Long-Cycle Stability of In Situ Ultraviolet Curable Organic/Inorganic Composite Electrolyte for Solid-State Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
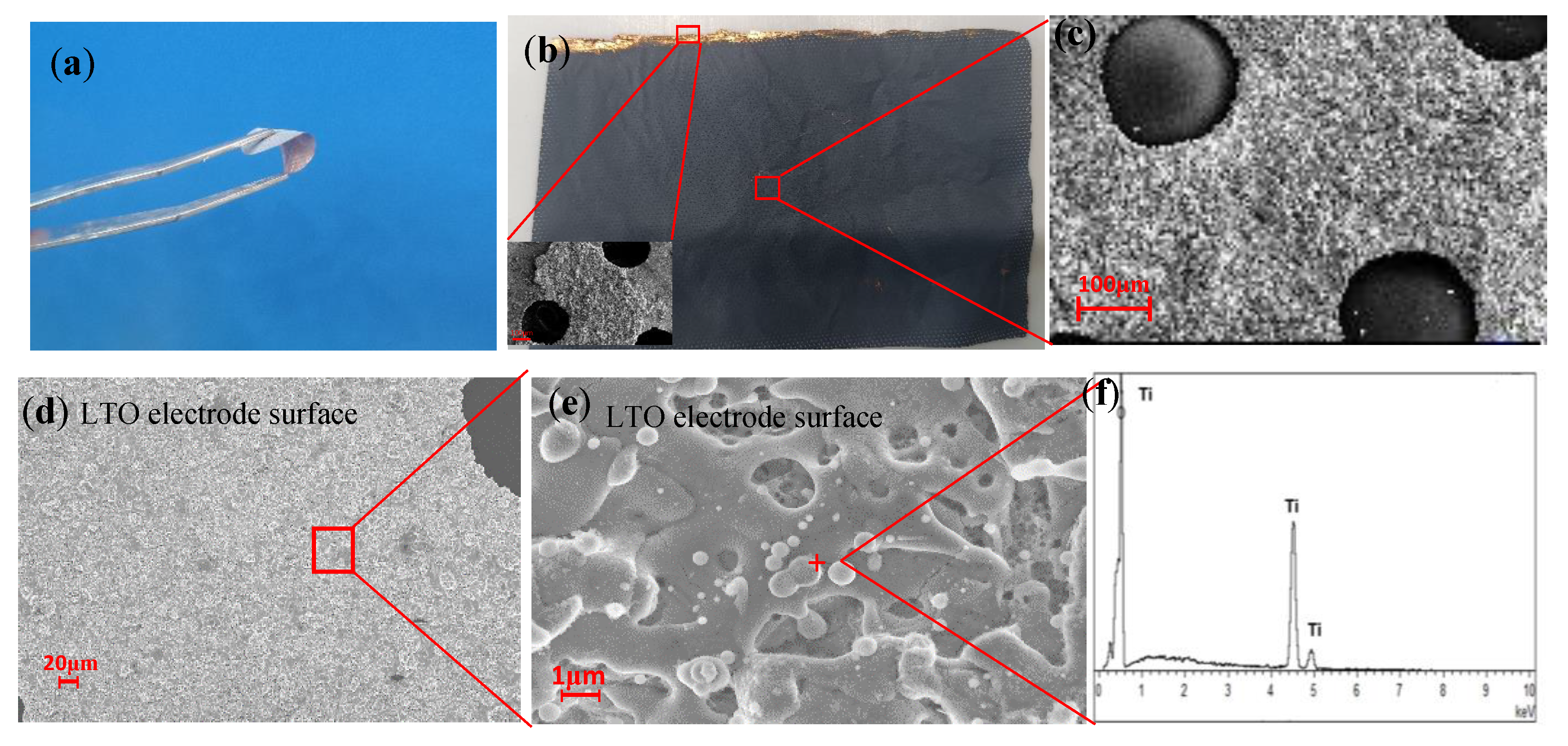
2.2. Preparation of LTO Electrodes
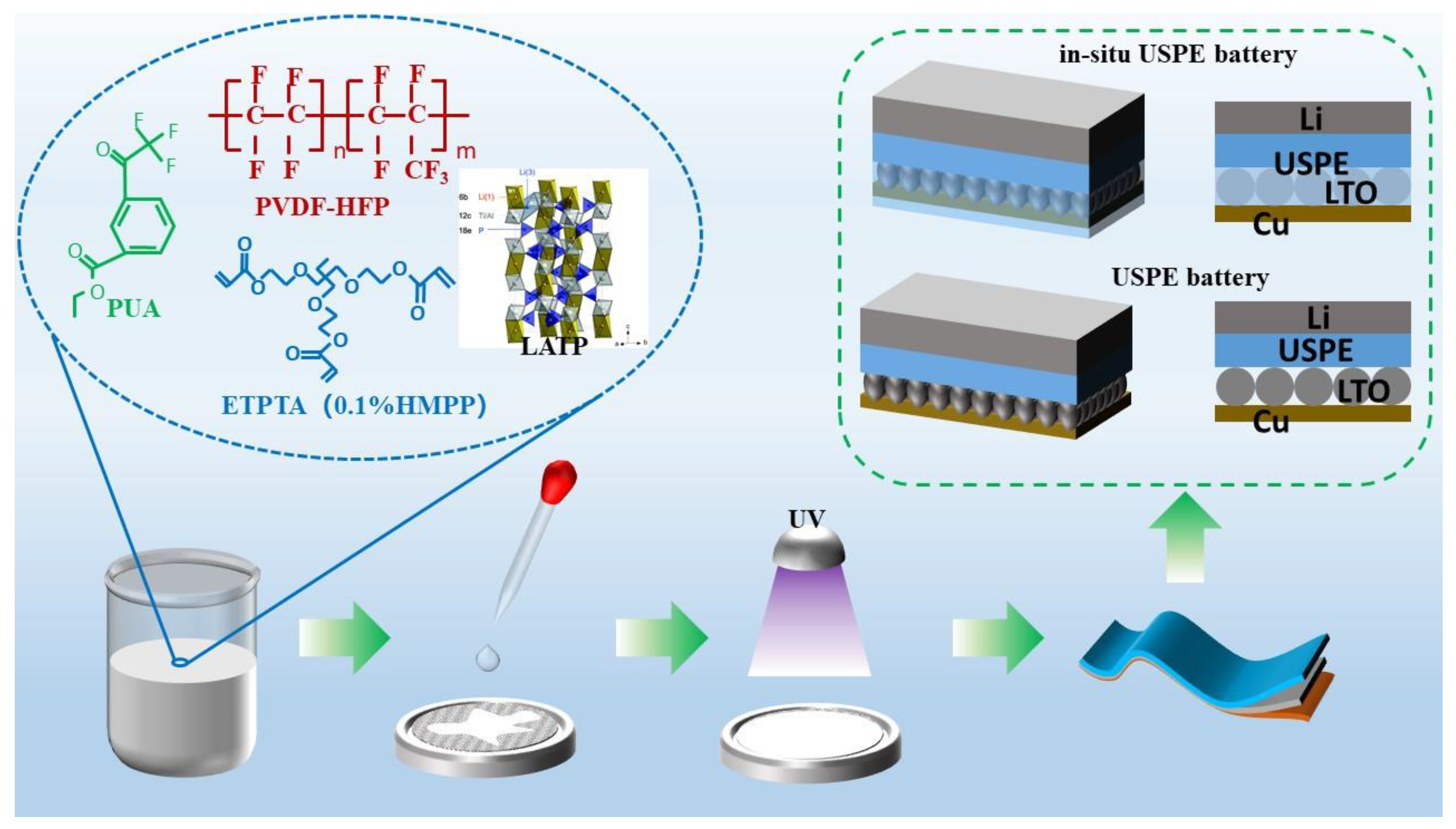
2.3. Preparation of Composite Solid State Electrolyte
2.4. LTO Battery Assembly
2.5. Characterization and Electrochemical Measurements
2.6. Electrochemical Measurements
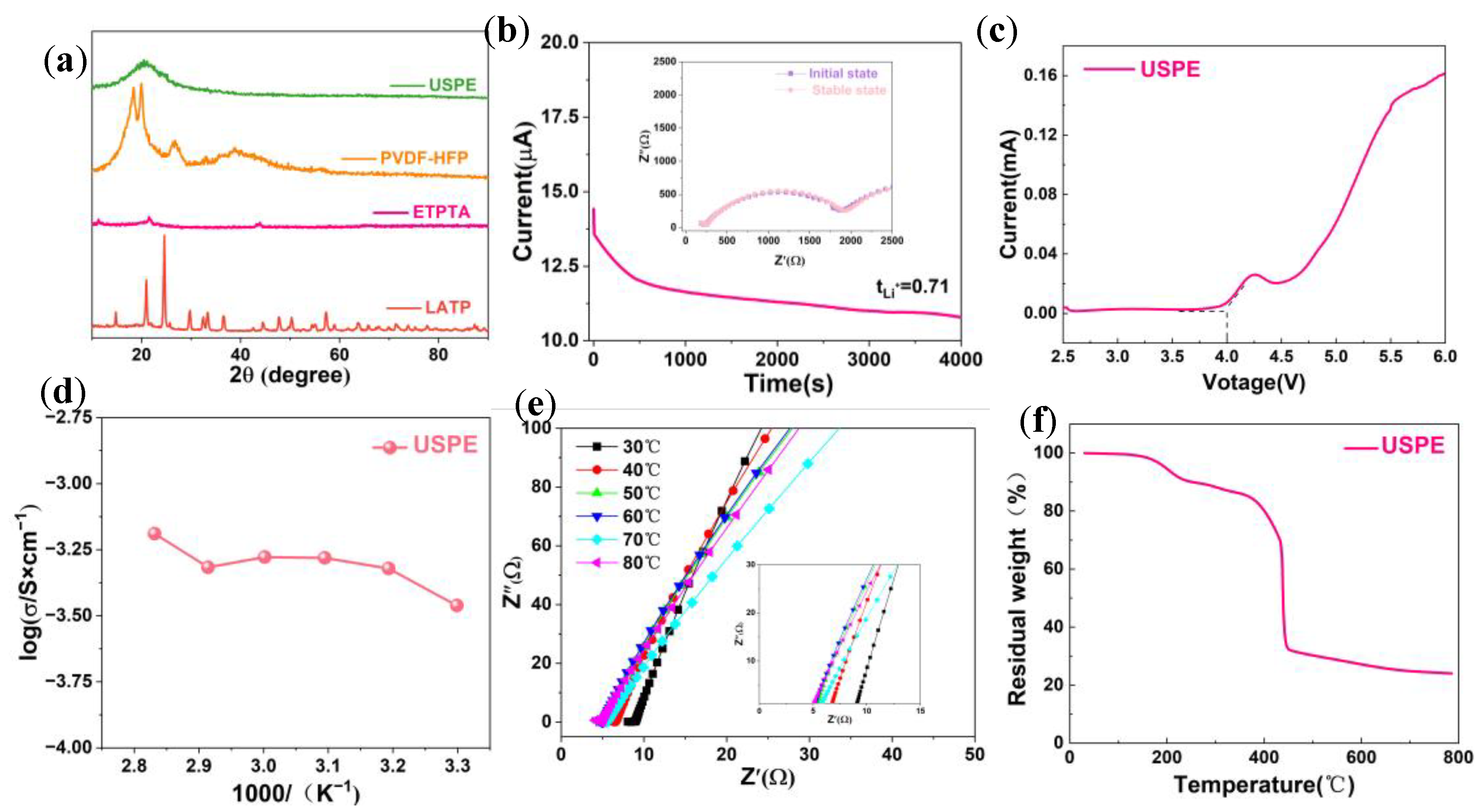
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zheng, F.; Kotobuki, M.; Song, S.; Lai, M.O.; Lu, L. Review on solid electrolytes for all-solid-state lithium-ion batteries. J. Power Sources 2018, 389, 198–213. [Google Scholar] [CrossRef]
- Nakamura, N.; Ahn, S.; Momma, T.; Osaka, T. Future potential for lithium-sulfur batteries. J. Power Sources 2023, 558, 232566. [Google Scholar] [CrossRef]
- Zhang, D.; Meng, X.; Hou, W.; Hu, W.; Mo, J.; Yang, T.; Zhang, W.; Fan, Q.; Liu, L.; Jiang, B.; et al. Solid polymer electrolytes: Ion conduction mechanisms and enhancement strategies. Nano Res. Energy 2023, 2, e9120050. [Google Scholar] [CrossRef]
- Dedryvère, R.; Foix, D.; Franger, S.; Patoux, S.; Daniel, L.; Gonbeau, D. Electrode/Electrolyte Interface Reactivity in High-Voltage Spinel LiMn1.6Ni0.4O4/Li4Ti5O12 Lithium-Ion Battery. J. Phys. Chem. C 2010, 114, 10999–11008. [Google Scholar] [CrossRef]
- Zhao, B.; Ran, R.; Liu, M.; Shao, Z. A comprehensive review of Li4Ti5O12-based electrodes for lithium-ion batteries: The latest advancements and future perspectives. Mater. Sci. Eng. R Rep. 2015, 98, 1–71. [Google Scholar] [CrossRef]
- Shi, Y.; Wen, L.; Li, F.; Cheng, H.-M. Nanosized Li4Ti5O12/graphene hybrid materials with low polarization for high rate lithium ion batteries. J. Power Sources 2011, 196, 8610–8617. [Google Scholar] [CrossRef]
- Zhao, C.-Z.; Zhao, B.-C.; Yan, C.; Zhang, X.-Q.; Huang, J.-Q.; Mo, Y.; Xu, X.; Li, H.; Zhang, Q. Liquid phase therapy to solid electrolyte–electrode interface in solid-state Li metal batteries: A review. Energy Storage Mater. 2020, 24, 75–84. [Google Scholar] [CrossRef]
- Dirican, M.; Yan, C.; Zhu, P.; Zhang, X. Composite solid electrolytes for all-solid-state lithium batteries. Mater. Sci. Eng. R Rep. 2019, 136, 27–46. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, Y.-H.; Shi, F.-N.; Zhang, L.-N. UV-Derived Double Crosslinked PEO-Based Solid Polymer Electrolyte for Room Temperature. J. Colloid Interface Sci. 2023, 629, 492–500. [Google Scholar] [CrossRef]
- Wang, X.; Hao, X.; Hengjing, Z.; Xia, X.; Tu, J. 3D ultraviolet polymerized electrolyte based on PEO modified PVDF-HFP electrospun membrane for high-performance lithium-sulfur batteries. Electrochim. Acta 2020, 329, 135108. [Google Scholar] [CrossRef]
- Xin, C.; Wen, K.; Guan, S.; Xue, C.; Wu, X.; Li, L.; Nan, C.-W. A Cross-Linked Poly(Ethylene Oxide)-Based Electrolyte for All-Solid-State Lithium Metal Batteries With Long Cycling Stability. Front. Mater. 2022, 9, 864478. [Google Scholar] [CrossRef]
- Fan, H.; Yang, C.; Wang, X.; Liu, L.; Wu, Z.; Luo, J.; Liu, R. UV-curable PVdF-HFP-based gel electrolytes with semi-interpenetrating polymer network for dendrite-free Lithium metal batteries. J. Electroanal. Chem. 2020, 871, 114308. [Google Scholar] [CrossRef]
- Luo, K.; Yi, L.; Chen, X.; Yang, L.; Zou, C.; Tao, X.; Li, H.; Wu, T.; Wang, X. PVDF-HFP-modified gel polymer electrolyte for the stable cycling lithium metal batteries. J. Electroanal. Chem. 2021, 895, 115462. [Google Scholar] [CrossRef]
- Li, D.; Chen, L.; Wang, T.; Fan, L.-Z. 3D Fiber-Network-Reinforced Bicontinuous Composite Solid Electrolyte for Dendrite-free Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2018, 10, 7069–7078. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tang, M.; Zhang, Q.; Li, B.; Ohno, S.; Walther, F.; Pan, R.; Xu, X.; Xin, C.; Zhang, W.; et al. Lithium Argyrodite as Solid Electrolyte and Cathode Precursor for Solid-State Batteries with Long Cycle Life. Adv. Energy Mater. 2021, 11, 2101370. [Google Scholar] [CrossRef]
- Jie, J.; Liu, Y.; Cong, L.; Zhang, B.; Lu, W.; Zhang, X.; Liu, J.; Xie, H.; Sun, L. High-performance PVDF-HFP based gel polymer electrolyte with a safe solvent in Li metal polymer battery. J. Energy Chem. 2020, 49, 80–88. [Google Scholar] [CrossRef]
- Wang, C.; Fu, K.; Kammampata, S.P.; McOwen, D.W.; Samson, A.J.; Zhang, L.; Hitz, G.T.; Nolan, A.M.; Wachsman, E.D.; Mo, Y.; et al. Garnet-Type Solid-State Electrolytes: Materials, Interfaces, and Batteries. Chem. Rev. 2020, 120, 4257–4300. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Ma, N.; Wu, Y.; Lu, Y.; Xiao, Q.; Li, Z.; Lei, G. Fabrication and electrochemical properties of LATP/PVDF composite electrolytes for rechargeable lithium-ion battery. Solid State Ion. 2018, 325, 112–119. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Lu, Y.; Xie, J.; Huang, C.; Xu, X.; Tu, J.; Zhao, X.; Zhu, T. LATP-coated LiNi0.8Co0.1Mn0.1O2 cathode with compatible interface with ultrathin PVDF-reinforced PEO-LLZTO electrolyte for stable solid-state lithium batteries. J. Mater. 2023in press.
- Zhang, Q.; Wang, Q.; Huang, S.; Jiang, Y.; Chen, Z. Preparation and electrochemical study of PVDF-HFP/LATP/g-C3N4 composite polymer electrolyte membrane. Inorg. Chem. Commun. 2021, 131, 108793. [Google Scholar] [CrossRef]
- Zallocco, V.M.; Freitas, J.M.; Bocchi, N.; Rodrigues, A.C.M. Electrochemical stability of a NASICON solid electrolyte from the lithium aluminum germanium phosphate (LAGP) series. Solid State Ion. 2022, 378, 115888. [Google Scholar] [CrossRef]
- Wu, Z.; Xie, Z.; Yoshida, A.; Wang, Z.; Hao, X.; Abudula, A.; Guan, G. Utmost limits of various solid electrolytes in all-solid-state lithium batteries: A critical review. Renew. Sustain. Energy Rev. 2019, 109, 367–385. [Google Scholar] [CrossRef]
- Siyal, S.H.; Li, M.; Li, H.; Lan, J.-L.; Yu, Y.; Yang, X. Ultraviolet irradiated PEO/LATP composite gel polymer electrolytes for lithium-metallic batteries (LMBs). Appl. Surf. Sci. 2019, 494, 1119–1126. [Google Scholar] [CrossRef]
- Liang, X.; Zhao, Y.; DiHan; Mao, J.; Lan, L. Synthesis and Electrochemical Characteristics of LiNi0.5Mn1.5O4 Coatings Prepared by Atmospheric Plasma Spray as Cathode Material for Lithium-Ion Batteries. Int. J. Electrochem. Sci. 2019, 14, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Z.; Xia, X.H.; Xie, D.; Xu, R.C.; Xu, Y.J.; Xia, Y.; Wu, J.B.; Yao, Z.J.; Wang, X.L.; Tu, J.P. Facile interfacial modification via in-situ ultraviolet solidified gel polymer electrolyte for high-performance solid-state lithium ion batteries. J. Power Sources 2019, 409, 31–37. [Google Scholar] [CrossRef]
- Aidoud, D.; Etiemble, A.; Guy-Bouyssou, D.; Maire, E.; Le Bideau, J.; Guyomard, D.; Lestriez, B. Interfacial stability and electrochemical behavior of Li/LiFePO4 batteries using novel soft and weakly adhesive photo-ionogel electrolytes. J. Power Sources 2016, 330, 92–103. [Google Scholar] [CrossRef]
- Shi, C.; Takeuchi, S.; Alexander, G.V.; Hamann, T.; O’Neill, J.; Dura, J.A.; Wachsman, E.D. High Sulfur Loading and Capacity Retention in Bilayer Garnet Sulfurized-Polyacrylonitrile/Lithium-Metal Batteries with Gel Polymer Electrolytes. Adv. Energy Mater. 2023, 13, 2301656. [Google Scholar] [CrossRef]
- Lv, F.; Liu, K.; Wang, Z.; Zhu, J.; Zhao, Y.; Yuan, S. Ultraviolet-cured polyethylene oxide-based composite electrolyte enabling stable cycling of lithium battery at low temperature. J. Colloid Interface Sci. 2021, 596, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wu, Z.; He, P.; Fan, H.; Huang, Z.; Zhang, L.; Chang, X.; Liu, H.; Wang, C.; Li, Y. A self-standing, UV-cured semi-interpenetrating polymer network reinforced composite gel electrolytes for dendrite-suppressing lithium ion batteries. J. Mater. 2019, 5, 185–194. [Google Scholar] [CrossRef]
- Xia, Y.; Liang, Y.F.; Xie, D.; Wang, X.L.; Zhang, S.Z.; Xia, X.H.; Gu, C.D.; Tu, J.P. A poly (vinylidene fluoride-hexafluoropropylene) based three-dimensional network gel polymer electrolyte for solid-state lithium-sulfur batteries. Chem. Eng. J. 2019, 358, 1047–1053. [Google Scholar] [CrossRef]
- Hao, X.; Wenren, H.; Wang, X.; Xia, X.; Tu, J. A gel polymer electrolyte based on PVDF-HFP modified double polymer matrices via ultraviolet polymerization for lithium-sulfur batteries. J. Colloid Interface Sci. 2020, 558, 145–154. [Google Scholar] [CrossRef]
- Liang, X.; Ning, Y.; Lan, L.; Yang, G.; Li, M.; Tang, S.; Huang, J. Electrochemical Performance of a PVDF-HFP-LiClO4-Li6.4La3.0Zr1.4Ta0.6O12 Composite Solid Electrolyte at Different Temperatures. Nanomaterials 2022, 12, 3390. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Huang, Y.; Zhang, Z.; Huang, J.; Li, C. Li6.7La3Zr1.7Ta0.15Nb0.15O12 enhanced UV-cured poly(ethylene oxide)-based composite gel polymer electrolytes for lithium metal batteries. Electrochim. Acta 2020, 360, 137014. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, J.; Xiang, Y.; Lin, M.; Wang, H.; Zheng, B.; Wu, Q.; Huang, J.Y.; Yang, Y. Chemo-Mechanical Failure Mechanism Study in NASICON-type Li1.3Al0.3Ti1.7(PO4)3 Solid-State Lithium Batteries. Chem. Mater. 2020, 32, 4998–5008. [Google Scholar] [CrossRef]




| Coating Material | Current/A | Ar/L min−1 | H2/L min−1 | Spray Gun Moving/mm s−1 | Spray Distance/mm |
|---|---|---|---|---|---|
| LTO | 500–600 | 40 | 11 | 1000 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Wang, Y.; Liang, Z.; Yan, G.; Lan, L.; Wang, Y.; Shi, X.; Yun, S.; Huang, M. Long-Cycle Stability of In Situ Ultraviolet Curable Organic/Inorganic Composite Electrolyte for Solid-State Batteries. Polymers 2024, 16, 55. https://doi.org/10.3390/polym16010055
Liang X, Wang Y, Liang Z, Yan G, Lan L, Wang Y, Shi X, Yun S, Huang M. Long-Cycle Stability of In Situ Ultraviolet Curable Organic/Inorganic Composite Electrolyte for Solid-State Batteries. Polymers. 2024; 16(1):55. https://doi.org/10.3390/polym16010055
Chicago/Turabian StyleLiang, Xinghua, Yuying Wang, Zhida Liang, Ge Yan, Lingxiao Lan, Yujiang Wang, Xueli Shi, Shuhong Yun, and Meihong Huang. 2024. "Long-Cycle Stability of In Situ Ultraviolet Curable Organic/Inorganic Composite Electrolyte for Solid-State Batteries" Polymers 16, no. 1: 55. https://doi.org/10.3390/polym16010055
APA StyleLiang, X., Wang, Y., Liang, Z., Yan, G., Lan, L., Wang, Y., Shi, X., Yun, S., & Huang, M. (2024). Long-Cycle Stability of In Situ Ultraviolet Curable Organic/Inorganic Composite Electrolyte for Solid-State Batteries. Polymers, 16(1), 55. https://doi.org/10.3390/polym16010055





