Biomimetic Materials Based on Poly-3-hydroxybutyrate and Chlorophyll Derivatives
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
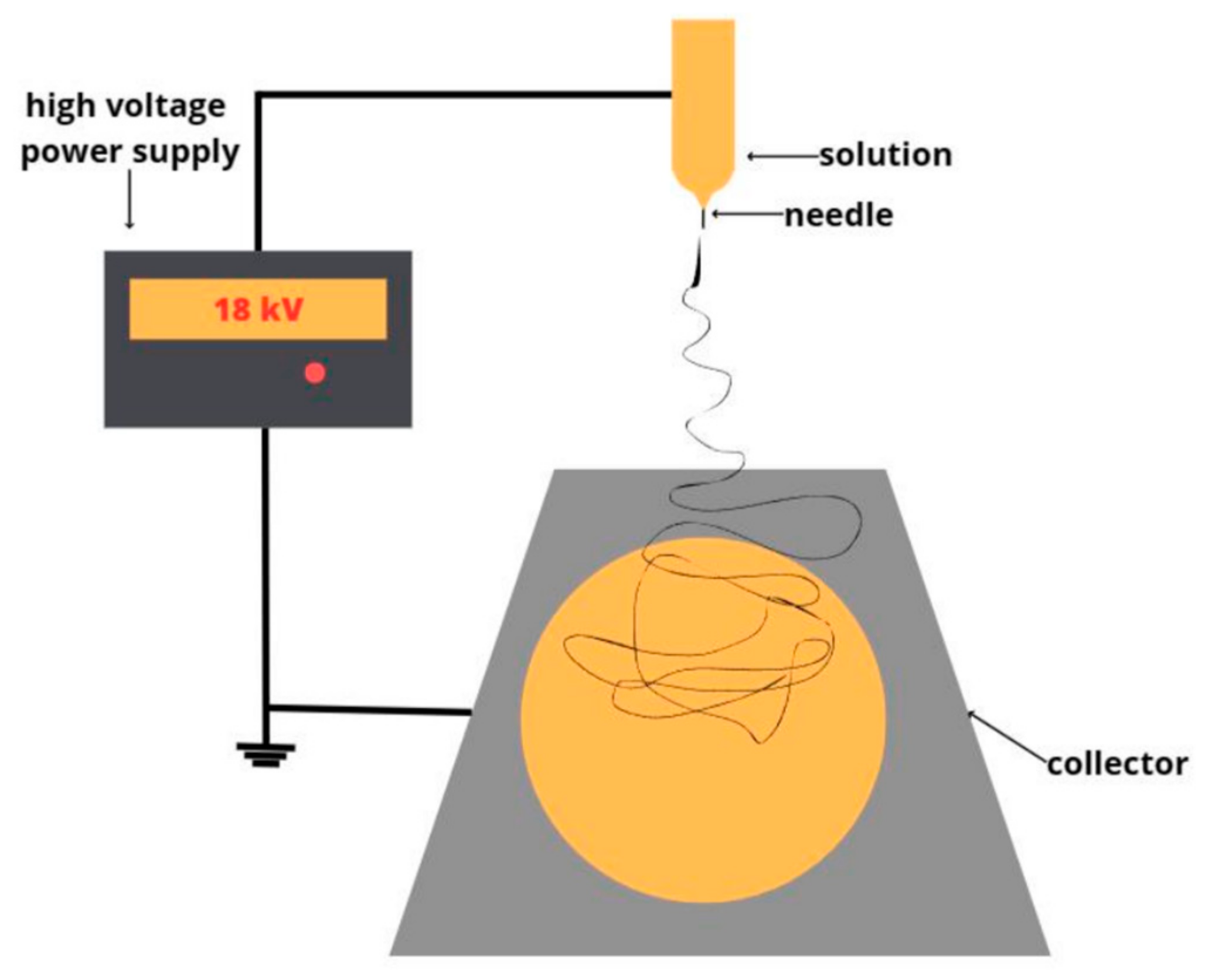
2.2.1. Obtaining of Nonwoven Fibrous Materials
2.2.2. Scanning Electron Microscopy (SEM)
2.2.3. Differential Scanning Calorimetry (DSC)
2.2.4. Fourier-Transform Infrared Spectroscopy (FTIR)
2.2.5. Wettability
2.2.6. Air Permeability
2.2.7. Antimicrobial Properties
2.2.8. Mechanical Properties
3. Results
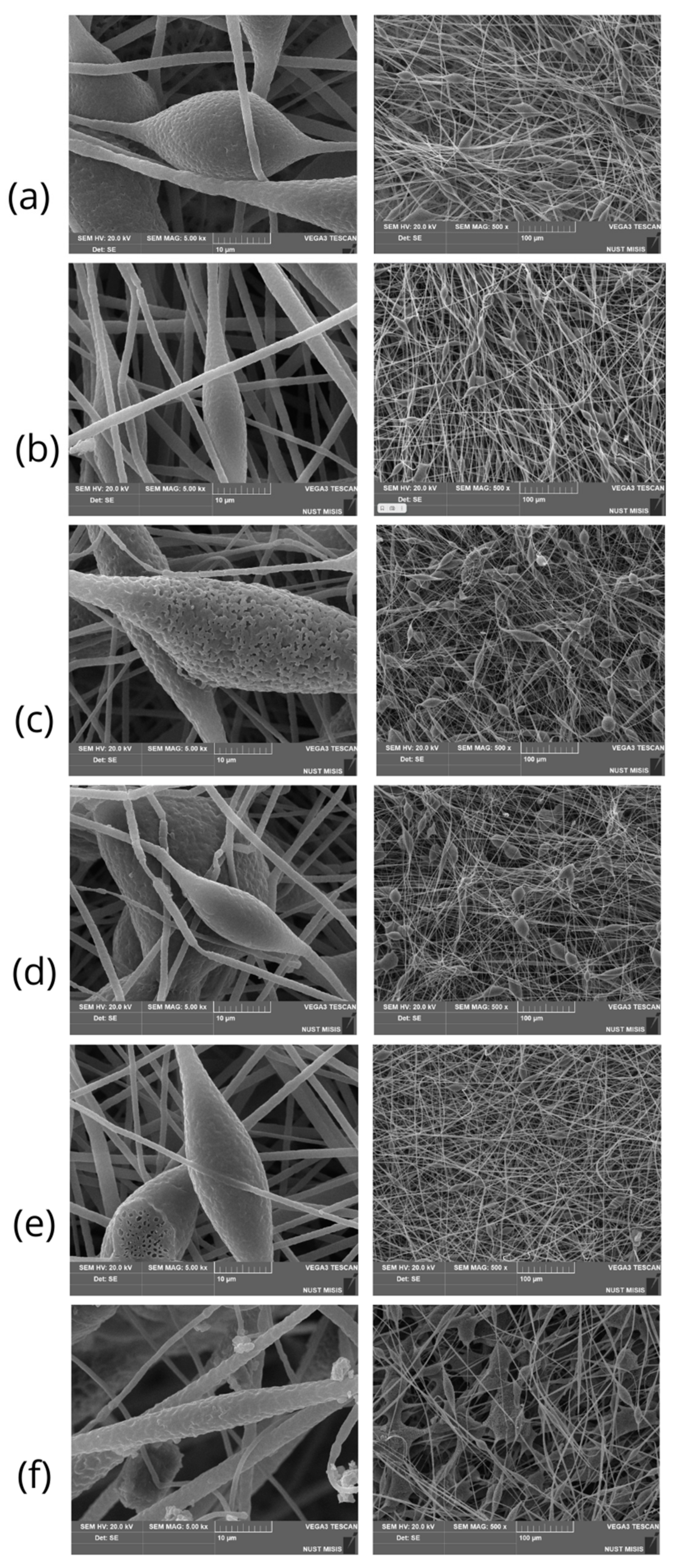
3.1. Morphology of PHB/Chlorophyll Derivatives Electrospun Materials
3.2. Chemical Structure PHB/Chlorophyll Derivatives Electrospun Materials
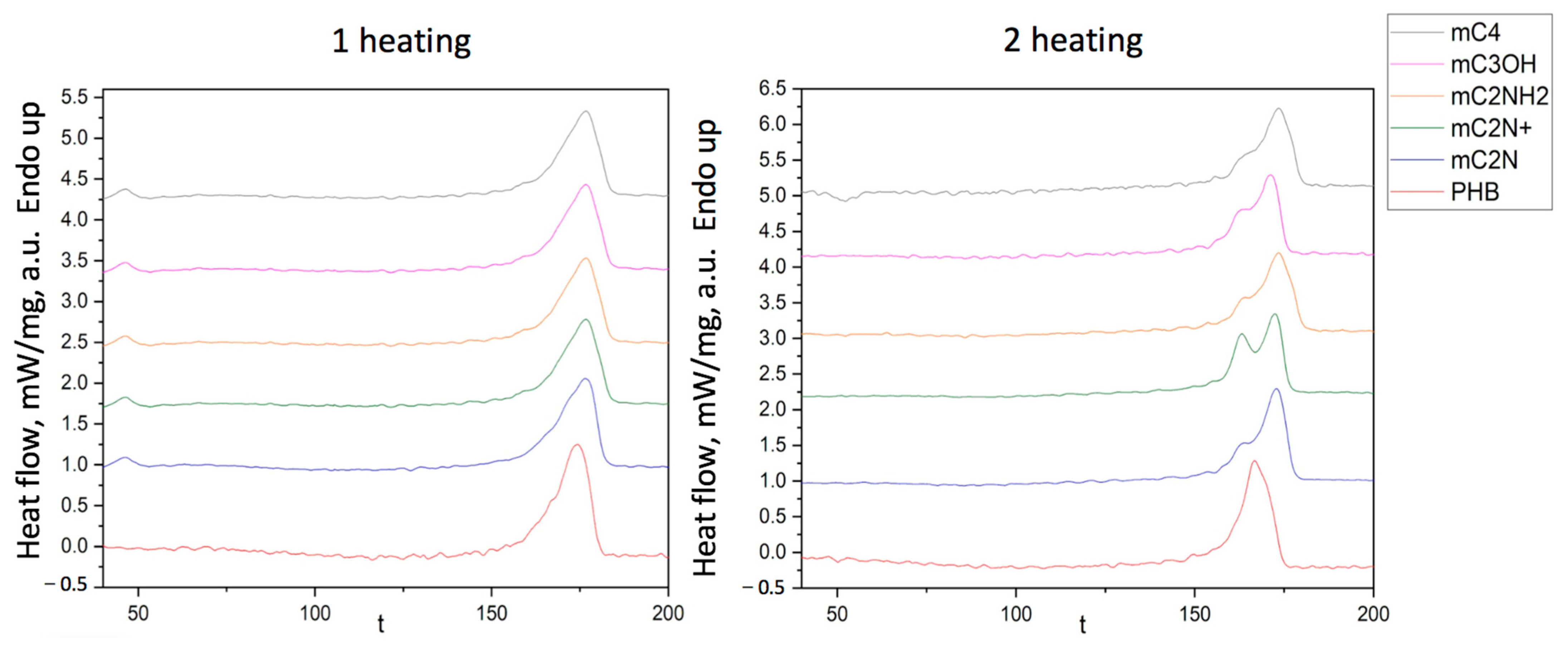
3.3. Thermophysical Characteristics of PHB/Chlorophyll Derivatives Electrospun Materials
3.4. Wettability of PHB/Chlorophyll Derivatives Electrospun Materials
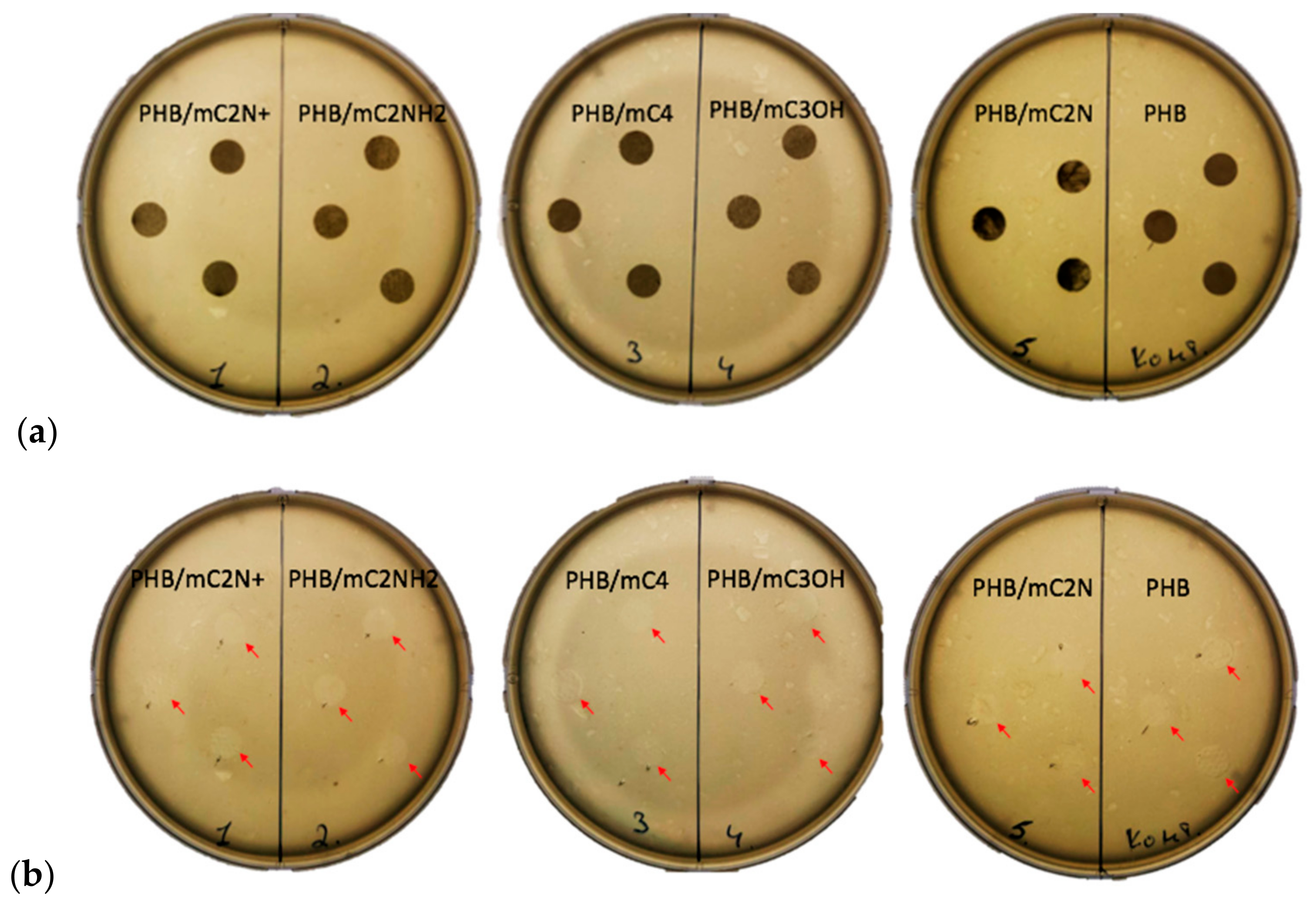
3.5. Antimicrobial Properties of PHB/Chlorophyll Derivatives Electrospun Materials
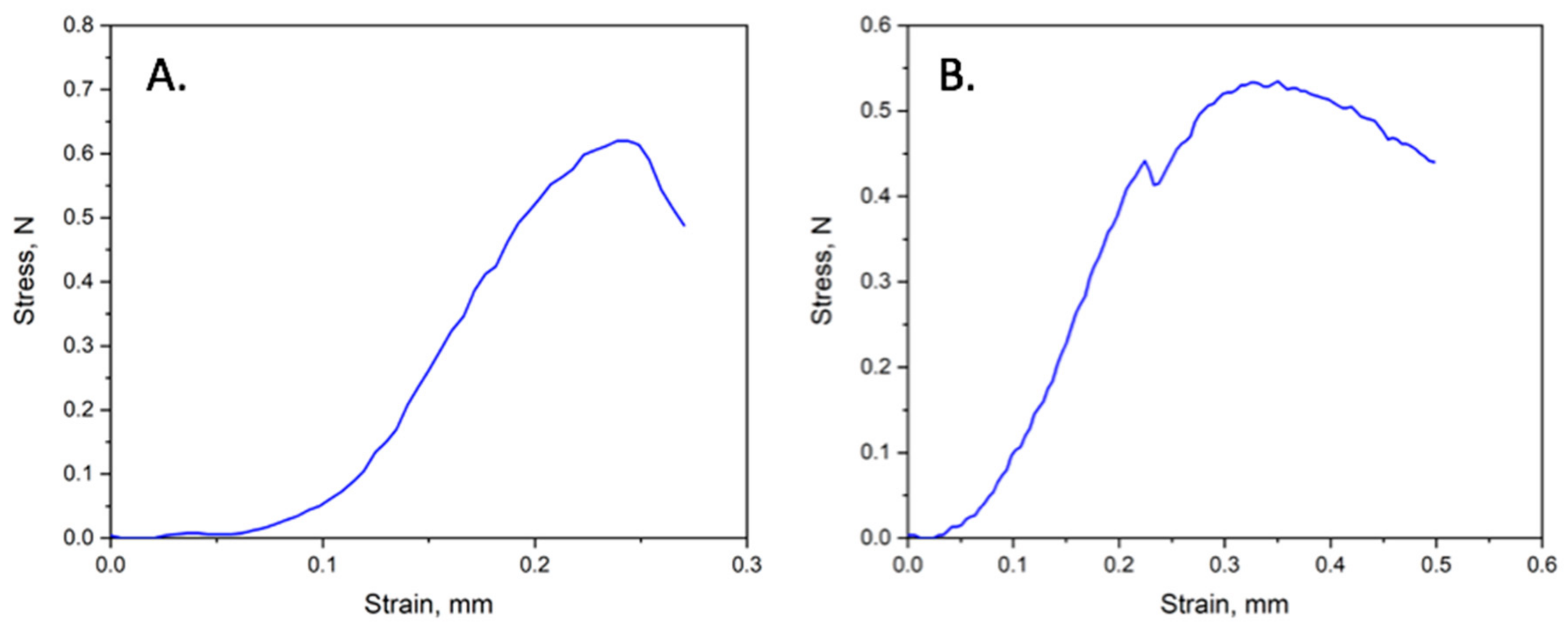
3.6. Mechanical Properties of PHB/Chlorophyll Derivatives Electrospun Materials
4. Discussion
5. Conclusions
- mC4, mC3OH, mC2NH2, and mC2N+ enable the decreasing of the average diameters of the electrospun materials by 25–40% and increase air permeability by 600–1040%. This can be an excellent achievement when planning highly porous permeable materials with membrane properties.
- The introduction of additives clearly accelerated the crystallization of the polymer, reducing the degree of crystallinity of PHB by 6–10%, and also had an effect on the state of the amorphous phase. Reducing the crystalline phase of natural polyesters such as PHB allows for the control of the rate of their bioresorption and biodegradation, which can be a valuable advantage when planning deliveries with a controlled life span.
- mC3OH enabled the significant hydrophobization of the surfaces of the nonwoven material. As an addition to the proportion of open pores, this aspect is important for planning deliveries to areas where it is important to increase cell proliferation by attaching them to a more hydrophilic surface.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pucci, C.; Martinelli, C.; Degl’Innocenti, A.; Desii, A.; De Pasquale, D.; Ciofani, G. Light-Activated Biomedical Applications of Chlorophyll Derivatives. Macromol. Biosci. 2021, 21, 19. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Ramzan, M.; Qureshi, A.K.; Khan, M.A.; Tariq, M. Emerging Applications of Porphyrins and Metalloporphyrins in Biomedicine and Diagnostic Magnetic Resonance Imaging. Biosensors 2018, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Tsolekile, N.; Nelana, S.; Oluwafemi, O.S. Porphyrin as Diagnostic and Therapeutic Agent. Molecules 2019, 24, 2669. [Google Scholar] [CrossRef] [PubMed]
- Lukyanets, E.A. The key role of peripheral substituents in the chemistry of phthalocyanines and their analogs. J. Porphyr. Phthalocyanines 2010, 14, 40. [Google Scholar] [CrossRef]
- Staron, J.; Boron, B.; Karcz, D.; Szczygieł, M.; Fiedor, L. Recent Progress in Chemical Modifications of Chlorophylls and Bacteriochlorophylls for the Applications in Photodynamic Therapy. Curr. Med. Chem. 2015, 22, 74. [Google Scholar] [CrossRef]
- Chen, M. Chlorophyll modifications and their spectral extension in oxygenic photosynthesis. Annu. Rev. Biochem. 2014, 83, 40. [Google Scholar] [CrossRef]
- Perez-Galvez, A.; Viera, I.; Roca, M. Chemistry in the Bioactivity of Chlorophylls: An Overview. Curr. Med. Chem. 2017, 24, 4515–4536. [Google Scholar] [CrossRef]
- Negishi, T.; Rai, H.; Hayatsu, H. Antigenotoxic activity of natural chlorophylls. Mutat. Res. 1997, 12, 97–100. [Google Scholar] [CrossRef]
- Solymosi, K.; Mysliwa-Kurdziel, B. Chlorophylls and their Derivatives Used in Food Industry and Medicine. Mini-Rev. Med. Chem. 2017, 17, 1194–1222. [Google Scholar] [CrossRef]
- Hoober, J.K.; Eggink, L.L.; Chen, M. Chlorophylls, ligands and assembly of light-harvesting complexes in chloroplasts. Photosynth. Res. 2007, 94, 387–400. [Google Scholar] [CrossRef]
- Pogorilyy, V.; Ostroverkhov, P.; Efimova, V.; Plotnikova, E.; Bezborodova, O.; Diachkova, E.; Vasil’ev, Y.; Pankratov, A.; Grin, M. Thiocarbonyl Derivatives of Natural Chlorins: Synthesis Using Lawesson’s Reagent and a Study of Their Properties. Molecules 2023, 20, 4215. [Google Scholar] [CrossRef] [PubMed]
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural Colorants: Food Colorants from Natural Sources. Annu. Rev. Food Sci. Technol. 2017, 28, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Wei, Y.; Wu, B.; Chen, Q.; Xing, D. Pyropheophorbide A and c(RGDyK) comodified chitosan-wrapped upconversion nanoparticle for targeted near-infrared photodynamic therapy. Mol. Pharm. 2012, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, T.J. Photosensitizers: Therapy and detection of malignant tumors. Photochem. Photobiol. 1987, 45, 89. [Google Scholar] [CrossRef] [PubMed]
- Thanasekaran, P.; Chu, C.H.; Wang, S.B.; Chen, K.Y.; Gao, H.D.; Lee, M.M.; Sun, S.S.; Li, J.P.; Chen, J.Y.; Chen, J.K.; et al. Lipid-Wrapped Upconversion Nanoconstruct/Photosensitizer Complex for Near-Infrared Light-Mediated Photodynamic Therapy. ACS Appl. Mater. Interfaces 2019, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Moan, J.; Nesland, J.M. Correlation of subcellular and intratumoral photosensitizer localization with ultrastructural features after photodynamic therapy. Ultrastruct. Pathol. 1996, 20, 109–129. [Google Scholar] [CrossRef] [PubMed]
- Pavani, C.; Uchoa, A.F.; Oliveira, C.S.; Iamamoto, Y.; Baptista, M.S. Effect of zinc insertion and hydrophobicity on the membrane interactions and PDT activity of porphyrin photosensitizers. Photochem. Photobiol. Sci. 2009, 8, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Schweizer, H.P. Bacterial resistance to antibiotics: Active efflux and reduced uptake. Adv. Drug Deliv. Rev. 2005, 57, 1486–1513. [Google Scholar] [CrossRef]
- Wang, E.; Braun, M.S.; Wink, M. Chlorophyll and Chlorophyll Derivatives Interfere with Multi-Drug Resistant Cancer Cells and Bacteria. Molecules 2019, 24, 2968. [Google Scholar] [CrossRef]
- Chu, M.; Li, H.; Wu, Q.; Wo, F.; Shi, D. Pluronic-encapsulated natural chlorophyll nanocomposites for in vivo cancer imaging and photothermal/photodynamic therapies. Biomaterials 2014, 35, 8357–8373. [Google Scholar] [CrossRef]
- Ferreira, D.P.; Conceição, D.S.; Calhelha, R.C.; Sousa, T.; Socoteanu, R.; Ferreira, I.C.F.R.; Vieira Ferreira, L.F. Porphyrin dye into biopolymeric chitosan films for localized photodynamic therapy of cancer. Carbohydr. Polym. 2016, 20, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.F.V.; Machado, I.F.; Gama, A.; Socoteanu, R.P.; Boscencu, R.; Manda, G.; Calhelha, R.C.; Ferreira, I.C.F.R. Photochemical/Photocytotoxicity Studies of New Tetrapyrrolic Structures as Potential Candidates for Cancer Theranostics. Curr. Drug Discov. Technol. 2020, 7, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Xu, X.; Zhang, X.; Yu, Y.; Wang, X. Photodynamic Treatment of Colorectal Cancer Using Chlorin e6-Loaded Poly(lactide-co-glycolide)-Based Nanoparticles. J. Biomed. Nanotechnol. 2021, 1, 1939–1950. [Google Scholar] [CrossRef] [PubMed]
- Mollaeva, M.R.; Nikolskaya, E.; Beganovskaya, V.; Sokol, M.; Chirkina, M.; Obydennyi, S.; Belykh, D.; Startseva, O.; Mollaev, M.D.; Yabbarov, N. Oxidative Damage Induced by Phototoxic Pheophorbide a 17-Diethylene Glycol Ester Encapsulated in PLGA Nanoparticles. Antioxidants 2021, 10, 1985. [Google Scholar] [CrossRef] [PubMed]
- McAdam, B.; Brennan Fournet, M.; McDonald, P.; Mojicevic, M. Production of Polyhydroxybutyrate (PHB) and Factors Impacting Its Chemical and Mechanical Characteristics. Polymers 2020, 4, 2908. [Google Scholar] [CrossRef] [PubMed]
- Tyubaeva, P.M.; Varyan, I.A.; Zykova, A.K.; Yarysheva, A.Y.; Ivchenko, P.V.; Olkhov, A.A.; Arzhakova, O.V. Bioinspired Electropun Fibrous Materials Based on Poly-3-Hydroxybutyrate and Hemin: Preparation, Physicochemical Properties, and Weathering. Polymers 2022, 12, 4878. [Google Scholar] [CrossRef] [PubMed]
- Kliem, S.; Kreutzbruck, M.; Bonten, C. Review on the Biological Degradation of Polymers in Various Environments. Materials 2020, 13, 4586. [Google Scholar] [CrossRef]
- Tyubaeva, P.M.; Varyan, I.A.; Nikolskaya, E.D.; Mollaeva, M.R.; Yabbarov, N.G.; Sokol, M.B.; Chirkina, M.V.; Popov, A.A. Biocompatibility and Antimicrobial Activity of Electrospun Fibrous Materials Based on PHB and Modified with Hemin. Nanomaterials 2023, 13, 236. [Google Scholar] [CrossRef]
- Dhilip Kumar, S.S.; Abrahamse, H. Biocompatible Nanocarriers for Enhanced Cancer Photodynamic Therapy Applications. Pharmaceutics 2021, 13, 1933. [Google Scholar] [CrossRef]
- Yu, Z.; Zhou, P.; Pan, W.; Li, N.; Tang, B. A biomimetic nanoreactor for synergistic chemiexcited photodynamic therapy and starvation therapy against tumor metastasis. Nat. Commun. 2018, 9, 5044. [Google Scholar] [CrossRef]
- Hwang, J.; Jeong, Y.; Park, J.M.; Lee, K.H.; Hong, J.W.; Choi, J. Biomimetics: Forecasting the future of science, engineering, and medicine. Int. J. Nanomed. 2015, 10, 5701–5713. [Google Scholar] [CrossRef]
- Zou, Q.; Bao, J.; Yan, X. Functional Nanomaterials Based on Self-Assembly of Endogenic NIR-Absorbing Pigments for Diagnostic and Therapeutic Applications. Small Methods 2022, 6, 2101359. [Google Scholar] [CrossRef]
- He, P.; Yang, G.; Zhu, D.; Kong, H.; Corrales-Ureña, Y.R.; Ciacchi, L.C.; Wei, G. Biomolecule-mimetic nanomaterials for photothermal and photodynamic therapy of cancers: Bridging nanobiotechnology and biomedicine. J. Nanobiotechnol. 2022, 20, 483. [Google Scholar] [CrossRef] [PubMed]
- Woods, I.; Flanagan, T.C. Electrospinning of biomimetic scaffolds for tissue-engineered vascular grafts: Threading the path. Expert Rev. Cardiovasc. Ther. 2014, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Pramual, S.; Assavanig, A.; Bergkvist, M.; Batt, C.A.; Sunintaboon, P.; Lirdprapamongkol, K.; Svasti, J.; Niamsiri, N. Development and characterization of bio-derived polyhydroxyalkanoate nanoparticles as a delivery system for hydrophobic photodynamic therapy agents. J. Mater. Sci. Mater. Med. 2016, 27, 40. [Google Scholar] [CrossRef] [PubMed]
- Avossa, J.; Paolesse, R.; Di Natale, C.; Zampetti, E.; Bertoni, G.; De Cesare, F.; Scarascia-Mugnozza, G.; Macagnano, A. Electrospinning of Polystyrene/Polyhydroxybutyrate Nanofibers Doped with Porphyrin and Graphene for Chemiresistor Gas Sensors. Nanomaterials 2019, 17, 280. [Google Scholar] [CrossRef]
- Tyubaeva, P.; Varyan, I.; Lobanov, A.; Olkhov, A.; Popov, A. Effect of the Hemin Molecular Complexes on the Structure and Properties of the Composite Electrospun Materials Based on Poly(3-hydroxybutyrate). Polymers 2021, 21, 4024. [Google Scholar] [CrossRef]
- Ol’khov, A.A.; Tyubaeva, P.M.; Zernova, Y.N.; Kurnosov, A.S.; Karpova, S.G.; Iordanskii, A.L. Structure and Properties of Biopolymeric Fibrous Materials Based on Polyhydroxybutyrate-Metalloporphyrin Complexes. Russ. J. Gen. Chem. 2021, 91, 546–553. [Google Scholar] [CrossRef]
- Gushchina, O.I.; Grishina, M.Y.; Larkina, E.A.; Lebedeva, V.S.; Mironov, A.F. Synthesis of Hydroxyl Derivatives of Chlorin e6. Macroheterocycles 2017, 10, 81–83. [Google Scholar] [CrossRef]
- Belykh, D.V.; Kopylov, E.A.; Gruzdev, I.V.; Kuchin, A.V. Opening of the extra ring in pheophorbide a methyl ester by the action of amines as a one-step method for introduction of additional fragments at the periphery of chlorin macroring. Russ. J. Org. Chem. 2010, 46, 577–585. [Google Scholar] [CrossRef]
- Rybkin, A.Y.; Belik, A.Y.; Goryachev, N.S.; Mikhaylov, P.A.; Kraevaya, O.A.; Filatova, N.V.; Parkhomenko, I.I.; Peregudov, A.S.; Terent’ev, A.A.; Larkina, E.A.; et al. Self-assembling nanostructures of water-soluble fullerene [60]–chlorin e6 dyads: Synthesis, photophysical properties, and photodynamic activity. Dye. Pigment. 2020, 180, 108411. [Google Scholar] [CrossRef]
- Gushchina, O.I.; Larkina, E.A.; Mironov, A.F. Makrogeterotsikly. Macroheterocycles 2014, 7, 414. (In Russian) [Google Scholar] [CrossRef][Green Version]
- Scandola, M.; Focarete, M.L.; Adamus, G.; Sikorska, W.; Baranowska, I.; Świerczek, S.; Gnatowski, M.; Kowalczuk, M.; Jedliński, Z. Polymer Blends of Natural Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and a Synthetic Atactic Poly(3-hydroxybutyrate). Characterization and Biodegradation Studies. Macromolecules 1997, 30, 2568–2574. [Google Scholar] [CrossRef]
- Hantel, M.M.; Armstrong, M.J.; DaRosa, F.; l’Abee, R. Characterization of Tortuosity in Polyetherimide Membranes Based on Gurley and Electrochemical Impedance Spectroscopy. J. Electrochem. Soc. 2016, 164, 334–339. [Google Scholar] [CrossRef]
- Rogers, L.; Majer, F.; Sergeeva, N.N.; Paszko, E.; Gilmer, J.F.; Senge, M.O. Synthesis and biological evaluation of Foscan® bile acid conjugates to target esophageal cancer cells. Bioorganic Med. Chem. Lett. 2013, 23, 2495–2499. [Google Scholar] [CrossRef] [PubMed]
- Kano, A.; Taniwaki, Y.; Nakamura, I.; Shimada, N.; Moriyama, K.; Maruyama, A. Tumor delivery of Photofrin® by PLL-g-PEG for photodynamic therapy. J. Control. Release 2013, 167, 315–321. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, B.; Zhao, R.; Zhang, Q.; Kong, X. Multifunctional nanoparticles as photosensitizer delivery carriers for enhanced photodynamic cancer therapy. Mater. Sci. Eng. C 2020, 115, 111099. [Google Scholar] [CrossRef]
- Majerník, M.; Jendželovský, R.; Vargová, J.; Jendželovská, Z.; Fedorocko, P. Multifunctional Nanoplatforms as a Novel Effective Approach in Photodynamic Therapy and Chemotherapy, to Overcome Multidrug Resistance in Cancer. Pharmaceutics 2022, 14, 1075. [Google Scholar] [CrossRef]
- Olkhov, A.A.; Tyubaeva, P.M.; Zernova, Y.N.; Markin, V.S.; Kosenko, R.; Filatova, A.G.; Gasparyan, K.G.; Iordanskii, A.L. The Influence of Technological Factors and Polar Molecules on the Structure of Fibrillar Matrices Based on Ultrafine Poly-3-hydroxybutyrate Fibers Obtained via Electrospinning. Technologies 2023, 11, 118. [Google Scholar] [CrossRef]
- Behere, I.; Ingavle, G. In vitro and in vivo advancement of multifunctional electrospun nanofiber scaffolds in wound healing applications: Innovative nanofiber designs, stem cell approaches, and future perspectives. J. Biomed. Mater. Res. A 2022, 110, 443–461. [Google Scholar] [CrossRef]
- Thanh, N.H.; Olekhnovich, R.; Sitnikova, V.; Kremleva, A.; Snetkov, P.; Uspenskaya, M. PHB/PEG Nanofiber Mat Obtained by Electrospinning and Their Performances. Technologies 2023, 11, 48. [Google Scholar] [CrossRef]
- Borisova, I.; Stoilova, O.; Manolova, N.; Rashkov, I. Modulating the Mechanical Properties of Electrospun PHB/PCL Materials by Using Different Types of Collectors and Heat Sealing. Polymers 2020, 20, 693. [Google Scholar] [CrossRef] [PubMed]
- El-hadi, A.M.; Al-Jabri, F.Y. Influence of Electrospinning Parameters on Fiber Diameter and Mechanical Properties of Poly(3-Hydroxybutyrate) (PHB) and Polyanilines (PANI). Blends Polymers 2016, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Katsogiannis, K.A.G.; Vladisavljević, G.T.; Georgiadou, S. Porous electrospun polycaprolactone (PCL) fibers by phase separation. Eur. Polym. J. 2015, 69, 284–295. [Google Scholar] [CrossRef]
- Innes, N.; Johnson, I.G.; Al-Yaseen, W.; Harris, R.; Jones, R.; Kc, S.; McGregor, S.; Robertson, M.; Wade, W.G.; Gallagher, J.E. A systematic review of droplet and aerosol generation in dentistry. J. Dent. 2021, 105, 103556. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Tobías, H.; Morales, G.; Ledezma, A.; Romero, J.; Saldívar, R.; Langlois, V.; Renard, E.; Grande, D. Electrospinning and electrospraying techniques for designing novel antibacterial poly(3-hydroxybutyrate)/zinc oxide nanofibrous composites. J. Mater. Sci. 2016, 51, 8593–8609. [Google Scholar] [CrossRef]
- Lim, L.T.; Mendes, A.C.; Chronakis, I.S. Electrospinning and electrospraying technologies for food applications. Adv. Food Nutr. Res. 2019, 88, 167–234. [Google Scholar]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.; Beck Tan, N.C. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Ol’khov, A.A.; Gorshenev, V.N.; Staroverova, O.V.; Bondarenko, L.V.; Perov, V.I.; Iordanskii, A.L. The Morphology of Fibrous Matrices for Medical Use from Poly-3-Oxybutyrate Obtained by Electrospinning. Polym. Sci. Ser. D 2019, 12, 58–63. [Google Scholar] [CrossRef]
- Wagner, A.; Poursorkhabi, V.; Mohanty, A.K.; Misra, M. Analysis of Porous Electrospun Fibers from Poly(l-lactic acid)/Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Blends. ACS Sustain. Chem. Eng. 2014, 2, 1976–1982. [Google Scholar] [CrossRef]
- Berezin, D.B.; Makarov, V.V.; Guseinov, S.S.; Romanenko, Y.V.; Khudyaeva, I.S.; Startseva, O.M.; Belykh, D.V.; Kustov, A.V. Thermal stability of chlorophyll a derivatives containing hydrophilic groups. Russ. J. Gen. Chem. 2017, 87, 1557–1561. [Google Scholar] [CrossRef]
- Yeo, J.C.C.; Muiruri, J.K.; Thitsartarn, W.; Li, Z.; He, C. Recent advances in the development of biodegradable PHB-based toughening materials: Approaches, advantages, and applications. Mater. Sci. Eng. C 2018, 92, 1092–1116. [Google Scholar] [CrossRef] [PubMed]
- Walle, G.A.; Koning, G.J.; Weusthuis, R.A.; Eggink, G. Properties, modifications and applications of biopolyesters. Adv. Biochem. Eng. Biotechnol. 2001, 71, 263–291. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Azari, P.; Jiménez-Moreno, M.F.; Rodriguez-Garcia, A.; Pingguan-Murphy, B.; Madou, M.J.; Martínez-Chapa, S.O. Polymethacrylate Coated Electrospun PHB Fibers as a Functionalized Platform for Bio-Diagnostics: Confirmation Analysis on the Presence of Immobilized IgG Antibodies against Dengue Virus. Materials 2017, 17, 2292. [Google Scholar] [CrossRef] [PubMed]
- Shamala, T.R.; Divyashree, M.S.; Davis, R.; Kumari, K.S.L.; Vijayendra, S.V.N.; Raj, B. Production and characterization of bacterial polyhydroxyalkanoate copolymers and evaluation of their blends by Fourier transform infrared spectroscopy and scanning electron microscopy. Indian J. Microbiol. 2009, 49, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Stuart, B. Infrared Spectroscopy: Fundamentals and Applications, 2nd ed.; University of Technology: Sydney, Australia, 2004; p. 208. [Google Scholar]
- Kovalcik, A.; Obruca, S.; Kalina, M.; Machovsky, M.; Enev, V.; Jakesova, M.; Sobkova, M.; Marova, I. Enzymatic Hydrolysis of Poly(3-Hydroxybutyrate-co-3-Hydroxyvalerate) Scaffolds. Materials 2020, 13, 2992. [Google Scholar] [CrossRef] [PubMed]
- Carter, G.A.; Spiering, B.A. Optical properties of intact leaves for estimating chlorophyll concentration. J. Environ. Qual. 2002, 31, 1424–1432. [Google Scholar] [CrossRef]
- Shamaei, A.; Mahmoudi, B.; Kazemnejadi, M.; Nasseri, M.A. Mg-catalyzed one-pot preparation of benzimidazoles and spirooxindoles by an immobilized chlorophyll b on magnetic nanoparticles. Organomet. Chem. 2020, 34, 5997. [Google Scholar] [CrossRef]
- Luo, S.; Netravali, A.N. A study of physical and mechanical properties of poly(hydroxybutyrate-co-hydroxyvalerate) during composting. Polym. Degrad. Stab. 2003, 80, 59–66. [Google Scholar] [CrossRef]
- Xu, J.; Guo, B.H.; Yang, R.; Wu, Q.; Chen, G.Q.; Zhang, Z.M. In situ FTIR study on melting and crystallization of polyhydroxyalkanoates. Polymer 2002, 43, 6893–6899. [Google Scholar] [CrossRef]
- Moreira, J.B.; Kuntzler, S.G.; Vaz, B.S.; da Silva, C.K.; Costa, J.A.V.; de Morais, M.G. Polyhydroxybutyrate (PHB)-based blends and composites. In Biodegradable Polymers, Blends and Composites; Woodhead Publishing: Sawston, UK, 2022; pp. 389–413. [Google Scholar]
- Pantani, R.; Turng, L.S. Manufacturing of advanced biodegradable polymeric components. J. Appl. Polym. Sci. 2015, 132, 48. [Google Scholar] [CrossRef]
- Suvorov, N.; Pogorilyy, V.; Diachkova, E.; Vasil’ev, Y.; Mironov, A.; Grin, M. Derivatives of Natural Chlorophylls as Agents for Antimicrobial Photodynamic Therapy. Int. J. Mol. Sci. 2021, 22, 6392. [Google Scholar] [CrossRef] [PubMed]
- Banfi, S.; Caruso, E.; Buccafurni, L.; Battini, V.; Zazzaron, S.; Barbieri, P.; Orlandi, V. Antibacterial activity of tetraaryl-porphyrin photosensitizers: An in vitro study on Gram-negative and Gram-positive bacteria. J. Photochem. Photobiol. B Biol. 2006, 85, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Nitzan, Y.; Ladan, H.; Gozansky, S.; Malik, Z. Characterization of hemin antibacterial action on Staphylococcus aureus. FEMS Microbiol. Lett. 1987, 48, 401–406. [Google Scholar] [CrossRef]
- Beyene, B.B.; Wassie, G.A. Antibacterial activity of Cu(II) and Co(II) porphyrins: Role of ligand modification. BMC Chem. 2020, 14, 51. [Google Scholar] [CrossRef] [PubMed]
- Gerola, A.P.; Santana, A.; Franc¸a, P.B.; Tsubone, T.M.; de Oliveira, H.P.M.; Caetano, W.; Kimura, E.; Hioka, N. Effects of Metal and the Phytyl Chain on Chlorophyll Derivatives: Physicochemical Evaluation for Photodynamic Inactivation of Microorganisms. Photochem. Photobiol. 2011, 87, 884–894. [Google Scholar] [CrossRef]
- Indrawati, R.; Zubaidah, E.; Sutrisno, A.; Limantara, L.; Yusuf, M.M.; Brotosudarmo, T.H.P. Visible Light-Induced Antibacterial Activity of Pigments Extracted from Dregs of Green and Black Teas. Scientifica 2021, 12, 5524468. [Google Scholar] [CrossRef]
- Turco, R.; Santagata, G.; Corrado, I.; Pezzella, C.; Di Serio, M. In Vivo and Post-Synthesis Strategies to Enhance the Properties of PHB-Based Materials: A Review. Front. Bioeng. Biotechnol. 2021, 8, 619266. [Google Scholar] [CrossRef]
- Czerniecka-Kubicka, A.; Frącz, W.; Jasiorski, M.; Błażejewski, W.; Pilch-Pitera, B.; Pyda, M.; Zarzyka, I. Thermal properties of poly(3-hydroxybutyrate) modified by nanoclay. J. Therm. Anal. Calorim. 2017, 128, 1513–1526. [Google Scholar] [CrossRef]
- Iordanskii, A.; Fomin, S.; Burkov, A.; Pankova, Y.; Zaikov, G. Characterization of the structure and properties of the polymer blends based on poly-3-hydroxybutyrate and polyisobutylene. J. Charact. Dev. Nov. Mater. 2015, 1937, 7975. [Google Scholar]
- Urbanek, O.; Sajkiewicz, P.; Pierini, F. The effect of polarity in the electrospinning process on PCL/chitosan nanofibres’ structure, properties and efficiency of surface modification. Polymer 2017, 124, 168–175. [Google Scholar] [CrossRef]



| Sample | Voltage, Kv | Viscosity, Pa s | Flow Rate, mL/min |
|---|---|---|---|
| PHB | 18 | 1.0 | 6.6 |
| PHB/mC2NH2 | 17 | 1.2 | 6.6 |
| PHB/mC2N | 19 | 1.2 | 7.2 |
| PHB/mC2N+ | 17 | 1.2 | 4.8 |
| PHB/mC4 | 17 | 1.2 | 6.4 |
| PHB/mC3OH | 17 | 1.2 | 6.6 |
| Sample | Average Diameter, µm Δ ±0.04 µm | Bulk Density, g/cm3 Δ ±0.01 g/cm3 | Air Permeability, mL Δ ±0.2 ml | Time by Gurley Method, s Δ ±0.6 s |
|---|---|---|---|---|
| PHB | 3.5 | 0.30 | 0.38 | 50.0 |
| PHB/mC2NH2 | 2.24 | 0.18 | 3.75 | 26.7 |
| PHB/mC2N | 4.86 | - | - | - |
| PHB/mC2N+ | 2.27 | 0.13 | 2.78 | 36.0 |
| PHB/mC4 | 2.63 | 0.16 | 2.35 | 42.6 |
| PHB/mC3OH | 2.08 | 0.16 | 4.35 | 23.0 |
| Sample | First Heating Run | Second Heating Run | ||||
|---|---|---|---|---|---|---|
| Tm, °C | ΔH, J/g | χ, % | Tm, °C | ΔH, J/g | χ, % | |
| PHB | 175 | 93.1 | 63.8 | 170 | 90.8 | 62.2 |
| PHB/mC2NH2 | 176 | 83.3 | 57.1 | 174 | 78.7 | 53.9 |
| PHB/mC2N | 176 | 85.8 | 58.8 | 173 | 81.2 | 55.6 |
| PHB/mC2N+ | 177 | 80.6 | 55.2 | 173 | 79.8 | 54.7 |
| PHB/mC4 | 176 | 82.0 | 56.2 | 174 | 78.9 | 54.0 |
| PHB/mC3OH | 175 | 77.4 | 53.0 | 171 | 74.9 | 51.3 |
| Sample | Tensile Strength, MPa Δ ±0.02 MPa | Elongation at Break, % Δ ±0.2% |
|---|---|---|
| PHB | 1.7 | 3.6 |
| PHB/mC2NH2 | - | - |
| PHB/mC2N | - | - |
| PHB/mC2N+ | 1.5 | 1.3 |
| PHB/mC4 | 1.5 | 0.6 |
| PHB/mC3OH | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyubaeva, P.M.; Gasparyan, K.G.; Romanov, R.R.; Kolesnikov, E.A.; Martirosyan, L.Y.; Larkina, E.A.; Tyubaev, M.A. Biomimetic Materials Based on Poly-3-hydroxybutyrate and Chlorophyll Derivatives. Polymers 2024, 16, 101. https://doi.org/10.3390/polym16010101
Tyubaeva PM, Gasparyan KG, Romanov RR, Kolesnikov EA, Martirosyan LY, Larkina EA, Tyubaev MA. Biomimetic Materials Based on Poly-3-hydroxybutyrate and Chlorophyll Derivatives. Polymers. 2024; 16(1):101. https://doi.org/10.3390/polym16010101
Chicago/Turabian StyleTyubaeva, Polina M., Kristina G. Gasparyan, Roman R. Romanov, Evgeny A. Kolesnikov, Levon Y. Martirosyan, Ekaterina A. Larkina, and Mikhail A. Tyubaev. 2024. "Biomimetic Materials Based on Poly-3-hydroxybutyrate and Chlorophyll Derivatives" Polymers 16, no. 1: 101. https://doi.org/10.3390/polym16010101
APA StyleTyubaeva, P. M., Gasparyan, K. G., Romanov, R. R., Kolesnikov, E. A., Martirosyan, L. Y., Larkina, E. A., & Tyubaev, M. A. (2024). Biomimetic Materials Based on Poly-3-hydroxybutyrate and Chlorophyll Derivatives. Polymers, 16(1), 101. https://doi.org/10.3390/polym16010101








