Brucine Sulfate, a Novel Bacteriostatic Agent in 3D Printed Bone Scaffold Systems
Abstract
1. Introduction
2. Experimental
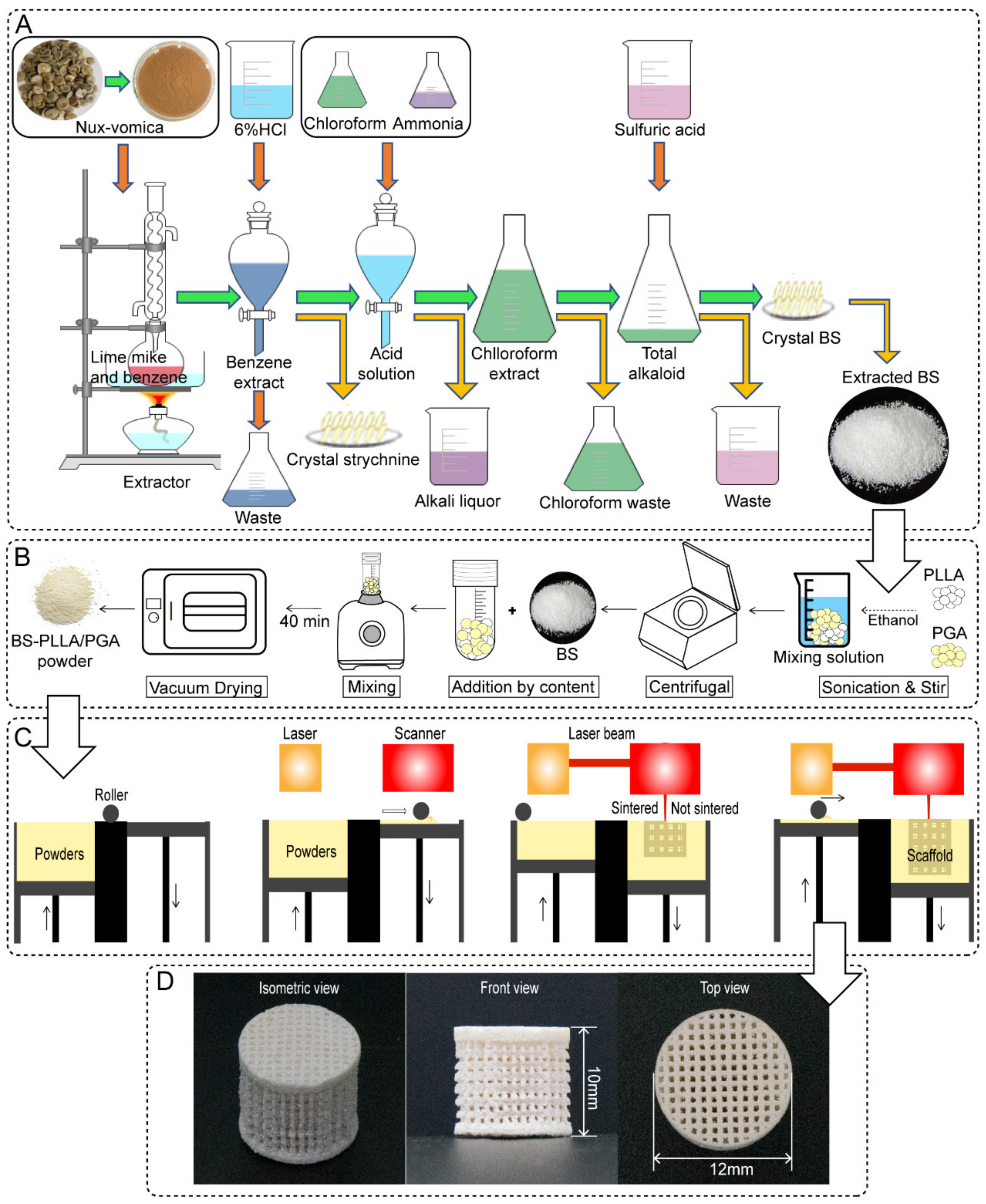
2.1. Materials and Preparation
2.2. Extraction and Preparation of BS
2.3. Antibacterial Property of BS
2.4. Microstructure and Slow-Release Ability of the Scaffold
2.5. Antibacterial Properties of Drug-Loaded Bone Scaffolds
2.6. Bacteriostatic Mechanism
2.7. Cytocompatibility Assay
2.8. Statistical Analysis
3. Results and Discussion
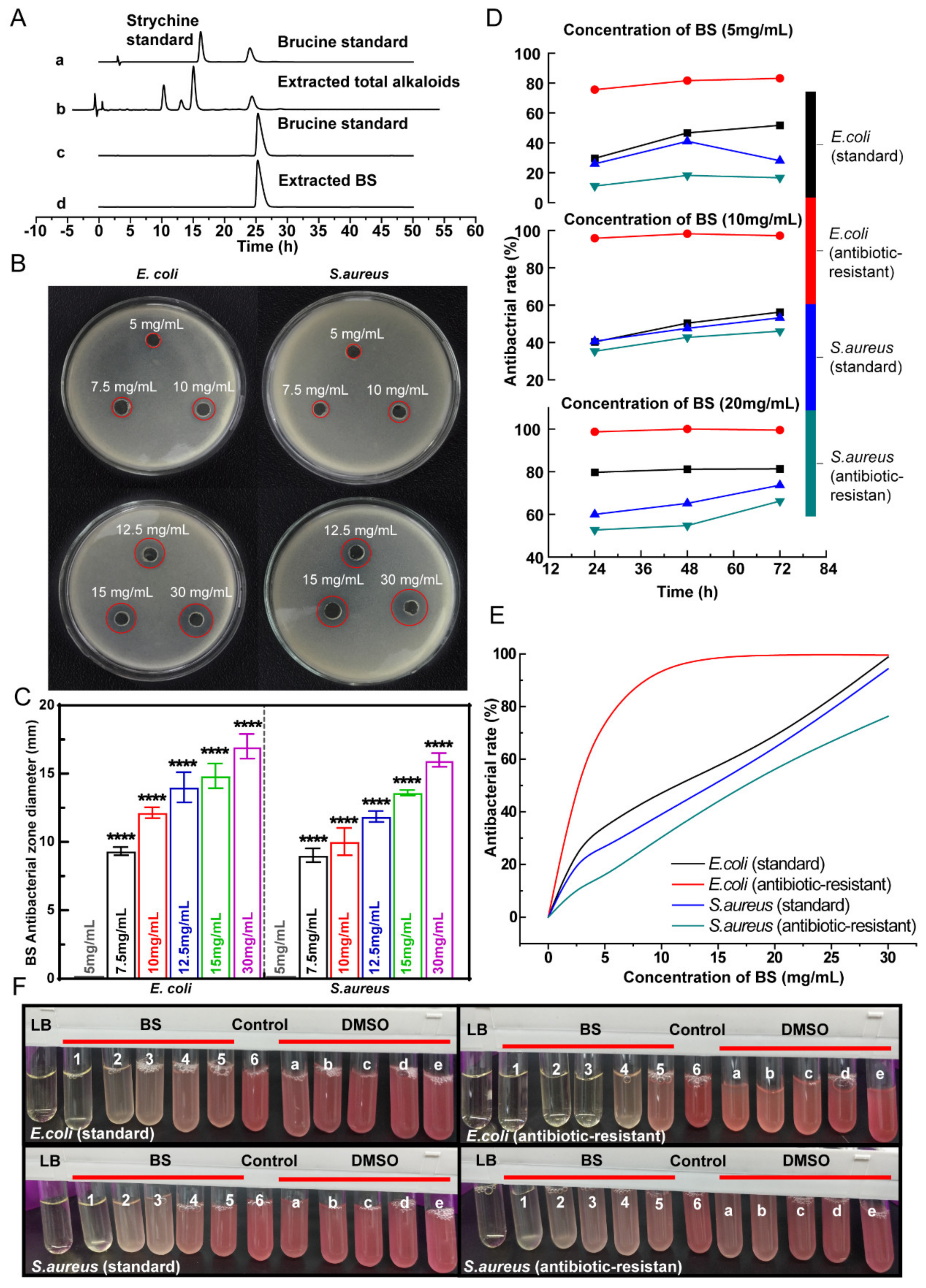
3.1. High-Purity BS Extracted from Semen Strychnine
3.2. BS Antibacterial Property
3.3. Physicochemical Properties and Drug Release Behaviors of the Scaffolds
3.4. Antibacterial Properties of the Scaffolds
3.5. Antibacterial Mechanism
3.6. Cytocompatibility
4. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, Y.; Chi, X.; Wang, Y.; Heng, B.C.; Wei, Y.; Zhang, X.; Zhao, H.; Yin, Y.; Deng, X. Mitochondria transfer enhances proliferation, migration, and osteogenic differentiation of bone marrow mesenchymal stem cell and promotes bone defect healing. Stem Cell Res. Ther. 2020, 11, 245. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yeung, K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Liu, H.; Tian, Y.; Fan, Y.; Li, S.; Wang, G.; Wang, Y.; Peng, C.; Wu, D. Dual-functional composite scaffolds for inhibiting infection and promoting bone regeneration. Mater. Today Bio 2022, 16, 100409. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.D.; Balestri, W.; Reinwald, Y. Biomedical implants for regenerative therapies. In Biomaterials; IntechOpen: London, UK, 2020; pp. 1–36. [Google Scholar]
- Lord, J.M.; Midwinter, M.J.; Chen, Y.F.; Belli, A.; Brohi, K.; Kovacs, E.J.; Koenderman, L.; Kubes, P.; Lilford, R.J. The systemic immune response to trauma: An overview of pathophysiology and treatment. Lancet 2014, 384, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Takahashi, Y.; Tabata, Y. Enhanced bone regeneration at a segmental bone defect by controlled release of bone morphogenetic protein-2 from a biodegradable hydrogel. Tissue Eng. 2006, 12, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Liu, Y.; Guo, J.; Wu, H.; Wang, J.; Wu, G. Biomaterials with Antibacterial and Osteoinductive Properties to Repair Infected Bone Defects. Int. J. Mol. Sci. 2016, 17, 334. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; See, C.W.; Li, X.; Zhu, D. Orthopedic implants and devices for bone fractures and defects: Past, present and perspective. Eng. Regen. 2020, 1, 6–18. [Google Scholar] [CrossRef]
- Branca Vergano, L.; Monesi, M. Bone Infections. In Textbook of Emergency General Surgery; Springer: Cham, Switzerland, 2023; pp. 1689–1712. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; Lozano, D.; González, B.; Izquierdo-Barba, I. Biomaterials against Bone Infection. Adv. Healthc. Mater. 2020, 9, e2000310. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, M.A.; Walsh, C.T. Antibiotics for emerging pathogens. Science 2009, 325, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.G.; Zhao, Y.; Li, B.; Huang, C.L.; Zhang, S.Y.; Yu, S.; Chen, Y.S.; Zhang, T.; Gillings, M.R.; Su, J.Q. Continental-scale pollution of estuaries with antibiotic resistance genes. Nat. Microbiol. 2017, 2, 16270. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Webster, T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J. Orthop. Res. 2018, 36, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.; Cerceo, E. Trends, Epidemiology, and Management of Multi-Drug Resistant Gram-Negative Bacterial Infections in the Hospitalized Setting. Antibiotics 2020, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Terreni, M.; Taccani, M.; Pregnolato, M. New Antibiotics for Multidrug-Resistant Bacterial Strains: Latest Research Developments and Future Perspectives. Molecules 2021, 26, 2671. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.D.; Wright, G.D. Antibacterial drug discovery in the resistance era. Nature 2016, 529, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Pu, H.; Li, X.; Du, Q.; Cui, H.; Xu, Y. Research Progress in the Application of Chinese Herbal Medicines in Aquaculture: A Review. Engineering 2017, 3, 731–737. [Google Scholar] [CrossRef]
- Singh, M.; Khatoon, S.; Singh, S.; Kumar, V.; Rawat, A.K.; Mehrotra, S. Antimicrobial screening of ethnobotanically important stem bark of medicinal plants. Pharmacogn. Res. 2010, 2, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.S.; Ramanadham, M.; Prasad, M.N. Anti-proliferative and cytotoxic effects of Strychnos nux-vomica root extract on human multiple myeloma cell line—RPMI 8226. Food Chem. Toxicol. 2009, 47, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Omar, F.; Tareq, A.M.; Alqahtani, A.M.; Dhama, K.; Sayeed, M.A.; Emran, T.B.; Simal-Gandara, J. Plant-Based Indole Alkaloids: A Comprehensive Overview from a Pharmacological Perspective. Molecules 2021, 26, 2297. [Google Scholar] [CrossRef] [PubMed]
- Ukwubile, C.A.; Ikpefan, E.O.; Dibal, M.Y.; Umeano, V.A.; Menkiti, D.N.; Kaosi, C.C.; Paul, S.; Famurewa, A.C.; Nettey, H.; Yerima, T.S. Pharmacognostic profiles, evaluation of analgesic, anti-inflammatory and anticonvulsant activities of Newbouldia laevis (P. Beauv.) Seem. ex Bureau leaf and root extracts in Wistar rats. J. Ethnopharmacol. 2023, 314, 116632. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Hu, S.; Liang, Q.; Guo, W.; Xia, Y.; Shuai, C.; Li, Y. A polymer scaffold with drug-sustained release and antibacterial activity. Int. J. Polym. Mater. Polym. Biomater. 2020, 69, 398–405. [Google Scholar] [CrossRef]
- Gao, Y.; Guo, L.; Han, Y.; Zhang, J.; Dai, Z.; Ma, S. A Combination of In Silico ADMET Prediction, In Vivo Toxicity Evaluation, and Potential Mechanism Exploration of Brucine and Brucine N-oxide-A Comparative Study. Molecules 2023, 28, 1341. [Google Scholar] [CrossRef] [PubMed]
- Kafle, A.; Luis, E.; Silwal, R.; Pan, H.M.; Shrestha, P.L.; Bastola, A.K. 3D/4D Printing of Polymers: Fused Deposition Modelling (FDM), Selective Laser Sintering (SLS), and Stereolithography (SLA). Polymers 2021, 13, 3101. [Google Scholar] [CrossRef] [PubMed]
- Raeisdasteh Hokmabad, V.; Davaran, S.; Ramazani, A.; Salehi, R. Design and fabrication of porous biodegradable scaffolds: A strategy for tissue engineering. J. Biomater. Sci. Polym. Ed. 2017, 28, 1797–1825. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Wu, P.; Zhong, Y.; Feng, P.; Gao, C.; Huang, W.; Zhou, Z.; Chen, L.; Shuai, C. Polyetheretherketone/poly (glycolic acid) blend scaffolds with biodegradable properties. J. Biomater. Sci. Polym. Ed. 2016, 27, 1434–1446. [Google Scholar] [CrossRef] [PubMed]
- Lehtonen, T.J.; Tuominen, J.U.; Hiekkanen, E. Resorbable composites with bioresorbable glass fibers for load-bearing applications. In vitro degradation and degradation mechanism. Acta Biomater. 2013, 9, 4868–4877. [Google Scholar] [CrossRef] [PubMed]
- Samantaray, P.K.; Little, A.; Haddleton, D.M.; McNally, T.; Tan, B.; Sun, Z.; Wan, C. Poly(glycolic acid) (PGA): A versatile building block expanding high performance and sustainable bioplastic applications. Green Chem. 2020, 22, 4055–4081. [Google Scholar] [CrossRef]
- Qu, X.J.; Moore, M.J.; Li, D.Z.; Yi, T.S. PGA: A software package for rapid, accurate, and flexible batch annotation of plastomes. Plant Methods 2019, 15, 50. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Qin, S.; He, M.; Zhou, D.; Qin, Q.; Wang, H. Current applications of poly (lactic acid) composites in tissue engineering and drug delivery. Compos. Part B Eng. 2020, 199, 108238. [Google Scholar] [CrossRef]
- Shuai, C.; Guo, W.; Wu, P.; Yang, W.; Hu, S.; Xia, Y.; Feng, P. A graphene oxide-Ag co-dispersing nanosystem: Dual synergistic effects on antibacterial activities and mechanical properties of polymer scaffolds. Chem. Eng. J. 2018, 347, 322–333. [Google Scholar] [CrossRef]
- Larsen, B.S.; Kaiser, M.A.; Botelho, M.; Wooler, G.R.; Buxton, L.W. Comparison of pressurized solvent and reflux extraction methods for the determination of perfluorooctanoic acid in polytetrafluoroethylene polymers using LC-MS-MS. Analyst 2005, 130, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Carrillo, N.; Aguilar-Santamaría, M.d.l.Á.; Vernon-Carter, E.J.; Jiménez-Alvarado, R.; Cruz-Sosa, F.; Román-Guerrero, A. Extraction of phenolic compounds from Satureja macrostema using microwave-ultrasound assisted and reflux methods and evaluation of their antioxidant activity and cytotoxicity. Ind. Crops Prod. 2017, 103, 213–221. [Google Scholar] [CrossRef]
- Gu, Z.P.; Zhang, S.M.; Wang, C.L.; Lian, W.Y.; Xiao, P.G.; Chen, J.M. Determation of strychnine and brucine in Strychnos by HPLC. Acta Pharm. Sin. 1997, 32, 791–794. [Google Scholar]
- Guo, Y.; Shao, S.; Zhang, W.; Li, C.; Meng, Z.; Sun, S.; Yang, D.; Lü, S. Content Determination and Release Characteristics of Six Components in the Different Phases of “Glycyrrhizaglabra-Nux vomica” Decoction by UPLC-MS/MS. Molecules 2022, 27, 6180. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, H.; Huo, K.; Cui, L.; Zhang, W.; Ni, H.; Zhang, Y.; Wu, Z.; Chu, P.K. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials 2011, 32, 5706–5716. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Li, X.; He, J.; Chen, Y.; Qi, L.; Yuan, P.; Ou, K.; Liu, F.; Zhou, Y.; Qin, X. Graphene oxide-silver nanocomposites embedded nanofiber core-spun yarns for durable antibacterial textiles. J. Colloid. Interface Sci. 2021, 584, 164–173. [Google Scholar] [CrossRef]
- Nakamura, Y.; Matsunaga, H.; Haginaka, J. Preparation of molecularly imprinted polymers for strychnine by precipitation polymerization and multistep swelling and polymerization and their application for the selective extraction of strychnine from nux-vomica extract powder. J. Sep. Sci. 2016, 39, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Zhao, W.; Zhang, L.; Wang, N. Microstructure, content and in vitro release of brucine and strychnine in Strychnos nux-vomica powder with different particle sizes. Trans. Tianjin Univ. 2014, 20, 444–450. [Google Scholar] [CrossRef]
- Jackson, N.; Sutton, T.; Bedford, L.; Ugrinovic, S.; Kumararatne, D.; Gkrania-Klotsas, E. A First Unexplained Invasive Encapsulated Bacterial Infection in Young Adults Associated With High Mortality and Readmission Rates. Clin. Infect. Dis. 2020, 70, 528–530. [Google Scholar] [CrossRef] [PubMed]
- Aliakbar Ahovan, Z.; Esmaeili, Z.; Eftekhari, B.S.; Khosravimelal, S.; Alehosseini, M.; Orive, G.; Dolatshahi-Pirouz, A.; Pal Singh Chauhan, N.; Janmey, P.A.; Hashemi, A.; et al. Antibacterial smart hydrogels: New hope for infectious wound management. Mater. Today Bio 2022, 17, 100499. [Google Scholar] [CrossRef] [PubMed]
- Banin, E.; Hughes, D.; Kuipers, O.P. Editorial: Bacterial pathogens, antibiotics and antibiotic resistance. FEMS Microbiol. Rev. 2017, 41, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic resistance and persistence—Implications for human health and treatment perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef] [PubMed]
- Tilahun, M.; Kassa, Y.; Gedefie, A.; Ashagire, M. Emerging Carbapenem-Resistant Enterobacteriaceae Infection, Its Epidemiology and Novel Treatment Options: A Review. Infect. Drug Resist. 2021, 14, 4363–4374. [Google Scholar] [CrossRef]
- Tiseo, G.; Brigante, G.; Giacobbe, D.R.; Maraolo, A.E.; Gona, F.; Falcone, M.; Giannella, M.; Grossi, P.; Pea, F.; Rossolini, G.M.; et al. Diagnosis and management of infections caused by multidrug-resistant bacteria: Guideline endorsed by the Italian Society of Infection and Tropical Diseases (SIMIT), the Italian Society of Anti-Infective Therapy (SITA), the Italian Group for Antimicrobial Stewardship (GISA), the Italian Association of Clinical Microbiologists (AMCLI) and the Italian Society of Microbiology (SIM). Int. J. Antimicrob. Agents 2022, 60, 106611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Zeng, L.; Zhang, J.; Zuo, J.; Zou, J.; Ding, J.; Chen, X. Polymer Fiber Scaffolds for Bone and Cartilage Tissue Engineering. Adv. Funct. Mater. 2019, 29, 1903279. [Google Scholar] [CrossRef]
- Feng, P.; Jia, J.; Peng, S.; Shuai, Y.; Pan, H.; Bai, X.; Shuai, C. Transcrystalline growth of PLLA on carbon fiber grafted with nano-SiO2 towards boosting interfacial bonding in bone scaffold. Biomater. Res. 2022, 26, 2. [Google Scholar] [CrossRef] [PubMed]
- Zan, J.; Qian, G.; Deng, F.; Zhang, J.; Zeng, Z.; Peng, S.; Shuai, C. Dilemma and breakthrough of biodegradable poly-l-lactic acid in bone tissue repair. J. Mater. Res. Technol. 2022, 17, 2369–2387. [Google Scholar] [CrossRef]
- Mauney, J.R.; Blumberg, J.; Pirun, M.; Volloch, V.; Vunjak-Novakovic, G.; Kaplan, D.L. Osteogenic differentiation of human bone marrow stromal cells on partially demineralized bone scaffolds in vitro. Tissue Eng. 2004, 10, 81–92. [Google Scholar] [CrossRef]
- Urist, M.R.; DeLange, R.J.; Finerman, G.A. Bone cell differentiation and growth factors. Science 1983, 220, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Glimcher, M.J. Characterization of matrix-induced osteogenesis in rat calvarial bone defects: II. Origins of bone-forming cells. Calcif. Tissue Int. 1999, 65, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Zeltinger, J.; Sherwood, J.K.; Graham, D.A.; Müeller, R.; Griffith, L.G. Effect of pore size and void fraction on cellular adhesion, proliferation, and matrix deposition. Tissue Eng. 2001, 7, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Akay, G.; Birch, M.A.; Bokhari, M.A. Microcellular polyHIPE polymer supports osteoblast growth and bone formation in vitro. Biomaterials 2004, 25, 3991–4000. [Google Scholar] [CrossRef] [PubMed]
- Akin, F.A.; Zreiqat, H.; Jordan, S.; Wijesundara, M.B.; Hanley, L. Preparation and analysis of macroporous TiO2 films on Ti surfaces for bone-tissue implants. J. Biomed. Mater. Res. 2001, 57, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.; Zhao, D.; Ren, Y.; Zhang, L.; Zhou, Z.; Li, Q. A convenient process to fabricate gelatin modified porous PLLA materials with high hydrophilicity and strength. Biomater. Sci. 2016, 4, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Guo, X.; Gao, C.; Gao, D.; Xiao, T.; Shuai, X.; Shuai, C.; Peng, S. Diopside modified porous polyglycolide scaffolds with improved properties. RSC Adv. 2015, 5, 54822–54829. [Google Scholar] [CrossRef]
- Du, Y.; Liu, H.; Yang, Q.; Wang, S.; Wang, J.; Ma, J.; Noh, I.; Mikos, A.G.; Zhang, S. Selective laser sintering scaffold with hierarchical architecture and gradient composition for osteochondral repair in rabbits. Biomaterials 2017, 137, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Mao, Z.; Lu, H.; Nie, Y.; Hu, H.; Peng, S. Fabrication of porous polyvinyl alcohol scaffold for bone tissue engineering via selective laser sintering. Biofabrication 2013, 5, 015014. [Google Scholar] [CrossRef] [PubMed]
- Celesti, C.; Gervasi, T.; Cicero, N.; Giofrè, S.V.; Espro, C.; Piperopoulos, E.; Gabriele, B.; Mancuso, R.; Lo Vecchio, G.; Iannazzo, D. Titanium Surface Modification for Implantable Medical Devices with Anti-Bacterial Adhesion Properties. Materials 2022, 15, 3283. [Google Scholar] [CrossRef]
- Yazdani-Ahmadabadi, H.; Felix, D.F.; Yu, K.; Yeh, H.H.; Luo, H.D.; Khoddami, S.; Takeuchi, L.E.; Alzahrani, A.; Abbina, S.; Mei, Y.; et al. Durable Surfaces from Film-Forming Silver Assemblies for Long-Term Zero Bacterial Adhesion without Toxicity. ACS Cent. Sci. 2022, 8, 546–561. [Google Scholar] [CrossRef] [PubMed]
- Uneputty, A.; Dávila-Lezama, A.; Garibo, D.; Oknianska, A.; Bogdanchikova, N.; Hernández-Sánchez, J.F.; Susarrey-Arce, A. Strategies applied to modify structured and smooth surfaces: A step closer to reduce bacterial adhesion and biofilm formation. Colloid Interface Sci. Commun. 2022, 46, 100560. [Google Scholar] [CrossRef]
- Lanno, G.; Ramos, C.; Preem, L.; Putrinš, M.; Laidmäe, I.; Tenson, T.; Kogermann, K. Antibacterial Porous Electrospun Fibers as Skin Scaffolds for Wound Healing Applications. ACS Omega 2020, 5, 30011–30022. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Shi, M.; Huang, L.; Shao, Q.; Chen, J. Growth of lactobacilli, Staphylococcus aureus and Escherichia coli in normal and mastitic milk and whey. Vet. Microbiol. 1993, 37, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Altun, E.; Yuca, E.; Ekren, N.; Kalaskar, D.M.; Ficai, D.; Dolete, G.; Ficai, A.; Gunduz, O. Kinetic Release Studies of Antibiotic Patches for Local Transdermal Delivery. Pharmaceutics 2021, 13, 613. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.C.; Yang, Y.T.; Wu, C.C.; Hsiao, J.K.; Huang, C.Y.; Chen, I.H.; Wang, C.C. Bioinspired collagen-gelatin-hyaluronic acid-chondroitin sulfate tetra-copolymer scaffold biomimicking native cartilage extracellular matrix facilitates chondrogenesis of human synovium-derived stem cells. Int. J. Biol. Macromol. 2023, 240, 124400. [Google Scholar] [CrossRef]
- Deng, F.; Wu, P.; Qian, G.; Shuai, Y.; Zhang, L.; Peng, S.; Shuai, C.; Wang, G. Silver-decorated black phosphorus: A synergistic antibacterial strategy. Nanotechnology 2022, 33, 245708. [Google Scholar] [CrossRef] [PubMed]
- Nourmohammadi, J.; Hadidi, M.; Nazarpak, M.H.; Mansouri, M.; Hasannasab, M. Physicochemical and antibacterial characterization of nanofibrous wound dressing from silk fibroin-polyvinyl alcohol-elaeagnus angustifolia extract. Fibers Polym. 2020, 21, 456–464. [Google Scholar] [CrossRef]
- Yang, M.; Shuai, Y.; Yang, Y.; Zeng, D.; Peng, S.; Tian, Z.; Shuai, C. In situ grown rare earth lanthanum on carbon nanofibre for interfacial reinforcement in Zn implants. Virtual Phys. Prototyp. 2022, 17, 700–717. [Google Scholar] [CrossRef]
- Hu, K.; Kong, X.; Zhong, M.; Wan, H.; Lin, N.; Pei, X. Brucine inhibits bone metastasis of breast cancer cells by suppressing Jagged1/Notch1 signaling pathways. Chin. J. Integr. Med. 2017, 23, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Wang, Z.; Peng, S.; Shuai, Y.; Chen, Y.; Zeng, D.; Feng, P. Water-responsive shape memory thermoplastic polyurethane scaffolds triggered at body temperature for bone defect repair. Mater. Chem. Front. 2022, 6, 1456–1469. [Google Scholar] [CrossRef]
- Qi, F.; Gao, X.; Shuai, Y.; Peng, S.; Deng, Y.; Yang, S.; Yang, Y.; Shuai, C. Magnetic-driven wireless electrical stimulation in a scaffold. Compos. Part B Eng. 2022, 237, 109864. [Google Scholar] [CrossRef]
- Shuai, C.; Yuan, X.; Shuai, Y.; Qian, G.; Yao, J.; Xu, W.; Peng, S.; Yang, W. Nitrogen-doped carbon-ZnO heterojunction derived from ZIF-8: A photocatalytic antibacterial strategy for scaffold. Mater. Today Nano 2022, 18, 100210. [Google Scholar] [CrossRef]
- Qi, F.; Liao, R.; Shuai, Y.; Pan, H.; Qian, G.; Peng, S.; Shuai, C. A conductive network enhances nerve cell response. Addit. Manuf. 2022, 52, 102694. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Hu, S.; Feng, P.; Xia, Y.; Pei, Z.; Tian, J.; Jiang, K.; Liu, L.; Cai, X.; Wu, P. Brucine Sulfate, a Novel Bacteriostatic Agent in 3D Printed Bone Scaffold Systems. Polymers 2024, 16, 1428. https://doi.org/10.3390/polym16101428
Li J, Hu S, Feng P, Xia Y, Pei Z, Tian J, Jiang K, Liu L, Cai X, Wu P. Brucine Sulfate, a Novel Bacteriostatic Agent in 3D Printed Bone Scaffold Systems. Polymers. 2024; 16(10):1428. https://doi.org/10.3390/polym16101428
Chicago/Turabian StyleLi, Jinying, Shi Hu, Pei Feng, Yang Xia, Zihan Pei, Jiaxuan Tian, Kun Jiang, Liang Liu, Xiong Cai, and Ping Wu. 2024. "Brucine Sulfate, a Novel Bacteriostatic Agent in 3D Printed Bone Scaffold Systems" Polymers 16, no. 10: 1428. https://doi.org/10.3390/polym16101428
APA StyleLi, J., Hu, S., Feng, P., Xia, Y., Pei, Z., Tian, J., Jiang, K., Liu, L., Cai, X., & Wu, P. (2024). Brucine Sulfate, a Novel Bacteriostatic Agent in 3D Printed Bone Scaffold Systems. Polymers, 16(10), 1428. https://doi.org/10.3390/polym16101428







