On the Unique Morphology and Elastic Properties of Multi-Jet Electrospun Cashew Gum-Based Fiber Mats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Polymeric Solutions
2.3. Physicochemical Characterization of the Solutions
2.4. Electrospinning Process
2.5. Characterization of the Electrospun Fibers
2.5.1. Scanning Electron Microscopy (SEM)
2.5.2. Thermogravimetric Analysis (TGA)
2.5.3. Differential Scanning Calorimetry (DSC)
2.5.4. Wide-Angle X-ray Scattering (WAXS)
2.5.5. Mechanical Tests
2.5.6. Statistical Analysis
3. Results and Discussion
3.1. Fiber Production and Physicochemical Characterization of the Solutions
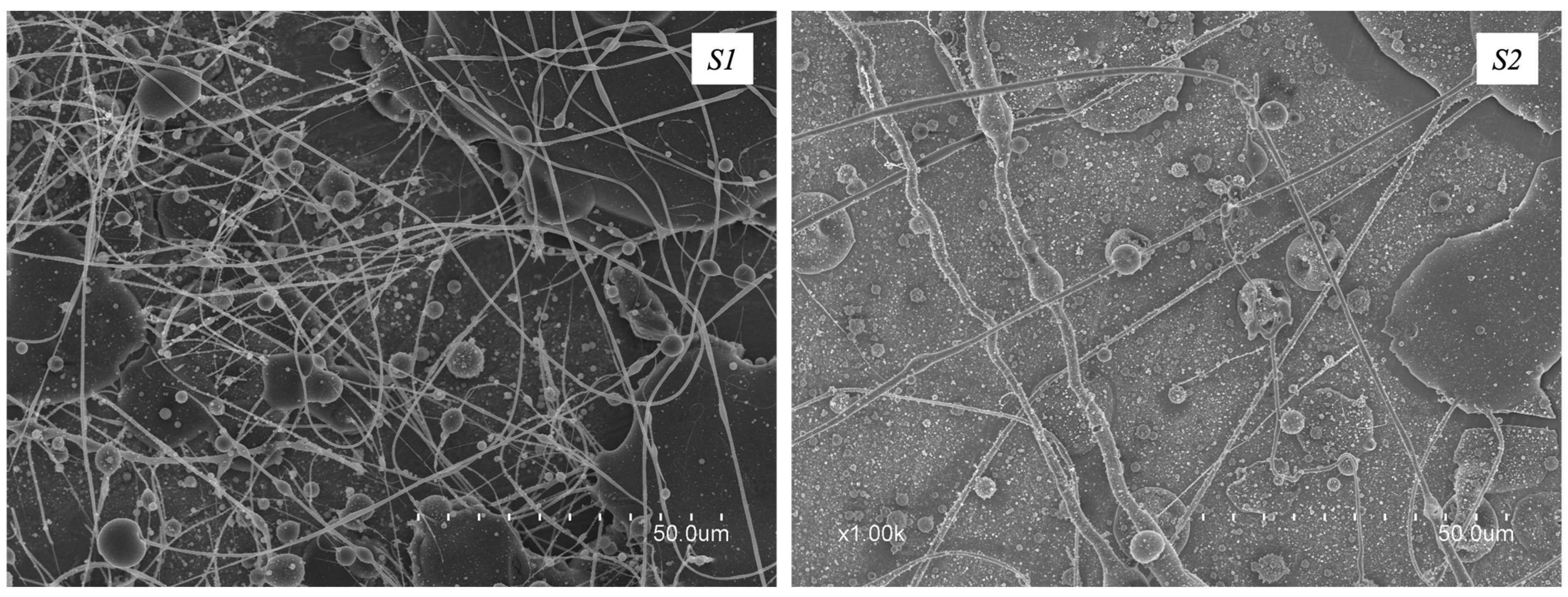
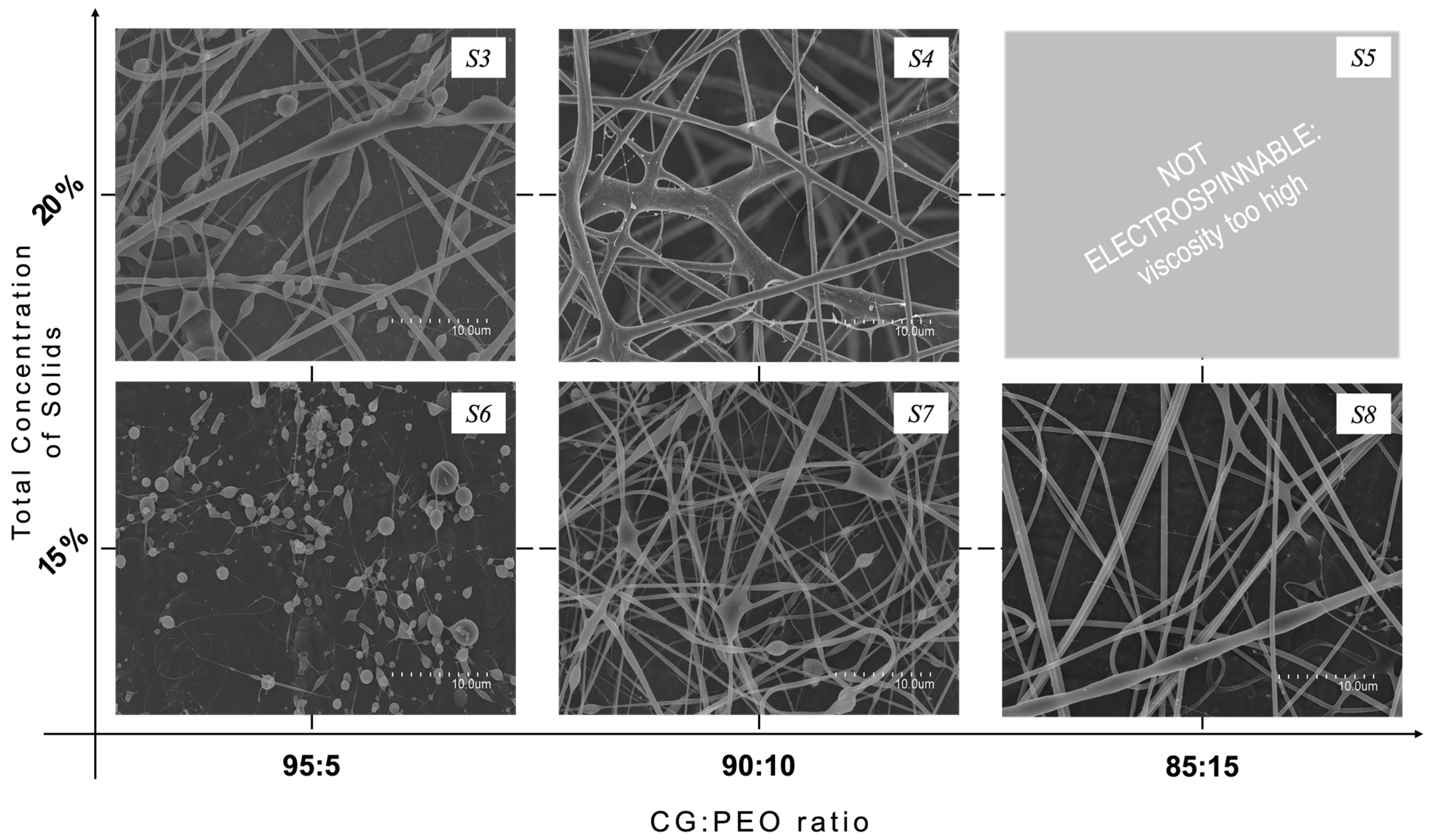
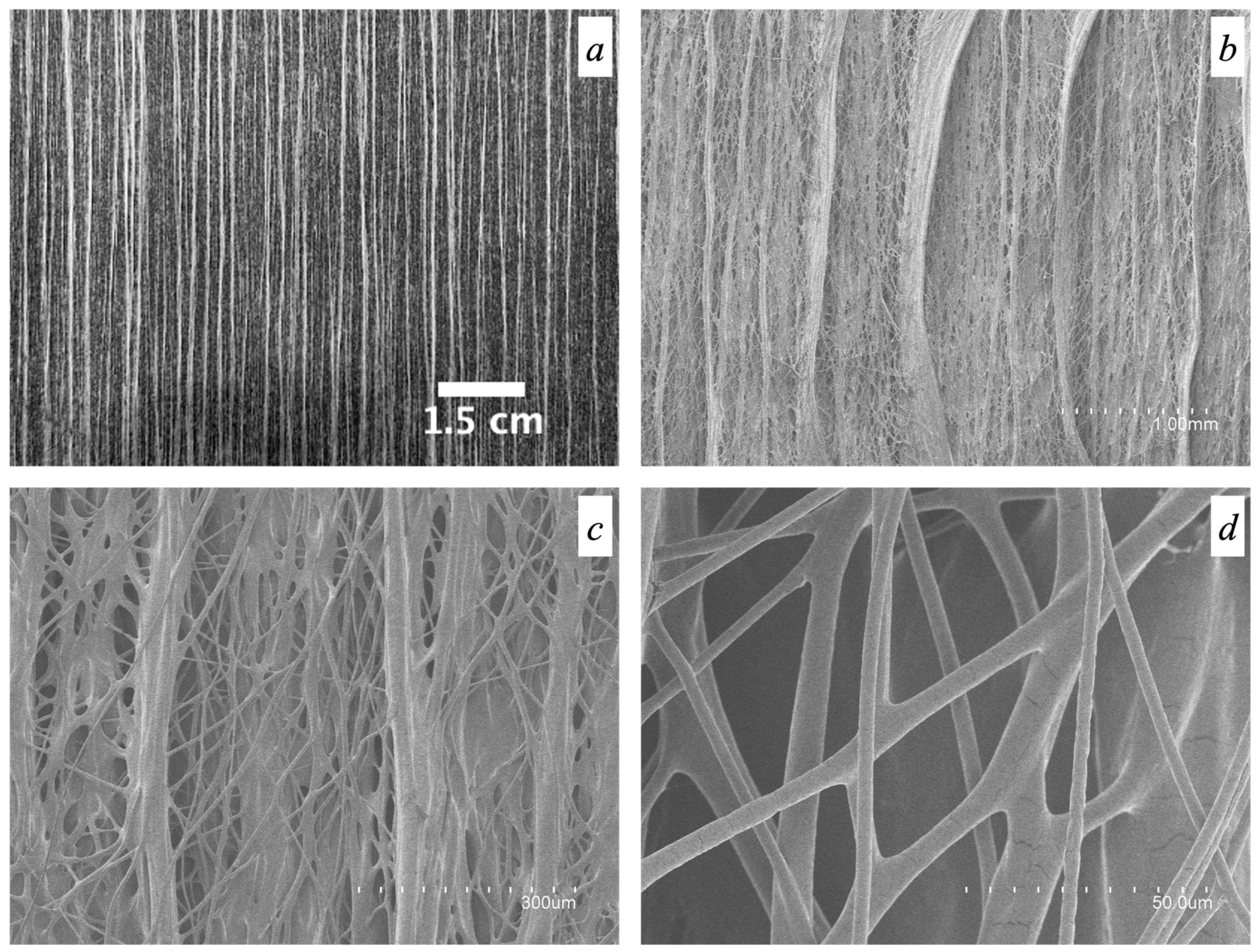
3.2. Morphology of Electrospun CG-PEO Fibers
3.3. Thermal Properties and Thermal Stability
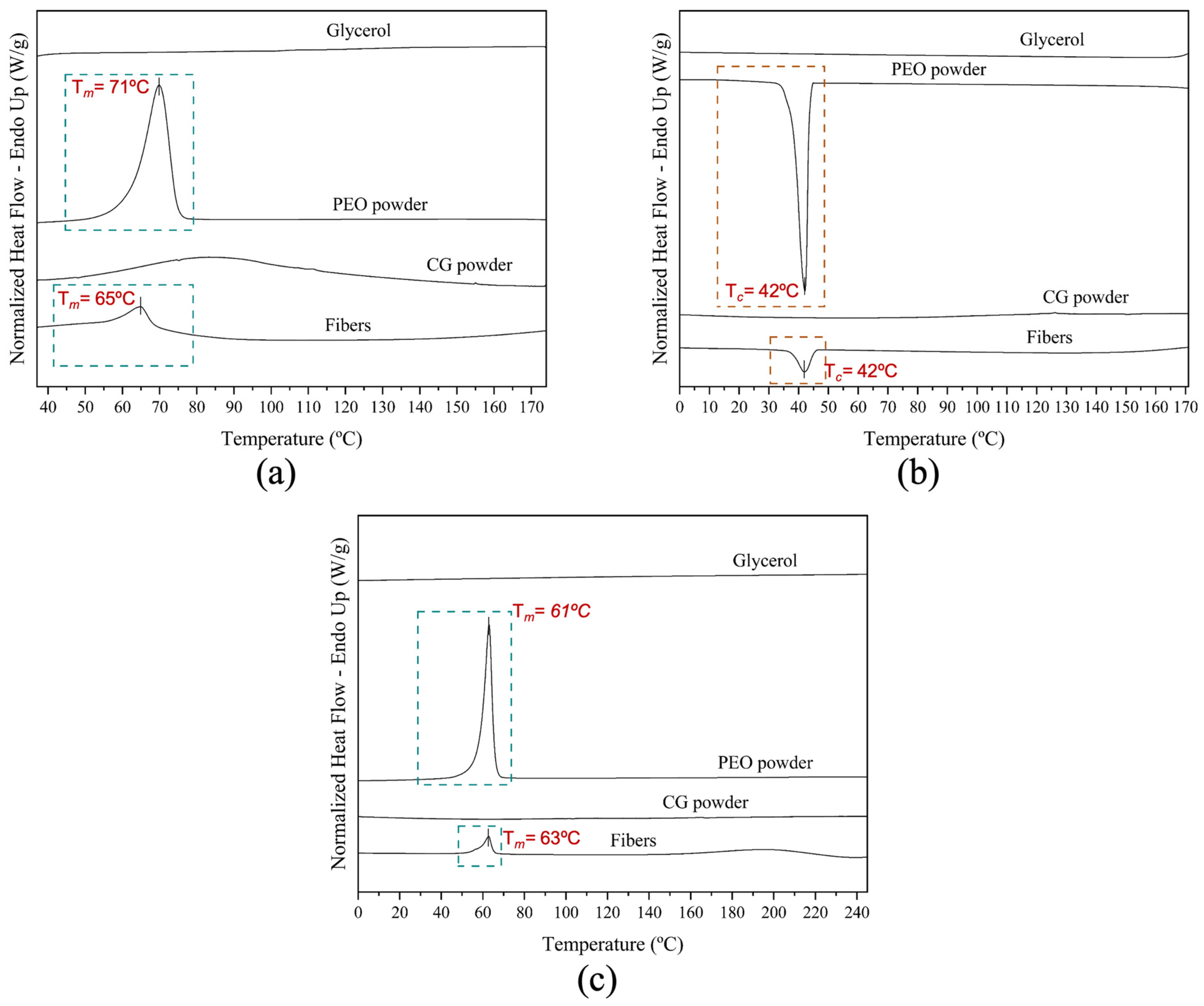
3.3.1. Differential Scanning Calorimetry (DSC)
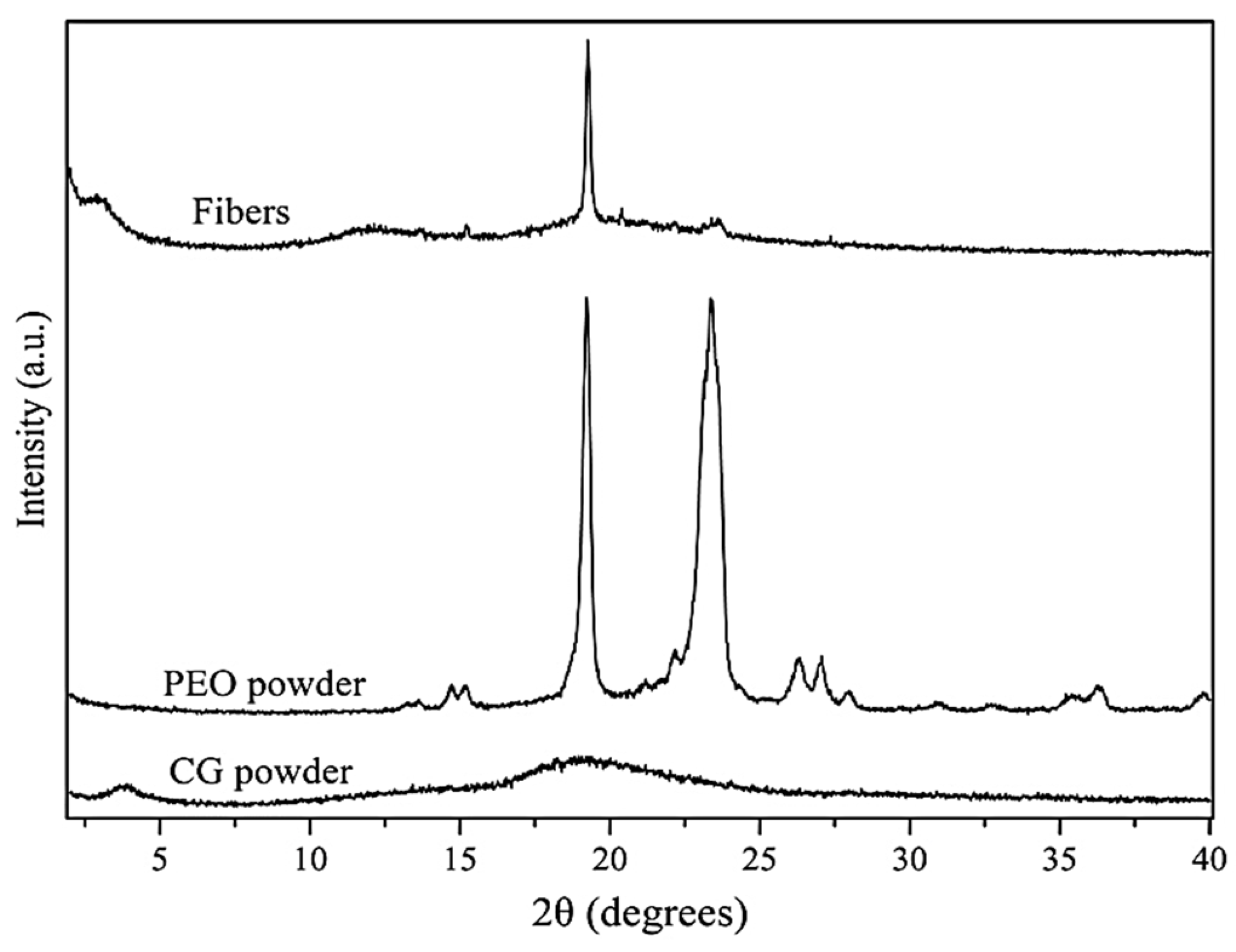
3.3.2. Wide-Angle X-ray Scattering (WAXS)
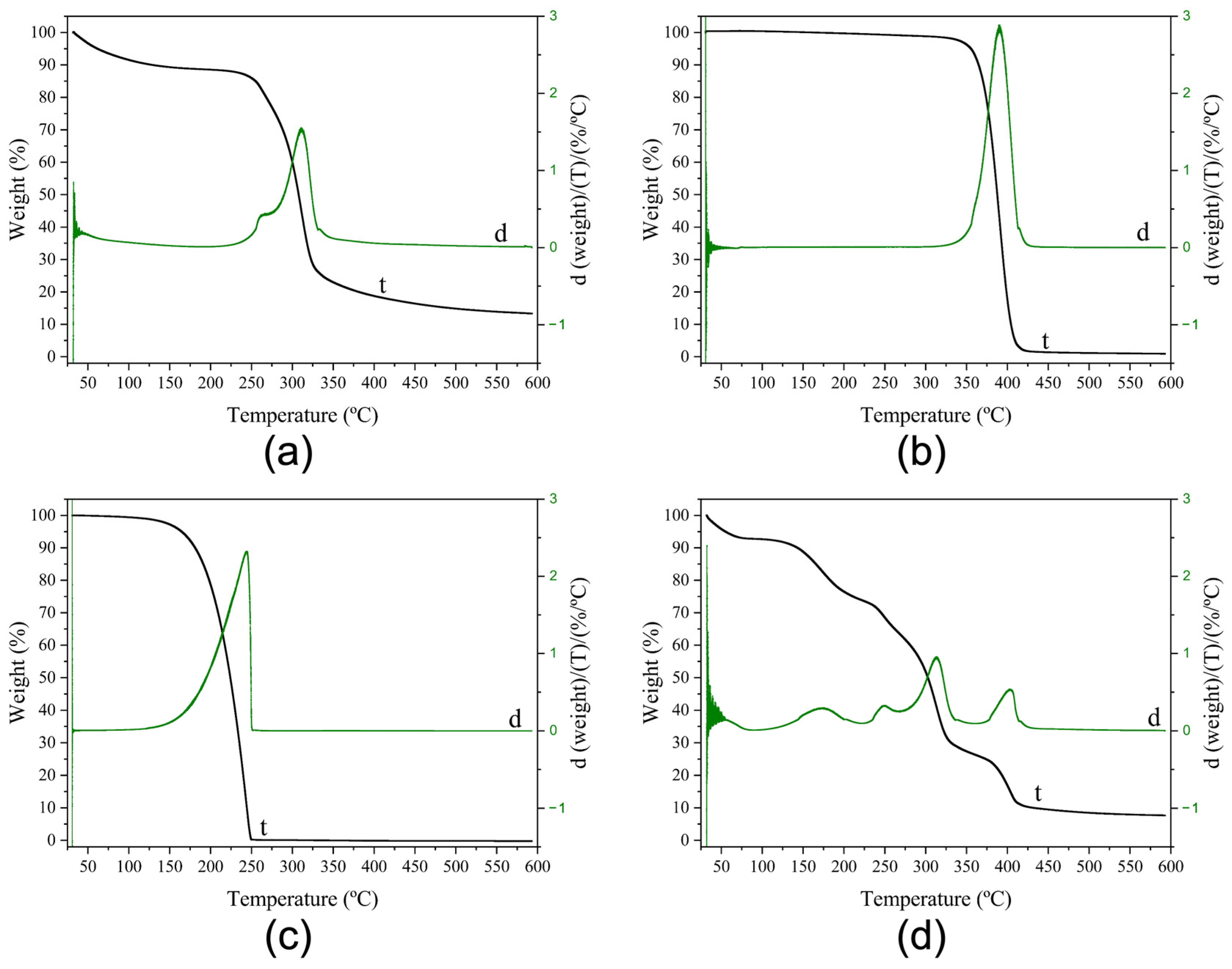
3.3.3. Thermogravimetric Analysis
3.4. ATR-FTIR Spectroscopy
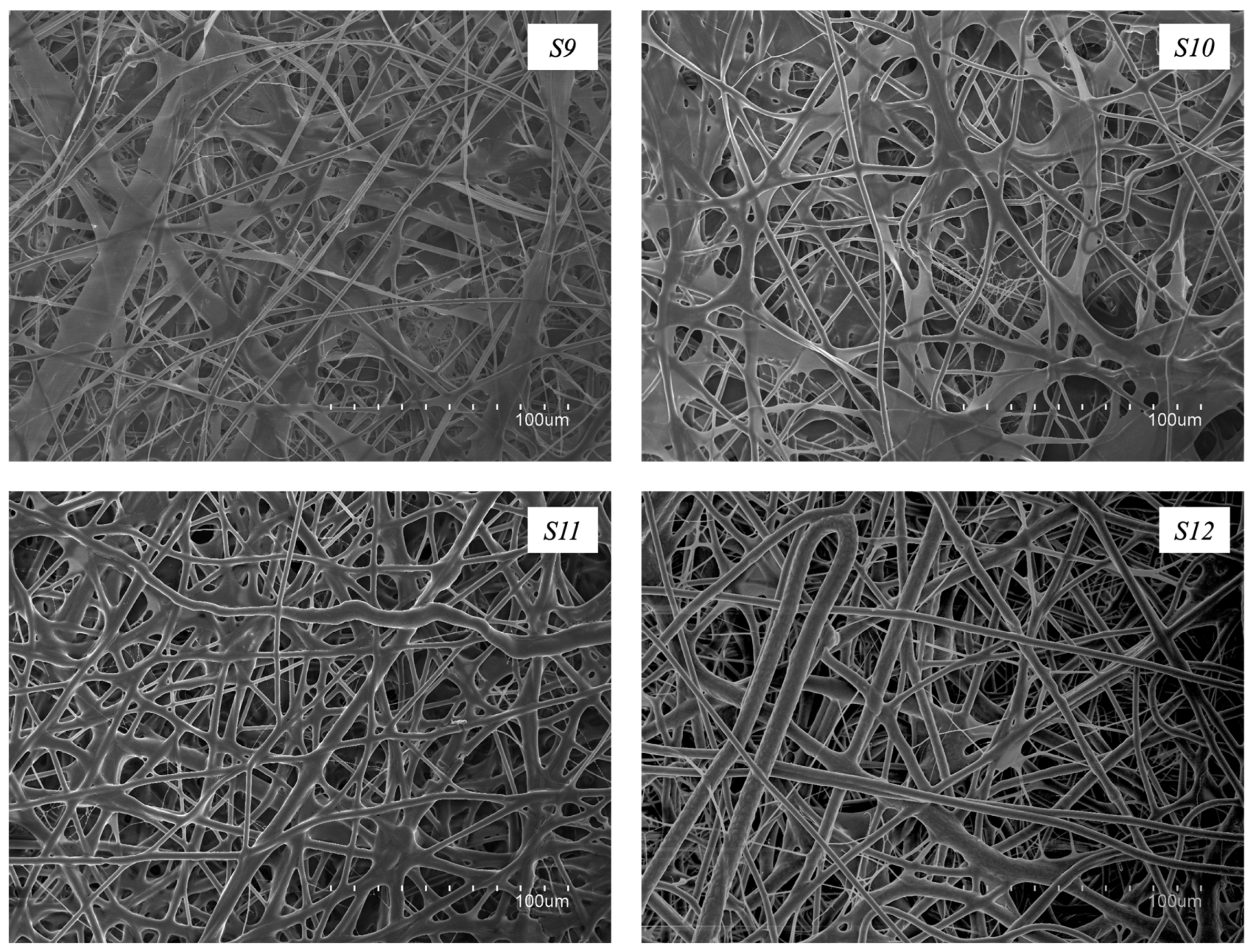
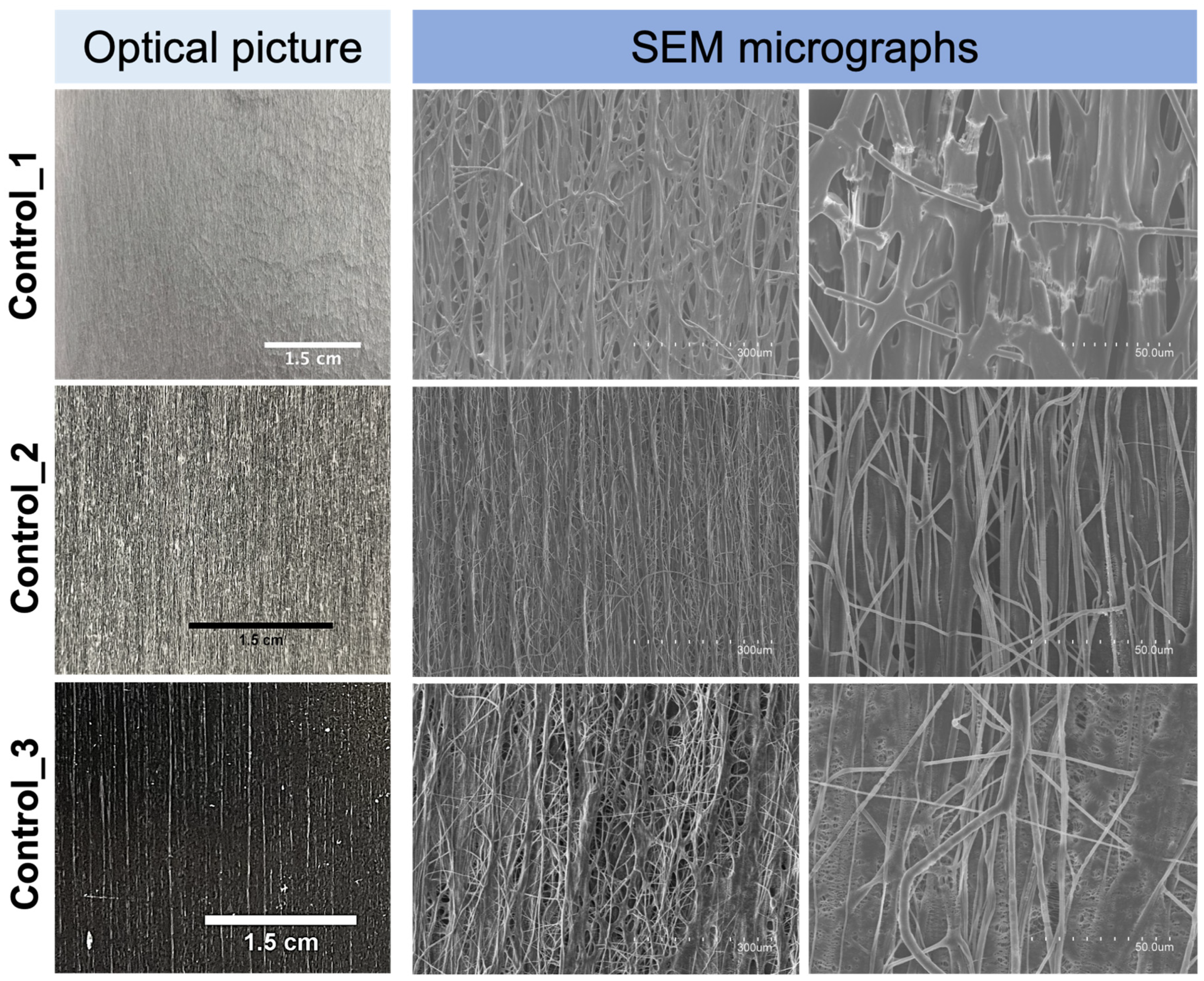
3.5. Morphology of the Fiber Mats Obtained with Multiple Emitters over a Drum Collector
3.6. Mechanical Properties
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chausali, N.; Saxena, J.; Prasad, R. Recent Trends in Nanotechnology Applications of Bio-Based Packaging. J. Agric. Food Res. 2022, 7, 100257. [Google Scholar] [CrossRef]
- Amaral, R.G.; de Andrade, L.R.M.; Andrade, L.N.; Loureiro, K.C.; Souto, E.B.; Severino, P. Cashew Gum: A Review of Brazilian Patents and Pharmaceutical Applications with a Special Focus on Nanoparticles. Micromachines 2022, 13, 1137. [Google Scholar] [CrossRef]
- Prajapati, V.D.; Jani, G.K.; Moradiya, N.G.; Randeria, N.P. Pharmaceutical Applications of Various Natural Gums, Mucilages and Their Modified Forms. Carbohydr. Polym. 2013, 92, 1685–1699. [Google Scholar] [CrossRef]
- Munir, H.; Bilal, M.; Khan, M.I.; Iqbal, H.M.N. Gums-Based Bionanostructures for Medical Applications. In Polysaccharides; Wiley Online Books; Wiley: Hoboken, NJ, USA, 2021; pp. 385–398. [Google Scholar]
- Ribeiro, A.J.; de Souza, F.R.L.; Bezerra, J.M.N.A.; Oliveira, C.; Nadvorny, D.; de La Roca Soares, M.F.; Nunes, L.C.C.; Silva-Filho, E.C.; Veiga, F.; Soares Sobrinho, J.L. Gums’ Based Delivery Systems: Review on Cashew Gum and Its Derivatives. Carbohydr. Polym. 2016, 147, 188–200. [Google Scholar] [CrossRef]
- Nehra, A.; Biswas, D.; Siracusa, V.; Roy, S. Natural Gum-Based Functional Bioactive Films and Coatings: A Review. Int. J. Mol. Sci. 2022, 24, 485. [Google Scholar] [CrossRef]
- Carvalho da Silva, L.; Alves do Nascimento, M.; Guabiraba Mendes, L.; Ferro Furtado, R.; Correia da Costa, J.M.; Luiz Herzog Cardoso, A. Optimization of Cashew Gum and Chitosan for Microencapsulation of Pequi Oil by Complex Coacervation. J. Food Process Preserv. 2018, 42, e13538. [Google Scholar] [CrossRef]
- Cheng, H.N.; Furtado, R.F.; Biswas, A.; Alves, C.; Prieto, C.; Lagaron, J.M. Chemical Modifications and Applications of Cashew Byproducts—A Selective Review. ACS Food Sci. Technol. 2022, 3, 546–552. [Google Scholar] [CrossRef]
- Silva, S.M.F.; Ribeiro, H.L.; Mattos, A.L.A.; de Borges, M.F.; de Rosa, M.F.; de Azeredo, H.M.C. Films from Cashew Byproducts: Cashew Gum and Bacterial Cellulose from Cashew Apple Juice. J. Food Sci. Technol. 2021, 58, 1979–1986. [Google Scholar] [CrossRef]
- Andrade, K.C.S.; de Carvalho, C.W.P.; Takeiti, C.Y.; de Azeredo, H.M.C.; da Corrêa, J.S.; Caldas, C.M. Goma de Cajueiro (Anacardium Occidentale): Avaliação Das Modificações Químicas e Físicas Por Extrusão Termoplástica. PolÍMeros CiÊNcia E Tecnol. 2013, 23, 667–671. [Google Scholar] [CrossRef]
- de Paula, R.C.M.; Rodrigues, J.F. Composition and Rheological Properties of Cashew Tree Gum, the Exudate Polysaccharide from Anacardium occidentale L. Carbohydr. Polym. 1995, 26, 177–181. [Google Scholar] [CrossRef]
- Kumar, A.; Moin, A.; Ahmed, A.; G Shivakumar, H. Cashew Gum A Versatile Hydrophyllic Polymer: A Review. Curr. Drug. Ther. 2012, 7, 2–12. [Google Scholar] [CrossRef]
- Silva, F.E.F.; Batista, K.A.; Di-Medeiros, M.C.B.; Silva, C.N.S.; Moreira, B.R.; Fernandes, K.F. A Stimuli-Responsive and Bioactive Film Based on Blended Polyvinyl Alcohol and Cashew Gum Polysaccharide. Mater. Sci. Eng. C 2016, 58, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, G.A.; Heinrichs, M.C.; Moraes, Â.M. Cashew Tree Gum for Biomaterials Engineering: A Versatile Raw Material in Consolidation. J. Appl. Polym. Sci. 2022, 139, e52484. [Google Scholar] [CrossRef]
- Gyedu-Akoto, E.; Amoah, F.M.; Oduro, I. Cashew Tree (Anarcadium Occidentale L.) Exudate Gum. Emerg. Nat.Hydrocoll. Rheol. Funct. 2019, 13, 327–346. [Google Scholar] [CrossRef]
- Vázquez-González, Y.; Prieto, C.; Filizoglu, M.F.; Ragazzo-Sánchez, J.A.; Calderón-Santoyo, M.; Furtado, R.F.; Cheng, H.N.; Biswas, A.; Lagaron, J.M. Electrosprayed Cashew Gum Microparticles for the Encapsulation of Highly Sensitive Bioactive Materials. Carbohydr. Polym. 2021, 264, 118060. [Google Scholar] [CrossRef] [PubMed]
- Porto, B.C.; Augusto, P.E.D.; Cristianini, M. A Comparative Study Between Technological Properties of Cashew Tree Gum and Arabic Gum. J. Polym. Environ. 2015, 23, 392–399. [Google Scholar] [CrossRef]
- Sameen, D.E.; Ahmed, S.; Lu, R.; Li, R.; Dai, J.; Qin, W.; Zhang, Q.; Li, S.; Liu, Y. Electrospun Nanofibers Food Packaging: Trends and Applications in Food Systems. Crit. Rev. Food Sci. Nutr. 2021, 62, 6238–6251. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Busolo, M.; Cherpinski, A.; Lagaron, J.M. CHAPTER 10 Electrospinning in the Packaging Industry. In Electrospinning: From Basic Research to Commercialization; The Royal Society of Chemistry: London, UK, 2018; pp. 238–260. ISBN 978-1-78801-100-6. [Google Scholar]
- Doshi, J.; Reneker, D.H. Electrospinning Process and Applications of Electrospun Fibers. J. Electrostat 1995, 35, 151–160. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.-K. A Comprehensive Review Summarizing the Effect of Electrospinning Parameters and Potential Applications of Nanofibers in Biomedical and Biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Hernaez, B.; Muñoz-Gómez, A.; Sanchiz, A.; Orviz, E.; Valls-Carbo, A.; Sagastagoitia, I.; Ayerdi, O.; Martín, R.; Puerta, T.; Vera, M.; et al. Monitoring Monkeypox Virus in Saliva and Air Samples in Spain: A Cross-Sectional Study. Lancet Microbe 2023, 4, e21–e28. [Google Scholar] [CrossRef]
- Yan, X.; Yao, H.; Luo, J.; Li, Z.; Wei, J. Functionalization of Electrospun Nanofiber for Bone Tissue Engineering. Polymers 2022, 14, 2940. [Google Scholar] [CrossRef]
- Teno, J.; Pardo-Figuerez, M.; Figueroa-Lopez, K.J.; Prieto, C.; Lagaron, J.M. Development of Multilayer Ciprofloxacin Hydrochloride Electrospun Patches for Buccal Drug Delivery. J. Funct. Biomater. 2022, 13, 170. [Google Scholar] [CrossRef]
- Prieto, C.; Talón, E.; Noreña, C.Z.; Lagaron, J.M. Effect of Whey Protein Purity on the Characteristics of Algae Oil-Loaded Encapsulates Obtained by Electrospraying Assisted by Pressurized Gas. Nanomaterials 2022, 12, 3096. [Google Scholar] [CrossRef]
- Vázquez-González, Y.; Prieto, C.; Calderón-Santoyo, M.; Ragazzo-Sánchez, J.A.; Lagarón, J.M. Development of Antifungal Electrospun Nanofiber Mats Containing Meyerozyma Caribbica. Food Hydrocoll. 2024, 147, 109343. [Google Scholar] [CrossRef]
- Rajora, A.D.; Bal, T. Evaluation of Cashew Gum-Polyvinyl Alcohol (CG-PVA) Electrospun Nanofiber Mat for Scarless Wound Healing in a Murine Model. Int. J. Biol. Macromol. 2023, 240, 124417. [Google Scholar] [CrossRef]
- Oliveira, M.A.; Furtado, R.F.; Bastos, M.S.R.; Leitão, R.C.; Benevides, S.D.; Muniz, C.R.; Cheng, H.N.; Biswas, A. Performance Evaluation of Cashew Gum and Gelatin Blend for Food Packaging. Food Packag. Shelf Life 2018, 17, 57–64. [Google Scholar] [CrossRef]
- de Souza, W.F.C.; de Lucena, F.A.; da Silva, K.G.; Martins, L.P.; de Castro, R.J.S.; Sato, H.H. Influence of Edible Coatings Composed of Alginate, Galactomannans, Cashew Gum, and Gelatin on the Shelf- Life of Grape Cultivar ‘Italia’: Physicochemical and Bioactive Properties. LWT 2021, 152, 112315. [Google Scholar] [CrossRef]
- da Silva, D.P.B.; Florentino, I.F.; da Silva Moreira, L.K.; Brito, A.F.; Carvalho, V.V.; Rodrigues, M.F.; Vasconcelos, G.A.; Vaz, B.G.; Pereira-Junior, M.A.; Fernandes, K.F.; et al. Chemical Characterization and Pharmacological Assessment of Polysaccharide Free, Standardized Cashew Gum Extract (Anacardium Occidentale L.). J. Ethnopharmacol. 2018, 213, 395–402. [Google Scholar] [CrossRef]
- Vázquez-González, Y.; Prieto, C.; Stojanovic, M.; Torres, C.A.V.; Freitas, F.; Ragazzo-Sánchez, J.A.; Calderón-Santoyo, M.; Lagaron, J.M. Preparation and Characterization of Electrospun Polysaccharide FucoPol-Based Nanofiber Systems. Nanomaterials 2022, 12, 498. [Google Scholar] [CrossRef] [PubMed]
- Melendez-Rodriguez, B.; Castro-Mayorga, J.L.; Reis, M.A.M.; Sammon, C.; Cabedo, L.; Torres-Giner, S.; Lagaron, J.M. Preparation and Characterization of Electrospun Food Biopackaging Films of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) Derived From Fruit Pulp Biowaste. Front. Sustain. Food Syst. 2018, 2, 38. [Google Scholar] [CrossRef]
- Akinalan Balik, B.; Argin, S.; Lagaron, J.M.; Torres-Giner, S. Preparation and Characterization of Electrospun Pectin-Based Films and Their Application in Sustainable Aroma Barrier Multilayer Packaging. Appl. Sci. 2019, 9, 5136. [Google Scholar] [CrossRef]
- Liang, Q.; Pan, W.; Gao, Q. Preparation of Carboxymethyl Starch/Polyvinyl-Alcohol Electrospun Composite Nanofibers from a Green Approach. Int. J. Biol. Macromol. 2021, 190, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Hernández, J.; Ragazzo-Sánchez, J.; Calderón-Santoyo, M.; Ortiz-Basurto, R.; Prieto, C.; Lagaron, J. Use of Electrosprayed Agave Fructans as Nanoencapsulating Hydrocolloids for Bioactives. Nanomaterials 2018, 8, 868. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Mascaraque, L.G.; Perez-Masiá, R.; González-Barrio, R.; Periago, M.J.; López-Rubio, A. Potential of Microencapsulation through Emulsion-Electrospraying to Improve the Bioaccesibility of β-Carotene. Food Hydrocoll. 2017, 73, 1–12. [Google Scholar] [CrossRef]
- Jaworek, A. Micro- and Nanoparticle Production by Electrospraying. Powder Technol. 2007, 176, 18–35. [Google Scholar] [CrossRef]
- Fong, H.; Chun, I.; Reneker, D.H. Beaded Nanofibers Formed during Electrospinning. Polymer 1999, 40, 4585–4592. [Google Scholar] [CrossRef]
- Yu, J.; Qiu, Y.; Zha, X.; Yu, M.; Yu, J.; Rafique, J.; Yin, J. Production of Aligned Helical Polymer Nanofibers by Electrospinning. Eur. Polym. J. 2008, 44, 2838–2844. [Google Scholar] [CrossRef]
- Gibis, M.; Pribek, F.; Kutzli, I.; Weiss, J. Influence of the Protein Content on Fiber Morphology and Heat Treatment of Electrospun Potato Protein–Maltodextrin Fibers. Appl. Sci. 2021, 11, 7896. [Google Scholar] [CrossRef]
- Cui, S.-S.; Sun, X.; Yao, B.; Peng, X.-X.; Zhang, X.-T.; Zhou, Y.-F.; Hu, J.-L.; Liu, Y.-C. Size-Tunable Low Molecular Weight Pectin-Based Electrospun Nanofibers Blended with Low Content of Poly(Ethylene Oxide). J. Nanosci. Nanotechnol. 2017, 17, 681–689. [Google Scholar] [CrossRef]
- Guerreiro, B.M.; Freitas, F.; Lima, J.C.; Silva, J.C.; Dionísio, M.; Reis, M.A.M. Demonstration of the Cryoprotective Properties of the Fucose-Containing Polysaccharide FucoPol. Carbohydr. Polym. 2020, 245, 116500. [Google Scholar] [CrossRef]
- Cohen, L.E.; Rocco, A.M. Study of the Crystallization Kinetics Poly(Ethylene Oxide) and a Blend of Poly(Ethylene Oxide) and Poly(Bisphenol A-Co-Epichlorohydrin). J. Therm. Anal. Calorim. 2000, 59, 625–632. [Google Scholar] [CrossRef]
- Medeiros, G.B.; Souza, P.R.; Retamiro, K.M.; Nakamura, C.V.; Muniz, E.C.; Corradini, E. Experimental Design to Evaluate Properties of Electrospun Fibers of Zein/Poly (Ethylene Oxide) for Biomaterial Applications. J. Appl. Polym. Sci. 2021, 138, 50898. [Google Scholar] [CrossRef]
- Kanis, L.A.; Viel, F.C.; Crespo, J.S.; Bertolino, J.R.; Pires, A.T.N.; Soldi, V. Study of Poly(Ethylene Oxide)/Carbopol Blends through Thermal Analysis and Infrared Spectroscopy. Polymer 2000, 41, 3303–3309. [Google Scholar] [CrossRef]
- Lu, C.; Chiang, S.W.; Du, H.; Li, J.; Gan, L.; Zhang, X.; Chu, X.; Yao, Y.; Li, B.; Kang, F. Thermal Conductivity of Electrospinning Chain-Aligned Polyethylene Oxide (PEO). Polymer 2017, 115, 52–59. [Google Scholar] [CrossRef]
- Arai, F.; Shinohara, K.; Nagasawa, N.; Takeshita, H.; Takenaka, K.; Miya, M.; Shiomi, T. Crystallization Behavior and Higher-Order Structure in Miscible Crystalline/Crystalline Polymer Blends. Polym. J. 2013, 45, 921–928. [Google Scholar] [CrossRef]
- Hubackova, J.; Dvorackova, M.; Svoboda, P.; Mokrejs, P.; Kupec, J.; Pozarova, I.; Alexy, P.; Bugaj, P.; Machovsky, M.; Koutny, M. Influence of Various Starch Types on PCL/Starch Blends Anaerobic Biodegradation. Polym. Test. 2013, 32, 1011–1019. [Google Scholar] [CrossRef]
- Abdollahi, S.; Ehsani, M.; Morshedian, J.; Khonakdar, H.A.; Reuter, U. Structural and Electrochemical Properties of PEO/PAN Nanofibrous Blends: Prediction of Graphene Localization. Polym. Compos. 2018, 39, 3626–3635. [Google Scholar] [CrossRef]
- Zhang, L.; Hsieh, Y. lo Nanoporous Ultrahigh Specific Surface Polyacrylonitrile Fibres. Nanotechnology 2006, 17, 4416–4423. [Google Scholar] [CrossRef]
- Lin, H.; Kai, T.; Freeman, B.D.; Kalakkunnath, S.; Kalika, D.S. The Effect of Cross-Linking on Gas Permeability in Cross-Linked Poly(Ethylene Glycol Diacrylate). Macromolecules 2005, 38, 8381–8393. [Google Scholar] [CrossRef]
- Sunderrajan, S.; Freeman, B.D.; Hall, C.K.; Pinnau, I. Propane and Propylene Sorption in Solid Polymer Electrolytes Based on Poly(Ethylene Oxide) and Silver Salts. J. Memb. Sci. 2001, 182, 1–12. [Google Scholar] [CrossRef]
- Khan, M.A.; Zhou, C.; Zheng, P.; Zhao, M.; Liang, L. Improving Physicochemical Stability of Quercetin-Loaded Hollow Zein Particles with Chitosan/Pectin Complex Coating. Antioxidants 2021, 10, 1476. [Google Scholar] [CrossRef]
- Pasini Cabello, S.D.; Takara, E.A.; Marchese, J.; Ochoa, N.A. Influence of Plasticizers in Pectin Films: Microstructural Changes. Mater. Chem. Phys. 2015, 162, 491–497. [Google Scholar] [CrossRef]
- Diener, M. Structural Hierarchy in Linear Polysaccharides from the Nano to Macroscale. Ph.D. Thesis, ETH Zurich, Zurich, Switzerland, 2020. [Google Scholar]
- Figueroa-Lopez, K.; Castro-Mayorga, J.; Andrade-Mahecha, M.; Cabedo, L.; Lagaron, J. Antibacterial and Barrier Properties of Gelatin Coated by Electrospun Polycaprolactone Ultrathin Fibers Containing Black Pepper Oleoresin of Interest in Active Food Biopackaging Applications. Nanomaterials 2018, 8, 199. [Google Scholar] [CrossRef]
- dos Ferreira, S.R.S.; Mesquita, M.V.N.; de Sá, L.L.F.; Nogueira, N.C.; dos Rizzo, M.S.; Silva-Filho, E.C.; da Costa, M.P.; Ribeiro, A.B. Sustainable Natural Gums for Industrial Application: Physiochemical and Texturometric Evaluation. J. Drug Deliv. Sci. Technol. 2019, 54, 101306. [Google Scholar] [CrossRef]
- Bozkaya, O.; Arat, E.; Gün Gök, Z.; Yiğitoğlu, M.; Vargel, İ. Production and Characterization of Hybrid Nanofiber Wound Dressing Containing Centella Asiatica Coated Silver Nanoparticles by Mutual Electrospinning Method. Eur. Polym. J. 2022, 166, 111023. [Google Scholar] [CrossRef]
- Costa, C.M.; MacHiavello, M.N.T.; Ribelles, J.L.G.; Lanceros-Méndez, S. Composition-Dependent Physical Properties of Poly[(Vinylidene Fluoride)-Co-Trifluoroethylene]-Poly(Ethylene Oxide) Blends. J. Mater. Sci. 2013, 48, 3494–3504. [Google Scholar] [CrossRef]
- Jakić, M.; Stipanelov Vrandečić, N.; Erceg, M. Thermal Degradation of Poly(3-Hydroxybutyrate)/Poly(Ethylene Oxide) Blends: Thermogravimetric and Kinetic Analysis. Eur. Polym. J. 2016, 81, 376–385. [Google Scholar] [CrossRef]
- García, N.L.; Famá, L.; Dufresne, A.; Aranguren, M.; Goyanes, S. A Comparison between the Physico-Chemical Properties of Tuber and Cereal Starches. Food Res. Int. 2009, 42, 976–982. [Google Scholar] [CrossRef]
- Vendruscolo, C.W.; Ferrero, C.; Pineda, E.A.G.; Silveira, J.L.M.; Freitas, R.A.; Jiménez-Castellanos, M.R.; Bresolin, T.M.B. Physicochemical and Mechanical Characterization of Galactomannan from Mimosa Scabrella: Effect of Drying Method. Carbohydr. Polym. 2009, 76, 86–93. [Google Scholar] [CrossRef]
- Tong, H.-W.; Wang, M. Electrospinning of Aligned Biodegradable Polymer Fibers and Composite Fibers for Tissue Engineering Applications. J. Nanosci. Nanotechnol. 2007, 7, 3834–3840. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Xia, Y. Electrospinning of Polymeric and Ceramic Nanofibers as Uniaxially Aligned Arrays. Nano Lett. 2003, 3, 1167–1171. [Google Scholar] [CrossRef]
- Bellan, L.M.; Craighead, H.G. Applications of Controlled Electrospinning Systems. Polym. Adv. Technol. 2011, 22, 304–309. [Google Scholar] [CrossRef]
- Alfaro De Prá, M.A.; Ribeiro-do-Valle, R.M.; Maraschin, M.; Veleirinho, B. Effect of Collector Design on the Morphological Properties of Polycaprolactone Electrospun Fibers. Mater. Lett. 2017, 193, 154–157. [Google Scholar] [CrossRef]
- Kiselev, P.; Rosell-Llompart, J. Highly Aligned Electrospun Nanofibers by Elimination of the Whipping Motion. J. Appl. Polym. Sci. 2012, 125, 2433–2441. [Google Scholar] [CrossRef]
- Liashenko, I.; Rosell-Llompart, J.; Cabot, A. Ultrafast 3D Printing with Submicrometer Features Using Electrostatic Jet Deflection. Nat. Commun. 2020, 11, 753. [Google Scholar] [CrossRef]
- Deitzel, J. Controlled Deposition of Electrospun Poly(Ethylene Oxide) Fibers. Polymer 2001, 42, 8163–8170. [Google Scholar] [CrossRef]
- Urbanek, O.; Sajkiewicz, P.; Pierini, F. The Effect of Polarity in the Electrospinning Process on PCL/Chitosan Nanofibres’ Structure, Properties and Efficiency of Surface Modification. Polymer 2017, 124, 168–175. [Google Scholar] [CrossRef]
- Csiszár, E.; Nagy, S. A Comparative Study on Cellulose Nanocrystals Extracted from Bleached Cotton and Flax and Used for Casting Films with Glycerol and Sorbitol Plasticisers. Carbohydr. Polym. 2017, 174, 740–749. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.P.B.; da Silva Moreira, L.K.; Cabral, I.B.; da Silva, C.N.S.; de Aleluia Batista, K.; Fajemiroye, J.O.; Costa, E.A. Chemistry, Biological Activities, and Uses of Cashew Gum. In Gums, Resins and Latexes of Plant Origin: Chemistry, Biological Activities and Uses; Murthy, H.N., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 291–305. [Google Scholar]
- Bose, S.; Biswas, M. The structure of the gum of anacardium occidentale. Acta Hortic. 1985, 108, 207–217. [Google Scholar] [CrossRef]
- Yu, H.; Li, Y.; Li, T.; Chen, B.; Li, P.; Wu, Y. Fabrication of Aligned Eu(TTA)3phen/PS Fiber Bundles from High Molecular Weight Polymer Solution by Electrospinning. Russ. J. Phys. Chem. A 2015, 89, 2455–2460. [Google Scholar] [CrossRef]
- Silva, T.M.; Santiago, P.O.; Purcena, L.L.A.; Fernandes, K.F. Study of the Cashew Gum Polysaccharide for the Horseradish Peroxidase Immobilization—Structural Characteristics, Stability and Recovery. Mater. Sci. Eng. C 2010, 30, 526–530. [Google Scholar] [CrossRef]
- Miranda, R.L. Cashew Tree Bark Secretion—Persectives for Its Use in Protein Isolation Strategies. Open Glycosci. 2009, 2, 16–19. [Google Scholar] [CrossRef]
- de Paula, R.C.M.; Heatley, F.; Budd, P.M. Characterization of Anacardium Occidentale Exudate Polysaccharide. Polym. Int. 1998, 45, 27–35. [Google Scholar] [CrossRef]
- Anderson, D.M.W.; Bell, P.C. Structural Analysis of the Gum Polysaccharide from Anacardium Occidentale. Anal. Chim. Acta 1975, 79, 185–197. [Google Scholar] [CrossRef]
- Anderson, D.M.W.; Bell, P.C.; Millar, J.R.A. Composition of Gum Exudates from Anacardium Occidentale. Phytochemistry 1974, 13, 2189–2193. [Google Scholar] [CrossRef]
- Vigani, B.; Rossi, S.; Milanesi, G.; Bonferoni, M.; Sandri, G.; Bruni, G.; Ferrari, F. Electrospun Alginate Fibers: Mixing of Two Different Poly(Ethylene Oxide) Grades to Improve Fiber Functional Properties. Nanomaterials 2018, 8, 971. [Google Scholar] [CrossRef]
- Martins, C.S.; Morgado, D.L.; Assi, O.B.G. Cashew Gum-Chitosan Blended Films: Spectral, Mechanical and Surface Wetting Evaluations. Macromol. Res. 2016, 24, 691–697. [Google Scholar] [CrossRef]
- Lyu, H.; Sun, Z.; Liu, Y.; Yu, X.; Guo, C. Processing-Structure-Properties Relationships of Glycerol-Plasticized Silk Films. Molecules 2022, 27, 1339. [Google Scholar] [CrossRef]
- Mohd Amin, A.M.; Mohd Sauid, S.; Musa, M.; Ku Hamid, K.H. The effect of glycerol content on mechanical properties, surface morphology and water absorption of thermoplastic films from tacca leontopetaloides starch. J. Teknol. 2017, 79, 53–59. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. How Glycerol and Water Contents Affect the Structural and Functional Properties of Starch-Based Edible Films. Polymers 2018, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Farshi, P.; Salarian, R.; Rabiee, M.; Alizadeh, S.; Gholipourmalekabadi, M.; Ahmadi, S.; Rabiee, N. Design, Preparation, and Characterization of Silk Fibroin/Carboxymethyl Cellulose Wound Dressing for Skin Tissue Regeneration Applications. Polym. Eng. Sci. 2022, 62, 2741–2749. [Google Scholar] [CrossRef]
- Nagakawa, Y.; Kato, M.; Suye, S.; Fujita, S. Fabrication of Tough, Anisotropic, Chemical-Crosslinker-Free Poly(Vinyl Alcohol) Nanofibrous Cryogels via Electrospinning. RSC Adv. 2020, 10, 38045–38054. [Google Scholar] [CrossRef]
- da Silva, D.A.; Feitosa, J.P.A.; Paula, H.C.B.; de Paula, R.C.M. Synthesis and characterization of cashew gum/acrylic acid nanoparticles. Mater. Sci. Eng. C 2009, 29, 437–441. [Google Scholar] [CrossRef]
- Silva, F.; Torres, L.; Silva, L.; Figueiredo, R.; Garruti, D.; Araújo, T.; Duarte, A.; Brito, D.; Ricardo, N. Cashew gum and maltrodextrin particles for green tea (Camellia sinensis var Assamica) extract encapsulation. Food Chem. 2018, 261, 169–175. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Rishishwar, P.; Rishishwar, S.; Ali, S.; Nayak, A.K. Extraction and characterization of cashew tree (Anacardium occidentale) gum; use in aceclofenac dental pastes. Int. J. Biol. Macromol. 2018, 116, 1074–1081. [Google Scholar] [CrossRef]
- Waly, A.L.; Abdelghany, A.M.; Tarabiah, A.E. Study the structure of selenium modified polyethylene oxide/polyvinyl alcohol (PEO/PVA) polymer blend. J. Mater. Res. Technol. 2021, 14, 2962–2969. [Google Scholar] [CrossRef]
- Ling, S.; Qi, Z.; Watts, B.; Shao, Z.; Chen, X. Structural determination of protein-based polymer blends with a promising tool: Combination of FTIR and STXM spectroscopic imaging. Phys. Chem. Chem. Phys. 2014, 16, 7741–7748. [Google Scholar] [CrossRef]
- Padmaja, S.; Jayakumar, S. Functional Group Analysis of CdS:PEO Nanocomposite Solid Films. Mater. Today Proc. 2018, 5, 14473–14480. [Google Scholar] [CrossRef]
- El-Sayed, S.; Saber, S.; el Sayed, A.M. Controlling the structural; optical, and electrical properties of PVA/PEO blend by clay nanoparticles content. Phys. Scr. 2021, 96, 125812. [Google Scholar] [CrossRef]
- Holser, R.A. Thermal analysis of glycerol citrate/starch blends. J. Appl. Polym. Sci. 2008, 110, 1498–1501. [Google Scholar] [CrossRef]
- Khaskheli, A.A.; Talpur, F.N.; Ashraf, M.A.; Cebeci, A.; Jawaid, S.; Afridi, H.I. Monitoring the Rhizopus oryzae lipase catalyzed hydrolysis of castor oil by ATR-FTIR spectroscopy. J. Mol. Catal. B Enzym. 2015, 113, 56–61. [Google Scholar] [CrossRef]
- Synytsya, A. Fourier transform Raman and infrared spectroscopy of pectins. Carbohydr Polym. 2003, 54, 97–106. [Google Scholar] [CrossRef]
| Solution | TCS (% w/v) | CG:PEO Ratio | PEO Mw (Da) | Span® 20 (% w/v) | Glycerol (% w/w) | CG Loss after Centrifuging (% w/v) |
|---|---|---|---|---|---|---|
| S0 | 120 | 100:0 | - | 3.00 | - | - |
| S1 | 50 | 95:5 | 6 × 105 | 1.00 | - | - |
| S2 | 50 | 90:10 | 1 × 106 | 1.00 | - | - |
| S3 | 20 | 95:5 | 5 × 106 | 1.00 | - | - |
| S4 | 20 | 90:10 | 5 × 106 | 1.00 | - | - |
| S5 | 20 | 85:15 | 5 × 106 | 1.00 | - | - |
| S6 | 15 | 95:5 | 5 × 106 | 1.00 | - | - |
| S7 | 15 | 90:10 | 5 × 106 | 1.00 | - | - |
| S8 | 15 | 85:15 | 5 × 106 | 1.00 | - | - |
| S9 | 15 | 82:18 | 5 × 106 | 1.00 | - | <2.30 |
| S10 | 15 | 82:18 | 5 × 106 | 1.00 | 1.5 | <2.30 |
| S11 | 15 | 82:18 | 5 × 106 | 1.00 | 2.5 | <2.30 |
| S12 | 15 | 82:18 | 5 × 106 | 1.00 | 3.5 | <2.30 |
| Control_1 | 15 | 82:18 | 5 × 106 | 1.00 | - | <2.30 |
| Control_2 | 2.7 | 0:100 | 5 × 106 | 1.00 | - | - |
| Control_3 | 2.7 | 0:100 | 5 × 106 | 1.00 | 3.5 | - |
| Sample | Viscosity (cP) | Conductivity (μS/cm) | Surface Tension (mN/m) |
|---|---|---|---|
| S8 | 24,647 ± 148 a | 815.1 ± 0.00 b | 36.0 ± 0.6 b |
| S9 | 21,849 ± 197 b | 657.1 ± 0.03 a | 39.4 ± 1.1 a |
| S12 | 24,189 ± 115 c | 645.4 ± 0.00 c | 37.0 ± 1.2 a,b |
| Solution | Voltage (V+/V−) (kV) | Flowrate (μL/h) | Tip-to-Collector Distance (cm) | Needle Gauge | Fiber Formation |
|---|---|---|---|---|---|
| S0 | +15/−0 | 250 | 12.0 | 27 | no |
| S1 | +25/−9 | 800 | 20.0 | 22 | no |
| S2 | +31/−0 | 500 | 20.0 | 22 | no |
| S3 | +29/−9 | 300 | 28.0 | 23 | yes |
| S4 | +29/−9 | 200 | 22.0 | 23 | yes |
| S5 | // | // | // | // | no |
| S6 | +29/−9 | 400 | 28.0 | 25 | no |
| S7 | +29/−9 | 300 | 28.0 | 23 | yes |
| S8 | +29/−9 | 300 | 20.5 | 23 | yes |
| S9 | +22/−9 | 450 | 20.0 | 22 | yes |
| S10 | +22/−9 | 450 | 28.5 | 22 | yes |
| S11 | +22/−9 | 450 | 28.5 | 22 | yes |
| S12 | +22/−9 | 450 | 30.0 | 22 | yes |
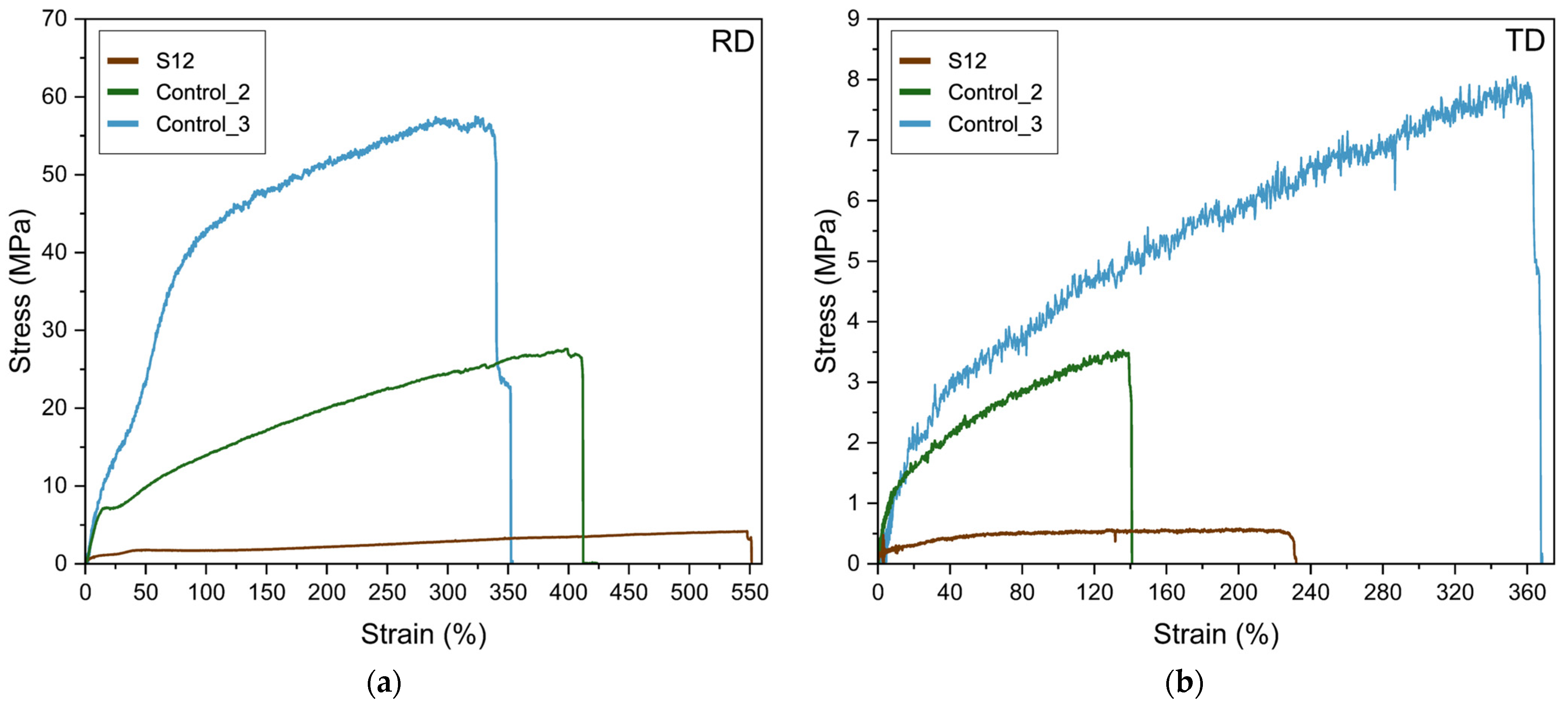
| Sample | Measurement Direction | E (MPa) | σb (MPa) | εb (%) | T (mJ/m3) |
|---|---|---|---|---|---|
| Fibers from S12 | RD | 78 ± 23 a | 3.7 ± 1.4 c | 550 ± 54 a | 14 ± 5 c |
| TD | 67 ± 39 a | 0.6 ± 0.1 c | 225 ± 17 b | 1 ± 0 c | |
| Control_1 | RD | n.a. | n.a. | n.a. | n.a. |
| TD | n.a. | n.a. | n.a. | n.a. | |
| Control_2 | RD | 64 ± 3 a | 26 ± 2 b | 380 ± 46 b | 69 ± 12 b |
| TD | 13 ± 1 a | 3.6 ± 0.2 b | 147 ± 18 b | 3.8 ± 0.6 b | |
| Control_3 | RD | 54 ± 4 a | 58 ± 5 a | 341 ± 7 b | 153 ± 6 a |
| TD | 24 ± 3 a | 7 ± 2 a | 332 ± 56 a | 20 ± 4 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grumi, M.; Prieto, C.; Furtado, R.F.; Cheng, H.N.; Biswas, A.; Limbo, S.; Cabedo, L.; Lagaron, J.M. On the Unique Morphology and Elastic Properties of Multi-Jet Electrospun Cashew Gum-Based Fiber Mats. Polymers 2024, 16, 1355. https://doi.org/10.3390/polym16101355
Grumi M, Prieto C, Furtado RF, Cheng HN, Biswas A, Limbo S, Cabedo L, Lagaron JM. On the Unique Morphology and Elastic Properties of Multi-Jet Electrospun Cashew Gum-Based Fiber Mats. Polymers. 2024; 16(10):1355. https://doi.org/10.3390/polym16101355
Chicago/Turabian StyleGrumi, Mattia, Cristina Prieto, Roselayne F. Furtado, Huai N. Cheng, Atanu Biswas, Sara Limbo, Luis Cabedo, and Jose M. Lagaron. 2024. "On the Unique Morphology and Elastic Properties of Multi-Jet Electrospun Cashew Gum-Based Fiber Mats" Polymers 16, no. 10: 1355. https://doi.org/10.3390/polym16101355
APA StyleGrumi, M., Prieto, C., Furtado, R. F., Cheng, H. N., Biswas, A., Limbo, S., Cabedo, L., & Lagaron, J. M. (2024). On the Unique Morphology and Elastic Properties of Multi-Jet Electrospun Cashew Gum-Based Fiber Mats. Polymers, 16(10), 1355. https://doi.org/10.3390/polym16101355











