Chemical Recycling of CFRP in an Environmentally Friendly Approach
Abstract
1. Introduction
2. Materials and Methods
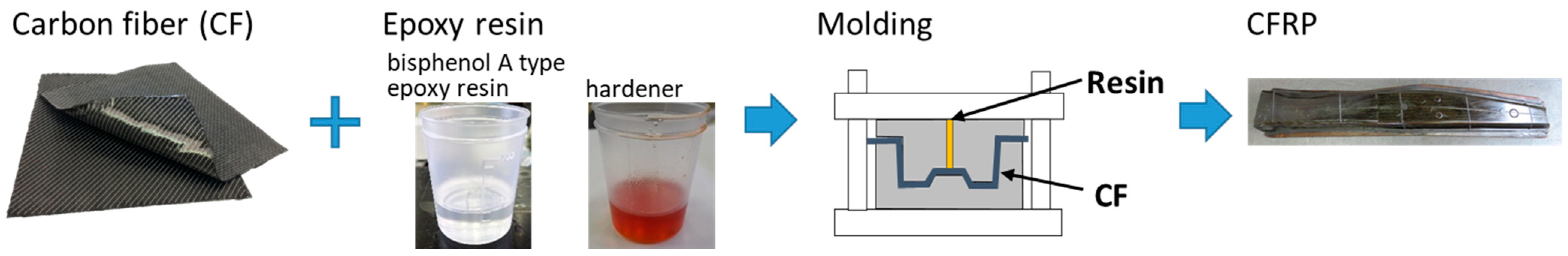
2.1. CFRP Materials
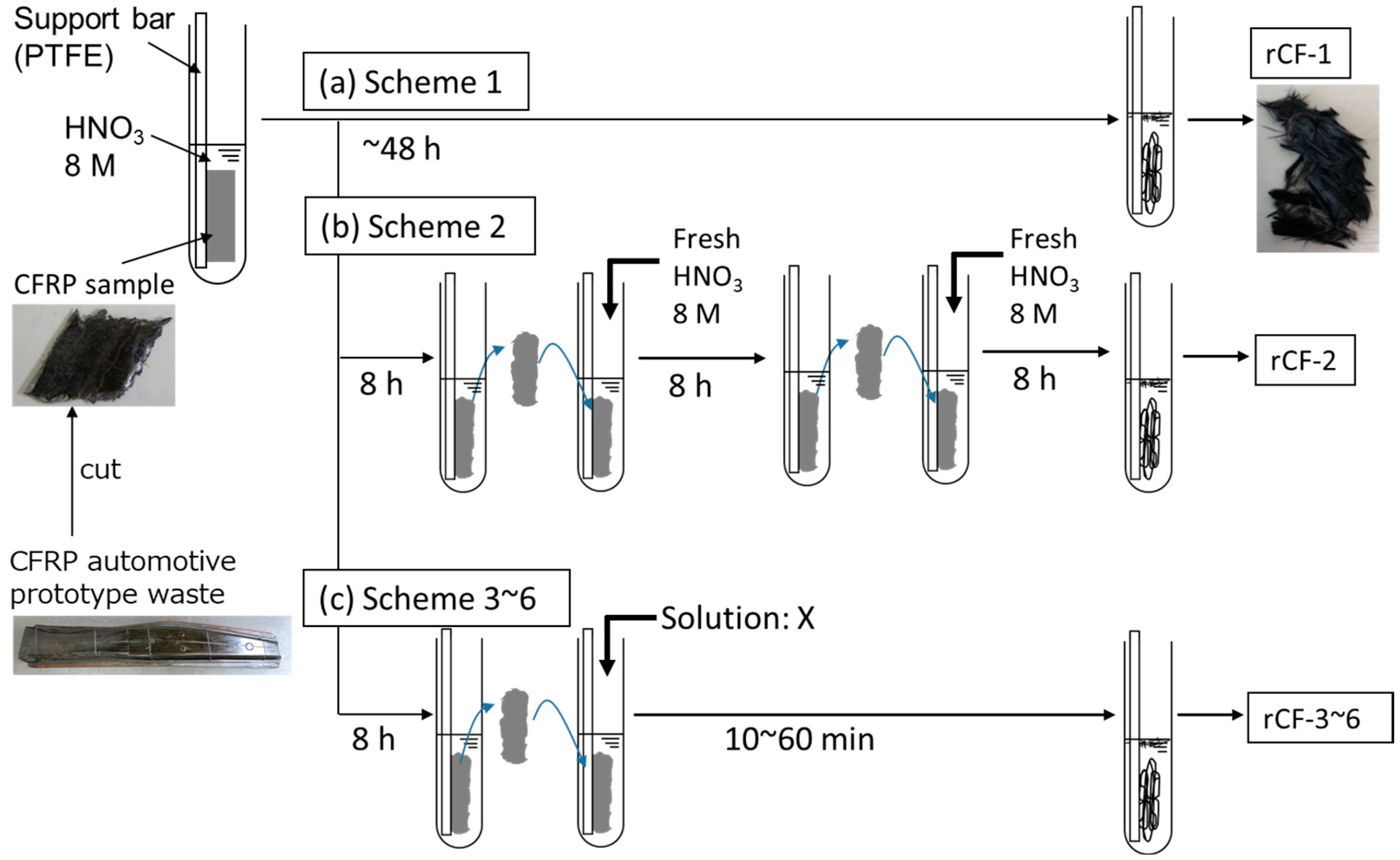
2.2. Recycling Scheme of CFRP
- (a)
- Scheme-1
- (b) Scheme-2
- (c) Scheme-3
- (d) Scheme-4
- (e) Scheme-5
- (f) Scheme-6
2.3. Method of Recovering Decomposed Resin
2.4. Evaluation
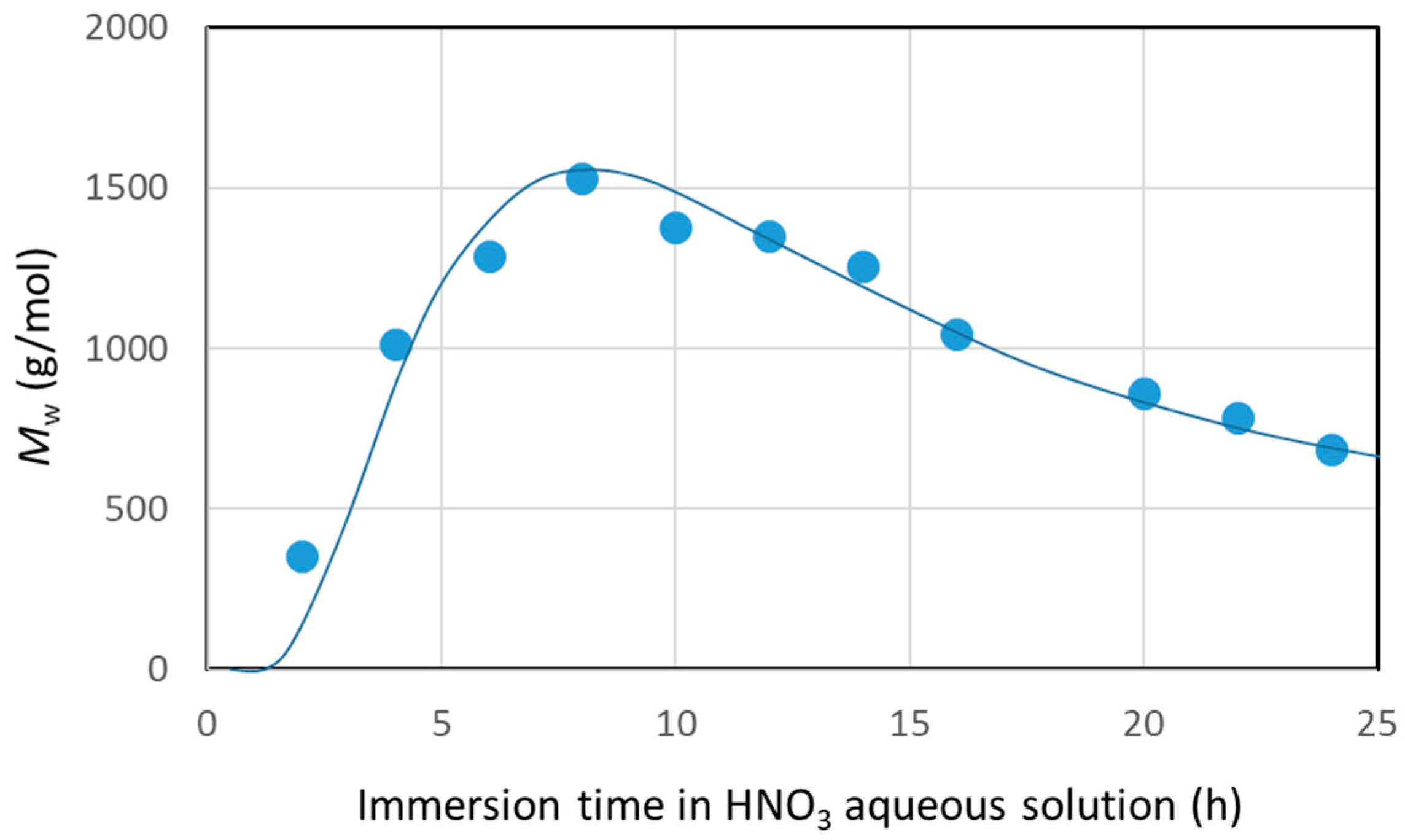
2.4.1. Time Change in Resin Decomposition Ratio
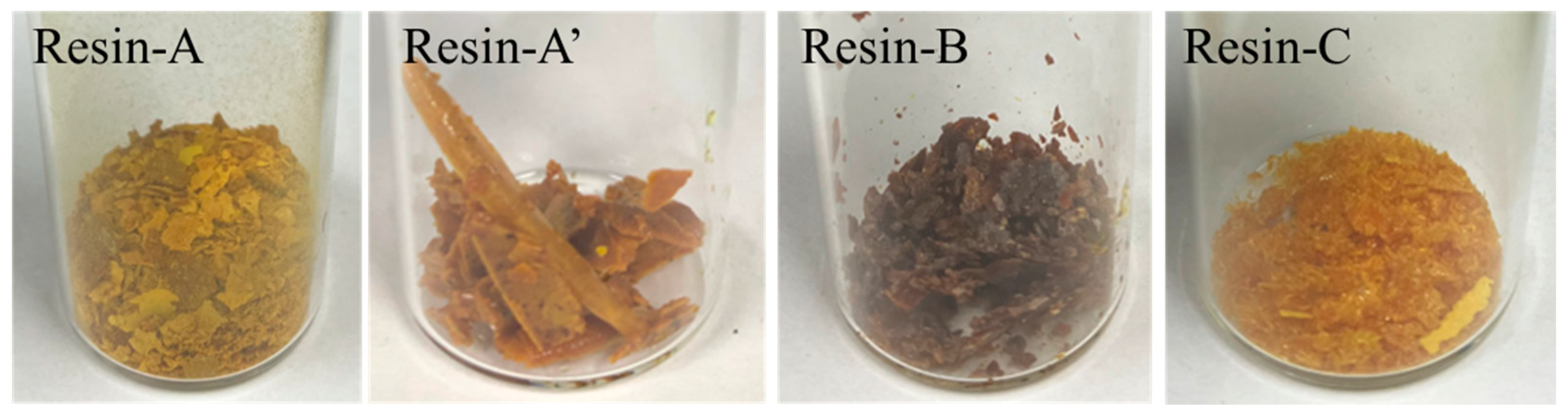
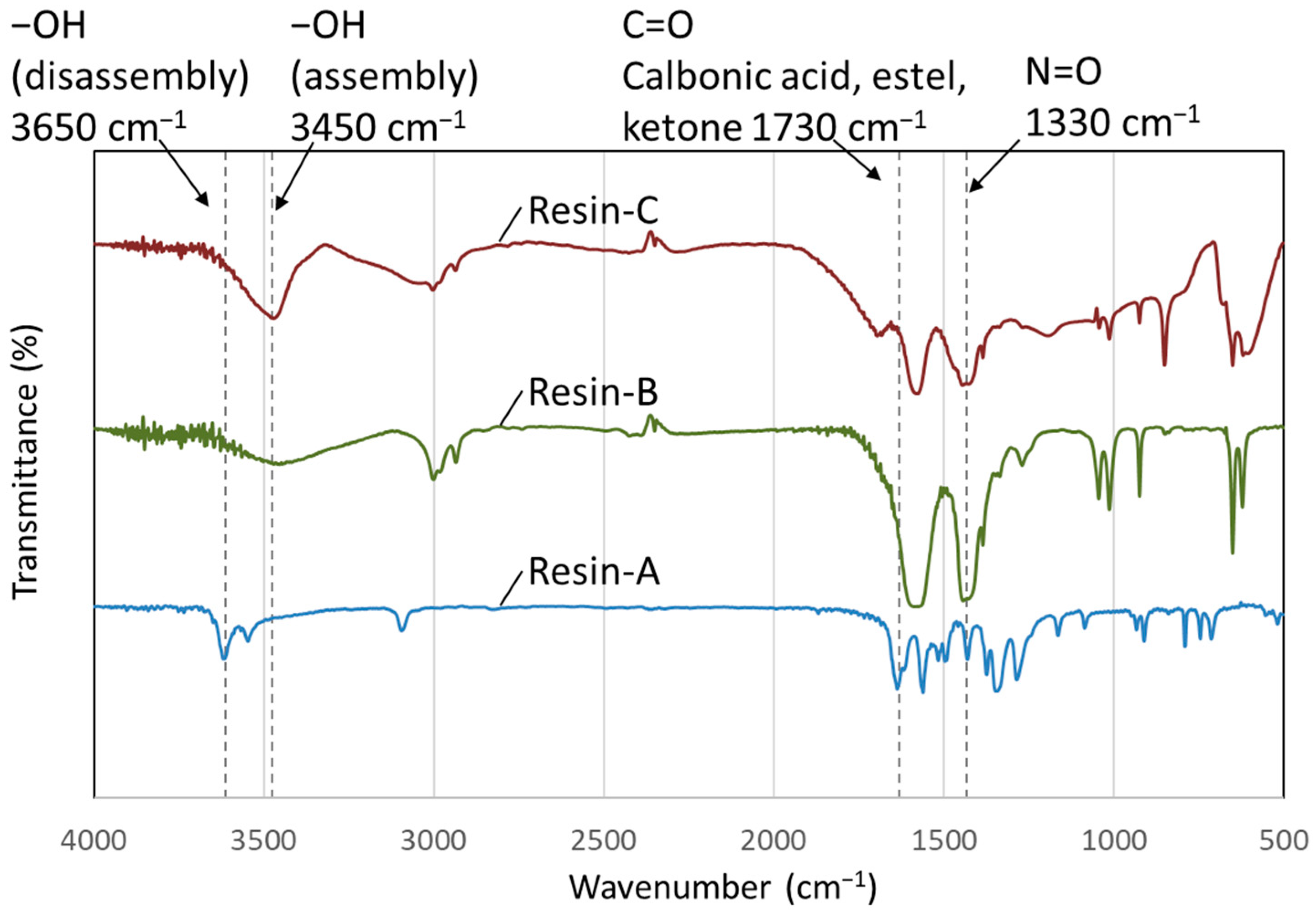
2.4.2. Analysis of the Decomposed Resin
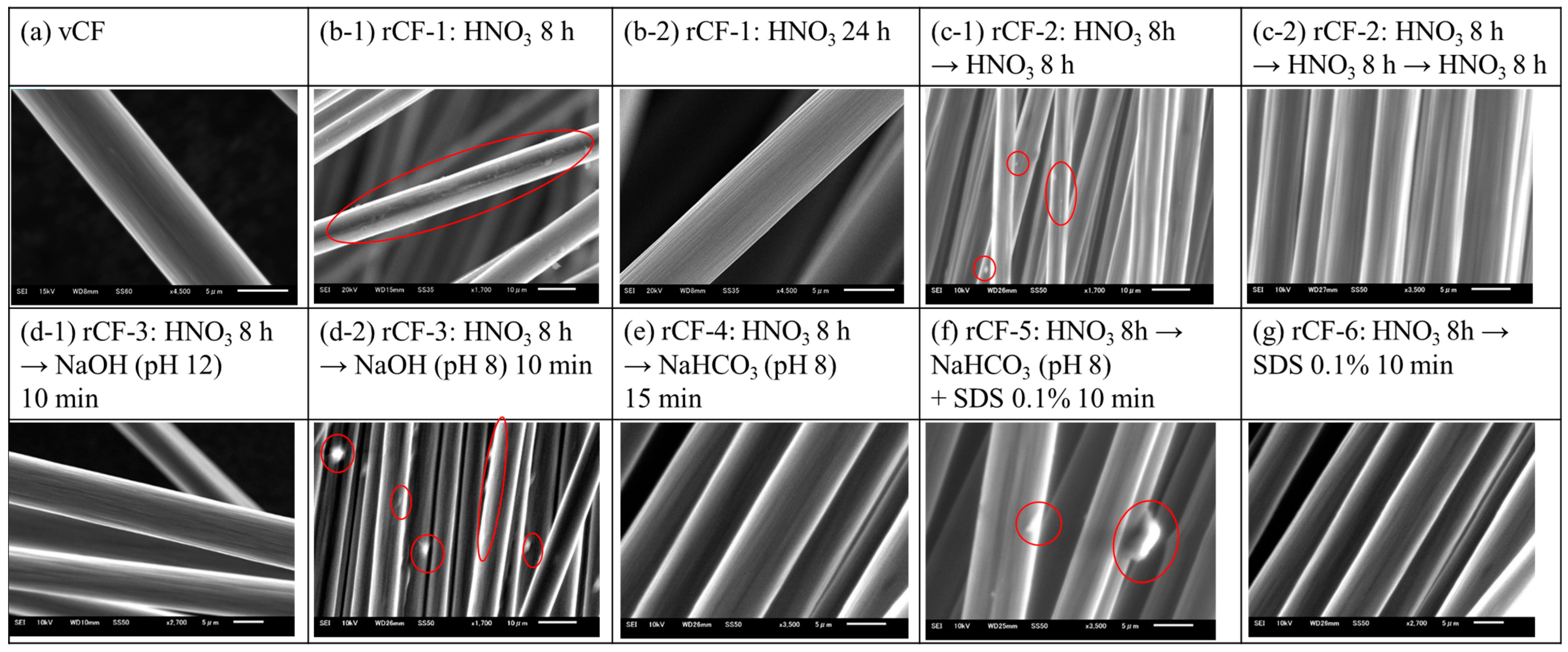
2.4.3. Analysis of the Carbon Fiber Surface
2.4.4. Adhesiveness of the Carbon Fiber and Resin
2.4.5. Tensile Strength of Carbon Fibers
3. Results and Discussion
3.1. Resin Decomposition Ratio of the Carbon Fiber and Surface Observation Results of Recycled Carbon Fiber
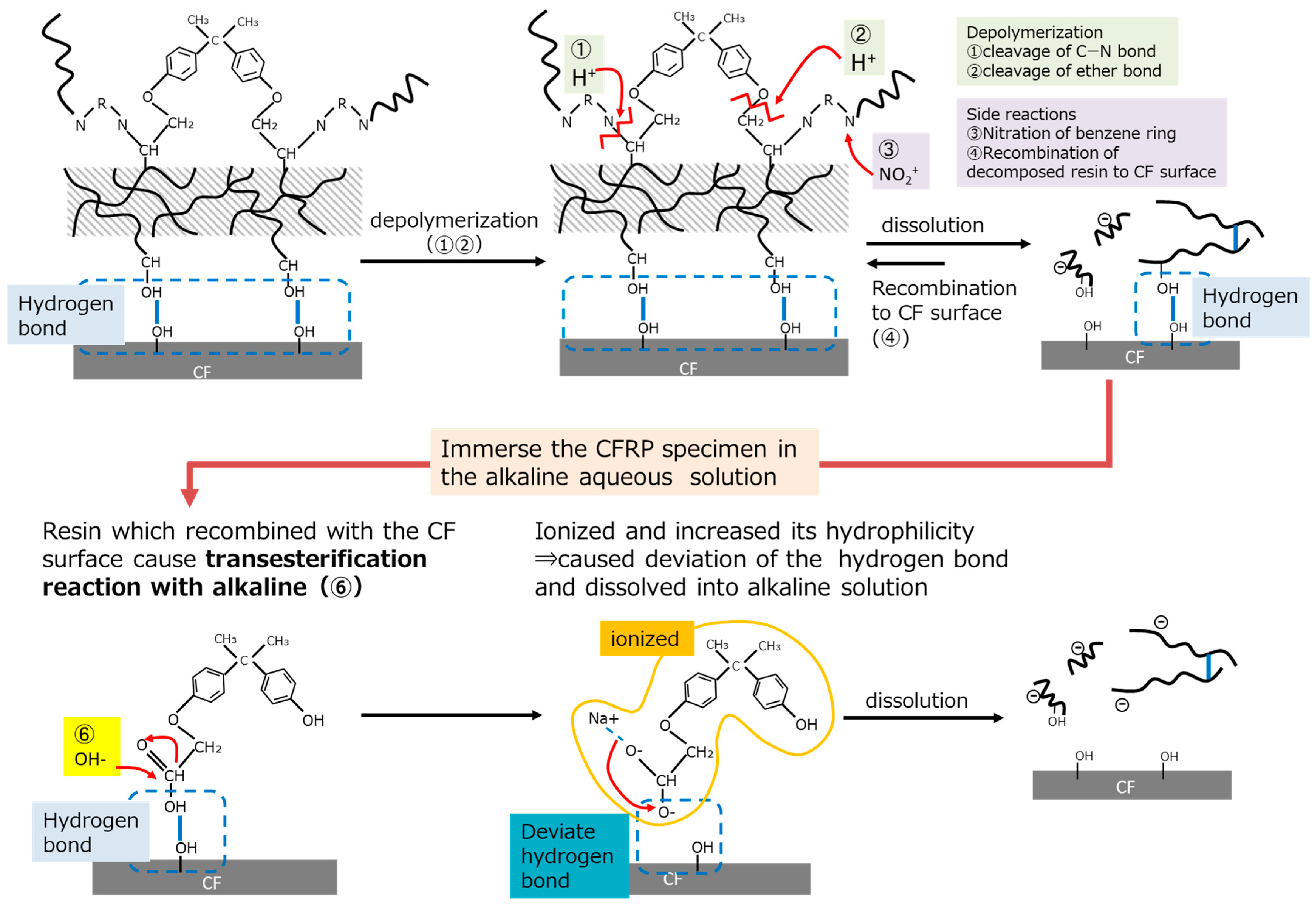
3.2. Consideration of the Mechanism to Remove Resin from the Carbon Fiber’s Surface by Using an Alkaline
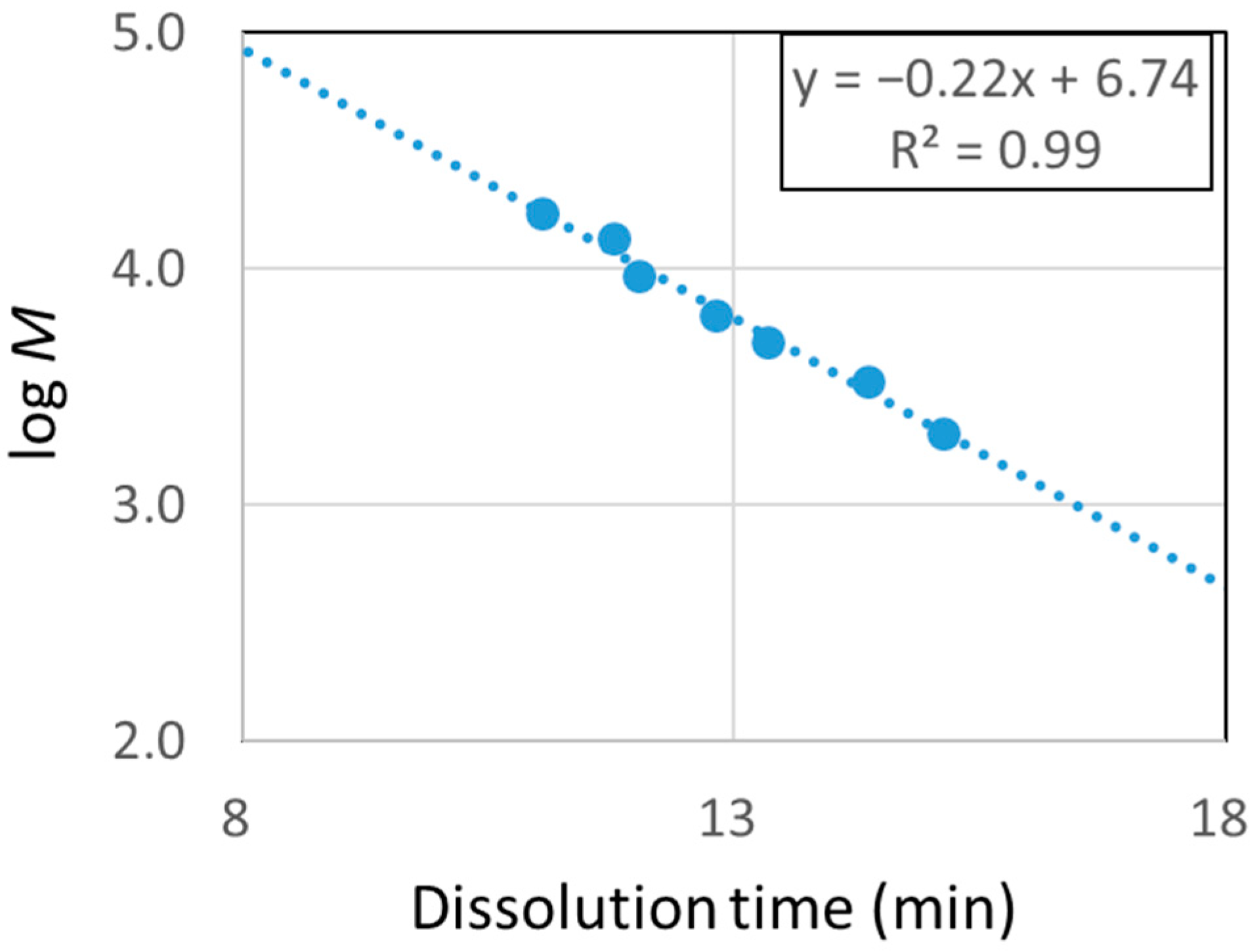
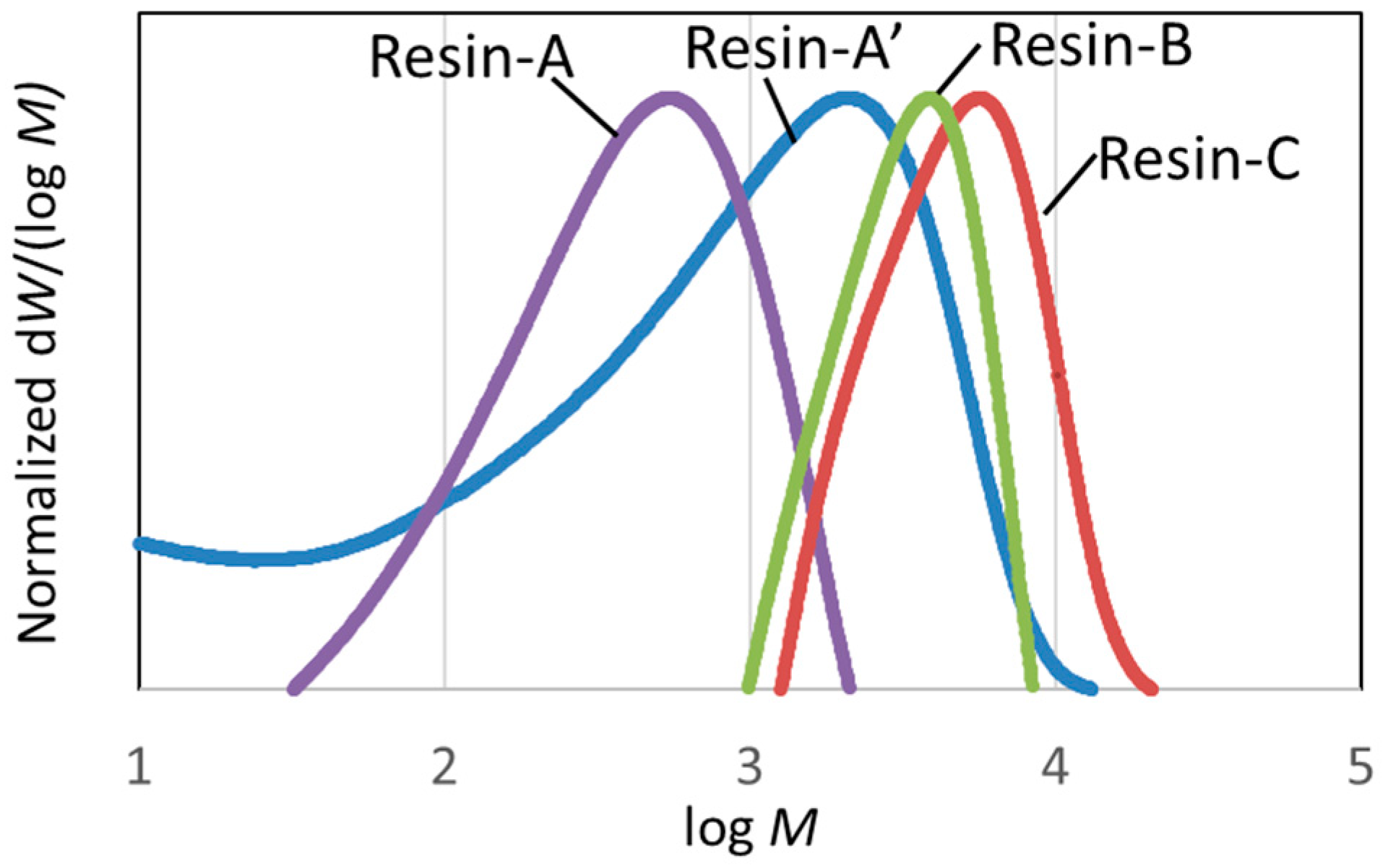
3.2.1. Molecular Weight Distribution of Decomposed Resin
3.2.2. FT-IR Measurement of Decomposed Resin
3.2.3. Resin Dissolution Mechanism Using an Alkaline
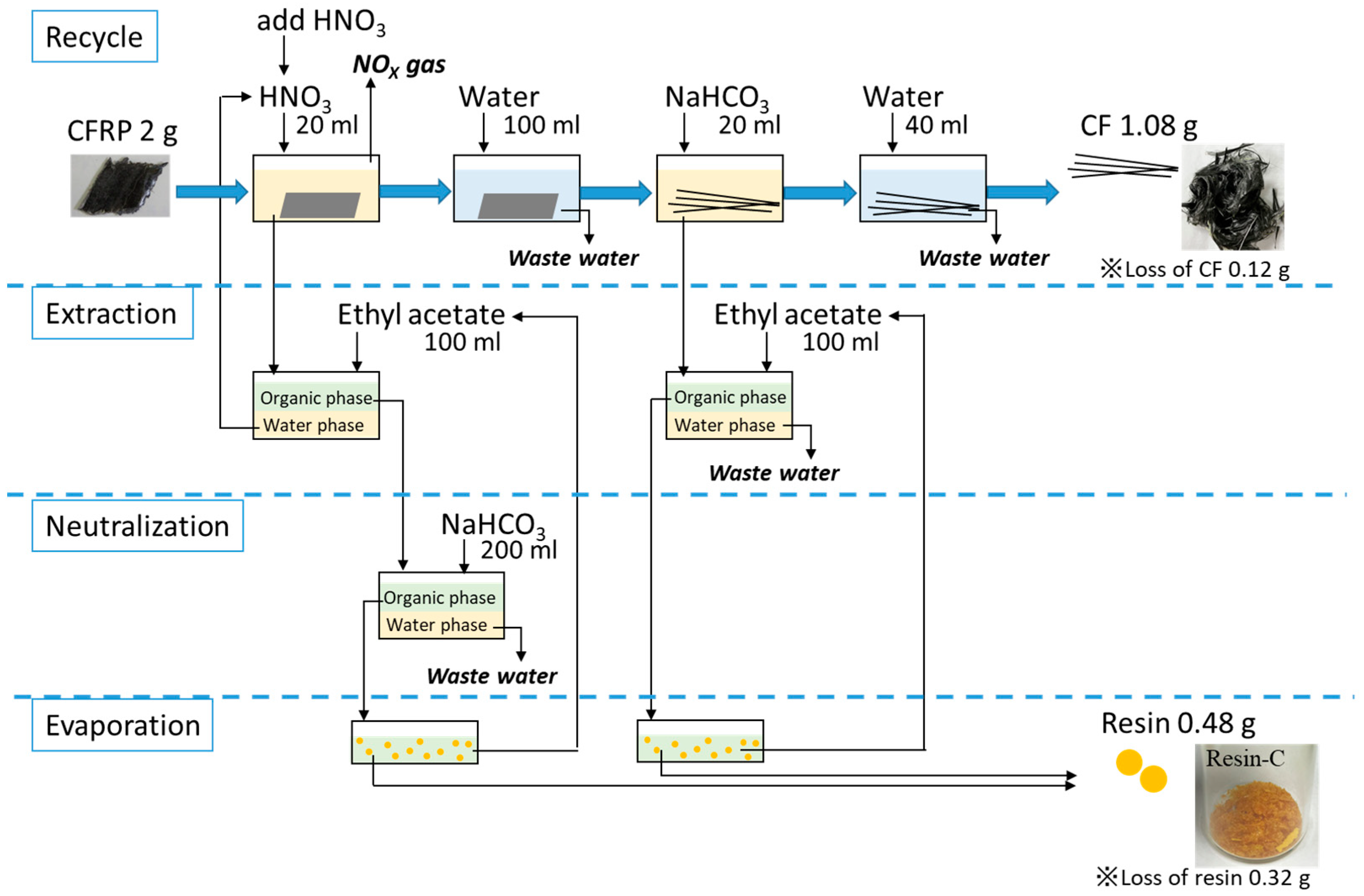
3.3. Mass Balance of the Input and Output Materials through Recycling Scheme- 4
3.4. Physical Properties of Carbon Fiber
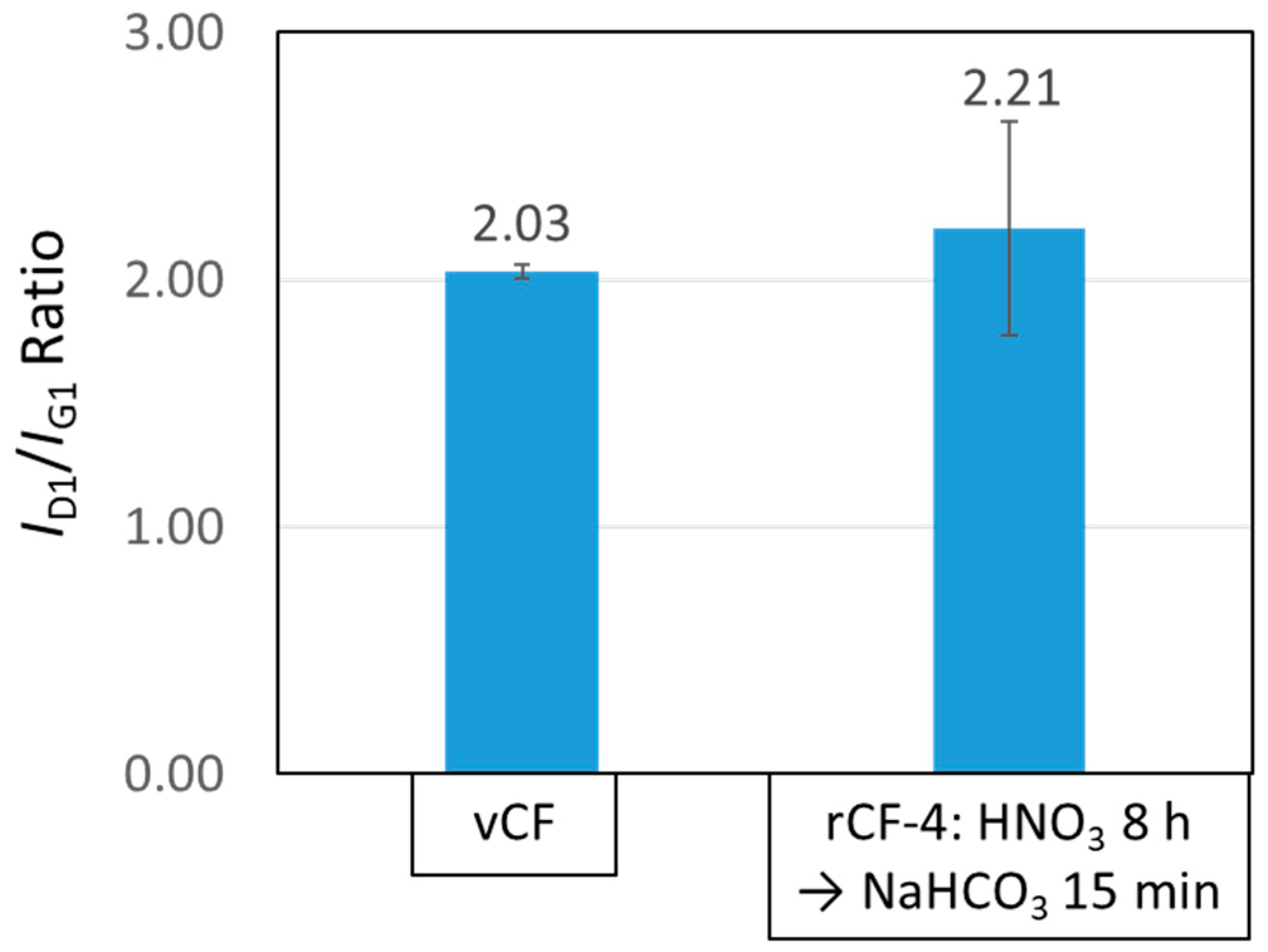
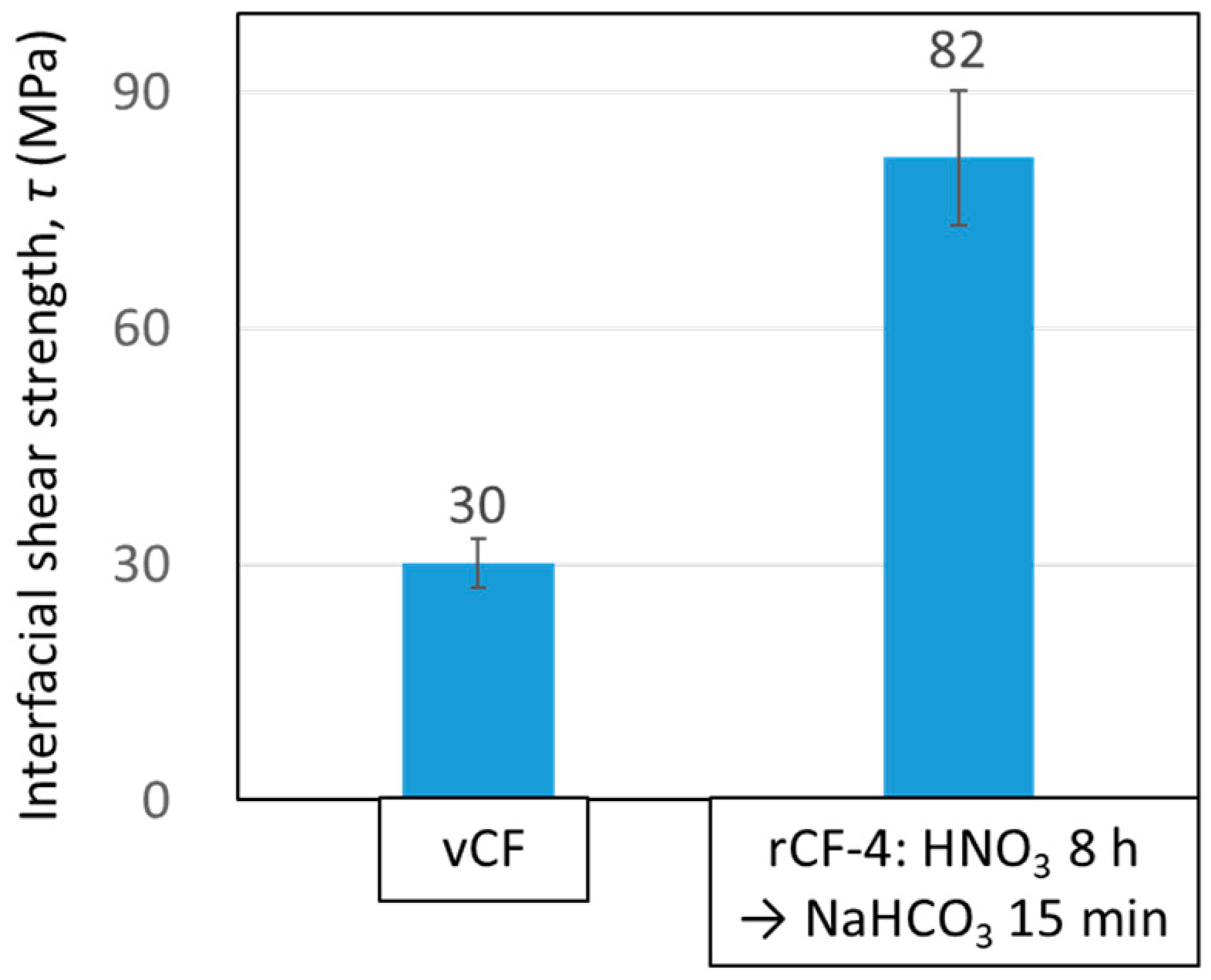
3.4.1. Interfacial Shear Strength between Carbon Fiber and Resin
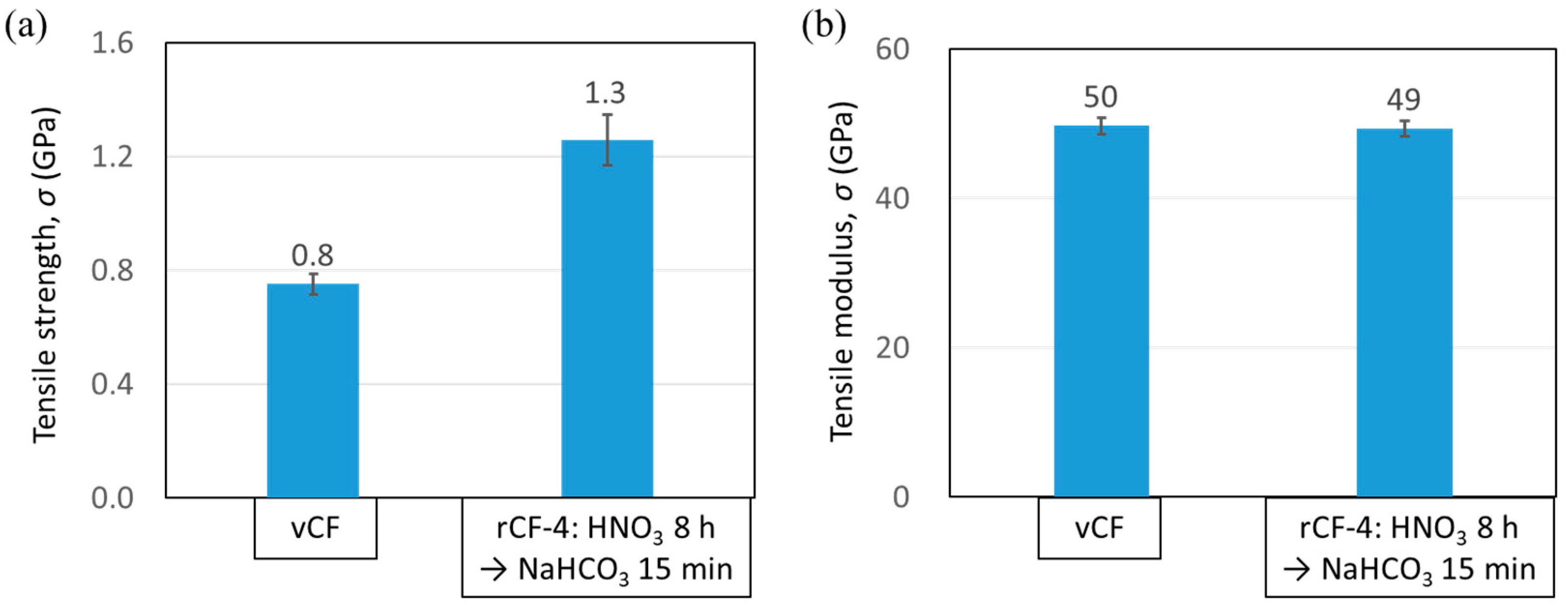
3.4.2. Tensile Strength of Carbon Fiber
4. Conclusions
- (1)
- A CFRP specimen immersed in nitric acid for 8 h followed by immersion in sodium hydrogen carbonate aqueous solution for 15 min resulted in a reduction in the recycling time, whereas it originally took 24 h.
- (2)
- This new recycling scheme was effective at quickly removing the epoxy resin from the CF surface, since the alkaline reacted with resin and dissolved it in an aqueous solution even if the epoxy resin remained in a long chain. Moreover, sodium hydrogen carbonate was the most effective alkaline to use because it produced carbon dioxide gas through the chemical reaction between sodium hydrogen carbonate and nitric acid, which helped removing the resin physically by the carbon dioxide gas bubbles. This was the first research that elucidated this mechanism.
- (3)
- The physical properties of rCFs, such as the fiber strength as well as the interfacial shear strength between the CF and the resin, which were recovered through this new recycling scheme expressed higher value than those of vCFs.5. Finally, the abovementioned findings show that the recycling method using sodium hydrogen carbonate, which can be safely handled, is an effective recycling method that reduces recycling time and enables the recovery of high-quality rCFs.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Recycling Scheme | 1st Step | 2nd Step | Recycled Carbon Fiber | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type of Solution | Conc. | Immersion Time | Type of Solution | Conc. | Immersion Time | Additives | Conc. | Immersion Time | Type of rCF | Rd (mass%) | |
| Scheme-1 | HNO3 | 8 M | 5 h | rCF-1 | 61.0 | ||||||
| 8 h | 75.8 | ||||||||||
| 9 h | 80.1 | ||||||||||
| 12 h | 89.1 | ||||||||||
| 24 h | 94.9 | ||||||||||
| 48 h | 95.3 | ||||||||||
| Scheme-2 | HNO3 | 8 M | 8 h | HNO3 | 8 M | 2 h | rCF-2 | 77.9 | |||
| 4 h | 82.8 | ||||||||||
| 6 h | 88.1 | ||||||||||
| 8 h | 94.8 | ||||||||||
| 16 h | 95.2 | ||||||||||
| Scheme-3 | HNO3 | 8 M | 8 h | NaOH | pH 12 | 1 min | rCF-3 | 91.3 | |||
| 10 min | 98.3 | ||||||||||
| pH 10 | 1 min | 96.4 | |||||||||
| pH 8 | 1 min | 81.3 | |||||||||
| 10 min | 82.0 | ||||||||||
| 20 min | 92.4 | ||||||||||
| 60 min | 96.6 | ||||||||||
| Scheme-4 | HNO3 | 8 M | 8 h | NaHCO3 | 0.1 M | 1 min | rCF-4 | 81.3 | |||
| 5 min | 89.0 | ||||||||||
| 10 min | 92.5 | ||||||||||
| 15 min | 96.4 | ||||||||||
| 20 min | 98.5 | ||||||||||
| 30 min | 99.6 | ||||||||||
| 0.01 M | 90 min | 96.6 | |||||||||
| Scheme-5 | HNO3 | 8 M | 8 h | NaHCO3 | 0.01 M | - | SDS | 0.10% | 10 min | rCF-5 | 85.0 |
| 60 min | 99.0 | ||||||||||
| 1.0% | 10 min | 97.0 | |||||||||
| PEG | 0.10% | 60 min | 95.9 | ||||||||
| 1.0% | 10 min | 97.6 | |||||||||
| PEG | 0.10% | 60 min | 96.9 | ||||||||
| 1.0% | 10 min | 99.7 | |||||||||
| SDS, PEG | 0.1% each | 1 min | 92.6 | ||||||||
| 0.1% each | 5 min | 91.5 | |||||||||
| 0.1% each | 10 min | 96.9 | |||||||||
| Cationic surfactant | 0.10% | 60 min | 85.4 | ||||||||
| 1.0% | 10 min | 97.9 | |||||||||
| Scheme-6 | HNO3 | 8 M | 8 h | - | SDS | 0.10% | 10 min | rCF-6 | 81.5 | ||
| 60 min | 81.6 | ||||||||||
| 0.30% | 20 min | 80.1 | |||||||||
| PEG | 0.10% | 60 min | 81.8 | ||||||||
| PEG | 0.10% | 60 min | 82.5 | ||||||||
| vCF | rCF-4 | ||
|---|---|---|---|
| C (%) | CHn | 45.1 | 35.8 |
| -C-OH, -C-O-C | 27.8 | 28.5 | |
| >C=O | 2.8 | 3.3 | |
| -CO-O- | 0.8 | 1.6 | |
| -C=C- | 0.7 | 0.8 | |
| Total | 77.2 | 70.1 | |
| N (%) | -NH2 | 0.0 | 3.3 |
| -NO/NH4 | 1.2 | 0.9 | |
| -NO2 | 0.0 | 4.1 | |
| Total | 1.2 | 8.3 | |
| O (%) | 19.5 | 21.5 | |
| Others (%) | 1.9 | 0.2 | |
Appendix B
References
- Alam, P.; Mamalis, D.; Robert, C.; Floreani, C.; Brádaigh, C.M.Ó. The fatigue of carbon fibre reinforced plastics—A review. Compos. Part B Eng. 2019, 166, 555–579. [Google Scholar] [CrossRef]
- Yano Research Institute Ltd. Global Automotive CFRP (Carbon-Fiber-Reinforced Plastics) Market Forecast 2021; Yano Research Institute Ltd.: Tokyo, Japan, 2021; pp. 27–38. [Google Scholar]
- Kishi, H. Introduction to FRP constituent materials Chapter 2 Constituent materials and types—Epoxy resin. J. Jpn. Soc. Compos. Mater. 2007, 33, 232–237. [Google Scholar] [CrossRef]
- Li, X.; Bai, R.; McKechnie, J. Environmental and financial performance of mechanical recycling of carbon fibre reinforced polymers and comparison with conventional disposal routes. J. Clean. Prod. 2016, 127, 451–460. [Google Scholar] [CrossRef]
- Yano Research Institute Ltd. FY 2015 Annual Report: New Energy and Industrial Technology Development Organization, Study of Recycling Technologies for Carbon Fiber Composite Materials Used in Automobiles: Innovative New Structural Materials R&D Project; Yano Research Institute Ltd.: Tokyo, Japan, 2016; pp. 10–21. [Google Scholar]
- Kanemasu, M.; Nishimura, A.; Yoshikawa, A.; Yamamori, T.; Kobayashi, R. Environmental Impact Reduction by Recycling of Composite in Aircraft Manufacturing. Mitsubishi Heavy Ind. Tech. Rev. 2018, 55, 1–5. Available online: https://www.mhi.co.jp/technology/review/pdf/552/552004.pdf (accessed on 1 October 2023).
- Yano Research Institute Ltd. Market Forecast and Strategy of Recycled Carbon-Fiber-Reinforced Plastics (2023); Yano Research Institute Ltd.: Tokyo, Japan, 2023; p. 20. [Google Scholar]
- Hale, R.C.; Seeley, M.E.; Guardia, M.J.L.; Mai, L.; Zeng, E.Y. A Global Perspective on Microplastics. JGR Oceans 2020, 125, 1. [Google Scholar] [CrossRef]
- Dang, W.; Kubouchi, M.; Yamamoto, S.; Sembokuya, H.; Tsuda, K. An Approach to Chemical Recycling of Epoxy Resin Cured with Amine Using Nitric Acid. Polymer 2002, 43, 2953–2958. [Google Scholar] [CrossRef]
- Hanaoka, T.; Ikematsu, H.; Takahashi, S.; Ito, N.; Ijuin, N.; Kawada, H.; Arao, Y.; Kubouchi, M. Recovery of Carbon Fiber from Prepreg Using Nitric Acid and Evaluation of Recycled CFRP. Compos. Part B 2022, 231, 109560. [Google Scholar] [CrossRef]
- Sakai, A.; Kurniawan, W.; Kubouchi, M. Recycled Carbon Fibers with Improved Physical Properties Recovered from CFRP by Nitric Acid. Appl. Sci. 2023, 13, 3957. [Google Scholar] [CrossRef]
- Li, J.; Xu, P.L.; Zhu, Y.K.; Ding, J.P. A promising strategy for chemical recycling of carbon fiber/thermoset composites: Self-accelertating decomposition in a mild oxidative system. Green Chem. 2012, 14, 3260–3263. [Google Scholar] [CrossRef]
- Xu, P.; Li, J.; Ding, J. Chemical recycling of carbon fibre/epoxy composites in a mixed solution of peroxide hydrogen and N,N-dimethylformamide. Compos. Sci. Technol. 2013, 82, 54–59. [Google Scholar] [CrossRef]
- Sharifi, M.; Ghorpade, K.A.; Raman, V.I.; Palmese, G.R. Sythisis and Swelling Behavior of Highly Porous Epoxy Polymers. ACS Omega 2020, 5, 31011–31018. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, M.; Guo, X.; Liu, C.; Liu, T.; Xin, J.; Zhang, J. Mild chemical recycling of aerospace fiber/epoxy composite wastes and utilization of the decomposed resin. Polym. Degrad. Stab. 2017, 139, 20–27. [Google Scholar] [CrossRef]
- Das, M.; Varughese, S. A novel sonochemical approach for enhanced recovery of carbon fiber from CFRP waste using mild acid-peroxide mixture. ACS Sustain. Chem. Eng. 2016, 4, 2080–2087. [Google Scholar] [CrossRef]
- Das, M.; Chacko, R.; Varughese, S. An Efficient Method of Recycling of CFRP waste Using Peracetic Acid. ACS Sustain. Chem. Eng. 2018, 6, 1564–1571. [Google Scholar] [CrossRef]
- Zabihi, O.; Ahmadi, M.; Liu, C.; Mahmoodi, R.; Li, Q.; Naebe, M. Development of a low cost and green microwave assisted approach towards the circular carbon fibre composites. Compos. Part B 2020, 184, 107750. [Google Scholar] [CrossRef]
- Tian, F.; Wang, X.L.; Yang, Y.; An, W.; Zhao, X.; Xu, S.; Wang, Y.Z. Energy-Efficient Conversion of Amine-Cured Epoxy Resins into Functional Chemicals Based on Swelling-Induced Nanopores. ACS Sustain. Chem. Eng. 2020, 8, 2226–2235. [Google Scholar] [CrossRef]
- Lin, H.R.; Sung, K.C. Carbopol/pluronic phase change solutions for opthalmic drug delivery. J. Control. Release 2000, 69, 379–388. [Google Scholar] [CrossRef]
- Kang, S.K.; Lee, D.B.; Choi, N.S. Fiber/Epoxy Interfacial Shear Strength Measured by the Microdroplet Test. Compos. Sci. Technol. 2009, 69, 245–251. [Google Scholar] [CrossRef]
- Glazer, J. Monolayer studies of some ethoxylin resin adhesives and related compounds. J. Polym. Sci. 1954, 13, 355–369. [Google Scholar] [CrossRef]
- Hanaoka, T.; Arao, Y.; Kayaki, Y.; Kuwata, S.; Kubouchi, M. Analysis of nitric acid decomposition of epoxy resin network structures for chemical recycling. Polym. Degrad. Stab. 2021, 186, 109537. [Google Scholar] [CrossRef]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment II: Hybrid method. Adv. Environ. Res. 2004, 8, 553–597. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakai, A.; Kurniawan, W.; Kubouchi, M. Chemical Recycling of CFRP in an Environmentally Friendly Approach. Polymers 2024, 16, 143. https://doi.org/10.3390/polym16010143
Sakai A, Kurniawan W, Kubouchi M. Chemical Recycling of CFRP in an Environmentally Friendly Approach. Polymers. 2024; 16(1):143. https://doi.org/10.3390/polym16010143
Chicago/Turabian StyleSakai, Asuka, Winarto Kurniawan, and Masatoshi Kubouchi. 2024. "Chemical Recycling of CFRP in an Environmentally Friendly Approach" Polymers 16, no. 1: 143. https://doi.org/10.3390/polym16010143
APA StyleSakai, A., Kurniawan, W., & Kubouchi, M. (2024). Chemical Recycling of CFRP in an Environmentally Friendly Approach. Polymers, 16(1), 143. https://doi.org/10.3390/polym16010143






