Description of Poly(aryl-ether-ketone) Materials (PAEKs), Polyetheretherketone (PEEK) and Polyetherketoneketone (PEKK) for Application as a Dental Material: A Materials Science Review
Abstract
1. Introduction
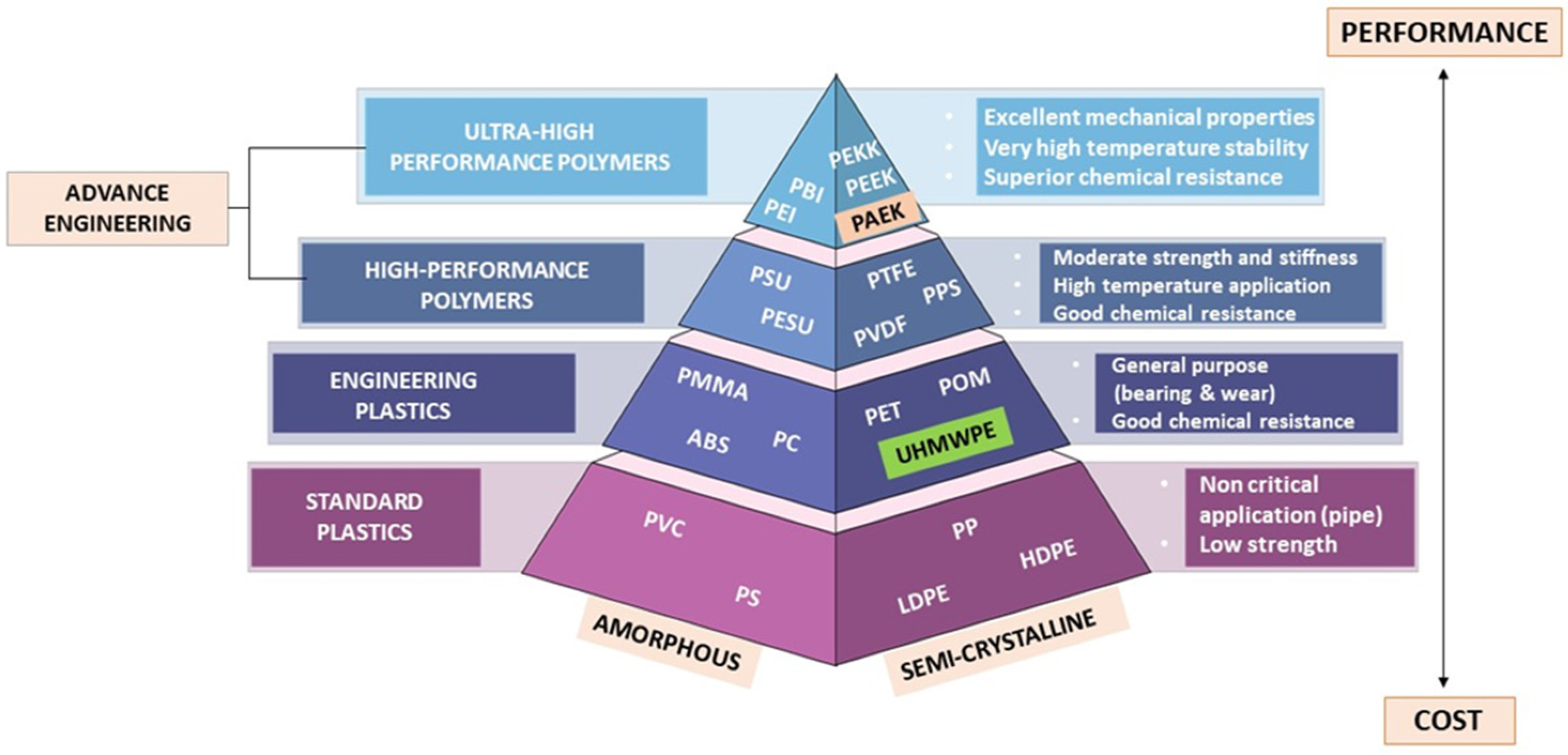
2. Properties of High-Performance Polymers (HPPs)
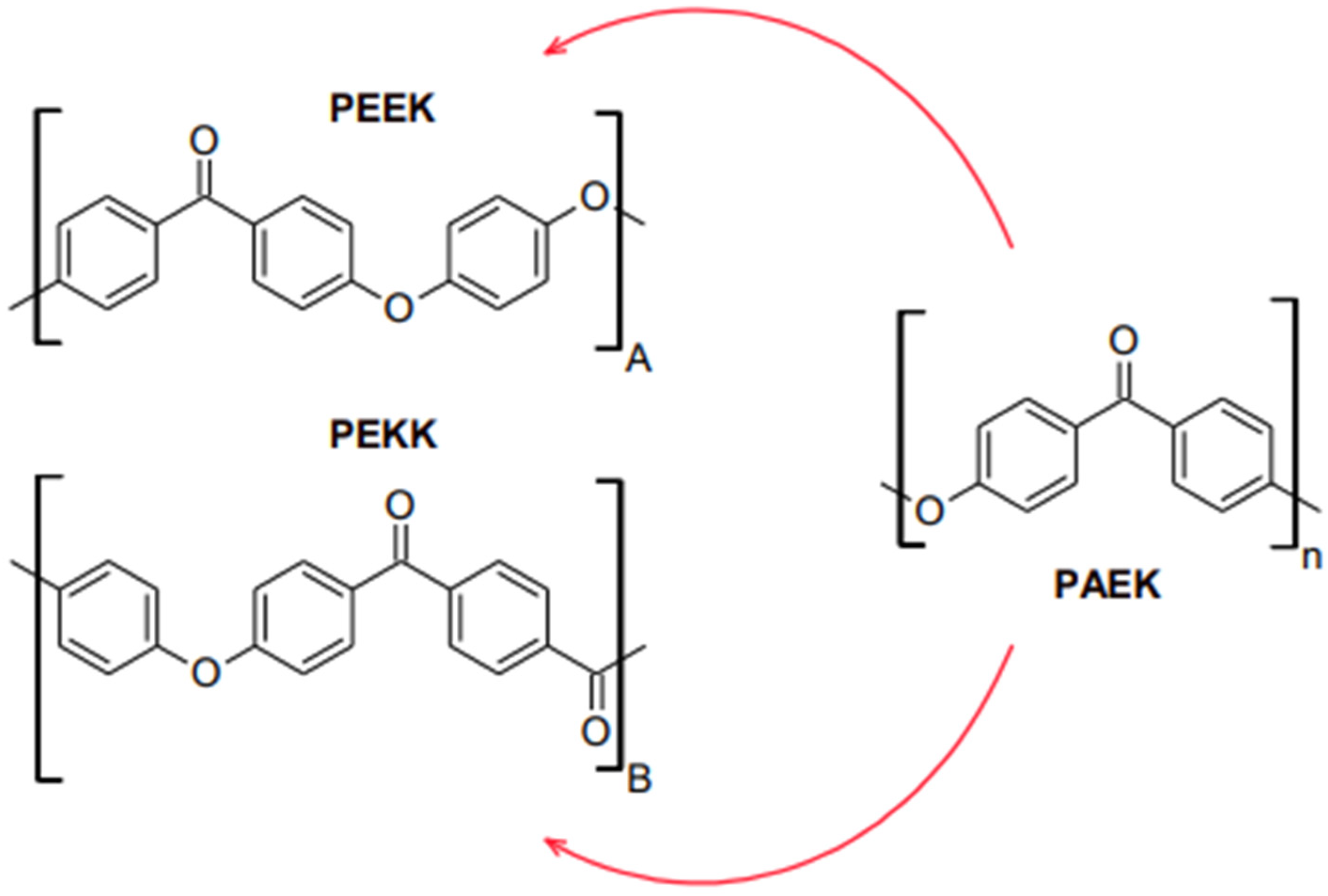
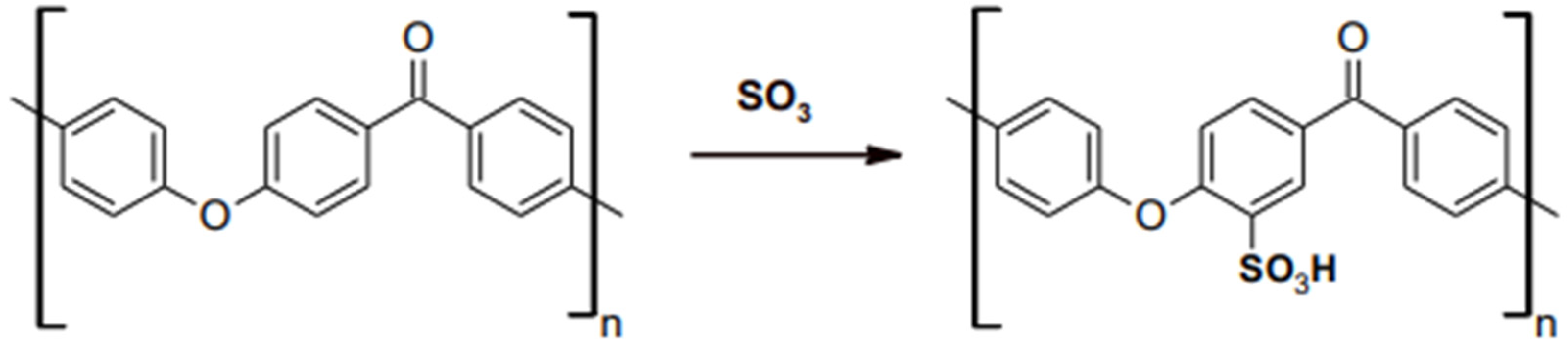
3. Properties of PEEK
4. Properties of PEKK
5. Application of High-Performance Polymer (HPPs) in Dentistry
6. Additive Manufacturing Application in Dentistry
7. Utilization of Ultra-Height Molecular Weight Polyethylene (UHMWPE) and High-Performance Polymer (HPPs) as Dental Materials
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Alla, R.; Raghavendra, K.N.; Vyas, R.; Konakanchi, A. Conventional and contemporary polymers for the fabrication of denture prosthesis: Part I–overview, composition and properties. Int. J. Appl. Dent. Sci. 2015, 1, 82–89. [Google Scholar]
- Rueggeberg, F.A. From vulcanite to vinyl, a history of resins in restorative dentistry. J. Prosthet. Dent. 2002, 87, 364–379. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, S.E.; Whincup, P.H.; Watt, R.G.; Tsakos, G.; Papacosta, A.O.; Lennon, L.T.; Wannamethee, S.G. Burden of poor oral health in older age: Findings from a population-based study of older British men. BMJ Open 2015, 5, e009476. [Google Scholar] [CrossRef]
- Uriciuc, W.A.; Vermesan, H.; Tiuc, A.E.; Ilea, A.; Bosca, A.B.; Popa, C.O. Casting over metal method used in manufacturing hybrid cobalt-chromium dental prosthetic frameworks assembles. Materials 2021, 14, 539. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Classification of Impairments, Disabilities, and Handicaps: A Manual of Classification Relating to the Consequences of Disease; WHO: Geneva, Switzerland, 1980. [Google Scholar]
- Kidd, E.A.; Giedrys-Leeper, E.; Simons, D. Take two dentists: A tale of root caries. Dent. Update 2000, 27, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Wiesli, M.G.; Özcan, M. High-performance polymers and their potential application as medical and oral implant materials: A review. Implant. Dent. 2015, 24, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Stawarczyk, B.; Wimmer, T.; Jahn, D.; Sener, B.; Roos, M.; Schmidlin, P.R. Polyetheretherketone(PEEK)—A suitable material for fixed dental prostheses? J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Prakoso, A.T.; Basri, H.; van der Heide, E. Computational Contact Pressure Prediction of CoCrMo, SS 316L and Ti6Al4V Femoral Head against UHMWPE Acetabular Cup under Gait Cycle. J. Funct. Biomater. 2022, 13, 64. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, G.S.M.; Freitag-Wolf, S.; Kern, M. Resin bonding to three types of polyaryletherketones (PAEKs)-durability and influence of surface conditioning. Dent. Mater. 2014, 30, 357–363. [Google Scholar] [CrossRef]
- Moon, S.M.; Ingalhalikar, A.; Highsmith, J.M.; Vaccaro, A.R. Biomechanical rigidity of an all-polyetheretherketone anterior thoracolumbar spinal reconstruction construct: An in vitro corpectomy model. Spine J. 2009, 9, 330–335. [Google Scholar] [CrossRef]
- Xin, H.; Shepherd, D.; Dearn, K. Strength of poly-etherether-ketone: Effects of sterilization and thermal ageing. Polym. Test. 2013, 32, 1001–1005. [Google Scholar] [CrossRef]
- Ates, S.M.; Caglar, I.; Duymus, Z. The effect of different surface pretreatments on the bond strength of veneering resin to polyetheretherketone. J. Adhes. Sci. Technol. 2018, 32, 2220–2231. [Google Scholar] [CrossRef]
- Zhou, L.Q.Y.; Zhu, Y.; Liu, H.; Gan, K.; Guo, J. The effect of different surface treatments on the bond strength of PEEK composite materials. Dent. Mater. 2014, 30, e209–e215. [Google Scholar] [CrossRef]
- Koutouzis, T.R.J.; Lundgren, T. Comparative soft and hard tissue responses to titanium and polymer healing abutments. J. Oral Implantol. 2011, 37, 174–182. [Google Scholar] [CrossRef]
- Najeeb, S.Z.M.; Khurshid, Z.; Siddiqui, F. Applications of polyetheretherketone (PEEK) in oral implantology and prosthodontics. J. Prosthodont. Res. 2016, 60, 12–19. [Google Scholar] [CrossRef]
- Tannous, F.; Steiner, M.; Shahin, R.; Kern, M. Retentive forces and fatigue resistance of thermoplastic resin clasps. Dent. Mater. 2012, 28, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Rosentritt, M.P.V.; Behr, M.; Sereno, N.; Kolbeck, C. Shear bond strength between veneering composite and PEEK after different surface modifications. Clin. Oral Investig. 2015, 19, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.M.; Devine, J.N. PEEK biomaterials in trauma, orthopedic, and spinal implants. Biomaterials 2007, 28, 4845–4869. [Google Scholar] [CrossRef]
- Elawadly, T.; Radi, I.A.W.; El Khadem, A.; Osman, R.B. Can PEEK Be an Implant Material? Evaluation of Surface Topography and Wettability of Filled Versus Unfilled PEEK with Different Surface Roughness. J. Oral Implantol. 2017, 43, 456–461. [Google Scholar] [CrossRef]
- Skinner, H.B. Composite technology for total hip arthroplasty. Clin. Orthop. Relat. Res. 1988, 235, 224–236. [Google Scholar] [CrossRef]
- Alqurashi, H.; Khurshid, Z.; Syed, A.U.Y.; Habib, S.R.; Rokaya, D.; Zafar, M.S. Polyetherketoneketone (PEKK): An emerging biomaterial for oral implants and dental prostheses. J. Adv. Res. 2021, 28, 87–95. [Google Scholar] [CrossRef]
- Song, C.H.; Choi, J.W.; Jeon, Y.C.; Jeong, C.M.; Lee, S.H.; Kang, E.S.; Yun, M.J.; Huh, J.B. Comparison of the microtensile bond strength of a polyetherketoneketone (PEKK) tooth post cemented with various surface treatments and various resin cements. Materials 2018, 11, 916. [Google Scholar] [CrossRef] [PubMed]
- Swier, S.; Gasa, J.; Shaw, M.T.; Weiss, R.A. Sulfonation Reaction Kinetics of Poly(Ether Ketone Ketone) (PEKK) Using a Mixture of Concentrated and Fuming Sulfuric Acid. Ind. Eng. Chem. Res. 2004, 43, 6948–6954. [Google Scholar] [CrossRef]
- Hussain, M.; Naqvi, R.A.; Abbas, N.; Khan, S.M.; Nawaz, S.; Hussain, A.; Zahra, N.; Khalid, M.W. Ultra-High-Molecular-Weight-Polyethylene (UHMWPE) as a Promising Polymer Material for Biomedical Applications: A Concise Review. Polymers 2020, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Falkensammer, F.; Arnetzl, G.V.; Wildburger, A.; Freudenthaler, J. Color stability of different composite resin materials. J. Prosthet. Dent. 2013, 109, 378–383. [Google Scholar] [CrossRef]
- Sinha, N.; Gupta, N.; Reddy, K.M.; Shastry, Y. Versatility of PEEK as a fixed partial denture framework. J. Indian Prosthodont. Soc. 2017, 17, 80. [Google Scholar] [CrossRef]
- Kessler, A.; Hickel, R.; Reymus, M. 3D printing in dentistry—State of the art. Oper. Dent. 2020, 45, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Perez-Martin, H.; Mackenzie, P.; Baidak, A.; Brádaigh, C.M.Ó.; Ray, D. Crystallinity studies of PEKK and carbon fibre/PEKK composites: A review. Compos. Part B 2021, 223, 109127. [Google Scholar] [CrossRef]
- Qin, L.; Yao, S.; Zhao, J.; Zhou, C.; Oates, T.W.; Weir, M.D.; Wu, J.; Xu, H.H. Review on development and dental applications of polyetheretherketone-based biomaterials and restorations. Materials 2021, 14, 408. [Google Scholar] [CrossRef]
- Kewekordes, T.; Wille, S.; Kern, M. Wear of polyetherketoneketones—Influence of titanium dioxide content and antagonistic material. Dent. Mater. 2018, 34, 560–567. [Google Scholar] [CrossRef]
- Guo, R.; McGrath, J.; Matyjaszewski, K.; Möller, M. (Eds.) Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2012; pp. 377–430. [Google Scholar]
- Stawarczyk, B.; Jordan, P.; Schmidlin, P.R.; Roos, M.; Eichberger, M.; Gernet, W.; Keul, C. PEEK surface treatment effects on tensile bond strength to veneering resins. J. Prosthet. Dent. 2014, 112, 1278–1288. [Google Scholar] [CrossRef]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010, A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Yuan, B.; Cheng, Q.; Zhao, R.; Zhu, X.; Yang, X.; Yang, X.; Zhang, K.; Song, Y.; Zhang, X. Comparison of osteointegration property between PEKK and PEEK: Effects of surface structure and chemistry. Biomaterials 2018, 170, 116–126. [Google Scholar] [CrossRef]
- Olivares-Navarrete, R.; Hyzy, S.L.; Gittens, R.A.; Schneider, J.M.; Haithcock, D.A.; Ullrich, P.F.; Slosar, P.J.; Schwartz, Z.; Boyan, B.D. Rough titanium alloys regulate osteoblast production of angiogenic factors. Spine J. 2013, 13, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.; Beredjiklian, P.; Rhoad, R.; Theiss, S.; Cuckler, J.; Ducheyne, P.; Baker, D.G. A comparison of the inflammatory potential of particulates derived from two composite materials. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. 1997, 34, 137–147. [Google Scholar] [CrossRef]
- Wada, J.; Fueki, K.; Yatabe, M.; Takahashi, H.; Wakabayashi, N. A comparison of the fitting accuracy of thermoplastic denture base resins used in non-metal clasp dentures to a conventional heat-cured acrylic resin. Acta Odontol. Scand. 2014, 73, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Chen, C.; Xu, X.; Wang, J.; Hou, X.; Li, K.; Lu, X.; Shi, H.; Lee, E.S.; Jiang, H.B. A review of 3D printing in dentistry: Technologies, affecting factors, and applications. Scanning 2021, 2021, 9950131. [Google Scholar] [CrossRef]
- Lesmes, D.; Laster, Z. Innovations in dental implant design for current therapy. Oral Maxillofac. Surg. Clin. N. Am. 2011, 23, 193–200. [Google Scholar] [CrossRef]
- Nakamura, K.; Kanno, T.; Milleding, P.; Örtengren, U. Zirconia as a dental implant abutment material: A systematic review. Int. J. Prosthodont. 2010, 23, 299–309. [Google Scholar]
- Özkurt, Z.; Kazazoğlu, E. Zirconia dental implants: A literature review. J. Oral Implantol. 2011, 37, 367–376. [Google Scholar] [CrossRef]
- Akagi, K.; Okamoto, Y.; Matsuura, T.; Horibe, T. Properties of test metal ceramic titanium alloys. J. Prosthet. Dent. 1992, 68, 462–467. [Google Scholar] [CrossRef]
- Kelly, J.R.; Denry, I. Stabilized zirconia as a structural ceramic: An overview. Dent. Mater. 2008, 24, 289–298. [Google Scholar] [CrossRef]
- Ma, R.; Tang, T. Current Strategies to Improve the Bioactivity of PEEK. Int. J. Mol. Sci. 2014, 15, 5426–5445. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, M.; Zhang, W.; Kwok, D.T.; Jiang, J.; Wu, Z.; Chu, P.K. Mechanical and biological characteristics of diamond-like carbon coated poly aryl-ether-ether-ketone. Biomaterials 2010, 31, 8181–8187. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Chowdhary, R. PEEK materials as an alternative to titanium in dental implants: A systematic review. Clin. Implant. Dent. Relat. Res. 2019, 21, 208–222. [Google Scholar] [CrossRef]
- Park, P.J.; Lehman, R.A. Optimizing the Spinal Interbody Implant: Current Advances in Material Modification and SurfaceTreatment Technologies. Curr. Rev. Musculoskelet. Med. 2020, 13, 688–695. [Google Scholar] [CrossRef]
- Deng, Y.; Zhou, P.; Liu, X.; Wang, L.; Xiong, X.; Tang, Z.; Wei, J.; Wei, S. Preparation, characterization, cellular response and invivo osseointegration of polyetheretherketone/nano-hydroxyapatite/carbon fiber ternary biocomposite. Colloids Surf. B Biointerfaces 2015, 136, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Wachtel, A.; Zimmermann, T.; Sutel, M.; Adali, U.; Abou-Emara, M.; Muller, W.D.; Muhlemann, S.; Schwitalla, A.D. Bacterialleakage and bending moments of screw-retained, composite-veneered PEEK implant crowns. J. Mech. Behav. Biomed. Mater. 2019, 91, 32–37. [Google Scholar] [CrossRef]
- Khalesi, R.; Abbasi, M.; Shahidi, Z.; Tabatabaei, M.H.; Moradi, Z. Interfacial Fracture Toughness Comparison of Three IndirectResin Composites to Dentin and Polyether Ether Ketone Polymer. Eur. J. Dent. 2020, 14, 456–461. [Google Scholar]
- Bathala, L.; Majeti, V.; Rachuri, N.; Singh, N.; Gedela, S. The Role of Polyether Ether Ketone (Peek) in Dentistry—A Review. J. Med. Life 2019, 12, 5–9. [Google Scholar] [CrossRef]
- Kaleli, N.; Sarac, D.; Kulunk, S.; Ozturk, O. Effect of different restorative crown and customized abutment materials on stressdistribution in single implants and peripheral bone: A three-dimensional finite element analysis study. J. Prosthet. Dent. 2018, 119, 437–445. [Google Scholar] [CrossRef]
- Gan, K.; Liu, H.; Jiang, L.; Liu, X.; Song, X.; Niu, D.; Chen, T.; Liu, C. Bioactivity and antibacterial effect of nitrogen plasmaimmersion ion implantation on polyetheretherketone. Dent. Mater. 2016, 32, e263–e274. [Google Scholar] [CrossRef] [PubMed]
- Knaus, J.; Schaffarczyk, D.; Colfen, H. On the Future Design of Bio-Inspired Polyetheretherketone Dental Implants. Macromol. Biosci. 2020, 20, e1900239.41. [Google Scholar] [CrossRef] [PubMed]
- Haralur, S.B. Fracture resistance of endodontically treated teeth restored with various esthetic posts. Technol. Health Care 2021, 29, 243–252. [Google Scholar] [CrossRef]
- Schwitalla, A.; Müller, W.-D. PEEK Dental Implants: A Review of the Literature. J. Oral Implantol. 2013, 39, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Luo, C.J.; Huang, J.; Edirisinghe, M. PEEK surface modification by fast ambient-temperature sulfonation for bone-implant applications. J. R. Soc. Interface 2019, 16, 20180955. [Google Scholar] [CrossRef]
- Stawarczyk, B.; Keul, C.; Beuer, F.; Roos, M.; Schmidlin, P.R. Tensile bond strength of veneering resins to PEEK: Impact of different adhesives. Dent. Mater. J. 2013, 32, 441–448. [Google Scholar] [CrossRef]
- Panayotov, I.V.; Orti, V.; Cuisinier, F.; Yachouh, J. Polyetheretherketone (PEEK) for medical applications. J. Mater. Sci. Mater. Med. 2016, 27, 118. [Google Scholar] [CrossRef]
- Khng, K.Y.K.; Ettinger, R.L.; Armstrong, S.R.; Lindquist, T.; Gratton, D.G.; Qian, F. In vitro evaluation of the marginal integrity of CAD/CAM interim crowns. J. Prosthet. Dent. 2016, 115, 617–623. [Google Scholar] [CrossRef]
- Lin, L.; Fang, Y.; Liao, Y.; Chen, G.; Gao, C.; Zhu, P. 3D printing and digital processing techniques in dentistry: A review of literature. Adv. Eng. Mater. 2019, 21, 1801013. [Google Scholar] [CrossRef]
- Prechtel, A.; Reymus, M.; Edelhoff, D.; Hickel, R.; Stawarczyk, B. Comparison of various 3D printed and milled PAEK materials: Effect of printing direction and artificial aging on Martens parameters. Dent. Mater. 2020, 36, 197–209. [Google Scholar] [CrossRef]
- Layani, M.; Wang, X.; Magdassi, S. Novel materials for 3D printing by photopolymerization. Adv. Mater. 2018, 30, 1706344. [Google Scholar] [CrossRef]
- Tasaka, A.; Matsunaga, S.; Odaka, K.; Ishizaki, K.; Ueda, T.; Abe, S.; Yoshinari, M.; Yamashita, S.; Sakurai, K. Accuracy and retention of denture base fabricated by heat curing and additive manufacturing. J. Prosthodont. Res. 2019, 63, 85–89. [Google Scholar] [CrossRef]
- Yoon, H.I.; Hwang, H.J.; Ohkubo, C.; Han, J.S.; Park, E.J. Evaluation of the trueness and tissue surface adaptation of CAD-CAM mandibular denture bases manufactured using digital light processing. J. Prosthet. Dent. 2018, 120, 919–926. [Google Scholar] [CrossRef]
- Masri, G.; Mortada, R.; Ounsi, H.; Alharbi, N.; Boulos, P.; Salameh, Z. Adaptation of complete denture base fabricated by conventional, milling, and 3-d printing techniques: An in vitro study. J. Contemp. Dent. Pract. 2020, 21, 367–371. [Google Scholar] [PubMed]
- Steinmassl, P.A.; Wiedemair, V.; Huck, C.; Klaunzer, F.; Steinmassl, O.; Grunert, I.; Dumfahrt, H. Do CAD/CAM dentures really release less monomer than conventional dentures? Clin. Oral Investig. 2017, 21, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-Y.; Koh, Y.-H.; Kim, H.-E. Digital Light Processing of Zirconia Suspensions Containing Photocurable Monomer/Camphor Vehicle for Dental Applications. Materials 2023, 16, 402. [Google Scholar] [CrossRef]
- Galante, R.; Figueiredo-Pina, C.G.; Serro, A.P. Additive manufacturing of ceramics for dental applications: A review. Dent. Mater. 2019, 35, 825–846. [Google Scholar] [CrossRef]
- Khanlar, L.N.; Rios, A.S.; Tahmaseb, A.; Zandinejad, A. Additive manufacturing of zirconia ceramic and its application in clinical dentistry: A review. Dent. J. 2021, 9, 104. [Google Scholar] [CrossRef] [PubMed]
- Zakeri, S.; Vippola, M.; Levänen, E. A comprehensive review of the photopolymerization of ceramic resins used in stereolithography. Addit. Manuf. 2020, 35, 101177. [Google Scholar] [CrossRef]
- De Camargo, I.L.; Morais, M.N.; Fortulan, C.A.; Branciforti, M.C. A review on the rheological behavior and formulations of ceramic suspensions for vat photopolymerization. Ceram. Inter. 2021, 47, 11906–11921. [Google Scholar] [CrossRef]
- Barazanchi, A.; Li, K.C.; Al-Amleh, B.; Lyons, K.; Waddell, J.N. Additive technology: Update on current materials and applications in dentistry. J. Prosthodont. 2017, 26, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, S.; Pelletier, A.; Al Dika, G. Adaptation of removable partial denture rest seats in prostheses made with selective laser sintering or casting techniques: A randomized clinical trial. J. Prosthet. Dent. 2022, in press. [CrossRef]
- Ramburrun, P.; Pringle, N.A.; Dube, A.; Adam, R.Z.; D’Souza, S.; Aucamp, M. Recent advances in the development of antimicrobial and antifouling biocompatible materials for dental applications. Materials 2021, 14, 3167. [Google Scholar] [CrossRef] [PubMed]
- Selvam, S.; Marimuthu, K. Development and Investigation of Mechanical Properties of PEEK Fine Particles Reinforced UHMWPE Composites. Int. J. Appl. Eng. Res. 2016, 11, 1298–1303. [Google Scholar]
- Villefort, R.F.; Diamantino, P.J.S.; Von Zeidler, S.L.V.; Borges, A.L.S.; Saavedra, G.D.F.A.; Tribst, J.P.M. Mechanical response of PEKK and PEEK as frameworks for implant-supported full-arch fixed dental prosthesis: 3D finite element analysis. Eur. J. Dent. 2022, 16, 115–121. [Google Scholar] [PubMed]
- Agrawal, M. Applications of ultrahigh molecular weight polyethylene fibres in dentistry: A review article. J. Adv. Med. Dent. Sci. Res. 2014, 2, 95–99. [Google Scholar]
- Choupin, T. Mechanical Performances of PEKK Thermoplastic Composites Linked to Their Processing Parameters. Ph.D. Thesis, ENSAM, Paris, France, 2017. [Google Scholar]
- Aldhuwayhi, S.; Alauddin, M.S.; Martin, N. The Structural Integrity and Fracture Behaviour of Teeth Restored with PEEK and Lithium-Disilicate Glass Ceramic Crowns. Polymers 2022, 14, 1001. [Google Scholar] [CrossRef] [PubMed]
- Kucher, M.; Dannemann, M.; Modler, N.; Hannig, C.; Weber, M.T. Effects of endodontic irrigants on material and surface properties of biocompatible thermoplastics. Dent. J. 2019, 7, 26. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Y.; Zhao, Y.; Liu, H. BMC Computer-aided design of polyetheretherketone for application to removable pediatric space maintainers. Oral Health 2020, 20, 201. [Google Scholar] [CrossRef]
- Choi, J.W.; Yun, B.H.; Jeong, C.M.; Huh, J.B. Retentive properties of two stud attachments with polyetherketoneketone or nylon insert in mandibular implant overdentures. Int. J. Oral Maxillofac. Implant. 2018, 33, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Shin, J.H.; Kim, J.E.; Kim, J.H.; Lee, W.C.; Shin, S.W.; Lee, J.Y. Biomechanical evaluation of a tooth restored with high performance polymer PEKK post-core system: A 3D finite element analysis. BioMed Res. Int. 2017, 2017, 1373127. [Google Scholar] [CrossRef] [PubMed]
- Güven, M.Ç.; Dayan, S.Ç.; Yıldırım, G.; Mumcu, E. Custom and prefabricated polyetherketoneketone (PEKK) post-core systems bond strength: Scanning electron microscopy evaluation. Microsc. Res. Tech. 2020, 83, 804–810. [Google Scholar] [CrossRef] [PubMed]



| Properties | PEKK | PEEK | UHMWPE |
|---|---|---|---|
| Specific Gravity | 1.27 g/cm3 | 1.30 g/cm3 | 0.933 g/cm3 |
| Glass Transition Temperature | 162 °C | 143 °C | −110 °C |
| Melting Point | 305 °C | 340 °C | 200–220 °C |
| Tensile Strength | 105 MPa | 90–100 MPa | 48 MPa |
| Young Modulus | 5.10 GPa | 3.70 GPa | 0.69 GPa |
| Operating temperature | 300 °C | 250 °C | <100 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zol, S.M.; Alauddin, M.S.; Said, Z.; Mohd Ghazali, M.I.; Hao-Ern, L.; Mohd Farid, D.A.; Zahari, N.A.H.; Al-Khadim, A.H.A.; Abdul Aziz, A.H. Description of Poly(aryl-ether-ketone) Materials (PAEKs), Polyetheretherketone (PEEK) and Polyetherketoneketone (PEKK) for Application as a Dental Material: A Materials Science Review. Polymers 2023, 15, 2170. https://doi.org/10.3390/polym15092170
Zol SM, Alauddin MS, Said Z, Mohd Ghazali MI, Hao-Ern L, Mohd Farid DA, Zahari NAH, Al-Khadim AHA, Abdul Aziz AH. Description of Poly(aryl-ether-ketone) Materials (PAEKs), Polyetheretherketone (PEEK) and Polyetherketoneketone (PEKK) for Application as a Dental Material: A Materials Science Review. Polymers. 2023; 15(9):2170. https://doi.org/10.3390/polym15092170
Chicago/Turabian StyleZol, Syazwani Mohamad, Muhammad Syafiq Alauddin, Zulfahmi Said, Mohd Ifwat Mohd Ghazali, Lee Hao-Ern, Durratul Aqwa Mohd Farid, Nur A’fifah Husna Zahari, Aws Hashim Ali Al-Khadim, and Azrul Hafiz Abdul Aziz. 2023. "Description of Poly(aryl-ether-ketone) Materials (PAEKs), Polyetheretherketone (PEEK) and Polyetherketoneketone (PEKK) for Application as a Dental Material: A Materials Science Review" Polymers 15, no. 9: 2170. https://doi.org/10.3390/polym15092170
APA StyleZol, S. M., Alauddin, M. S., Said, Z., Mohd Ghazali, M. I., Hao-Ern, L., Mohd Farid, D. A., Zahari, N. A. H., Al-Khadim, A. H. A., & Abdul Aziz, A. H. (2023). Description of Poly(aryl-ether-ketone) Materials (PAEKs), Polyetheretherketone (PEEK) and Polyetherketoneketone (PEKK) for Application as a Dental Material: A Materials Science Review. Polymers, 15(9), 2170. https://doi.org/10.3390/polym15092170






