridine- and 2-Oxazoline-Functionalized Vinyl Polymers by Alane-Based Frustrated Lewis Pairs. Synlett 2014, 25, 1534–1538. [Google Scholar]
- Altenbuchner, P.T.; Soller, B.S.; Kissling, S.; Bachmann, T.; Kronast, A.; Vagin, S.I.; Rieger, B. Versatile 2-Methoxyethylaminobis(Phenolate)Yttrium Catalysts: Catalytic Precision Polymerization of Polar Monomers via Rare Earth Metal-Mediated Group Transfer Polymerization. Macromolecules 2014, 47, 7742–7749. [Google Scholar] [CrossRef]
- Feng, H.; Changez, M.; Hong, K.; Mays, J.W.; Kang, N.-G. 2-Isopropenyl-2-oxazoline: Well-Defined Homopolymers and Block Copolymers via Living Anionic Polymerization. Macromolecules 2017, 50, 54–62. [Google Scholar] [CrossRef]
- Zhang, T.; Du, Y.; Gieseler, D.; Schneider, M.; Hafner, D.; Sheng, W.; Li, W.; Lange, F.; Wegener, E.; Amin, I.; et al. Facile Fabrication of Bio- and Dual-Functional Poly(2-oxazoline) Bottle-Brush Brush Surfaces. Chem. Eur. J. 2020, 26, 2749–2753. [Google Scholar]
- Jerca, F.A.; Anghelache, A.M.; Ghibu, E.; Cecoltan, S.; Stancu, I.C.; Trusca, R.; Vasile, E.; Teodorescu, M.; Vuluga, D.M.; Hoogenboom, R.; et al. Poly(2-Isopropenyl-2-Oxazoline) Hydrogels for Biomedical Applications. Chem. Mater. 2018, 30, 7938–7949. [Google Scholar] [CrossRef]
- Kroneková, Z.; Majerčíková, M.; Paulovičová, E.; Danko, M.; Markus, J.; Letašiová, S.; Kronek, J. Cytotoxicity and bioimmunological activity of poly(2-isopropenyl-2-oxazoline) conjugates with ibuprofen using 3D reconstructed tissue models. Biomacromolecules 2024, 25, 3288–3301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Vancoillie, G.; Mees, M.A.; Hoogenboom, R. Thermoresponsive polymeric temperature sensors with broad sensing regimes. Polym. Chem. 2015, 6, 2396–2400. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Y.; Wang, K.; Zhou, H.; Zhang, W. N-Ester-substituted polyacrylamides with a tunable lower critical solution temperature (LCST): The N-ester-substitute dependent thermoresponse. Polym. Chem. 2016, 7, 3509–3519. [Google Scholar] [CrossRef]
- Wang, K.; Song, Z.; Liu, C.; Zhang, W. RAFT synthesis of triply responsive poly[N-[2-(dialkylamino)ethyl]acrylamide]s and their N-substitute determined response. Polym. Chem. 2016, 7, 3423–3433. [Google Scholar] [CrossRef]
- Jin, N.; Woodcock, J.W.; Xue, C.; O’Lenick, T.G.; Jiang, X.; Jin, S.; Dadmun, M.D.; Zhao, B. Tuning of Thermo-Triggered Gel-to-Sol Transition of Aqueous Solution of Multi-Responsive Diblock Copolymer Poly(methoxytri(ethylene glycol) acrylate-co-acrylic acid)-b-poly(ethoxydi(ethylene glycol) acrylate). Macromolecules 2011, 44, 3556–3566. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Schlaad, H. Thermoresponsive poly(2-oxazoline)s, polypeptoids, and polypeptides. Polym. Chem. 2017, 8, 24–40. [Google Scholar] [CrossRef]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive Polymers for Biomedical Applications. Polymers 2011, 3, 1215. [Google Scholar] [CrossRef]
- Allen, T.M.; Curris, P.M. Drug Delivery Systems: Entering the Mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef]
- Khandare, J.; Minko, T. Polymer–drug conjugates: Progress in polymeric prodrugs. Prog. Polym. Sci. 2006, 31, 359–397. [Google Scholar] [CrossRef]
- Junyaprasert, V.B.; Thummarati, P. Innovative Design of Targeted Nanoparticles: Polymer–Drug Conjugates for Enhanced Cancer Therapy. Pharmaceutics 2023, 15, 2216. [Google Scholar] [CrossRef]
- Kolate, A.; Baradia, D.; Patil, S.; Vhora, I.; Kore, G.; Misra, A. PEG—A versatile conjugating ligand for drugs and drug delivery systems. J. Control. Release 2014, 192, 67–81. [Google Scholar] [CrossRef]
- Etrych, T.; Chytil, P.; Jelínková, M.; Říhová, B.; Ulbrich, K. Synthesis of HPMA Copolymers Containing Doxorubicin Bound via a Hydrazone Linkage. Effect of Spacer on Drug Release and in vitro Cytotoxicity. Macromol. Biosci. 2002, 2, 43–52. [Google Scholar] [CrossRef]
- Cai, X.; Dou, R.; Guo, C.; Tang, J.; Li, X.; Chen, J.; Zhang, J. Cationic Polymers as Transfection Reagents for Nucleic Acid Delivery. Pharmaceutics 2023, 15, 1502. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Skwarczynski, M.; Toth, I. Polyelectrolyte-Based Platforms for the Delivery of Peptides and Proteins. ACS Biomater. Sci. Eng. 2019, 5, 4937–4950. [Google Scholar] [CrossRef] [PubMed]
- Mok, H.; Park, T.G. Functional polymers for targeted delivery of nucleic acid drugs. Macromol. Biosci. 2009, 9, 731–743. [Google Scholar] [CrossRef]
- Breen, C.; Watson, R. Polycation-Exchanged Clays as Sorbents for Organic Pollutants: Influence of Layer Charge on Pollutant Sorption Capacity. J. Colloid Interface Sci. 1998, 208, 422–442. [Google Scholar] [CrossRef]
- Fattahi, N.; Gorgannezhad, L.; Masoule, S.F.; Babanejad, N.; Ramazani, A.; Raoufi, M.; Sharifikolouei, E.; Foroumadi, A.; Khoobi, M. PEI-based functional materials: Fabrication techniques, properties, and biomedical applications. Adv. Colloid Interface Sci. 2024, 325, 103119. [Google Scholar] [CrossRef]
- Casper, J.; Schenk, S.H.; Parhizkar, E.; Detampel, P.; Dehshahri, A.; Huwyler, J. Polyethylenimine (PEI) in gene therapy: Current status and clinical applications. J. Control. Release 2023, 362, 667–691. [Google Scholar] [CrossRef]
- Shen, W.; He, P.; Xiao, C.; Chen, X. From Antimicrobial Peptides to Antimicrobial Poly(α-amino acid)s. Adv. Healthc. Mater. 2018, 7, 1800354. [Google Scholar] [CrossRef]
- El-Araby, A.; Janati, W.; Ullah, R.; Ercisli, S.; Errachidi, F. Chitosan, chitosan derivatives, and chitosan-based nanocomposites: Eco-friendly materials for advanced applications (a review). Front. Chem. 2024, 11, 1327426. [Google Scholar] [CrossRef] [PubMed]
- Mittal, H.; Ray, S.S.; Kaith, B.S.; Bhatia, J.K.; Sukriti; Sharma, J.; Alhassan, S.M. Recent progress in the structural modification of chitosan for applications in diversified biomedical fields. Eur. Polym. J. 2018, 109, 402–434. [Google Scholar] [CrossRef]
- Shah, R.; Kroneková, Z.; Zahoranová, A.; Roller, L.; Saha, N.; Saha, P.; Kronek, J. In vitro study of partially hydrolyzed poly(2-ethyl-2-oxazolines) as materials for biomedical applications. J. Mater. Sci. Mater. Med. 2015, 26, 157. [Google Scholar] [CrossRef]
- Haladjova, E.; Smolíček, M.; Ugrinova; Momekova, D.; Shestakova, P.; Kroneková, Z.; Kronek, J.; Rangelov, S. DNA delivery systems based on copolymers of poly (2-methyl-2-oxazoline) and polyethyleneimine: Effect of polyoxazoline moieties on the endo-lysosomal escape. J. Appl. Polym. Sci. 2020, 137, e49400. [Google Scholar] [CrossRef]
- Mees, M.; Haladjova, E.; Momekova, D.; Momekov, G.; Shestakova, P.; Tsvetanov, C.; Hoogenboom, R.; Rangelov, S. Partially Hydrolyzed Poly(n-propyl-2-oxazoline): Synthesis, Aqueous Solution Properties, and Preparation of Gene Delivery Systems. Biomacromolecules 2016, 17, 3580. [Google Scholar] [CrossRef] [PubMed]
- Van Kuringen, H.P.C.; Lenoir, J.; Adriens, E.; Bender, J.; Geest, B.G.D.; Hoogenboom, R. Partial hydrolysis of poly(2-ethyl-2-oxazoline) and potential implications for biomedical applications. Macromol. Biosci. 2012, 12, 1114–1123. [Google Scholar] [CrossRef] [PubMed]
- Kuskov, A.; Selina, O.; Kulikov, P.; Imatdinov, I.; Balysheva, V.; Kryukov, A.; Shtilman, M.; Markvicheva, E. Amphiphilic Poly(N-Vinylpyrrolidone) Nanoparticles Loaded with DNA Plasmids Encoding Gn and Gc Glycoproteins of the Rift Valley Fever Virus: Preparation and In Vivo Evaluation. ACS Appl. Bio. Mater. 2021, 4, 6084–6092. [Google Scholar] [CrossRef]
- Lin, C.-C.; Anseth, K.S. PEG Hydrogels for the Controlled Release of Biomolecules in Regenerative Medicine. Pharm. Res. 2009, 26, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Teodorescu, M.; Bercea, M. Poly(Vinylpyrrolidone)—A Versatile Polymer for Biomedical and Beyond Medical Applications. Polym.-Plast. Technol. Eng. 2015, 54, 923–943. [Google Scholar] [CrossRef]
- Kumar, A.; Han, S.S. PVA-Based Hydrogels for Tissue Engineering: A Review. Int. J. Polym. Mater. Polym. Biomat. 2017, 66, 159–182. [Google Scholar] [CrossRef]
- Macková, H.; Plichta, Z.; Hlídková, H.; Sedláček, O.; Konefal, R.; Sadakbayeva, Z.; Dušková-Smrčková, M.; Horák, D.; Kubinová, Š. Reductively Degradable Poly(2-Hydroxyethyl Methacrylate) Hydrogels with Oriented Porosity for Tissue Engineering Applications. ACS Appl. Mater. Interfaces 2017, 9, 10544–10553. [Google Scholar] [CrossRef] [PubMed]
- Zahoranová, A.; Kroneková, Z.; Zahoran, M.; Chorvát, D.; Janigová, I.; Kronek, J. Poly(2-oxazoline) Hydrogels Crosslinked with Aliphatic bis(2-oxazoline)s: Properties, Cytotoxicity, and Cell Cultivation. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 1548–1559. [Google Scholar] [CrossRef]
- Šrámková, P.; Zahoranová, A.; Kroneková, Z.; Šišková, A.; Kronek, J. Poly(2-oxazoline) hydrogels by photoinduced thiol-ene “click” reaction using different dithiol crosslinkers. J. Polym. Res. 2017, 24, 82. [Google Scholar] [CrossRef]
- Dargaville, T.R.; Forster, R.; Farrugia, B.L.; Kempe, K.; Voorhaar, L.; Schubert, U.S.; Hoogenboom, R. Poly(2-oxazoline) hydrogel monoliths via thiol-ene coupling. Macromol. Rapid Commun. 2012, 33, 1695–1700. [Google Scholar] [CrossRef]
- Fimberger, M.; Behrendt, A.; Jakopic, G.; Stelzer, F.; Kumbaraci, V.; Wiesbrock, F. Modification Pathways for Copoly (2-oxazoline) s Enabling Their Application as Antireflective Coatings in Photolithography. Macromol. Rapid Commun. 2016, 37, 233–238. [Google Scholar] [CrossRef]
- Xu, X.; Jerca, F.A.; Jerca, V.V.; Hoogenboom, R. Self-healing and moldable poly (2-isopropenyl-2-oxazoline) supramolecular hydrogels based on a transient metal coordination network. Macromolecules 2020, 53, 6566–6575. [Google Scholar] [CrossRef]
- Cegłowski, M.; Marien, Y.W.; Smeets, S.; De Smet, L.; D’hooge, D.R.; Schroeder, G.; Hoogenboom, R. Molecularly Imprinted Polymers with Enhanced Selectivity Based on 4-(Aminomethyl)pyridine-Functionalized Poly(2-oxazoline)s for Detecting Hazardous Herbicide Contaminants. Chem. Mater. 2022, 34, 84–96. [Google Scholar] [CrossRef]
- Cegłowski, M.; Schroeder, G.; Hoogenboom, R. Porous Poly(2-oxazoline)-Based Polymers for Removal and Quantification of Phenolic Compounds. Chem. Mater. 2020, 32, 6425–6436. [Google Scholar] [CrossRef]
- Cegłowski, M.; Hoogenboom, R. Molecularly Imprinted Poly(2-oxazoline) Based on Cross-Linking by Direct Amidation of Methyl Ester Side Chains. Macromolecules 2018, 51, 6468–6475. [Google Scholar] [CrossRef]
- Hucknall, A.; Rangarajan, S.; Chilkoti, A. In pursuit of zero: Polymer brushes that resist the adsorption of proteins. Adv. Mater. 2009, 21, 2441–2446. [Google Scholar] [CrossRef]
- Ma, H.; Li, D.; Sheng, X.; Zhao, B.; Chilkoti, A. Protein-resistant polymer coatings on silicon oxide by surface-initiated atom transfer radical polymerization. Langmuir 2006, 22, 3751–3756. [Google Scholar] [CrossRef] [PubMed]
- Vogler, E.A. Protein adsorption in three dimensions. Biomaterials 2012, 33, 1201–1237. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Cordero, R.; Tran, H.Q.; Ober, C.K. 50th Anniversary Perspective: Polymer Brushes: Novel Surfaces for Future Materials. Macromolecules 2017, 50, 4089–4113. [Google Scholar] [CrossRef]
- Prime, K.L.; Whitesides, G.M. Adsorption of proteins onto surfaces containing end-attached oligo (ethylene oxide): A model system using self-assembled monolayers. J. Am. Chem. Soc. 1993, 115, 10714–10721. [Google Scholar] [CrossRef]
- Sheth, S.R.; Leckband, D. Measurements of attractive forces between proteins and end-grafted poly (ethylene glycol) chains. Proc. Natl. Acad. Sci. USA 1997, 94, 8399–8404. [Google Scholar] [CrossRef] [PubMed]
- Herrwerth, S.; Eck, W.; Reinhardt, S.; Grunze, M. Factors that determine the protein resistance of oligoether self-assembled monolayers—Internal hydrophilicity, terminal hydrophilicity, and lateral packing density. J. Am. Chem. Soc. 2003, 125, 9359–9366. [Google Scholar] [CrossRef]
- Balamurugan, S.; Ista, L.K.; Yan, J.; Lopez, G.P.; Fick, J.; Himmelhaus, M.; Grunze, M. Reversible protein adsorption and bioadhesion on monolayers terminated with mixtures of oligo(ethylene glycol) and methyl groups. J. Am. Chem. Soc. 2005, 127, 14548–14549. [Google Scholar] [CrossRef]
- Sebra, R.P.; Reddy, S.K.; Masters, K.S.; Bowman, C.N.; Anseth, K.S. Controlled polymerization chemistry to graft architectures that influence cell-material interactions. Acta. Biomater. 2007, 3, 151–161. [Google Scholar] [CrossRef]
- Ionov, L.; Synytska, A.; Kaul, E.; Diez, S. Protein-Resistant Polymer Coatings Based on Surface-Adsorbed Poly(aminoethyl methacrylate)/Poly(ethylene glycol) Copolymers. Biomacromolecules 2012, 11, 233–237. [Google Scholar] [CrossRef]
- Matos-Perez, C.R.; Wilker, J.J. Ambivalent adhesives: Combining biomimetic cross-linking with antiadhesive oligo (ethylene glycol). Macromolecules 2012, 45, 6634–6639. [Google Scholar]
- Herold, A.; Keil, K.; Bruns, D.E. Oxidation of polyethylene glycols by alcohol dehydrogenase. Biochem. Pharmacol. 1989, 38, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.C.; Martinson, L.; Wagner, M.S.; Castner, D.G.; Ratner, B.D.; Horbett, T.A. PEO-like plasma polymerized tetraglyme surface interactions with leukocytes and proteins: In vitro and in vivo studies. J. Biomater. Sci. Polym. Ed. 2002, 13, 367–390. [Google Scholar] [CrossRef] [PubMed]
- Garay, R.P.; El-Gewely, R.; Armstrong, J.K.; Garratty, G.; Richette, P. Antibodies against polyethylene glycol in healthy subjects and in patients treated with PEG-conjugated agents. Expert Opin. Drug Deliv. 2012, 9, 1319–1323. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Steenackers, M.; Luxenhofer, R.; Jordan, R. Bottle-Brush Brushes: Cylindrical Molecular Brushes of Poly(2-oxazoline) on Glassy Carbon. Macromolecules 2009, 42, 5345–5351. [Google Scholar] [CrossRef]
- Zanini, S.; Lehocky, M.; Lopez-Garcia, J.; Riccardi, C. Plasma polymerization of 2-isopropenyl-2-oxazoline: Improvement of the coating stability by co-polymerization with 1-octene. Thin Solid Film 2019, 677, 55–61. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kronek, J.; Minarčíková, A.; Kroneková, Z.; Majerčíková, M.; Strasser, P.; Teasdale, I. Poly(2-isopropenyl-2-oxazoline) as a Versatile Functional Polymer for Biomedical Applications. Polymers 2024, 16, 1708. https://doi.org/10.3390/polym16121708
Kronek J, Minarčíková A, Kroneková Z, Majerčíková M, Strasser P, Teasdale I. Poly(2-isopropenyl-2-oxazoline) as a Versatile Functional Polymer for Biomedical Applications. Polymers. 2024; 16(12):1708. https://doi.org/10.3390/polym16121708
Chicago/Turabian StyleKronek, Juraj, Alžbeta Minarčíková, Zuzana Kroneková, Monika Majerčíková, Paul Strasser, and Ian Teasdale. 2024. "Poly(2-isopropenyl-2-oxazoline) as a Versatile Functional Polymer for Biomedical Applications" Polymers 16, no. 12: 1708. https://doi.org/10.3390/polym16121708
APA StyleKronek, J., Minarčíková, A., Kroneková, Z., Majerčíková, M., Strasser, P., & Teasdale, I. (2024). Poly(2-isopropenyl-2-oxazoline) as a Versatile Functional Polymer for Biomedical Applications. Polymers, 16(12), 1708. https://doi.org/10.3390/polym16121708








