A Study on the Dilational Modulus Measurement of Polyacrylic Acid Films at Air–Water Interface by Pendant Bubble Tensiometry
Abstract
1. Introduction
2. Materials and Methods
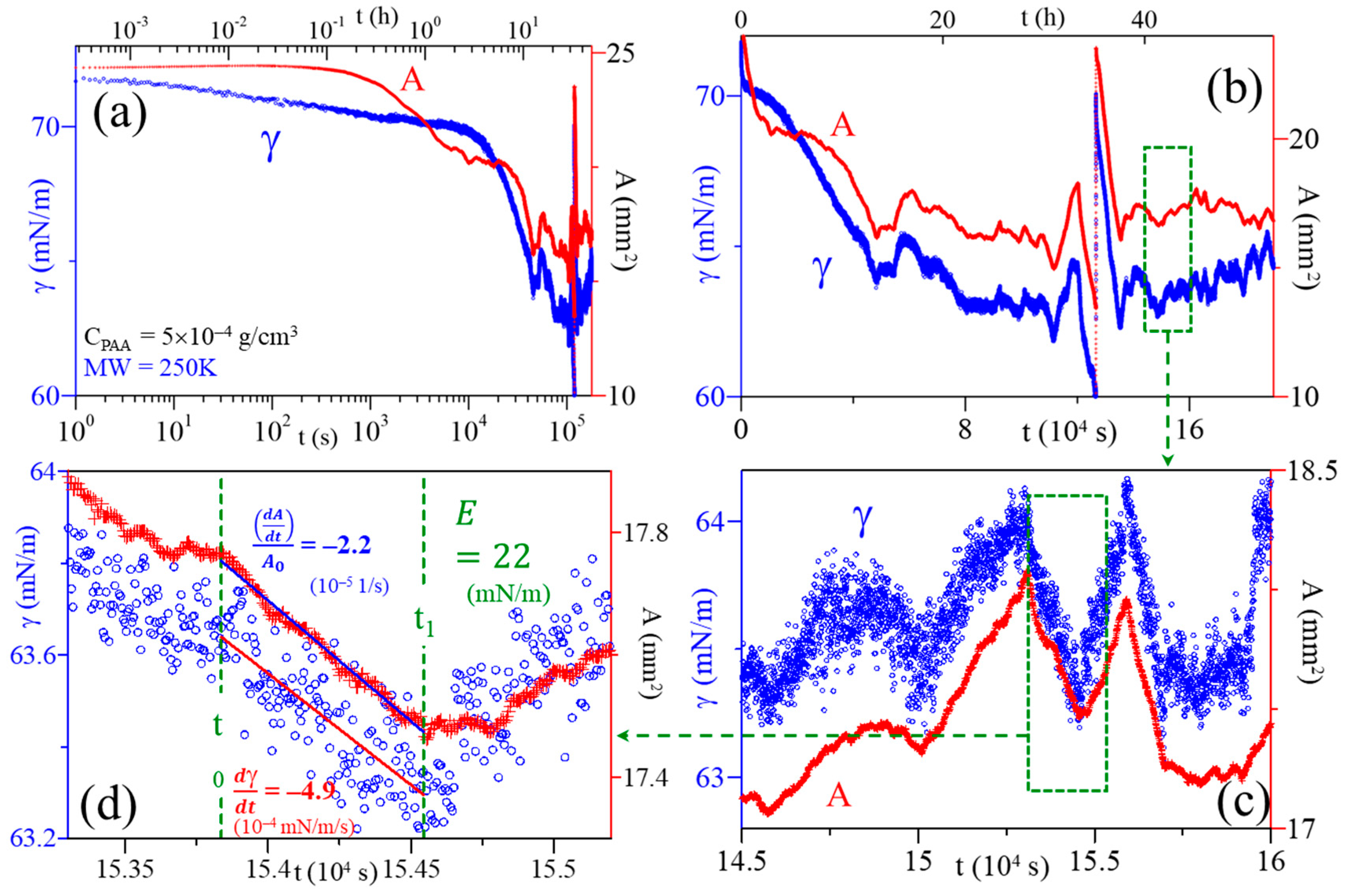
- The individual perturbances are identified in regions where SA and ST relaxations both have nearly linear changes (∆A > ~0.05 mm2) and ST (∆γ > ~0.1 mN/m);
- The onset and end of the perturbance are carefully selected (t0 and t1), which in turn will correspond to specific A0, A1, γ0, γ1 values, respectively;
- A linear regression is applied to the SA and ST data, respectively, in the selected time interval. The slopes from each linear fit provide the values for dγ/dt and dA/dt; and
- The surface dilational rate (dlnA/dt) is then obtained by evaluating dlnA/dt = (dA/dt)/(A0) and the dilational modulus (Ei) for an individual perturbance is calculated from Ei = (dγ/dt)/(dlnA/dt).
3. Results
3.1. Dynamic ST
3.2. Forced Perturbation
3.3. SA and ST Response to Temperature Variation
3.4. Equilibrium ST
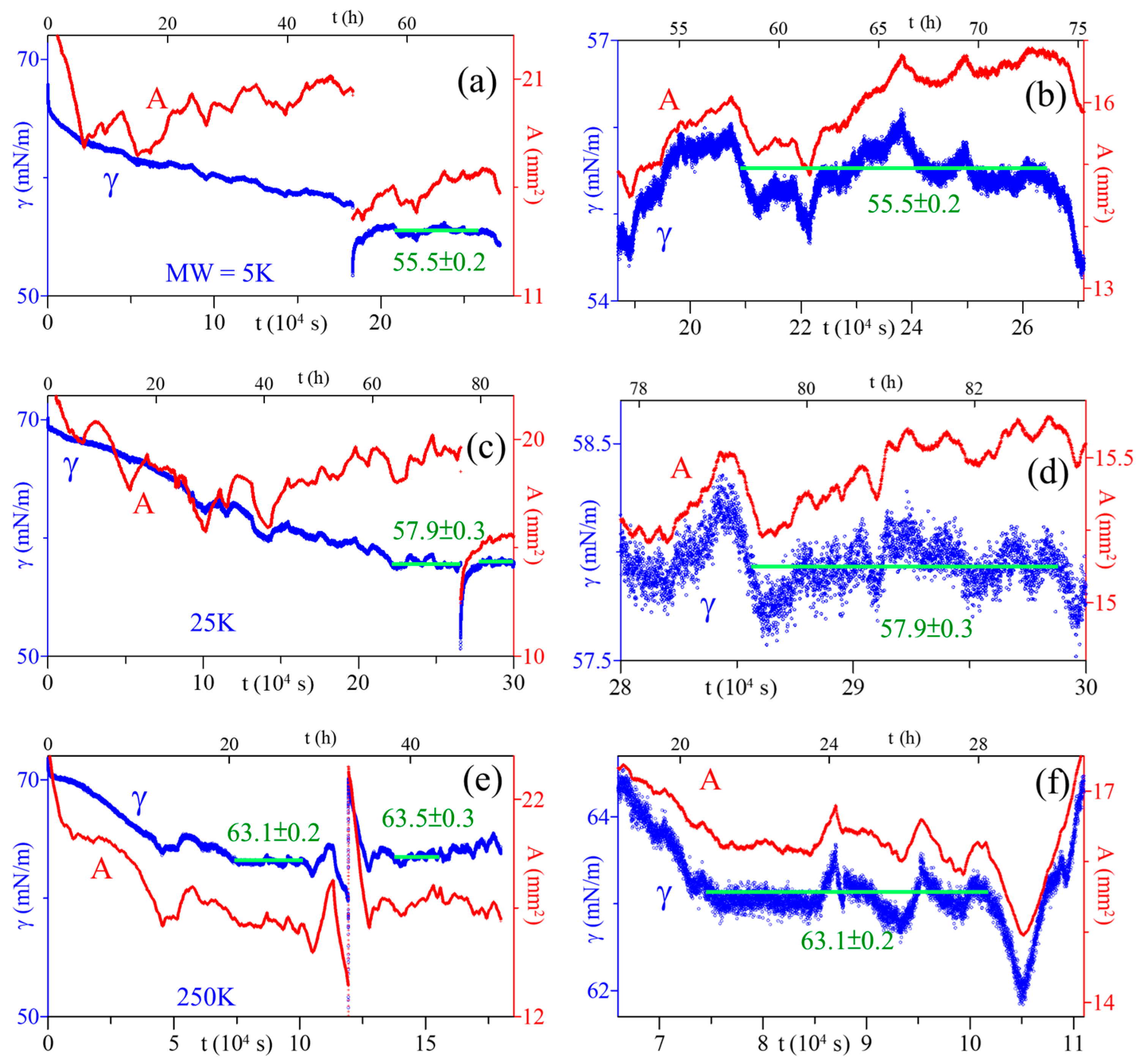
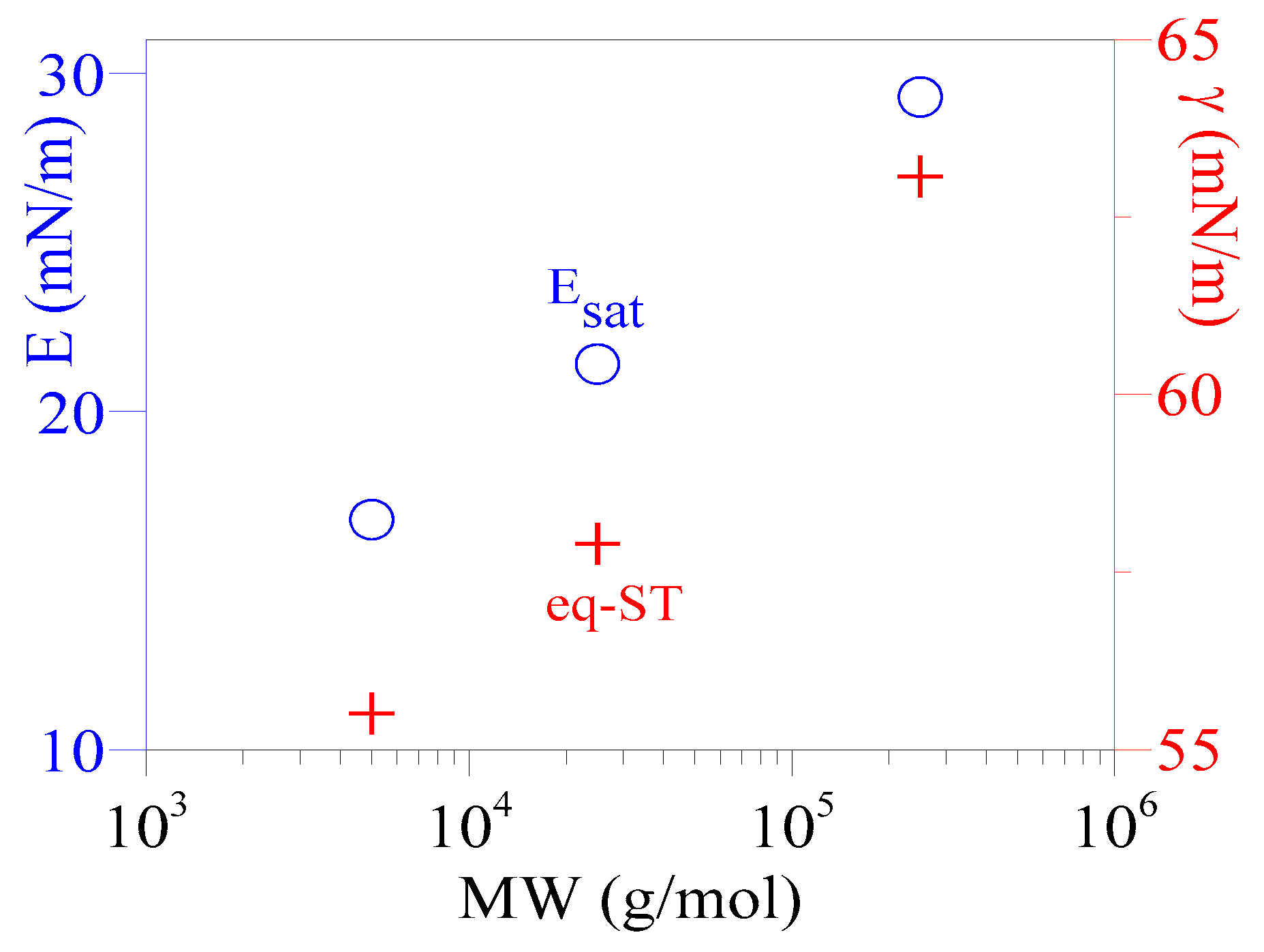
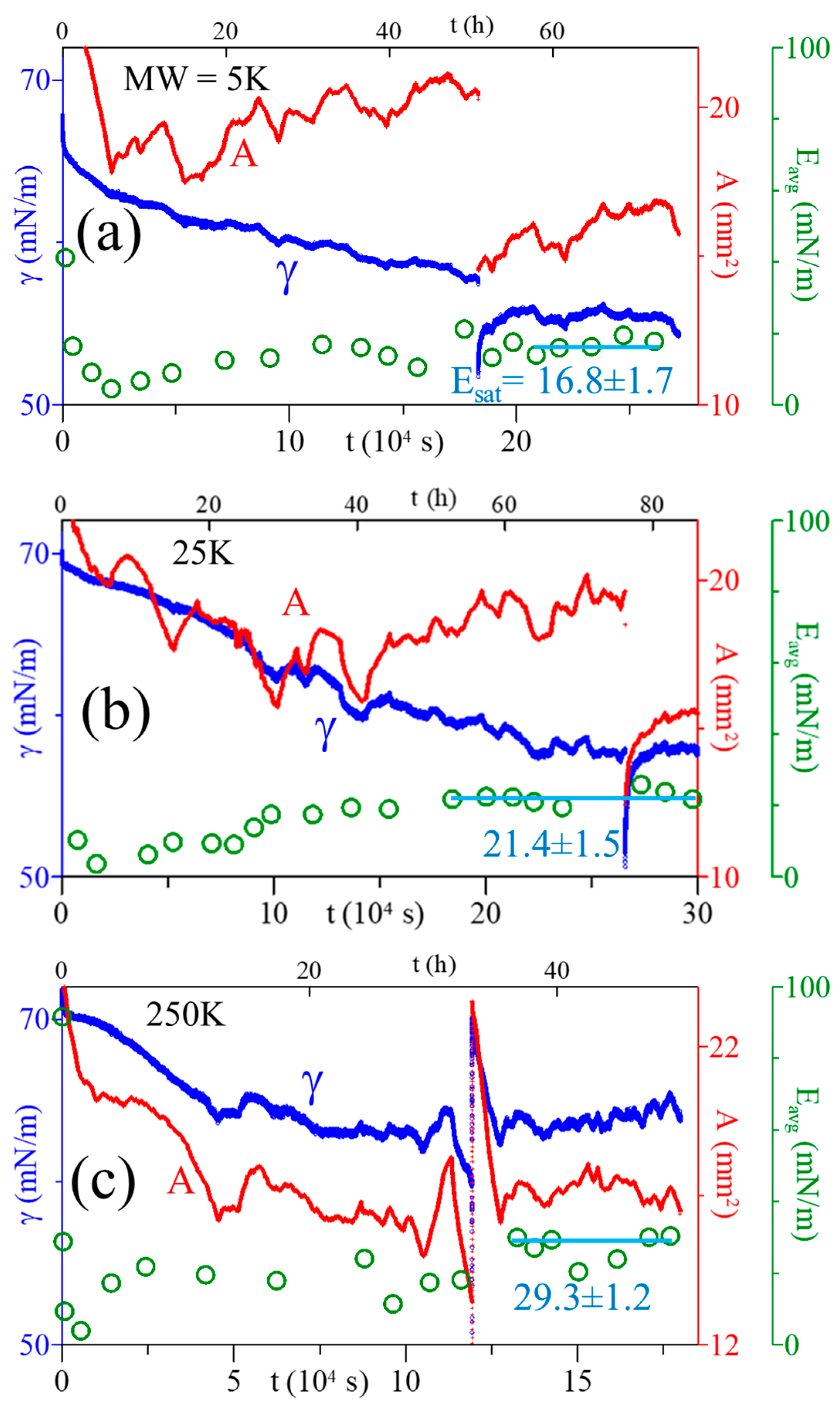
3.5. Esat of Adsorbed PAA Films
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbhuiya, S.; Das, B.B. Water-soluble polymers in cementitious materials: A comprehensive review of roles, mechanisms and applications. Case Stud. Constr. Mater. 2023, 19, e02312. [Google Scholar] [CrossRef]
- Arkaban, H.; Barani, M.; Akbarizadeh, M.R.; Chauhan, N.P.S.; Jadoun, S.; Soltani, M.D.; Zarrintaj, P. Polyacrylic Acid Nanoplatforms: Antimicrobial, Tissue Engineering, and Cancer Theranostic Applications. Polymers 2022, 14, 1259. [Google Scholar] [CrossRef]
- Kadajji, V.G.; Betageri, G.V. Water Soluble Polymers for Pharmaceutical Applications. Polymers 2011, 3, 1972–2009. [Google Scholar] [CrossRef]
- Lin, F.; Jian, H.R.; Wu, F.G. Glycol Chitosan: A Water-Soluble Polymer for Cell Imaging and Drug Delivery. Molecules 2019, 24, 4371. [Google Scholar] [CrossRef]
- Saitoh, T.; Ono, N.; Hiraide, M. Effective collection of hydrophobic organic pollutants in water with aluminum hydroxide and hydrophobically modified polyacrylic acid. Chemosphere 2012, 89, 759–763. [Google Scholar] [CrossRef]
- Vajihinejad, V.; Gumfekar, S.P.; Bazoubandi, B.; Najafabadi, Z.R.; Soares, J.B.P. Water Soluble Polymer Flocculants: Synthesis, Characterization, and Performance Assessment. Macromol. Mater. Eng. 2019, 304, 1800526. [Google Scholar] [CrossRef]
- Cao, S.C.; Bate, B.; Hu, J.W.; Jung, J. Engineering Behavior and Characteristics of Water-Soluble Polymers: Implication on Soil Remediation and Enhanced Oil Recovery. Sustainability 2016, 8, 205. [Google Scholar] [CrossRef]
- Liu, P.; Mu, Z.; Wang, C.; Wang, Y. Experimental Study of Rheological Properties and Oil Displacement Efficiency in Oilfields for a Synthetic Hydrophobically Modified Polymer. Sci. Rep. 2017, 7, 8791. [Google Scholar] [CrossRef]
- Pal, N.; Babu, K.; Mandal, A. Surface tension, dynamic light scattering and rheological studies of a new polymeric surfactant for application in enhanced oil recovery. J. Pet. Sci. Eng. 2016, 146, 591–600. [Google Scholar] [CrossRef]
- Sennakesavan, G.; Mostakhdemin, M.; Dkhar, L.K.; Seyfoddin, A.; Fatihhi, S.J. Acrylic Acid/Acrylamide Based Hydrogels and Its Properties—A Review. Polym. Degrad. Stab. 2020, 180, 109308. [Google Scholar] [CrossRef]
- Nigro, V.; Angelini, R.; Bertoldo, M.; Buratti, E.; Franco, S.; Ruzicka, B. Chemical-Physical Behaviour of Microgels Made of Interpenetrating Polymer Networks of PNIPAM and Poly(Acrylic Acid). Polymers 2021, 13, 1353. [Google Scholar] [CrossRef]
- Serrano-Aroca, Á.; Deb, S. (Eds.) pH Dependence of Acrylate-Derivative Polyelectrolyte Properties. In Acrylate Polymers for Advanced Applications; BoD—Books on Demand: Norderstedt, Germany, 2020; pp. 3–23. ISBN 9781789851830. [Google Scholar]
- Hammouda, B.; Horkay, F.; Becker, M.L. Clustering and Solvation in Poly(Acrylic Acid) Polyelectrolyte Solutions. Macromolecules 2005, 38, 2019–2021. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Bratek, A.; Jachimska, B.; Jasiński, T.; Warszyński, P. Structure of Poly(Acrylic Acid) in Electrolyte Solutions Determined from Simulations and Viscosity Measurements. J. Phys. Chem. B 2006, 110, 22426–22435. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, H.; Xiao, C. pH-Sensitive Cationic Guar Gum/Poly (Acrylic Acid) Polyelectrolyte Hydrogels: Swelling and in Vitro Drug Release. Carbohydr. Polym. 2007, 69, 774–783. [Google Scholar] [CrossRef]
- Jin, S.; Liu, M.; Chen, S.; Gao, C. A Drug-Loaded Gel Based on Polyelectrolyte Complexes of Poly (Acrylic Acid) with Poly (Vinylpyrrolidone) and Chitosan. Mater. Chem. Phys. 2010, 123, 463–470. [Google Scholar] [CrossRef]
- Paker, E.S.; Senel, M. Polyelectrolyte Multilayers Composed of Polyethyleneimine-Grafted Chitosan and Polyacrylic Acid for Controlled-Drug-Delivery Applications. J. Funct. Biomater. 2022, 13, 131. [Google Scholar] [CrossRef]
- Jaquet, B.; Wei, D.; Reck, B.; Reinhold, F.; Zhang, X.; Wu, H.; Morbidelli, M. Stabilization of Polymer Colloid Dispersions with pH-Sensitive Poly-Acrylic Acid Brushes. Colloid Polym. Sci. 2013, 291, 1659–1667. [Google Scholar] [CrossRef][Green Version]
- Lim, V.H.; Yamashita, Y.; Thi, Y.; Adachi, Y. Inhibition of Cationic Polymer-Induced Colloid Flocculation by Polyacrylic Acid. Water 2018, 10, 1215. [Google Scholar] [CrossRef]
- Gümüşoğlu, T.; Arı, G.A.; Deligöz, H. Investigation of Salt Addition and Acid Treatment Effects on the Transport Properties of Ionically Cross-Linked Polyelectrolyte Complex Membranes Based on Chitosan and Polyacrylic Acid. J. Membr. Sci. 2011, 376, 25–34. [Google Scholar] [CrossRef]
- Ennigrou, D.J.; Sik Ali, M.B.; Dhahbi, M.; Ferid, M. Removal of Heavy Metals from Aqueous Solution by Polyacrylic Acid Enhanced Ultrafiltration. Desalination Water Treat. 2014, 56, 2682–2688. [Google Scholar] [CrossRef]
- Monteux, C.; Williams, C.E.; Bergeron, V. Interfacial Microgels Formed by Oppositely Charged Polyelectrolytes and Surfactants. Part 2. Influence of Surfactant Chain Length and Surfactant/Polymer Ratio. Langmuir 2004, 20, 5367–5374. [Google Scholar] [CrossRef]
- Bykov, A.G.; Lin, S.Y.; Loglio, G.; Lyadinskaya, V.V.; Miller, R.; Noskov, B.A. Impact of surfactant chain length on dynamic surface properties of alkyltrimethylammonium bromide/polyacrylic acid solutions. Colloids Surf. A Physicochem. Eng. Asp. 2010, 354, 382–389. [Google Scholar] [CrossRef]
- Aricov, L.; Petkova, H.; Arabadzhieva, D.; Iovescu, A.; Mileva, E.; Khristov, K.; Stinga, G.; Mihailescu, C.F.; Anghel, D.F.; Todorov, R. Aqueous solutions of associative poly(acrylates): Bulk and interfacial properties. Colloids Surf. A Physicochem. Eng. Asp. 2016, 505, 138–149. [Google Scholar] [CrossRef]
- Okumura, Y.; Kawaguchi, M. Surface pressure–area isotherms and surface dilational moduli of poly (N-isopropyl acrylamide) monolayers spread at air–water interface. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 275–280. [Google Scholar] [CrossRef]
- Noskov, B.A.; Bykov, A.G. Dilational surface rheology of polymer solutions. Russ. Chem. Rev. 2015, 84, 634–652. [Google Scholar] [CrossRef]
- Diez-Pascual, A.; Monroy, F.; Ortega, F.; Rubio, R.; Miller, R.; Noskov, B. Adsorption of Water-Soluble Polymers with Surfactant Character. Dilational Viscoelasticity. Langmuir 2007, 23, 3802–3808. [Google Scholar] [CrossRef]
- Bykov, A.G.; Lin, S.Y.; Loglio, G.; Miller, R.; Noskov, B.A. Kinetics of Adsorption Layer Formation in Solutions of Polyacid/Surfactant Complexes. J. Phys. Chem. C 2009, 113, 5664–5671. [Google Scholar] [CrossRef]
- Gyurova, A.Y.; Halacheva, S.; Mileva, E. Aqueous solutions of random poly(methyl methacrylate-co-acrylic acid): Effect of the acrylic acid content. RSC Adv. 2017, 7, 13372–13382. [Google Scholar] [CrossRef]
- Noskov, B.A.; Loglio, G.; Miller, R. Dilational Viscoelasticity of Polyelectolyte/Surfactant Adsorption Films at the Air/Water Interface: Dodecyltrimethylammonium Bromide and Sodium Poly(styrenesulfonate). J. Phys. Chem. B 2004, 108, 18615–18622. [Google Scholar] [CrossRef]
- Noskov, B.A.; Nuzhnov, S.N.; Loglio, G.; Miller, R. Dynamic Surface Properties of Sodium Poly(styrenesulfonate) Solutions. Macromolecules 2004, 37, 2519–2526. [Google Scholar] [CrossRef]
- Lyadinskaya, V.V.; Bykov, A.G.; Campbell, R.A.; Varga, I.; Lin, S.Y.; Loglio, G.; Miller, R.; Noskov, B.A. Dynamic surface elasticity of mixed poly(diallyldimethylammonium chloride)/sodium dodecyl sulfate/NaCl solutions. Colloids Surf. A Physicochem. Eng. Asp. 2014, 460, 3–10. [Google Scholar] [CrossRef]
- Noskov, B.A.; Akentiev, A.V.; Bilibin, A.Y.; Grigoriev, D.O.; Loglio, G.; Zorin, I.M.; Miller, R. Dynamic Surface Properties of Poly(N-isopropylacrylamide) Solutions. Langmuir 2004, 20, 9669–9676. [Google Scholar] [CrossRef] [PubMed]
- Monteux, C.; Llauro, M.; Baigl, D.; Williams, C.E.; Anthony, O.; Bergeron, V. Interfacial Microgels Formed by Oppositely Charged Polyelectrolytes and Surfactants. 1. Influence of Polyelectrolyte Molecular Weight. Langmuir 2004, 20, 5358–5366. [Google Scholar] [CrossRef] [PubMed]
- Milling, A.J.; Baines, F.L.; Armes, S.P.; Billingham, N.C.; Richards, R.W. Surface Viscoelastic Parameters of Poly((dimethylamino)ethyl methacrylate-methyl methacrylate) Diblock Copolymer Solutions: pH Dependence of the Evolution of the Equilibrium Values. Macromolecules 2001, 34, 4173–4179. [Google Scholar] [CrossRef]
- Li, H.; Cui, C.Z.; Guo, L.L.; Yuan, F.Q.; Xu, Z.C.; Gong, Q.T.; Jin, Z.Q.; Zhang, L.; Zhang, L. Dynamic interfacial tensions of sulfobetaine and polymers solutions: Effect of structures. J. Mol. Liq. 2022, 356, 119018. [Google Scholar] [CrossRef]
- Lin, S.Y.; McKeigue, K.; Maldarelli, C. Diffusion-controlled surfactant adsorption studied by pendant drop digitization. AIChE J. 1990, 36, 1785–1795. [Google Scholar] [CrossRef]
- Hussain, S.; Le, T.T.Y.; Tsay, R.Y.; Lin, S.Y. Solubility determination of surface-active components from dynamic surface tension data. J. Ind. Eng. Chem. 2020, 92, 297–302. [Google Scholar] [CrossRef]
- Lin, S.Y.; Hwang, H.F. Measurement of Low Interfacial Tension by Pendant Drop Digitization. Langmuir 1994, 10, 4703–4709. [Google Scholar] [CrossRef]
- Tseng, W.C.; Tsay, R.Y.; Le, T.T.Y.; Hussain, S.; Noskov, B.A.; Akentiev, A.; Yeh, H.H.; Lin, S.Y. Evaluation of the dilational modulus of protein films by pendant bubble tensiometry. J. Mol. Liq. 2022, 349, 118113. [Google Scholar] [CrossRef]
- Hussain, S.; Rivas, J.E.M.; Tseng, W.C.; Tsay, R.Y.; Noskov, B.; Loglio, G.; Lin, S.Y. Measurement of Dilational Modulus of an Adsorbed BSA Film Using Pendant Bubble Tensiometry: From a Clean Interface to Saturation. Coll. Interf. 2024, 8, 4. [Google Scholar] [CrossRef]
- Le, T.T.Y.; Hussain, S.; Tsay, R.Y.; Noskov, B.; Akentiev, A.; Lin, S.Y. On the equilibrium surface tension of aqueous protein solutions—Bovine serum albumin. J. Mol. Liq. 2022, 347, 118305. [Google Scholar]
- Tran, V.N.; Rivas, J.E.M.; Hussain, S.; Tseng, W.C.; Akentiev, A.; Noskov, B.; Loglio, G.; Lin, S.Y. A comparison of surface properties for bovine serum albumin and human serum albumin—Dynamic/equilibrium surface tension and dilational modulus. J. Mol. Liq. 2023, 391, 123285. [Google Scholar] [CrossRef]


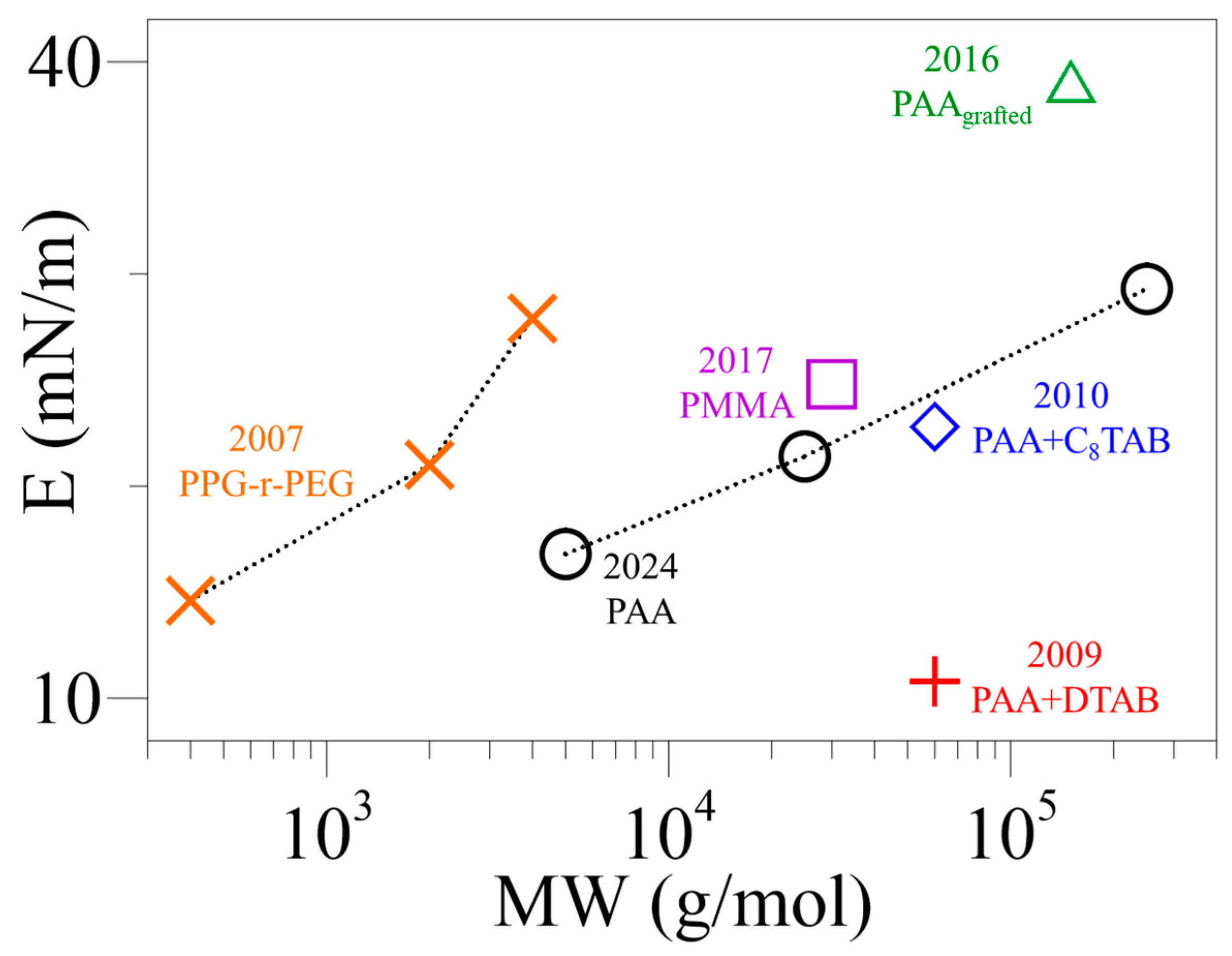
| Year [Ref.] | Compound | Instrument | ST | E | ||
|---|---|---|---|---|---|---|
| Polymer 1,2 | MW (kDa) | Conc. | tlife (h) | Result (mN/m) | ||
| (10−4 g/cm3) | ||||||
| 2001 [35] | poly(DMAEMA-b-MMA) | 42 | - | SQELS, osc. ring | ≤15 | pH ↑→ E ↑ (10–25) |
| 2004 [30] | PSS + DTAB | 70 | 0.01, 0.05 | osc. barrier + drop + Wilhelmy plate | ≤8 | Csurf ↑→ E ↓ then E ↑ (0–100) |
| 2004 [31] | PSS + NaCl | 70 | 500–1100 | osc. barrier and Wilhelmy plate | ≤5 | CNaCl ↑, Cpol ↑ → E ↑ (20–100) |
| 2004 [33] | PNIPAM | 300 | 0.09–1 | osc. barrier | ≤8 | Cpol ↑→ E ↓ then E ↑ (50–60) |
| 2007 [27] | PPG, copolymer (PPG + PEG) | PPG = 0.4, 2, 4, COP = 12 | - | osc. barrier and Wilhelmy plate | - | MW ↑ → E ↑ (15–26) |
| 2009 [28] | PAA, PMA + DTAB | 60(PAA), 100(PMA) | 0.5 | osc. ring | 5 | CDTAB ↑ → E ↑ (5–50) |
| 2010 [23] | PAA + CnTAB | 60 | 0.5 | osc. ring | 5 | CCTAB ↑ → E ↑ (20–75) |
| 2014 [25] | PNIPAM | 46.7 | - | osc. barrier and Wilhelmy plate | - | Π ↑→ Emax ↑, plateaus, then ↓ (40–60) |
| 2014 [32] | PDADMAC/SDS/NaCl | 100–200 | 0.1, 1.0 | osc. barrier | ≤5 | Csurf ↑→ E ↓, then E ↑ (0–70) |
| 2015 [26] | PEO76-PPO29-PEO76 | 8.35 | 0.19–0.48 | osc. barrier and Wilhelmy plate | - | Emax (MW) (10–20) |
| 2016 [24] | PAA (grafted)+ NaCl, HC1/C10/12-NH2 | ~150 | 7.5, 0.75 | osc. bubble | ~21 | E (pH, additive), at γeq (35–60) |
| 2017 [29] | modified PAA + PMMA | ~30 (PMMA-AA) | 0.03–300 | osc. drop + bubble | ~22 | f ↑→ E ↑, at γeq (10–25) |
| 2024 | PAA | 5, 25, 250 | 5 | pendant bubble tensiometer | 30+ | MW ↑ → Esat ↑, beyond γeq (16–30) |
| t0 (104 s) | t1 (104 s) | ∆A/A0 (%) | ∆γ (mN/m) | dlnA/dt (10−5 s−1) | dγ/dt (10−4 mN/m·s) | Ei (mN/m) | |
|---|---|---|---|---|---|---|---|
| 1 | 20.47 | 20.61 | 9.09 | 0.16 | 0.63 | 1.07 | 17 |
| 2 | 20.68 | 20.74 | 7.89 | 0.11 | 1.10 | 1.46 | 13 |
| 3 | 20.75 | 20.78 | −8.10 | −0.11 | −2.81 | −4.55 | 16 |
| 4 | 20.88 | 20.93 | −14.0 | −0.11 | −3.02 | −3.46 | 11 |
| 5 | 21.00 | 21.05 | −12.7 | −0.18 | −2.21 | −3.04 | 14 |
| 6 | 21.10 | 21.22 | −16.1 | −0.21 | −1.10 | −1.41 | 13 |
| 7 | 21.23 | 21.35 | 6.02 | 0.13 | 0.52 | 1.17 | 22 |
| [Ref.], Year | Compound/ MW (kDa) | Conc. (10−4 g/cm3) | Additive Conc. (mol/cm3) | E (mN/m) | Remarks |
|---|---|---|---|---|---|
| [27], 2007 | PPG-r-PEG/ 0.4, 2, 4 | - | - | 15, 21, 28 at MW = 0.4, 2, 4 kDa | Emax |
| [28], 2009 | PAA + DTAB/60 | 0.5 | 1, 7, 8.6 (10−5) | 11, 46, 51 at Cadd. = 1, 7, 8.6 10−5 mol/cm3 | E at t = 5 h |
| [23], 2010 | PAA + CnTAB/60 n = 8, 10, 12, 14, 16 | 0.5 | 4.0 × 10−7 | 23, 49, 69, 76, 70 at n = 8, 10, 12, 14, 16 | Emax |
| [24], 2016 | Grafted PAA + NaCl/150 | 7.5 | - | 39 | pH = 7.7 |
| 5.0 × 10−4 | 62 | pH = 5.9 | |||
| 1.0 × 10−4 | 64 | pH = 6.4 | |||
| [29], 2017 | Modified PAA (with PMMA)/30 | 0.15 | - | 25 | - |
| 2024 | PAA/ 5, 25, 250 | 5 | - | 17, 21, 29 at MW = 5, 25, 250 kDa | Esat |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maradiaga Rivas, J.E.; Chen, L.-J.; Lin, S.-Y.; Hussain, S. A Study on the Dilational Modulus Measurement of Polyacrylic Acid Films at Air–Water Interface by Pendant Bubble Tensiometry. Polymers 2024, 16, 1359. https://doi.org/10.3390/polym16101359
Maradiaga Rivas JE, Chen L-J, Lin S-Y, Hussain S. A Study on the Dilational Modulus Measurement of Polyacrylic Acid Films at Air–Water Interface by Pendant Bubble Tensiometry. Polymers. 2024; 16(10):1359. https://doi.org/10.3390/polym16101359
Chicago/Turabian StyleMaradiaga Rivas, Johann Eduardo, Li-Jen Chen, Shi-Yow Lin, and Siam Hussain. 2024. "A Study on the Dilational Modulus Measurement of Polyacrylic Acid Films at Air–Water Interface by Pendant Bubble Tensiometry" Polymers 16, no. 10: 1359. https://doi.org/10.3390/polym16101359
APA StyleMaradiaga Rivas, J. E., Chen, L.-J., Lin, S.-Y., & Hussain, S. (2024). A Study on the Dilational Modulus Measurement of Polyacrylic Acid Films at Air–Water Interface by Pendant Bubble Tensiometry. Polymers, 16(10), 1359. https://doi.org/10.3390/polym16101359





