AlveoMPU: Bridging the Gap in Lung Model Interactions Using a Novel Alveolar Bilayer Film
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Foamed Porous Polyurethane Film
2.2. Electron Microscopy
2.3. Atomic Force Microscopy
2.4. Pore Size Measuring with Laser Microscopy Observations
2.5. Measurement of Dynamic Contact Angle
2.6. Tensile Testing (Stress–Strain Curves)
2.7. Cell Culture and AlveoMPU Preparation
2.8. Immunostaining Cells on AlveoMPU
2.9. Intervention of AlveoMPU Using Proinflammatory and Anti-Inflammatory Substances and Permeability Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. Surface Structure of the Porous Polyurethane Film Used in AlveoMPU
3.2. Hardness Measurement
3.3. Wettability of P-PU
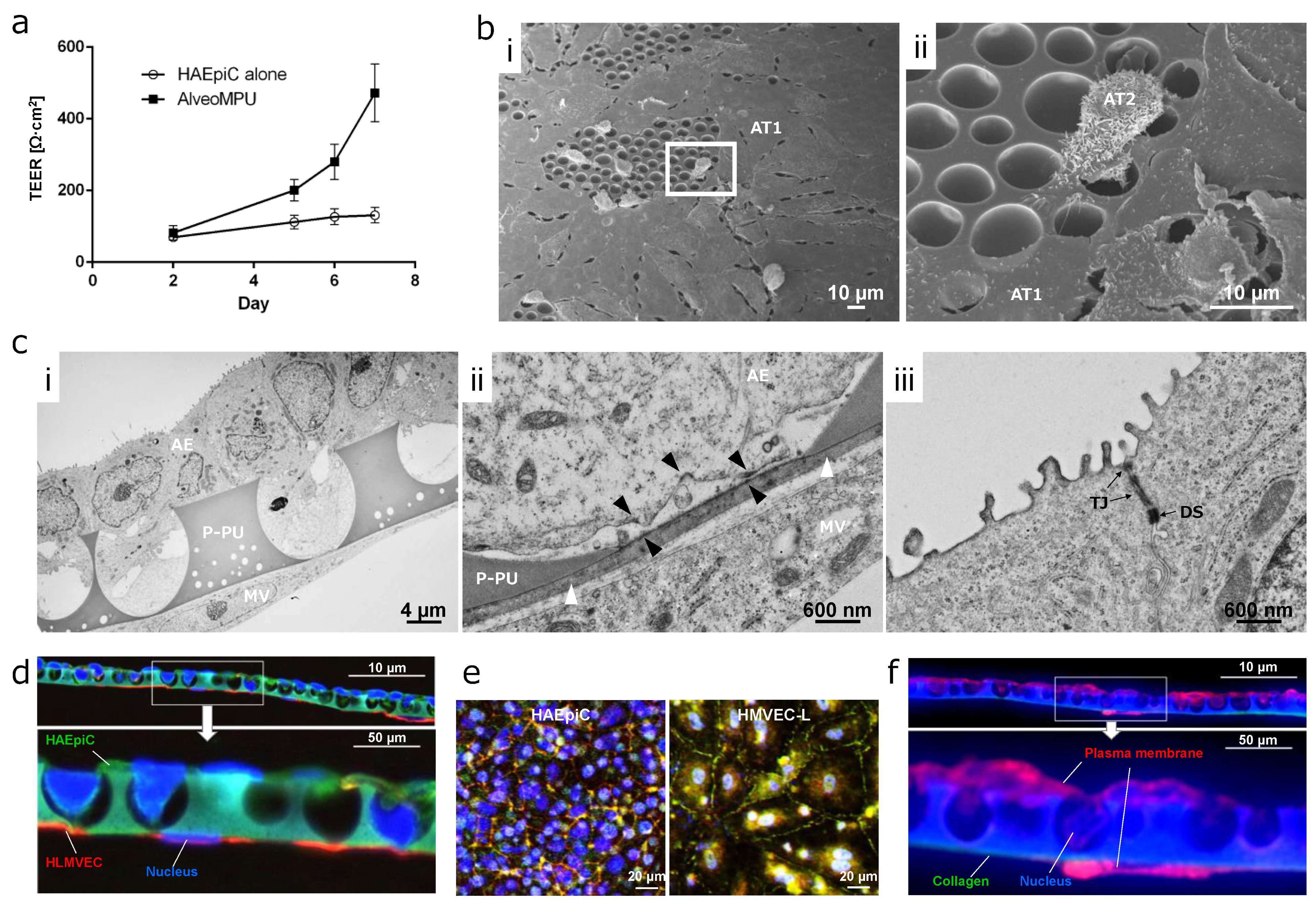
3.4. Time Course Transition of Barrier Function in AlveoMPU
3.5. Distribution of Alveolar Epithelial and Endothelial Cells on AlveoMPU
3.6. Inflammation Induction and Therapeutic Effect Trials Using AlveoMPU
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petersson, J.; Glenny, R.W. Gas Exchange in the Lung. Semin. Respir. Crit. Care Med. 2023, 44, 555–568. [Google Scholar] [CrossRef] [PubMed]
- West, J.B.; Mathieu-Costello, O. Structure, strength, failure, and remodeling of the pulmonary blood-gas barrier. Annu. Rev. Physiol. 1999, 61, 543–572. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, D.K.; Hirata, F.; Rishi, A.K.; Gairola, C.G. Cigarette smoke, inflammation, and lung injury: A mechanistic perspective. J. Toxicol. Environ. Health B Crit. Rev. 2009, 12, 45–64. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, L.; Ochs, M. The micromechanics of lung alveoli: Structure and function of surfactant and tissue components. Histochem. Cell Biol. 2018, 150, 661–676. [Google Scholar] [CrossRef]
- Hermanns, M.I.; Unger, R.E.; Kehe, K.; Peters, K.; Kirkpatrick, C.J. Lung epithelial cell lines in coculture with human pulmonary microvascular endothelial cells: Development of an alveolo-capillary barrier in vitro. Lab. Investig. 2004, 84, 736–752. [Google Scholar] [CrossRef]
- Hermanns, M.I.; Fuchs, S.; Bock, M.; Wenzel, K.; Mayer, E.; Kehe, K.; Bittinger, F.; Kirkpatrick, C.J. Primary human coculture model of alveolo-capillary unit to study mechanisms of injury to peripheral lung. Cell Tissue Res. 2009, 336, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef]
- Jain, P.; Nishiguchi, A.; Linz, G.; Wessling, M.; Ludwig, A.; Rossaint, R.; Moller, M.; Singh, S. Reconstruction of Ultra-thin Alveolar-capillary Basement Membrane Mimics. Adv. Biol. 2021, 5, e2000427. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Matsushita, S.I.; Kurono, N.; Sawadaishi, T.; Shimomura, M. Hierarchical honeycomb structures utilized a dissipative process. Synth. Met. 2004, 147, 237–240. [Google Scholar] [CrossRef]
- Nakazono, Y.; Arakawa, H.; Nishino, M.; Yamaki, I.; Oba, T.; Tomotoshi, K.; Kakinuma, C.; Ogihara, T.; Tamai, I. Drug Transcellular Transport Assay Using a High Porosity Honeycomb Film. Biol. Pharm. Bull. 2021, 44, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Nishikawa, M.; Mori, T.; Takeya, J.; Shrestha, L.K.; Hill, J.P. Self-assembly as a key player for materials nanoarchitectonics. Sci. Technol. Adv. Mater. 2019, 20, 51–95. [Google Scholar] [CrossRef] [PubMed]
- Lamberton, P.; Lipsky, M.; McMillan, P. Use of semipermeable polyurethane hollow fibers for pituitary organ culture. In Vitro Cell Dev. Biol. 1988, 24, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Noureddine, B.; Zitouni, S.; Achraf, B.; Houssém, C.; Jannick, D.-R.; Jean-François, G. Development and Characterization of Tailored Polyurethane Foams for Shock Absorption. Appl. Sci. 2022, 12, 2206. [Google Scholar] [CrossRef]
- Gonzalez-Torres, M.; Becerra-Gonzalez, M.; Leyva-Gomez, G.; Lima, E.; Gonzalez Mendoza, O.; Ruvalcaba-Paredes, E.K.; Cortes, H.; Pineda, C.; Martinez-Torres, A. A poly (saccharide-ester-urethane) scaffold for mammalian cell growth. Cell. Mol. Biol. 2021, 67, 113–117. [Google Scholar] [CrossRef]
- Zang, L.; Cheng, Q.; Bai, S.; Wang, K.; Yuan, X. Electrospun membranes of carboxylated poly(ester urethane)urea/gelatin encapsulating pterostilbene for adaptive and antioxidative purposes. J. Biomater. Sci. Polym. Ed. 2023, 34, 1171–1194. [Google Scholar] [CrossRef] [PubMed]
- Szycher, M.; Siciliano, A.A.; Reed, A.M. Polyurethanes in medical devices. Med. Des. Mater. 1991, 1, 18–25. [Google Scholar]
- He, M.; Ro, L.; Liu, J.; Chu, C.C. Folate-decorated arginine-based poly(ester urea urethane) nanoparticles as carriers for gambogic acid and effect on cancer cells. J. Biomed. Mater. Res. A 2017, 105, 475–490. [Google Scholar] [CrossRef]
- Ciardelli, G.; Chiono, V. Materials for peripheral nerve regeneration. Macromol. Biosci. 2006, 6, 13–26. [Google Scholar] [CrossRef]
- Taki, S.; Mizuno, H.; Nakagawa, H.; Hagiyama, T.; Yoshimura, A.; Tanaka, M.; Sasaki, A.; Takahashi, T.; Ohta, T. Production method of polyurethane porous membrane to be used for at least one of applications of cell culture and cancer cell growth inhibition. U.S. Patent 9,637,722, 2 May 2017. [Google Scholar]
- Adams, T.; Grant, C.; Watson, H. A Simple Algorithm to Relate Measured Surface Roughness to Equivalent Sand-grain Roughness. Int. J. Mech. Eng. Mechatron. 2012, 1, 66–71. [Google Scholar] [CrossRef]
- Saad, S.M.; Neumann, A.W. Axisymmetric Drop Shape Analysis (ADSA): An Outline. Adv. Colloid. Interface Sci. 2016, 238, 62–87. [Google Scholar] [CrossRef]
- Kim, D.C.; Burton, P.S.; Borchardt, R.T. A correlation between the permeability characteristics of a series of peptides using an in vitro cell culture model (Caco-2) and those using an in situ perfused rat ileum model of the intestinal mucosa. Pharm. Res. 1993, 10, 1710–1714. [Google Scholar] [CrossRef]
- Majhy, B.; Priyadarshini, P.; Sen, A.K. Effect of surface energy and roughness on cell adhesion and growth—Facile surface modification for enhanced cell culture. RSC Adv. 2021, 11, 15467–15476. [Google Scholar] [CrossRef] [PubMed]
- Yi, B.; Xu, Q.; Liu, W. An overview of substrate stiffness guided cellular response and its applications in tissue regeneration. Bioact. Mater. 2022, 15, 82–102. [Google Scholar] [CrossRef]
- Handorf, A.M.; Zhou, Y.; Halanski, M.A.; Li, W.J. Tissue stiffness dictates development, homeostasis, and disease progression. Organogenesis 2015, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Kucinska-Lipka, J.; Gubanska, I.; Skwarska, A. Microporous Polyurethane Thin Layer as a Promising Scaffold for Tissue Engineering. Polymers 2017, 9, 277. [Google Scholar] [CrossRef]
- Tamada, Y.; Ikada, Y. Cell adhesion to plasma-treated polymer surfaces. Polymer 1993, 34, 2208–2212. [Google Scholar] [CrossRef]
- Al-Azzam, N.; Alazzam, A. Micropatterning of cells via adjusting surface wettability using plasma treatment and graphene oxide deposition. PLoS ONE 2022, 17, e0269914. [Google Scholar] [CrossRef]
- van Wachem, P.B.; Beugeling, T.; Feijen, J.; Bantjes, A.; Detmers, J.P.; van Aken, W.G. Interaction of cultured human endothelial cells with polymeric surfaces of different wettabilities. Biomaterials 1985, 6, 403–408. [Google Scholar] [CrossRef]
- Lerman, M.J.; Lembong, J.; Muramoto, S.; Gillen, G.; Fisher, J.P. The Evolution of Polystyrene as a Cell Culture Material. Tissue Eng. Part B Rev. 2018, 24, 359–372. [Google Scholar] [CrossRef]
- Mishra, H.; Schrader, A.M.; Lee, D.W.; Gallo, A., Jr.; Chen, S.Y.; Kaufman, Y.; Das, S.; Israelachvili, J.N. Time-Dependent Wetting Behavior of PDMS Surfaces with Bioinspired, Hierarchical Structures. ACS Appl. Mater. Interfaces 2016, 8, 8168–8174. [Google Scholar] [CrossRef] [PubMed]
- Elbert, K.J.; Schafer, U.F.; Schafers, H.J.; Kim, K.J.; Lee, V.H.; Lehr, C.M. Monolayers of human alveolar epithelial cells in primary culture for pulmonary absorption and transport studies. Pharm. Res. 1999, 16, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.J.; Aljayyoussi, G.; Mansour, O.; Griffiths, P.; Gumbleton, M. Endocytic Uptake, Transport and Macromolecular Interactions of Anionic PAMAM Dendrimers within Lung Tissue. Pharm. Res. 2017, 34, 2517–2531. [Google Scholar] [CrossRef] [PubMed]
- Suresh, V. Permeability Properties of an In Vitro Model of the Alveolar Epithelium. Cell Mol. Bioeng. 2021, 14, 653–659. [Google Scholar] [CrossRef]
- Vaccaro, C.A.; Brody, J.S. Structural features of alveolar wall basement membrane in the adult rat lung. J. Cell Biol. 1981, 91, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Merker, H.J. Morphology of the basement membrane. Microsc. Res. Tech. 1994, 28, 95–124. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. Basement membranes: Structure, assembly and role in tumour angiogenesis. Nat. Rev. Cancer 2003, 3, 422–433. [Google Scholar] [CrossRef]
- Fick, A. Ueber Diffusion. Ann. Der Phys. 2006, 170, 59–86. [Google Scholar] [CrossRef]
- Hosoya, O.; Chono, S.; Saso, Y.; Juni, K.; Morimoto, K.; Seki, T. Determination of diffusion coefficients of peptides and prediction of permeability through a porous membrane. J. Pharm. Pharmacol. 2004, 56, 1501–1507. [Google Scholar] [CrossRef]
- Poschl, E.; Schlotzer-Schrehardt, U.; Brachvogel, B.; Saito, K.; Ninomiya, Y.; Mayer, U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 2004, 131, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Yurchenco, P.D. Basement membranes: Cell scaffoldings and signaling platforms. Cold Spring Harb. Perspect. Biol. 2011, 3, a004911. [Google Scholar] [CrossRef] [PubMed]
- Walko, G.; Castanon, M.J.; Wiche, G. Molecular architecture and function of the hemidesmosome. Cell Tissue Res. 2015, 360, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.S.; Taeusch, H.W., Jr.; Thurlbeck, W.W.; Avery, M.E. A combined scanning and transmission electron microscopic study of alveolar epithelial development of the fetal rabbit lung. Am. J. Pathol. 1973, 73, 365–376. [Google Scholar] [PubMed]
- Schutt, C. Fighting infection: The role of lipopolysaccharide binding proteins CD14 and LBP. Pathobiology 1999, 67, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Takeda, K.; Kaisho, T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat. Immunol. 2001, 2, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Palucka, K.; Banchereau, J. Sensing pathogens and tuning immune responses. Science 2001, 293, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulou, L.; Holt, A.C.; Medzhitov, R.; Flavell, R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 2001, 413, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Eixarch, H.; Haltner-Ukomadu, E.; Beisswenger, C.; Bock, U. Drug Delivery to the Lung: Permeability and Physicochemical Characteristics of Drugs as the Basis for a Pulmonary Biopharmaceutical Classification System (pBCS). J. Epithel. Biol. Pharmacol. 2010, 3, 1–14. [Google Scholar]
- Boivin, M.A.; Ye, D.; Kennedy, J.C.; Al-Sadi, R.; Shepela, C.; Ma, T.Y. Mechanism of glucocorticoid regulation of the intestinal tight junction barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G590–G598. [Google Scholar] [CrossRef]
- Metz, J.K.; Wiegand, B.; Schnur, S.; Knoth, K.; Schneider-Daum, N.; Gross, H.; Croston, G.; Reinheimer, T.M.; Lehr, C.M.; Hittinger, M. Modulating the Barrier Function of Human Alveolar Epithelial (hAELVi) Cell Monolayers as a Model of Inflammation. Altern. Lab. Anim. 2020, 48, 252–267. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Pan, X.; Wang, L.; Yu, G. Alveolar cells under mechanical stressed niche: Critical contributors to pulmonary fibrosis. Mol. Med. 2020, 26, 95. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Ueno, K. Apparent contact angle calculated from a water repellent model with pinning effect. Langmuir 2017, 44, 138–143. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirano, M.; Iwata, K.; Yamada, Y.; Shinoda, Y.; Yamazaki, M.; Hino, S.; Ikeda, A.; Shimizu, A.; Otsuka, S.; Nakagawa, H.; et al. AlveoMPU: Bridging the Gap in Lung Model Interactions Using a Novel Alveolar Bilayer Film. Polymers 2024, 16, 1486. https://doi.org/10.3390/polym16111486
Hirano M, Iwata K, Yamada Y, Shinoda Y, Yamazaki M, Hino S, Ikeda A, Shimizu A, Otsuka S, Nakagawa H, et al. AlveoMPU: Bridging the Gap in Lung Model Interactions Using a Novel Alveolar Bilayer Film. Polymers. 2024; 16(11):1486. https://doi.org/10.3390/polym16111486
Chicago/Turabian StyleHirano, Minoru, Kosuke Iwata, Yuri Yamada, Yasuhiko Shinoda, Masateru Yamazaki, Sayaka Hino, Aya Ikeda, Akiko Shimizu, Shuhei Otsuka, Hiroyuki Nakagawa, and et al. 2024. "AlveoMPU: Bridging the Gap in Lung Model Interactions Using a Novel Alveolar Bilayer Film" Polymers 16, no. 11: 1486. https://doi.org/10.3390/polym16111486
APA StyleHirano, M., Iwata, K., Yamada, Y., Shinoda, Y., Yamazaki, M., Hino, S., Ikeda, A., Shimizu, A., Otsuka, S., Nakagawa, H., & Watanabe, Y. (2024). AlveoMPU: Bridging the Gap in Lung Model Interactions Using a Novel Alveolar Bilayer Film. Polymers, 16(11), 1486. https://doi.org/10.3390/polym16111486




