Influence of Electrospinning Parameters on the Morphology of Electrospun Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Fibrous Membranes and Their Application as Potential Air Filtration Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrospinning Process
2.2. Characterization
3. Results
3.1. Effect of Applied Voltage
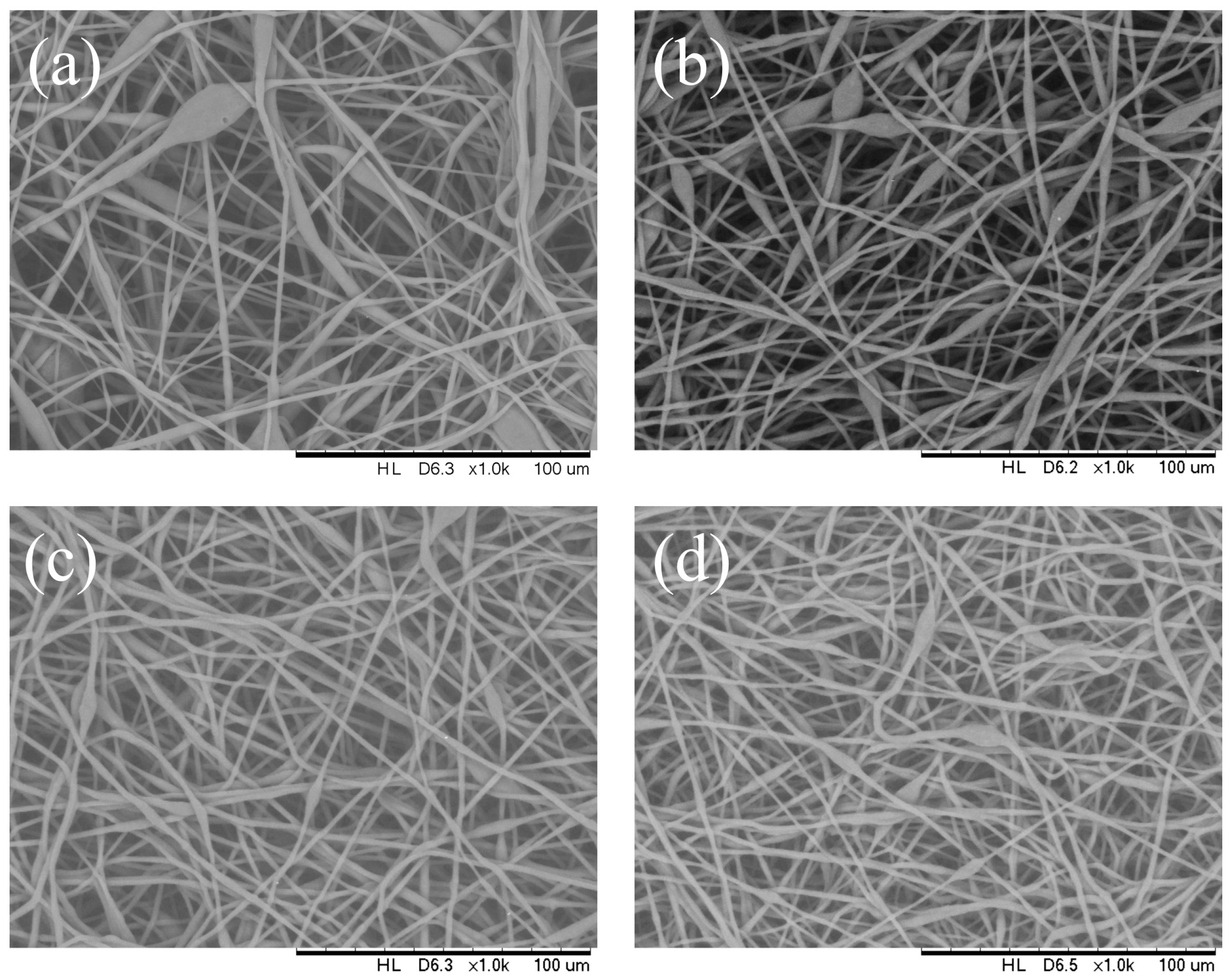
3.2. Effect of Solution Flow Rate
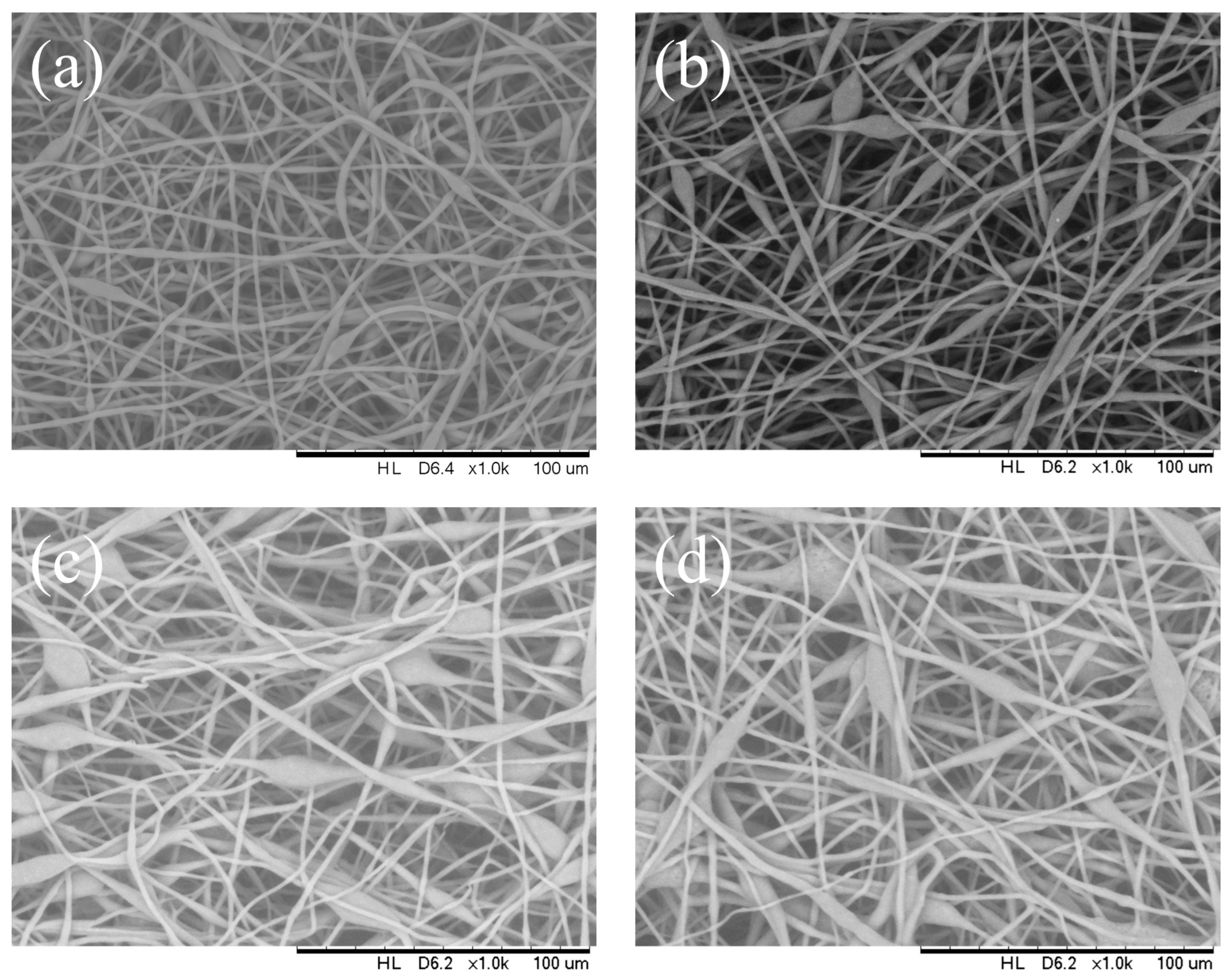
3.3. Effect of Capillary Inner Diameter of Needle
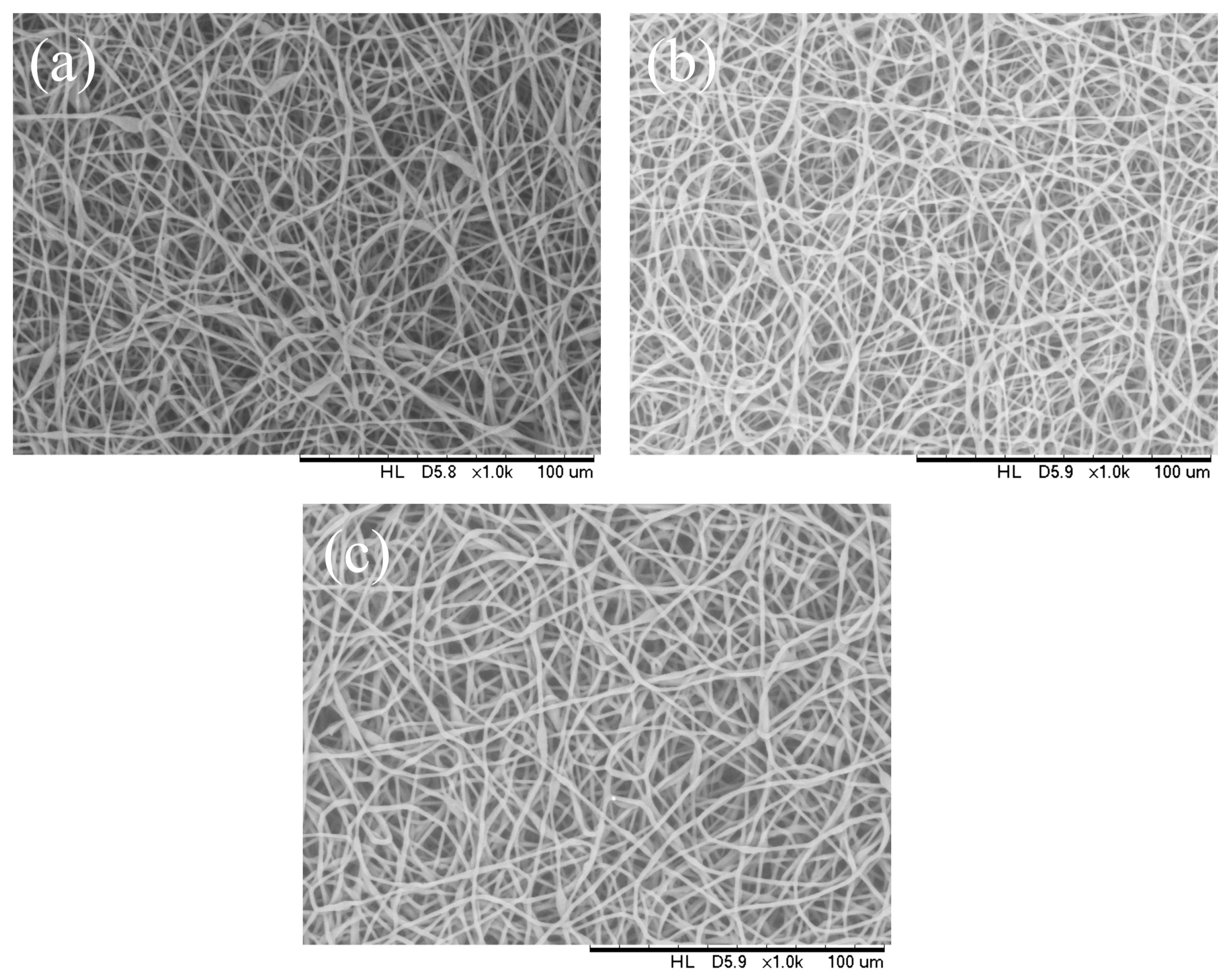
3.4. Effect of Solution Concentration
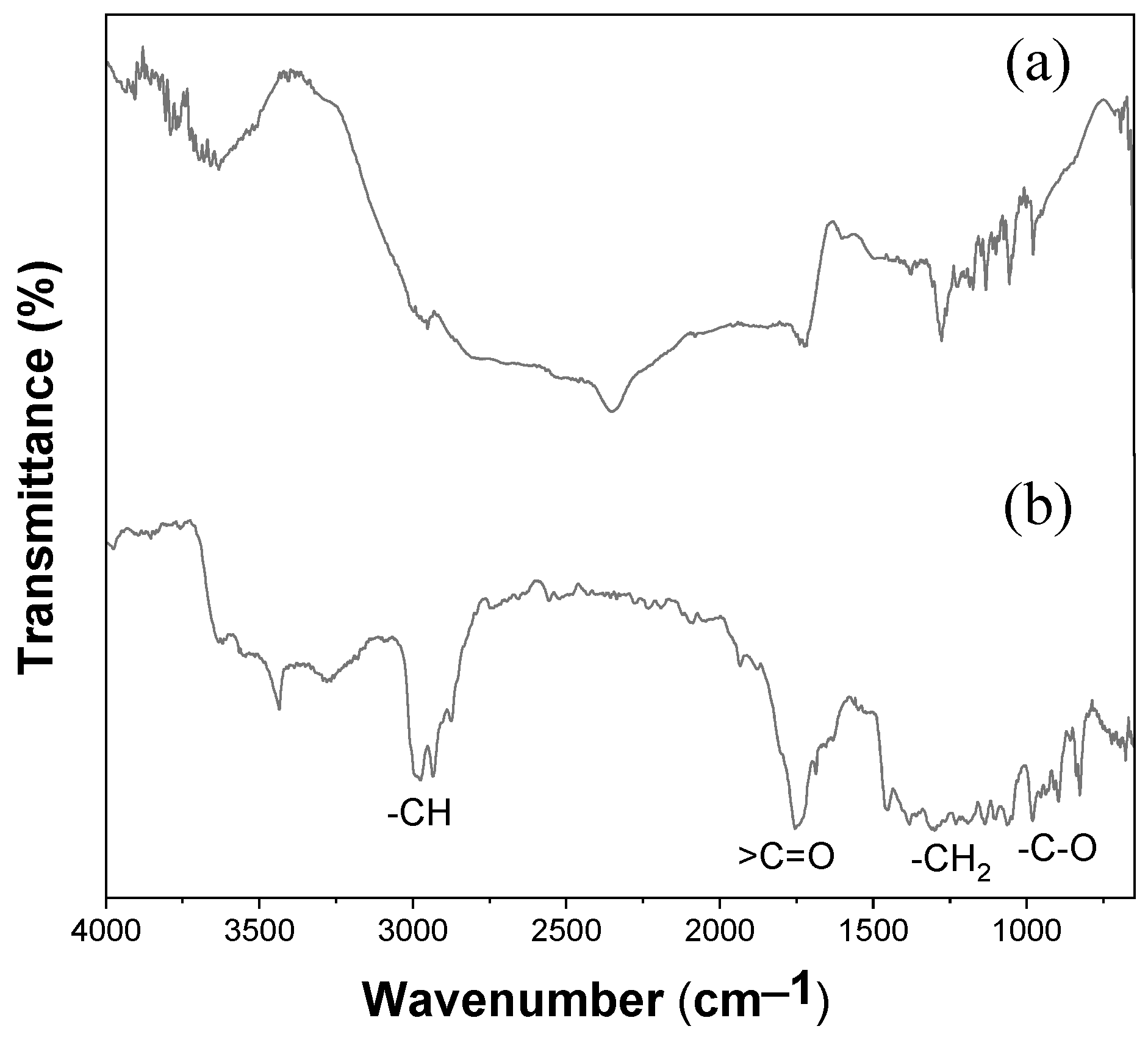
3.5. ATR-FTIR Spectroscopy of Electrospun PHBV Fiber Membrane
3.6. Raman Spectroscopy of Electrospun PHBV Fiber Membrane
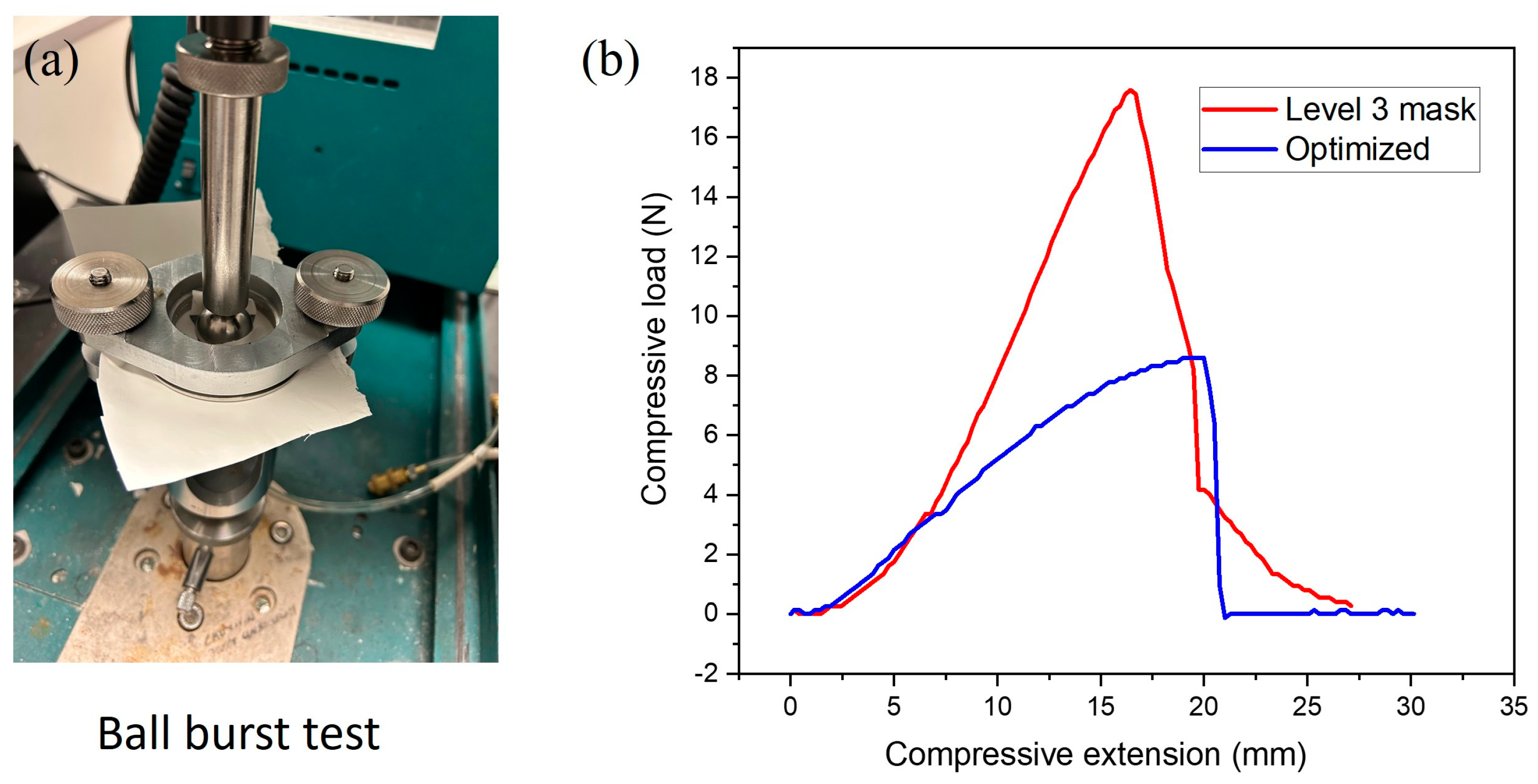
3.7. Ball Burst Strength (BBS)
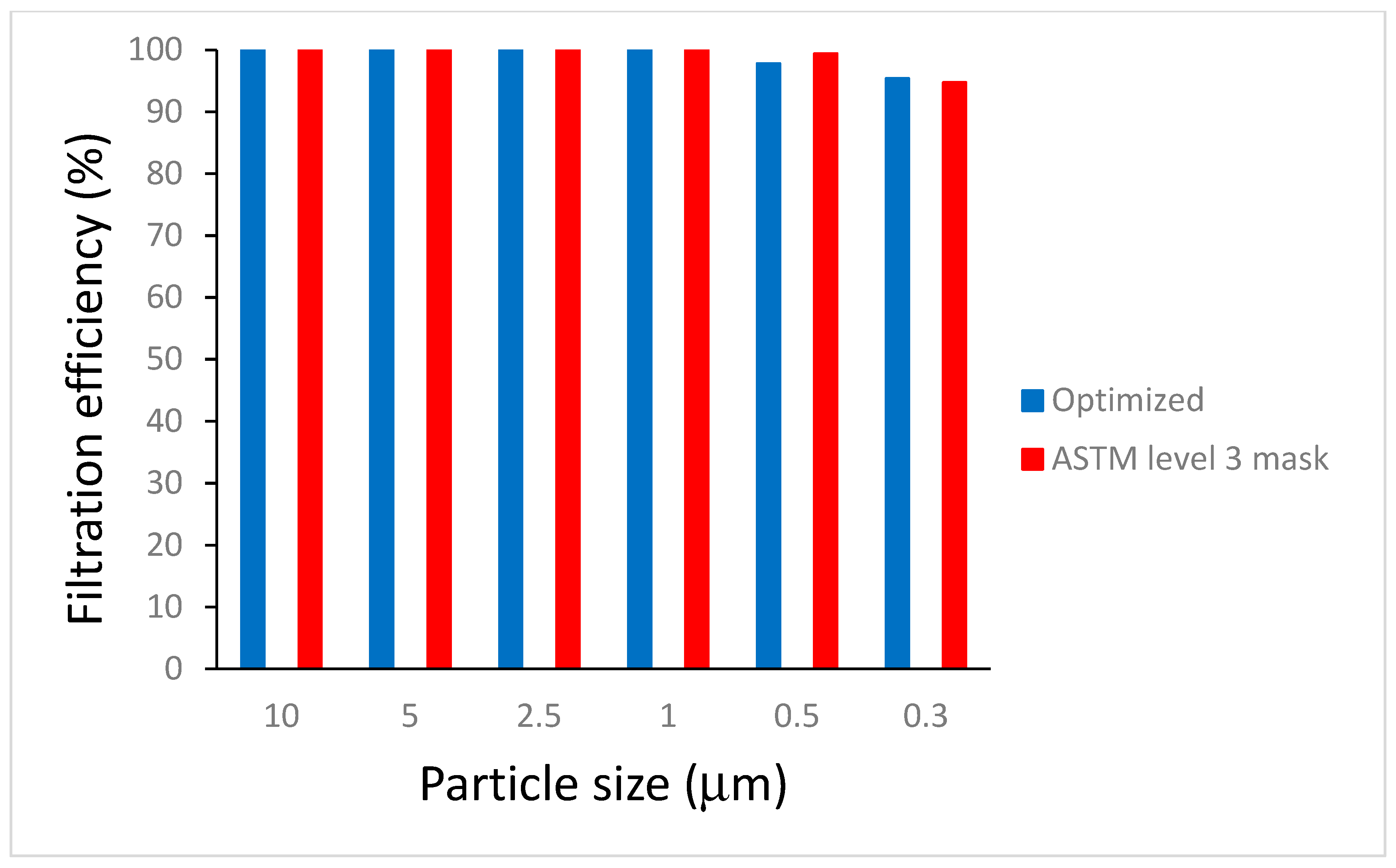
3.8. Filtration Efficiency
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Weng, Y.X.; Wang, Y.; Wang, X.L.; Wang, Y.Z. Biodegradation behavior of PHBV films in a pilot-scale composting condition. Polym. Test. 2010, 29, 579–587. [Google Scholar] [CrossRef]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A. Recent advances and challenges towards sustainable polyhydroxyalkanoate (PHA) production. Bioresour. Technol. 2017, 4, 55. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Q.; Patel, M.K. Plastics derived from biological sources: Present and future: A technical and environmental review. Chem. Rev. 2012, 112, 2082–2099. [Google Scholar] [CrossRef] [PubMed]
- Rujnić-Sokele, M.; Pilipović, A. Challenges and opportunities of biodegradable plastics: A mini review. Waste Manag. Res. 2017, 35, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Q. A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem. Soc. Rev. 2009, 38, 2434–2446. [Google Scholar] [CrossRef]
- Koller, M. Biodegradable and biocompatible polyhydroxy-alkanoates (PHA): Auspicious microbial macromolecules for pharmaceutical and therapeutic applications. Molecules 2018, 23, 362. [Google Scholar] [CrossRef] [PubMed]
- Holland, C.; Numata, K.; Rnjak-Kovacina, J.; Seib, F.P. The biomedical use of silk: Past, present, future. Adv. Healthc. Mater. 2019, 8, 1800465. [Google Scholar] [CrossRef]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Koller, M.; Braunegg, G. Advanced approaches to produce polyhydroxyalkanoate (PHA) biopolyesters in a sustainable and eco nomic fashion. EuroBiotech J. 2018, 2, 89–103. [Google Scholar] [CrossRef]
- Koller, M.; Maršálek, L.; de Sousa Dias, M.M.; Braunegg, G. Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. New Biotechnol. 2017, 37, 24–38. [Google Scholar] [CrossRef]
- Policastro, G.; Panico, A.; Fabbricino, M. Improving Biological Production of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) Co-Polymer: A Critical Review. Rev. Environ. Sci. Bio/Technol. 2021, 20, 479–513. [Google Scholar] [CrossRef]
- Tebaldi, M.L.; Maia, A.L.C.; Poletto, F.; de Andrade, F.V.; Soares, D.C.F. Poly(-3-hydroxybutyrate-Co-3-hydroxyvalerate) (PHBV): Current Advances in Synthesis Methodologies, Antitumor Applications and Biocompatibility. J. Drug Deliv. Sci. Technol. 2019, 51, 115–126. [Google Scholar] [CrossRef]
- Rivera-Briso, A.L.; Serrano-Aroca, Á. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate): Enhancement Strategies for Advanced Applications. Polymers 2018, 10, 732. [Google Scholar] [CrossRef]
- Amaro, L.; Correia, D.M.; Marques-Almeida, T.; Martins, P.M.; Pérez, L.; Vilas, J.L.; Botelho, G.; Lanceros-Mendez, S.; Ribeiro, C. Tailored Biodegradable and Electroactive Poly(hydroxybutyrate-Co-hydroxyvalerate) Based Morphologies for Tissue Engineering Applications. Int. J. Mol. Sci. 2018, 19, 2149. [Google Scholar] [CrossRef]
- Kaniuk, L.; Stachewicz, U. Development and Advantages of Biodegradable PHA Polymers Based on Electrospun PHBV Fibers for Tissue Engineering and Other Biomedical Applications. ACS Biomater. Sci. Eng. 2021, 7, 5339–5362. [Google Scholar] [CrossRef]
- Li, X.; Liu, T.; Zhang, Y.; Cai, J.; He, M.; Li, M.; Chen, Z.; Zhang, L. Growth of BiOBr/ZIF-67 Nanocomposites on Carbon Fibre Cloth as Filter-Membrane-Shaped Photocatalyst for Degrading Pollutants in Flowing Wastewater. Adv. Fibre Mater. 2022, 4, 1620–1631. [Google Scholar] [CrossRef]
- Keshel, S.H.; Biazar, E.; Rezaei Tavirani, M.; Rahmati Roodsari, M.; Ronaghi, A.; Ebrahimi, M.; Rad, H.; Sahebalzamani, A.; Rakhshan, A.; Afsordeh, K. The Healing Effect of Unrestricted Somatic Stem Cells Loaded in Collagen-Modified Nanofibrous PHBV Scaffold on Full-Thickness Skin Defects. Artif. Cells Nanomed. Biotechnol. 2014, 42, 210–216. [Google Scholar] [CrossRef]
- Bloembergen, S.; Holden, D.A.; Hamer, G.K.; Bluhm, T.L.; Marchessault, R.H. Studies of Composition and Crystallinity of Bacterial Poly(β-Hydroxybutyrate-co-β-Hydroxyvalerate). Macromolecules 1986, 19, 2865–2871. [Google Scholar] [CrossRef]
- Jun, D.; Guomin, Z.; Mingzhu, P.; Leilei, Z.; Dagang, L.; Rui, Z. Crystallization and Mechanical Properties of Reinforced PHBV Composites Using Melt Compounding: Effect of CNCs and CNFs. Carbohydr. Polym. 2017, 168, 255–262. [Google Scholar] [CrossRef]
- Zhang, X.; Ru, Z.; Sun, Y.; Zhang, M.; Wang, J.; Ge, M.; Liu, H.; Wu, S.; Cao, C.; Ren, X.; et al. Recent advances in applications for air pollutants purification and perspectives of electrospun nanofibres. J. Clean. Prod. 2022, 378, 134567. [Google Scholar] [CrossRef]
- Shao, Z.; Chen, H.; Wang, Q.; Kang, G.; Wang, X.; Li, W.; Liu, Y.; Zheng, G. High-performance multifunctional electrospun fibrous air filter for personal protection: A review. Sep. Purif. Technol. 2022, 302, 122175. [Google Scholar] [CrossRef]
- Matulevicius, J.; Kliucininkas, L.; Martuzevicius, D.; Krugly, E.; Tichonovas, M.; Baltrusaitis, J. Design and Characterization of Electrospun Polyamide Nanofiber Media for Air Filtration Applications. J. Nanomater. 2014, 2014, 859656. [Google Scholar] [CrossRef]
- Strain, I.N.; Wu, Q.; Pourrahimi, A.M.; Hedenqvist, M.S.; Olsson, R.T.; Andersson, R.L. Electrospinning of recycled PET to generate tough mesomorphic fibre membranes for smoke filtration. J. Mater. Chem. A 2014, 3, 1632–1640. [Google Scholar] [CrossRef]
- Yun, K.M.; Hogan, C.J., Jr.; Matsubayashi, Y.; Kawabe, M.; Iskandar, F.; Okuyama, K. Nanoparticle filtration by electrospun polymer fibers. Chem. Eng. Sci. 2007, 62, 4751–4759. [Google Scholar] [CrossRef]
- Zhang, J.; Gong, S.; Wang, C.; Jeong, D.-Y.; Wang, Z.L.; Ren, K. Biodegradable Electrospun Poly(lactic acid) Nanofibers for Effective PM 2.5 Removal. Macromol. Mater. Eng. 2019, 304, 1900259. [Google Scholar] [CrossRef]
- Qin, X.-H.; Wang, S.-Y. Electrospun nanofibers from crosslinked poly(vinyl alcohol) and its filtration efficiency. J. Appl. Polym. Sci. 2008, 109, 951–956. [Google Scholar] [CrossRef]
- Li, J.; Gao, F.; Liu, L.Q.; Zhang, Z. Needleless electro-spun nanofibers used for filtration of small particles. Express Polym. Lett. 2013, 7, 683–689. [Google Scholar] [CrossRef]
- Rampichova, M.; Chvojka, J.; Jencova, V.; Kubikova, T.; Tonar, Z.; Erben, J.; Buzgo, M.; Dankova, J.; Litvinec, A.; Vocetkova, K.; et al. The combination of nanofibrous and microfibrous materials for enhancement of cell infiltration and in vivo bone tissue formation. Biomed. Mater. 2017, 13, 025004. [Google Scholar] [CrossRef]
- Gu, J.; Yuan, L.; Zhang, Z.; Yang, X.; Luo, J.; Gui, Z.; Chen, S. Non-leaching bactericidal cotton fabrics with well-preserved physical properties, no skin irritation and no toxicity. Cellulose 2018, 25, 5415–5426. [Google Scholar] [CrossRef]
- Zhu, M.; Han, J.; Wang, F.; Shao, W.; Xiong, R.; Zhang, Q.; Pan, H.; Yang, Y.; Samal, S.K.; Zhang, F.; et al. Electrospun Nanofibers Membranes for Effective Air Filtration. Macromol. Mater. Eng. 2016, 302, 1600353. [Google Scholar] [CrossRef]
- Cheng, X.; Zhao, L.; Zhang, Z.; Deng, C.; Li, C.; Du, Y.; Shi, J.; Zhu, M. Highly efficient, low-resistant, well-ordered PAN nanofiber membranes for air filtration. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 655, 130302. [Google Scholar] [CrossRef]
- Kadam, V.; Kyratzis, I.L.; Truong, Y.B.; Schutz, J.; Wang, L.; Padhye, R. Electrospun bilayer nanomembrane with hierarchical placement of bead-on-string and fibers for low resistance respiratory air filtration. Sep. Purif. Technol. 2019, 224, 247–254. [Google Scholar] [CrossRef]
- Kimmer, D.; Vincent, I.; Sambaer, W.; Zatloukal, M.; Ondráček, J. The effect of combination electrospun and meltblown filtration materials on their filtration efficiency. AIP Conf. Proc. 2015, 1662, 050001. [Google Scholar]
- Babaahmadi, V.; Amid, H.; Naeimirad, M.; Ramakrishna, S. Biodegradable and multifunctional surgical face masks: A brief review on demands during COVID-19 pandemic, recent developments, and future perspectives. Sci. Total Environ. 2021, 798, 149233. [Google Scholar] [CrossRef]
- Correia, D.M.; Ribeiro, C.; Ferreira, J.C.C.; Botelho, G.; Ribelles, J.L.G.; Lanceros-Méndez, S.; Sencadas, V. Influence of electrospinning parameters on poly(hydroxybutyrate) electrospun membranes fiber size and distribution. Polym. Eng. Sci. 2013, 54, 1608–1617. [Google Scholar] [CrossRef]
- Nezarati, R.M.; Eifert, M.B.; Cosgriff-Hernandez, E. Effects of humidity and solution viscosity on electrospun fiber morphology. Tissue Eng. Part C Methods 2013, 19, 810–819. [Google Scholar] [CrossRef]
- Iordanskii, A.L. Electrospinning of biodegradable poly-3-hydroxybutyrate. Effect of the characteristics of the polymer solution. Russ. J. Phys. Chem. B 2016, 10, 830–838. [Google Scholar]
- ASTM F2100; Standard Specification for Performance of Materials Used in Med-ical Face Masks. ASTM International: West Conshohocken, PA, USA, 2023.
- ASTM D3787-07; Standard Test Method for Bursting Strength of Textiles-Constant-Rate-of-Traverse (CRT) Ball Burst Test. ASTM International: West Conshohocken, PA, USA, 2011.
- Huang, Z.M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their ap-plications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, R89. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Medeiros, G.B.; Lima, F.d.A.; de Almeida, D.S.; Guerra, V.G.; Aguiar, M.L. Modification and Functionalization of Fibers Formed by Electrospinning: A Review. Membranes 2022, 12, 861. [Google Scholar] [CrossRef]
- Oliveira, J.E.; Mattoso, L.H.C.; Orts, W.J.; Medeiros, E.S. Structural and Morphological Characterization of Micro and Nano fibers Produced by Electrospinning and Solution Blow Spinning: A Comparative Study. Adv. Mater. Sci. Eng. 2013, 2013, 409572. [Google Scholar] [CrossRef]
- Wei, L.; McDonald, A.G.; Stark, N.M. Grafting of Bacterial Polyhydroxybutyrate (PHB) onto Cellulose via In Situ Reactive Extrusion with Dicumyl Peroxide. Biomacromolecules 2015, 16, 1040–1049. [Google Scholar] [CrossRef]
- Aguiar, G.P.S.; Magro, C.D.; Oliveira, J.V.; Lanza, M. Poly(hydroxybutyrate-co-hydroxyvalerate) micronization by solution enhanced dispersion by supercritical fluids technique. Braz. J. Chem. Eng. 2018, 35, 1275–1282. [Google Scholar] [CrossRef]
- Bai, J.; Dai, J.; Li, G. Electrospun composites of PHBV/pearl powder for bone repairing. Prog. Nat. Sci. 2015, 25, 327–333. [Google Scholar] [CrossRef]
- Kovalcik, A.; Obruca, S.; Fritz, I.; Marova, I. Polyhydroxyalkanoates: Their Importance and Future. BioResources 2019, 14, 2468–2471. [Google Scholar]
- Zhou, Y.; Zhao, M.; Guo, H.; Li, Y.; Liu, Q.; Deng, B. Morphology and crystallization behavior of poly(3-hydroxy butyrate-Co-3-hydroxyvalerate)/polyhedral oligomeric silsesquioxane hybrids. RSC Adv. 2019, 9, 8146–8158. [Google Scholar] [CrossRef]
- Guho, N.M.; Pokhrel, D.; Abbasi, M.; McDonald, A.G.; Alfaro, M.; Brinkman, C.K.; Coats, E.R. Pilot-scale production of poly3-hydroxybutyrate-co-3-hydroxyvalerate from fermented dairy manure: Process performance, polymer characteriza-tion, and scale-up implications. Bioresour. Technol. Rep. 2020, 12, 100588. [Google Scholar]
- Pramanik, N.; DAS, R.; Rath, T.; Kundu, P.P. Microbial Degradation of Linseed Oil-Based Elastomer and Subse quent Accu-mulation of Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) Copolymer. Appl. Biochem. Biotechnol. 2014, 174, 1613–1630. [Google Scholar] [CrossRef]
- Izumi, C.M.S.; Temperini, M.L.A. FT-Raman investigation of biodegradable polymers: Poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Vib. Spectrosc. 2010, 54, 127–132. [Google Scholar] [CrossRef]
- Marlina, D.; Sato, H.; Hoshina, H.; Ozaki, Y. Intermolecular interactions of poly(3-hydroxybutyrate-co-3-hy droxyvalerate) (P(HB-co-HV)) with PHB-type crystal structure and PHV-type crystal structure studied by low-frequency Raman and te-rahertz spectroscopy. Polymer 2018, 135, 331–337. [Google Scholar] [CrossRef]
- Deeken, C.R.; Abdo, M.S.; Frisella, M.M.; Matthews, B.D. Physicomechanical evaluation of polypropylene, poly ester, and poly tetrafluoroethylene meshes for inguinal hernia repair. J. Am. Coll. Surg. 2011, 212, 68–79. [Google Scholar] [CrossRef]
- Pölöskei, K.; Csézi, G.; Hajba, S.; Tábi, T. Investigation of the thermoformability of various D-Lactide content poly(lactic acid) films by ball burst test. Polym. Eng. Sci. 2020, 60, 1266–1277. [Google Scholar] [CrossRef]
- GB 19083-2010; Standard Test Method: Technical Requirements for Protective Face Mask for Medical Use. National Standard of the People’s Republic of China: Beijing, China, 2010.
- Li, Y.; Yin, X.; Yu, J.; Ding, B. Electrospun nanofibers for high-performance air filtration. Compos. Commun. 2019, 15, 6–19. [Google Scholar]
- Zhou, Y.; Liu, Y.; Zhang, M.; Feng, Z.; Yu, D.-G.; Wang, K.; Arias, J.L.; Scherf, U.; El-Hammadi, M.M.; Zhou, Y.; et al. Electro-spun nanofiber membranes for air filtration: A review. Nanomaterial 2022, 12, 1077. [Google Scholar] [CrossRef]
- Deng, Y.; Lu, T.; Cui, J.; Keshari Samal, S.; Xiong, R.; Huang, C. Bio-based electrospun nanofiber as building blocks for a novel eco-friendly air filtration membrane: A review. Sep. Purif. Technol. 2021, 277, 119623. [Google Scholar] [CrossRef]
- Qin, X.-H.; Wang, S.-Y. Filtration properties of electrospinning nanofibers. J. Appl. Polym. Sci. 2006, 102, 1285–1290. [Google Scholar] [CrossRef]
- Lei, L.; Shang, L.; Li, Y.; Yang, C. Three-layer composite filter media containing electrospun polyimide nanofibers for the removal of fine particles. Fibers Polym. 2017, 18, 749–757. [Google Scholar] [CrossRef]
- Bidabadi, M.; Fereidooni, J.; Tavakoli, R.; Mafi, M. Premixed filtration combustion of micron and Sub-micronparticles in inert porous media: A theoretical analysis. Korean J. Chem. Eng. 2011, 28, 461–469. [Google Scholar] [CrossRef]
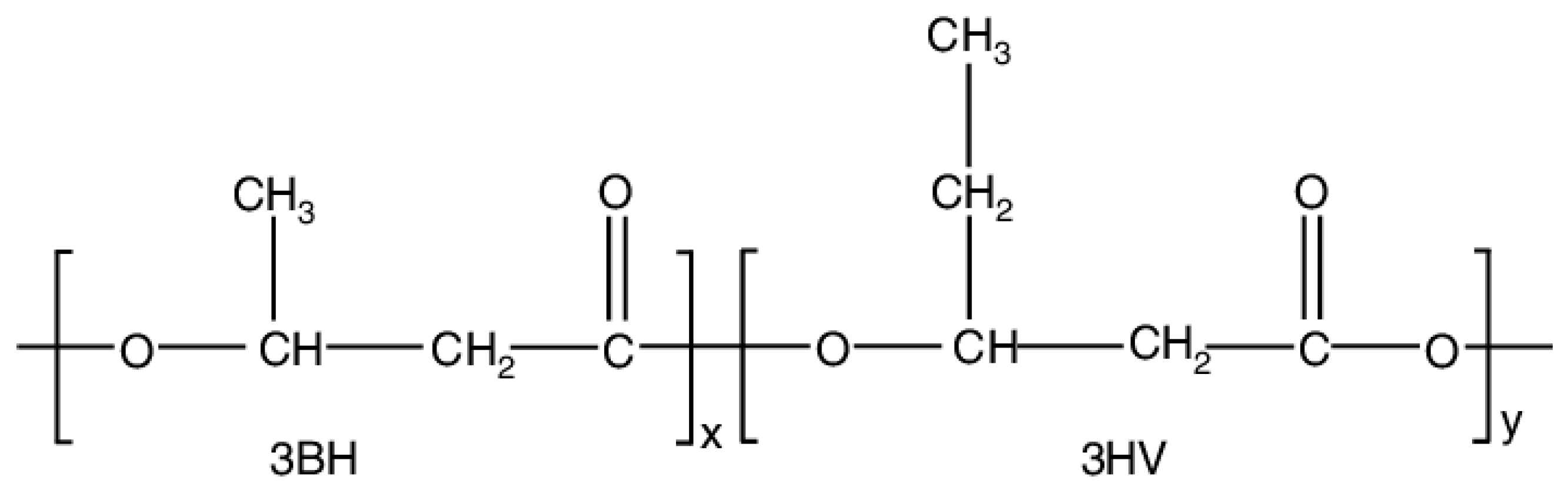



| Applied voltage (kV) | 9 | 12 | 18 | 20 |
| Mean fiber diameter (μm) | 2 | 1.4 | 1.26 | 1 |
| Injection flow rate (mL/h) | 0.5 | 1 | 3 | 5 |
| Mean fiber diameter (μm) | 2 | 2.57 | 3.6 | 4.6 |
| Capillary inner diameter (mm) | 0.5 | 0.33 | 0.21 | 0.16 |
| Mean fiber diameter (μm) | 2.55 | 2.47 | 2.36 | 1.83 |
| PHBV concentration (wt.%) | 12 | 13 | 14 |
| Mean fiber diameter (μm) | 2.55 | 2.53 | 2.54 |
| Maximum Compressive Load (N) | Compression Extension at Burst (mm) | |
|---|---|---|
| PHBV membrane | 8.6 | 19.99 |
| ASTM F2100 level 3 mask | 17.59 | 16.44 |
| Parameters | SEM Image of Electrospun Fiber Membrane | Particle Filtration Efficiency (PFE) | % Crystallinity | |
|---|---|---|---|---|
| 0.5 mL/h 20 kV 0.21 mm | 12 wt.% |  | 98.9% | 46.4 |
| 13 wt.% | 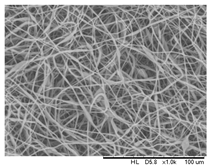 | 96.1% | 43.5 | |
| 12 wt.% 20 kV 0.21 mm | 5 mL/h |  | 91.1 % | 41.7 |
| 12 wt.% 0.5 mL/h 0.21 mm | 9 kV |  | 94.2% | 45.4 |
| 12 wt.% 0.5 mL/h 20 kV | 0.5 mm | 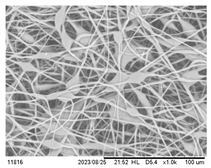 | 93.6% | 42.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, Y.; Lee, C.-H.; Kan, C.-W.; Lu, X. Influence of Electrospinning Parameters on the Morphology of Electrospun Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Fibrous Membranes and Their Application as Potential Air Filtration Materials. Polymers 2024, 16, 154. https://doi.org/10.3390/polym16010154
Liu Y, Wang Y, Lee C-H, Kan C-W, Lu X. Influence of Electrospinning Parameters on the Morphology of Electrospun Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Fibrous Membranes and Their Application as Potential Air Filtration Materials. Polymers. 2024; 16(1):154. https://doi.org/10.3390/polym16010154
Chicago/Turabian StyleLiu, Yaohui, Yanming Wang, Cheng-Hao Lee, Chi-Wai Kan, and Xiaoying Lu. 2024. "Influence of Electrospinning Parameters on the Morphology of Electrospun Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Fibrous Membranes and Their Application as Potential Air Filtration Materials" Polymers 16, no. 1: 154. https://doi.org/10.3390/polym16010154
APA StyleLiu, Y., Wang, Y., Lee, C.-H., Kan, C.-W., & Lu, X. (2024). Influence of Electrospinning Parameters on the Morphology of Electrospun Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Fibrous Membranes and Their Application as Potential Air Filtration Materials. Polymers, 16(1), 154. https://doi.org/10.3390/polym16010154







