Fabrication of Waterborne Silicone-Modified Polyurethane Nanofibers for Nonfluorine Elastic Waterproof and Breathable Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Spinning Solutions
2.3. Fabrication of Fluorine-Free Waterborne PUSX Nanofibrous Membranes
2.4. Characterization
2.5. Characterization of Waterproofness and Breathability
3. Results and Discussion
3.1. Design of Environmentally Friendly PUSX Nanofibrous WBMs via Waterborne Electrospinning
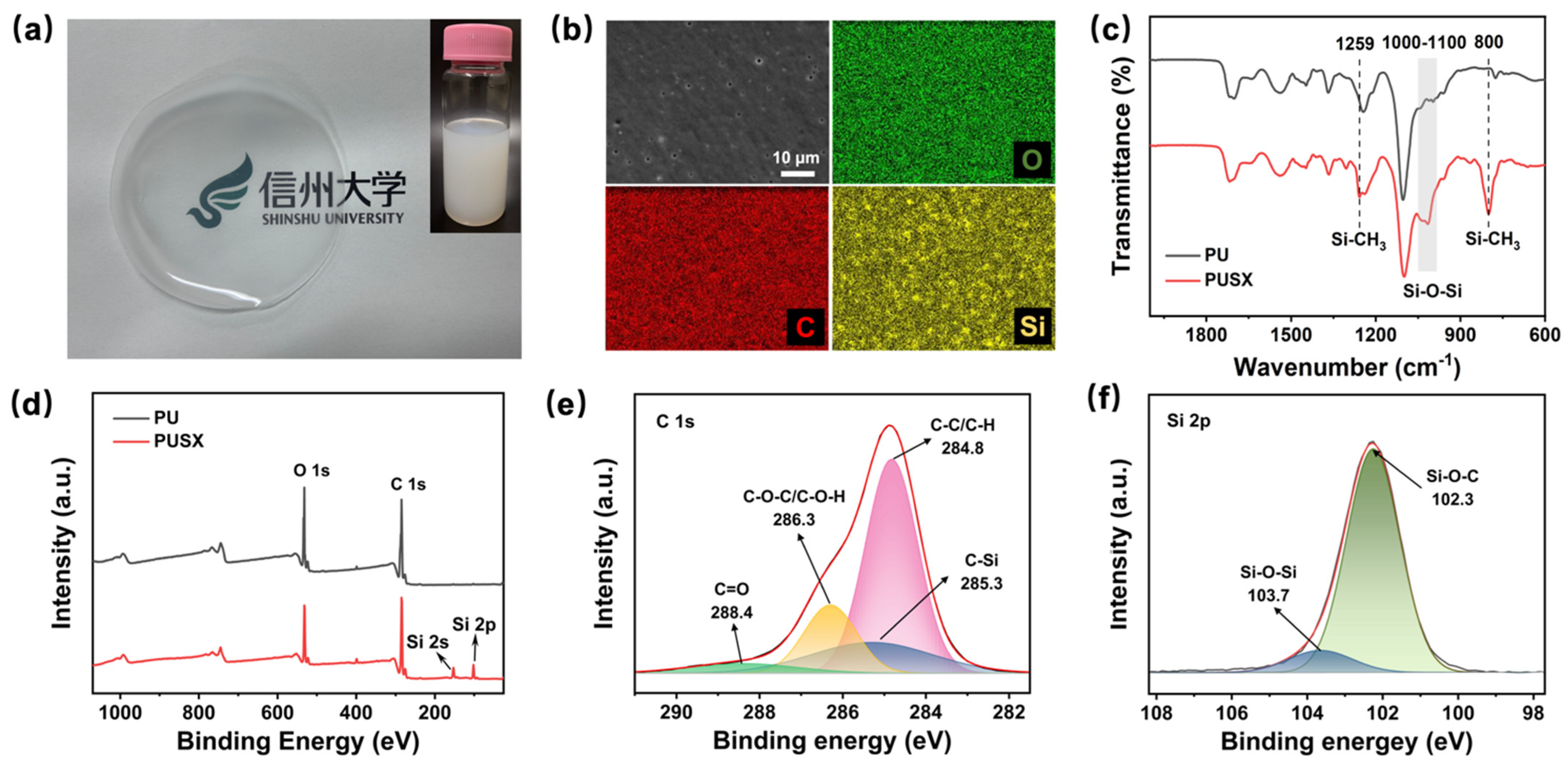
3.2. Characterization of Waterborne PUSX Dispersion
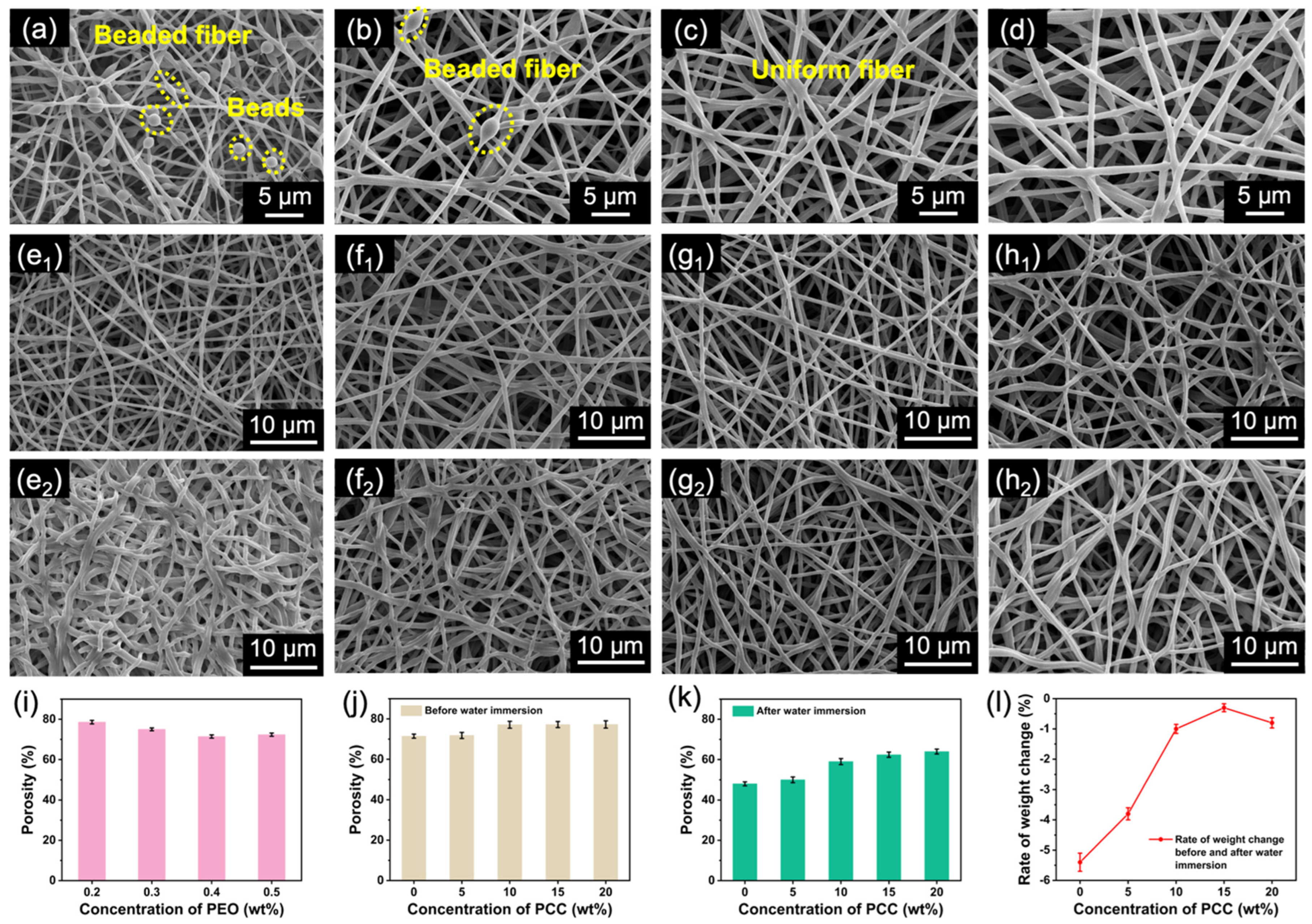
3.3. Formation and Structural Stability of Waterborne Electrospun PUSX Nanofibers
3.4. Improvement of Mechanical Properties of WE PUSX Nanofibrous Membrane with Crosslinking and Hot-Pressing
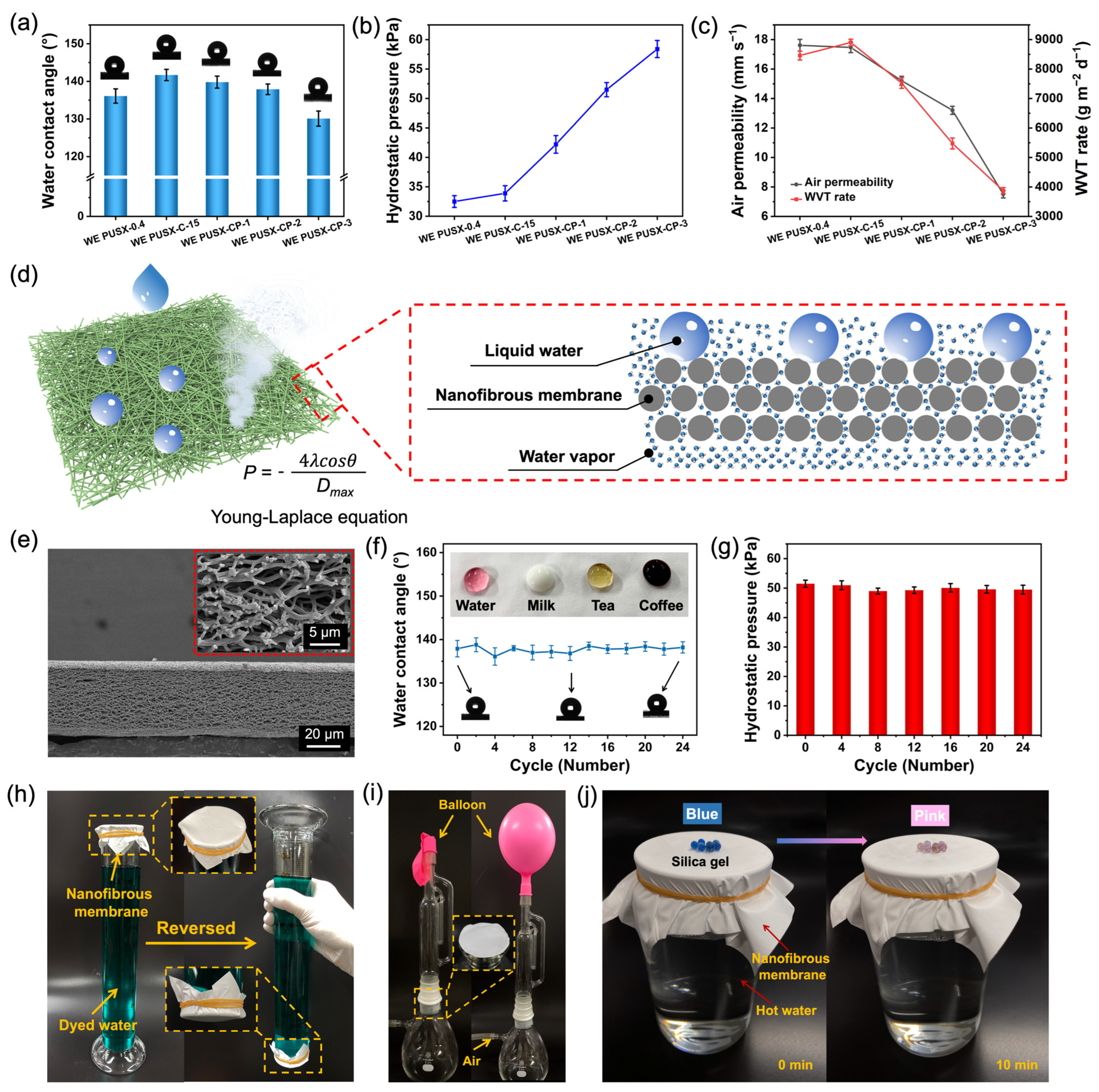
3.5. Waterproof and Breathable Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, W.; Gong, X.; Li, Y.; Si, Y.; Zhang, S.; Yu, J.; Ding, B. Waterborne electrospinning of fluorine-free stretchable nanofiber membranes with waterproof and breathable capabilities for protective textiles. J. Colloid Interface Sci. 2021, 602, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Luo, L.; Tan, L.; Wang, J.; Zhang, S.; Zhang, F.; Jin, J. Microsphere-fiber interpenetrated superhydrophobic PVDF microporous membranes with improved waterproof and breathable performance. ACS Appl. Mater. Interfaces 2018, 10, 28210–28218. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Yin, X.; Wang, F.; Liu, X.; Yu, J.; Zhang, S.; Ding, B. Electrospun nanofibrous membranes: A versatile medium for waterproof and breathable application. Small 2023, 19, 2205067. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Li, G.; Li, P.; Liu, H.; Chadyagondo, T.T.; Li, N.; Xiong, J. Superhydrophobic and breathable SiO2/polyurethane porous membrane for durable water repellent application and oil-water separation. Appl. Surf. Sci. 2020, 512, 144837. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Xu, Y.; He, P.; Si, Y.; Liu, L.; Yu, J.; Ding, B. Multifunctional, waterproof, and breathable nanofibrous textiles based on fluorine-free, all-water-based coatings. ACS Appl. Mater. Interfaces 2020, 12, 15911–15918. [Google Scholar] [CrossRef] [PubMed]
- Tehrani-Bagha, A.R. Waterproof breathable layers—A review. Adv. Colloid Interface Sci. 2019, 268, 114–135. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Liu, F. Review of Waterproof Breathable Membranes: Preparation, Performance and Applications in the Textile Field. Materials 2023, 16, 5339. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhu, W.; Yan, W.; Wang, X.; Liu, L.; Yu, J.; Ding, B. Tailoring waterproof and breathable properties of environmentally friendly electrospun fibrous membranes by optimizing porous structure and surface wettability. Compos. Commun. 2019, 15, 40–45. [Google Scholar] [CrossRef]
- Lao, L.; Shou, D.; Wu, Y.; Fan, J. “Skin-like” fabric for personal moisture management. Sci. Adv. 2020, 6, eaaz0013. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, J.; Zhang, S. Environmentally friendly waterproof and breathable membranes via electrospinning. J. Text. Inst. 2024, 115, 504–526. [Google Scholar] [CrossRef]
- Soto, D.; Ugur, A.; Farnham, T.A.; Gleason, K.K.; Varanasi, K.K. Short-Fluorinated iCVD Coatings for Nonwetting Fabrics. Adv. Funct. Mater. 2018, 28, 1707355. [Google Scholar] [CrossRef]
- Zhou, W.; Gong, X.; Li, Y.; Si, Y.; Zhang, S.; Yu, J.; Ding, B. Environmentally friendly waterborne polyurethane nanofibrous membranes by emulsion electrospinning for waterproof and breathable textiles. Chem. Eng. J. 2022, 427, 130925. [Google Scholar]
- Zhang, J.; Liu, L.; Si, Y.; Zhang, S.; Yu, J. Tailoring electrospun nanofibrous materials for oil/water emulsion separation. J. Text. Inst. 2022, 113, 2285–2298. [Google Scholar] [CrossRef]
- Sheng, J.; Zhang, M.; Xu, Y.; Yu, J.; Ding, B. Tailoring water-resistant and breathable performance of polyacrylonitrile nanofibrous membranes modified by polydimethylsiloxane. ACS Appl. Mater. Interfaces 2016, 8, 27218–27226. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhang, T.; Li, Y.; Huang, L.; Tang, Y. Fluorine-free, highly durable waterproof and breathable fibrous membrane with self-clean performance. Nanomaterials 2023, 13, 516. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhao, J.; Wang, X.; Liu, X.; Yu, J.; Ding, B. Facile fabrication of fluorine-free breathable poly (methylhydrosiloxane)/polyurethane fibrous membranes with enhanced water-resistant capability. J. Colloid Interface Sci. 2019, 556, 541–548. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, W.; Wang, X.; Liu, L.; Yu, J.; Ding, B. Fluorine-free waterborne coating for environmentally friendly, robustly water-resistant, and highly breathable fibrous textiles. ACS Nano 2019, 14, 1045–1054. [Google Scholar] [CrossRef]
- Gu, X.; Li, N.; Gu, H.; Xia, X.; Xiong, J. Polydimethylsiloxane-modified polyurethane–poly (ε-caprolactone) nanofibrous membranes for waterproof, breathable applications. J. Appl. Polym. Sci. 2018, 135, 46360. [Google Scholar] [CrossRef]
- Gu, X.; Li, N.; Cao, J.; Xiong, J. Preparation of electrospun polyurethane/hydrophobic silica gel nanofibrous membranes for waterproof and breathable application. Polym. Eng. Sci. 2018, 58, 1381–1390. [Google Scholar] [CrossRef]
- You, X.; Wang, H.; He, J.; Qi, K. Fluorine-free and breathable polyethylene terephthalate/polydimethylsiloxane (PET/PDMS) fibrous membranes with robust waterproof property. Compos. Commun. 2023, 40, 101621. [Google Scholar] [CrossRef]
- Curzons, A.; Constable, D.; Cunningham, V. Solvent selection guide: A guide to the integration of environmental, health and safety criteria into the selection of solvents. Clean Prod. Process. 1999, 1, 82–90. [Google Scholar] [CrossRef]
- Joshi, D.R.; Adhikari, N. An overview on common organic solvents and their toxicity. J. Pharm. Res. Int. 2019, 28, 1–18. [Google Scholar] [CrossRef]
- Capello, C.; Fischer, U.; Hungerbühler, K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007, 9, 927–934. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, J.; Zhang, S.; Ding, B. Direct electrospinning of fluorine-free waterproof polyamide/polydimethylsiloxane nanofibrous membranes with highly breathable performance. Compos. Commun. 2022, 35, 101337. [Google Scholar] [CrossRef]
- Alder, C.M.; Hayler, J.D.; Henderson, R.K.; Redman, A.M.; Shukla, L.; Shuster, L.E.; Sneddon, H.F. Updating and further expanding GSK’s solvent sustainability guide. Green Chem. 2016, 18, 3879–3890. [Google Scholar] [CrossRef]
- Li, F.; Weng, K.; Nakamura, A.; Ono, K.; Tanaka, T.; Noda, D.; Tanaka, M.; Irifune, S.; Sato, H. Preparation of waterborne silicone-modified polyurethane nanofibers and the effect of crosslinking agents on physical properties. Polymers 2024, 16, 1500. [Google Scholar] [CrossRef]
- Mandal, B.B.; Kundu, S.C. Cell proliferation and migration in silk fibroin 3D scaffolds. Biomaterials 2009, 30, 2956–2965. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Yu, X.; Li, Y.; Jiao, W.; Si, Y.; Yu, J.; Ding, B. Green-solvent-processed fibrous membranes with water/oil/dust-resistant and breathable performances for protective textiles. ACS Appl. Mater. Interfaces 2020, 13, 2081–2090. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.L.; Ji, F.L.; Wong, Y.W. Dependency of the shape memory properties of a polyurethane upon thermomechanical cyclic conditions. Polym. Int. 2005, 54, 600–605. [Google Scholar] [CrossRef]
- Li, P.; Feng, Q.; Chen, L.; Zhao, J.; Lei, F.; Yu, H.; Yi, N.; Gan, F.; Han, S.; Wang, L. Environmentally friendly, durably waterproof, and highly breathable fibrous fabrics prepared by one-step fluorine-free waterborne coating. ACS Appl. Mater. Interfaces 2022, 14, 8613–8622. [Google Scholar] [CrossRef]
- GB/T 12704-1991; Fabrics−Determination of Water Vapour Transmission Rate−Dish Method. China Standards Press: Beijing, China, 1991.
- Choi, C.K. Comparison between SiOC Thin Film by plasma enhance chemical vapor deposition and SiO2 Thin Film by Fourier Transform Infrared Spectroscopy? J. Korean Phys. Soc. 2010, 56, 1150–1155. [Google Scholar] [CrossRef]
- Dana, M.; Zohuri, G.; Ramezanian, N. Determination of gel content of silane cross-linked polyethylene copolymers using FTIR technique. Polyolefins J. 2023, 11, 1–10. [Google Scholar] [CrossRef]
- Gómez-Montaño, F.; Orduña-Díaz, A.; Avelino-Flores, M.; Avelino-Flores, F.; Ramos-Collazo, F.; Reyes-Betanzo, C.; López-Gayou, V. Detección de Salmonella enterica sobre sustratos de silicio biofuncionalizados con IgG anti-Salmonella, analizados por espectroscopía FTIR. Rev. Mex. Ing. Quim. 2020, 19, 1175–1185. [Google Scholar] [CrossRef]
- Feng, W.; Zhang, Y.-S.; Shao, Y.-W.; Huang, T.; Zhang, N.; Yang, J.-H.; Qi, X.-D.; Wang, Y. Coaxial electrospun membranes with thermal energy storage and shape memory functions for simultaneous thermal/moisture management in personal cooling textiles. Eur. Polym. J. 2021, 145, 110245. [Google Scholar] [CrossRef]
- Liakos, I.L.; D’autilia, F.; Garzoni, A.; Bonferoni, C.; Scarpellini, A.; Brunetti, V.; Carzino, R.; Bianchini, P.; Pompa, P.P.; Athanassiou, A. All natural cellulose acetate—Lemongrass essential oil antimicrobial nanocapsules. Int. J. Pharm. 2016, 510, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-J.; Xue, C.-H.; Jia, S.-T.; Ma, J.-Z. Mechanically durable superamphiphobic surfaces via synergistic hydrophobization and fluorination. Chem. Eng. J. 2017, 320, 330–341. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, J.; Ma, L.; Shi, X.; Li, L. Biomimetic preparation of a polycaprolactone membrane with a hierarchical structure as a highly efficient oil–water separator. J. Mater. Chem. A 2019, 7, 24532–24542. [Google Scholar] [CrossRef]
- Munir, M.M.; Suryamas, A.B.; Iskandar, F.; Okuyama, K. Scaling law on particle-to-fiber formation during electrospinning. Polymer 2009, 50, 4935–4943. [Google Scholar] [CrossRef]
- Posthumus, W.; Derksen, A.; Van den Goorbergh, J.; Hesselmans, L. Crosslinking by polycarbodiimides. Prog. Organ. Coat. 2007, 58, 231–236. [Google Scholar] [CrossRef]
- Forsythe, J.G.; Yu, S.S.; Mamajanov, I.; Grover, M.A.; Krishnamurthy, R.; Fernández, F.M.; Hud, N.V. Ester-mediated amide bond formation driven by wet–dry cycles: A possible path to polypeptides on the prebiotic earth. Angew. Chem. Int. Ed. 2015, 54, 9871–9875. [Google Scholar] [CrossRef]
- Xiong, W.; Chen, B.; Zhang, H.; Peng, J.; Pan, X.; Guo, M.; Luo, X.; Zhou, C.; Liu, Y. A bio-based waterborne polyurethane with high toughness, superior wear resistance, and water resistance enabled by sorbitol monooleate. Prog. Organ. Coat. 2023, 185, 107895. [Google Scholar] [CrossRef]
- Cui, J.; Xu, J.; Li, J.; Qiu, H.; Zheng, S.; Yang, J. A crosslinkable graphene oxide in waterborne polyurethane anticorrosive coatings: Experiments and simulation. Compos. Part B Eng. 2020, 188, 107889. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Xie, Y.; Wang, L.; Yang, L.; Yu, J.; Miyamoto, A.; Sun, F. Development of FGF-2-loaded electrospun waterborne polyurethane fibrous membranes for bone regeneration. Regen. Biomater. 2021, 8, rbaa046. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhuang, X.; Chen, X.; Wang, X.; Yang, L.; Jing, X. Preparation of core-sheath composite nanofibers by emulsion electrospinning. Macromol. Rapid Commun. 2006, 27, 1637–1642. [Google Scholar] [CrossRef]
- Cheng, F.; Fan, Y.; He, N.; Song, Y.; Shen, J.; Gong, Z.; Tong, X.; Yang, X. Castor oil based high transparent UV cured silicone modified polyurethane acrylate coatings with outstanding tensile strength and good chemical resistance. Prog. Organ. Coat. 2022, 163, 106624. [Google Scholar] [CrossRef]
- Liu, J.; Yao, Y.; Chen, S.; Li, X.; Zhang, Z. A new nanoparticle-reinforced silicone rubber composite integrating high strength and strong adhesion. Compos. Part A Appl. Sci. Manuf. 2021, 151, 106645. [Google Scholar] [CrossRef]
- Yin, C.; Rozet, S.; Okamoto, R.; Kondo, M.; Tamada, Y.; Tanaka, T.; Hattori, H.; Tanaka, M.; Sato, H.; Iino, S. Physical properties and in vitro biocompatible evaluation of silicone-modified polyurethane nanofibers and films. Nanomaterials 2019, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Li, Z.; Tian, L.; Lu, D.; Jin, Y.; Zhang, Y.; Li, B.; Yu, H.; He, J.; Sun, D. Environmentally friendly waterproof and breathable electrospun nanofiber membranes via post-heat treatment. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130643. [Google Scholar] [CrossRef]
- Liu, C.; Liao, X.; Shao, W.; Liu, F.; Ding, B.; Ren, G.; Chu, Y.; He, J. Hot-melt adhesive bonding of polyurethane/fluorinated polyurethane/alkylsilane-functionalized graphene nanofibrous fabrics with enhanced waterproofness, breathability, and mechanical properties. Polymers 2020, 12, 836. [Google Scholar] [CrossRef]
- Li, X.; Chen, J.; Liu, J.; Chen, Q. Preparation and Characterization of Cellulose Nanofibril-Waterborne Polyurethane Composite Films. Paper Biomater. 2023, 8, 26–34. [Google Scholar] [CrossRef]
- Santamaria-Echart, A.; Ugarte, L.; Gonzalez, K.; Martin, L.; Irusta, L.; Gonzalez, A.; Corcuera, M.A.; Eceiza, A. The role of cellulose nanocrystals incorporation route in waterborne polyurethane for preparation of electrospun nanocomposites mats. Carbohydr. Polym. 2017, 166, 146–155. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Q.; Chadyagondo, T.T.; Li, G.; Gu, H.; Li, N. Designing waterproof and breathable fabric based on polyurethane/silica dioxide web fabricated by electrospinning. Fibers Polym. 2020, 21, 1444–1452. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, Z.; Zhao, J.; Huang, L. Design the SBS elastomer electrospun fibermat/polyester composite textiles: Morphology effect on waterproof-breathable performance. Macromol. Mater. Eng. 2020, 305, 2000370. [Google Scholar] [CrossRef]
- Maksoud, F.J.; Lameh, M.; Fayyad, S.; Ismail, N.; Tehrani-Bagha, A.R.; Ghaddar, N.; Ghali, K. Electrospun waterproof breathable membrane with a high level of aerosol filtration. J. Appl. Polym. Sci. 2018, 135, 45660. [Google Scholar] [CrossRef]
- Xu, Y.; Sheng, J.; Yin, X.; Yu, J.; Ding, B. Functional modification of breathable polyacrylonitrile/polyurethane/TiO2 nanofibrous membranes with robust ultraviolet resistant and waterproof performance. J. Colloid Interface Sci. 2017, 508, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Park, Y.; Park, C.H. Preparation of breathable and superhydrophobic polyurethane electrospun webs with silica nanoparticles. Text. Res. J. 2016, 86, 1816–1827. [Google Scholar] [CrossRef]
- Zhang, M.; Sheng, J.; Yin, X.; Yu, J.; Ding, B. Polyvinyl butyral modified polyvinylidene fluoride breathable–waterproof nanofibrous membranes with enhanced mechanical performance. Macromol. Mater. Eng. 2017, 302, 1600272. [Google Scholar] [CrossRef]
- Ren, G.; Lu, D.; Zhang, Y.; Cui, Z.; Li, Z.; Yu, H.; He, J. Poly (methyl methacrylate) embedded fluorine-free polyurethane electrospun nanofiber membranes with enhanced waterproof and breathable performance. Compos. Commun. 2023, 43, 101698. [Google Scholar] [CrossRef]
- Gu, J.; Gu, H.; Zhang, Q.; Zhao, Y.; Li, N.; Xiong, J. Sandwich-structured composite fibrous membranes with tunable porous structure for waterproof, breathable, and oil-water separation applications. J. Colloid Interface Sci. 2018, 514, 386–395. [Google Scholar] [CrossRef]
- Kang, Y.K.; Park, C.H.; Kim, J.; Kang, T.J. Application of electrospun polyurethane web to breathable water-proof fabrics. Fibers Polym. 2007, 8, 564–570. [Google Scholar] [CrossRef]
- Guo, Y.; Zhou, W.; Wang, L.; Dong, Y.; Yu, J.; Li, X.; Ding, B. Stretchable PDMS embedded fibrous membranes based on an ethanol solvent system for waterproof and breathable applications. ACS Appl. Bio Mater. 2019, 2, 5949–5956. [Google Scholar] [CrossRef] [PubMed]



| Samples | PEO Concentration (wt%) | PCC Concentration (wt%) | Hot-Pressing Temperature (°C) |
|---|---|---|---|
| WE PUSX-0.2 | 0.2 | ----- | ----- |
| WE PUSX-0.3 | 0.3 | ||
| WE PUSX-0.4 | 0.4 | ||
| WE PUSX-0.5 | 0.5 | ||
| WE PUSX-C-5 | 0.4 | 5 | ----- |
| WE PUSX-C-10 | 10 | ||
| WE PUSX-C-15 | 15 | ||
| WE PUSX-C-20 | 20 | ||
| WE PUSX-CP-0 | 0.4 | 15 | Room temperature |
| WE PUSX-CP-1 | 100 | ||
| WE PUSX-CP-2 | 120 | ||
| WE PUSX-CP-3 | 140 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Weng, K.; Tanaka, T.; He, J.; Zheng, H.; Noda, D.; Irifune, S.; Sato, H. Fabrication of Waterborne Silicone-Modified Polyurethane Nanofibers for Nonfluorine Elastic Waterproof and Breathable Membranes. Polymers 2024, 16, 1505. https://doi.org/10.3390/polym16111505
Li F, Weng K, Tanaka T, He J, Zheng H, Noda D, Irifune S, Sato H. Fabrication of Waterborne Silicone-Modified Polyurethane Nanofibers for Nonfluorine Elastic Waterproof and Breathable Membranes. Polymers. 2024; 16(11):1505. https://doi.org/10.3390/polym16111505
Chicago/Turabian StyleLi, Fang, Kai Weng, Toshihisa Tanaka, Jianxin He, Haimin Zheng, Daisuke Noda, Shinji Irifune, and Hiromasa Sato. 2024. "Fabrication of Waterborne Silicone-Modified Polyurethane Nanofibers for Nonfluorine Elastic Waterproof and Breathable Membranes" Polymers 16, no. 11: 1505. https://doi.org/10.3390/polym16111505
APA StyleLi, F., Weng, K., Tanaka, T., He, J., Zheng, H., Noda, D., Irifune, S., & Sato, H. (2024). Fabrication of Waterborne Silicone-Modified Polyurethane Nanofibers for Nonfluorine Elastic Waterproof and Breathable Membranes. Polymers, 16(11), 1505. https://doi.org/10.3390/polym16111505






