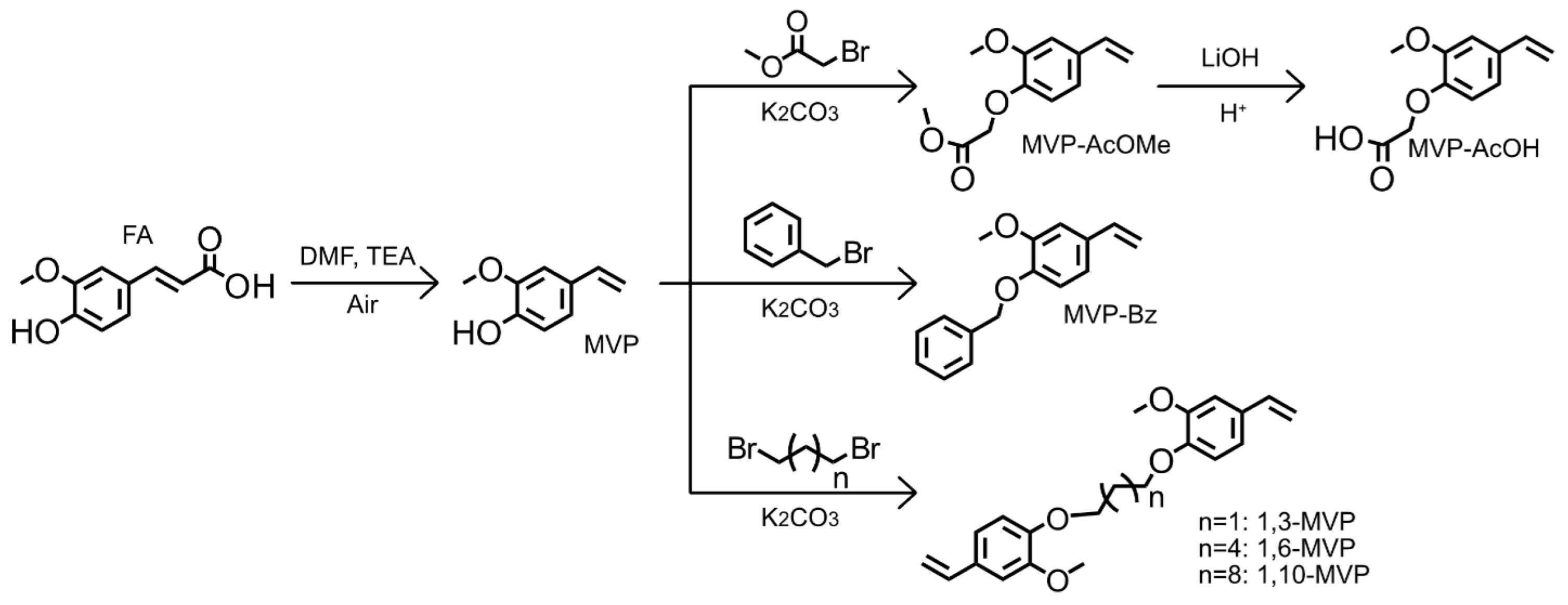
2-Methoxy-4-Vinylphenol as a Biobased Monomer Precursor for Thermoplastics and Thermoset Polymers
Abstract
1. Introduction
2. Materials and Methods
2.1. 1-(Benzyloxy)-2-Methoxy-4-Vinylbenzene (MVP-Bz)
2.2. Solution Polymers P(MVP-AcOMe), P(MVP-AcOH), and P(MVP-Bz)
2.3. Emulsion Copolymers
2.4. Preparation of Thiol-Ene Thermoset Films
2.5. Chemical Stability Test
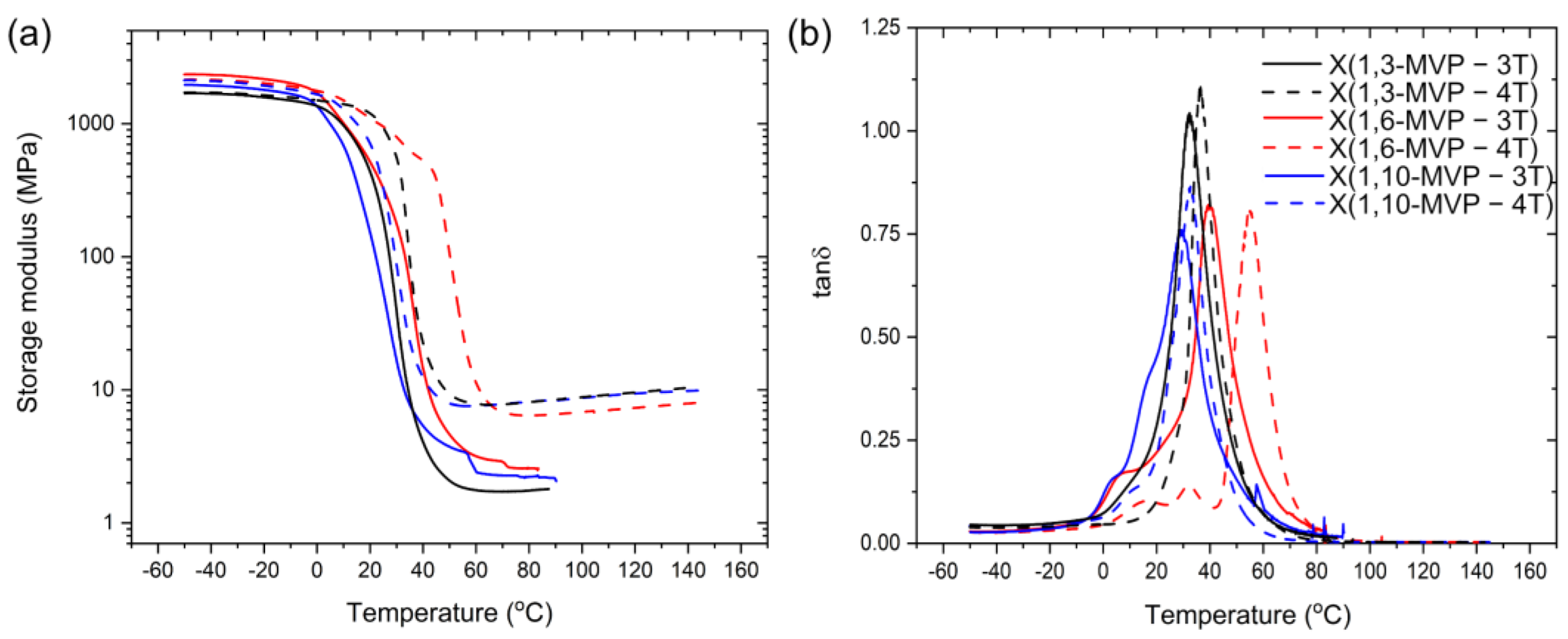
3. Results
3.1. Thermoplastic Applications
3.2. Thermosetting Applications
| Sample Name | (MPa) 1 | (MPa) 2 | (°C) 3 | (°C) 4 | (mol/m3) 5 | Gel Content (%) 6 |
|---|---|---|---|---|---|---|
| X(1,3-MVP–3T) | 1350 ± 300 | 2.4 ± 0.8 | 31 ± 2 | 29 ± 2 | 0.3 ± 0.1 | 98 |
| X(1,3-MVP–4T) | 2000 ± 560 | 7.5 ± 1.1 | 38 ± 1 | 34 ± 1 | 0.9 ± 0.2 | 67 |
| X(1,6-MVP–3T) | 2370 ± 40 | 2.3 ± 0.3 | 40 ± 1 | 22 ± 2 | 0.3 ± 0.1 | 81 |
| X(1,6-MVP–4T) | 2030 ± 290 | 6.3 ± 0.3 | 53 ± 2 | 36 ± 2 | 0.7 ± 0.1 | 78 |
| X(1,10-MVP–3T) | 2120 ± 230 | 2.8 ± 0.8 | 30 ± 1 | 4.5 ± 0.1 | 0.3 ± 0.1 | 94 |
| X(1,10-MVP–4T) | 2040 ± 75 | 7.0 ± 1.2 | 33 ± 1 | 18.9 ± 0.4 | 0.8 ± 0.2 | 95 |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrady, A.L.; Neal, M.A. Applications and societal benefits of plastics. Philos. Trans. R. Soc. B 2009, 364, 1977–1984. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.J.; Caldeira, K.; Matthews, H.D. Future CO2 Emissions and Climate Change from Existing Energy Infrastructure. Science 2010, 329, 1330–1333. [Google Scholar] [CrossRef] [PubMed]
- Rayner, T. Keeping it in the ground? Assessing global governance for fossil-fuel supply reduction. Earth Syst. Gov. 2021, 8, 100061. [Google Scholar] [CrossRef]
- Rosenboom, J.-G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef]
- Wu, X.F.; Chen, G.Q. Global overview of crude oil use: From source to sink through inter-regional trade. Energ. Policy 2019, 128, 476–486. [Google Scholar] [CrossRef]
- Sun, X.; Xie, M.; Mai, L.; Zeng, E.Y. Biobased plastic: A plausible solution toward carbon neutrality in plastic industry? J. Hazard. Mater. 2022, 435, 129037. [Google Scholar] [CrossRef]
- Machado, P.G.; Cunha, M.; Walter, A.; Faaij, A.; Guilhoto, J.J.M. Biobased economy for Brazil: Impacts and strategies for maximizing socioeconomic benefits. Renew. Sustain. Energy Rev. 2021, 139, 110573. [Google Scholar] [CrossRef]
- Rogge, K.S.; Johnstone, P. Exploring the role of phase-out policies for low-carbon energy transitions: The case of the German Energiewende. Energy Res. Soc. Sci. 2017, 33, 128–137. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for the production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Hatton, F.L. Recent advances in RAFT polymerization of monomers derived from renewable resources. Polym. Chem. 2020, 11, 220–229. [Google Scholar] [CrossRef]
- Molina-Gutierrez, S.; Ladmiral, V.; Bongiovanni, R.; Caillol, S.; Lacroix-Desmazes, P. Radical polymerization of biobased monomers in aqueous dispersed media. Green Chem. 2019, 21, 36–53. [Google Scholar] [CrossRef]
- Zago, E.; Dubreucq, E.; Lecomte, J.; Villeneuve, P.; Fine, F.; Fulcrand, H.; Aouf, C. Synthesis of bio-based epoxy monomers from natural allyl- and vinyl phenols and the estimation of their affinity to the estrogen receptor alpha by molecular docking. New J. Chem. 2016, 40, 7701–7710. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green and sustainable manufacture of chemicals from biomass: State of the art. Green Chem. 2014, 16, 950–963. [Google Scholar] [CrossRef]
- Dodds, D.R.; Gross, R.A. Chemicals from biomass. Science 2007, 318, 1250–1251. [Google Scholar] [CrossRef] [PubMed]
- Kungliska Tekniska Högskolan. The Ljungberg Textbook. Wood Chemistry and Wood Biotechnology; KTH Chemical Science and Engineering: Stockholm, Sweden, 2008. [Google Scholar]
- Ralph, J.; Lapierre, C.; Boerjan, W. Lignin structure and its engineering. Curr. Opin. Biotechnol. 2019, 56, 240–249. [Google Scholar] [CrossRef]
- Sapouna, I.; Lawoko, M. Deciphering lignin heterogeneity in ball milled softwood: Unravelling the synergy between the supramolecular cell wall structure and molecular events. Green Chem. 2021, 23, 3348–3364. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Moghadasian, M.H. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: A review. Food Chem. 2008, 109, 691–702. [Google Scholar] [CrossRef]
- Van Schijndel, J.; Molendijk, D.; van Beurden, K.; Canalle, L.A.; Noel, T.; Meuldijk, J. Preparation of bio-based styrene alternatives and their free radical polymerization. Eur. Polym. J. 2020, 125, 109534. [Google Scholar] [CrossRef]
- Buranov, A.U.; Mazza, G. Extraction and purification of ferulic acid from flax shives, wheat and corn bran by alkaline hydrolysis and pressurised solvents. Food Chem. 2009, 115, 1542–1548. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E. Bioconversions of ferulic acid, an hydroxycinnamic acid. Crit. Rev. Microbiol. 2006, 32, 115–125. [Google Scholar] [CrossRef]
- Uraji, M.; Kimura, M.; Inoue, Y.; Kawakami, K.; Kumagai, Y.; Harazono, K.; Hatanaka, T. Enzymatic Production of Ferulic Acid from Defatted Rice Bran by Using a Combination of Bacterial Enzymes. Appl. Biochem. Biotechnol. 2013, 171, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Overhage, J.; Steinbuchel, A.; Priefert, H. Biotransformation of eugenol to ferulic acid by a recombinant strain of Ralstonia eutropha H16. Appl. Environ. Microbiol. 2002, 68, 4315–4321. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.precedenceresearch.com/ferulic-acid-market (accessed on 29 September 2022).
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef]
- Pospiech, D.; Korwitz, A.; Komber, H.; Jehnichen, D.; Arnhold, K.; Brunig, H.; Scheibner, H.; Muller, M.T.; Voit, B. Polyesters with bio-based ferulic acid units: Crosslinking paves the way to property consolidation. Polym. Chem. 2021, 12, 5139–5148. [Google Scholar] [CrossRef]
- Bazin, A.; Averous, L.; Pollet, E. Ferulic Acid as Building Block for the Lipase-Catalyzed Synthesis of Biobased Aromatic Polyesters. Polymers 2021, 13, 3693. [Google Scholar] [CrossRef] [PubMed]
- Mialon, L.; Pemba, A.G.; Miller, S.A. Biorenewable polyethylene terephthalate mimics derived from lignin and acetic acid. Green Chem. 2010, 12, 1704–1706. [Google Scholar] [CrossRef]
- Nomura, E.; Hosoda, A.; Mori, H.; Taniguchi, H. Rapid base-catalyzed decarboxylation and amide-forming reaction of substituted cinnamic acids via microwave heating. Green Chem. 2005, 7, 863–866. [Google Scholar] [CrossRef]
- Cohen, L.A.J.; Jones, W.M. A Study of pH Dependence in the Decarboxylation of p-Hydroxycinnamic Acid. J. Am. Chem. Soc. 1960, 82, 1907–1911. [Google Scholar] [CrossRef]
- Takeshima, H.; Satoh, K.; Kamigaito, M. Bio-based vinylphenol family: Synthesis via decarboxylation of naturally occurring cinnamic acids and living radical polymerization for functionalized polystyrenes. J. Polym. Sci. 2020, 58, 91–100. [Google Scholar] [CrossRef]
- Takeshima, H.; Satoh, K.; Kamigaito, M. Naturally-Derived Amphiphilic Polystyrenes Prepared by Aqueous Controlled/Living Cationic Polymerization and Copolymerization of Vinylguaiacol with R-OH/BF3 center dot OEt2. Polymers 2018, 10, 1404. [Google Scholar] [CrossRef]
- McGuire, T.M.; Miyajima, M.; Uchiyama, M.; Buchard, A.; Kamigaito, M. Epoxy-functionalised 4-vinylguaiacol for the synthesis of bio-based, degradable star polymers via a RAFT/ROCOP strategy. Polym. Chem. 2020, 11, 5844–5850. [Google Scholar] [CrossRef]
- Takeshima, H.; Satoh, K.; Kamigaito, M. Bio-Based Functional Styrene Monomers Derived from Naturally Occurring Ferulic Acid for Poly(vinylcatechol) and Poly(vinylguaiacol) via Controlled Radical Polymerization. Macromolecules 2017, 50, 4206–4216. [Google Scholar] [CrossRef]
- Barbara, I.; Flourat, A.L.; Allais, F. Renewable polymers derived from ferulic acid and biobased diols via ADMET. Eur. Polym. J. 2015, 62, 236–243. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E.; Zakaria, Z.A. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J. Food Sci. Technol. 2015, 52, 5790–5798. [Google Scholar] [CrossRef]
- Cywar, R.M.; Rorrer, N.A.; Hoyt, C.B.; Beckham, G.T.; Chen, E.Y.X. Bio-based polymers with performance-advantaged properties. Nat. Rev. Mater. 2022, 7, 83–103. [Google Scholar] [CrossRef]
- Trost, B.M. The atom economy—A search for synthetic efficiency. Science 1991, 254, 1471–1477. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E factor 25 years on: The rise of green chemistry and sustainability. Green Chem. 2017, 19, 18–43. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E Factor: Fifteen years on. Green Chem. 2007, 9, 1273–1283. [Google Scholar] [CrossRef]
- Wan, J.; Gan, B.; Li, C.; Molina-Aldareguia, J.; Kalali, E.N.; Wang, X.; Wang, D.-Y. A sustainable, eugenol-derived epoxy resin with high biobased content, modulus, hardness and low flammability: Synthesis, curing kinetics and structure–property relationship. Chem. Eng. J. 2016, 284, 1080–1093. [Google Scholar] [CrossRef]
- Fadlallah, S.; Sinha Roy, P.; Garnier, G.; Saito, K.; Allais, F. Are lignin-derived monomers and polymers truly sustainable? An in-depth green metrics calculations approach. Green Chem. 2021, 23, 1495–1535. [Google Scholar] [CrossRef]
- Imada, M.; Takenaka, Y.; Hatanaka, H.; Tsuge, T.; Abe, H. Unique acrylic resins with aromatic side chains by homopolymerization of cinnamic monomers. Commun. Chem. 2019, 2, 109. [Google Scholar] [CrossRef]
- Hoogenboom, R. Poly(2-oxazoline)s based on fatty acids. Eur. J. Lipid Sci. Technol. 2011, 113, 59–71. [Google Scholar] [CrossRef]
- Minhas, M.U.; Ahmad, M.; Anwar, J.; Khan, S. Synthesis and Characterization of Biodegradable Hydrogels for Oral Delivery of 5-Fluorouracil Targeted to Colon: Screening with Preliminary In Vivo Studies. Adv. Polym. Technol. 2018, 37, 221–229. [Google Scholar] [CrossRef]
- Rudin, A.; Samanta, M.C.; Reilly, P.M. Thermal-Decomposition of Anionic and Emulsion Polymerized Polystyrenes. J. Appl. Polym. Sci. 1979, 24, 171–185. [Google Scholar] [CrossRef]
- Stutz, H.; Illers, K.-H.; Mertes, J. A generalized theory for the glass transition temperature of crosslinked and uncrosslinked polymers. J. Polym. Sci. Part B Polym. Phys. 1990, 28, 1483–1498. [Google Scholar] [CrossRef]
- Pezzana, L.; Mousa, M.; Malmström, E.; Johansson, M.; Sangermano, M. Bio-based monomers for UV-curable coatings: Allylation of ferulic acid and investigation of photocured thiol-ene network. Prog. Org. Coat. 2021, 150, 105986. [Google Scholar] [CrossRef]
- Krongauz, V.V. Crosslink density dependence of polymer degradation kinetics: Photocrosslinked acrylates. Thermochim. Acta 2010, 503–504, 70–84. [Google Scholar] [CrossRef]
- Li, F.; Hanson, M.V.; Larock, R.C. Soybean oil-divinylbenzene thermosetting polymers: Synthesis, structure, properties and their relationships. Polymer 2001, 42, 1567–1579. [Google Scholar] [CrossRef]
- Kim, S.-S.; Lau, C.M.; Lillie, L.M.; Tolman, W.B.; Reineke, T.M.; Ellison, C.J. Degradable Thermoset Fibers from Carbohydrate-Derived Diols via Thiol-Ene Photopolymerization. ACS Appl. Polym. Mater. 2019, 1, 2933–2942. [Google Scholar] [CrossRef]
- Ribca, I.; Jawerth, M.E.; Brett, C.J.; Lawoko, M.; Schwartzkopf, M.; Chumakov, A.; Roth, S.V.; Johansson, M. Exploring the Effects of Different Cross-Linkers on Lignin-Based Thermoset Properties and Morphologies. ACS Sustain. Chem. Eng. 2021, 9, 1692–1702. [Google Scholar] [CrossRef]
- Kasetaite, S.; De la Flor, S.; Serra, A.; Ostrauskaite, J. Effect of Selected Thiols on Cross-Linking of Acrylated Epoxidized Soybean Oil and Properties of Resulting Polymers. Polymers 2018, 10, 439. [Google Scholar] [CrossRef]
- Xu, Y.; Odelius, K.; Hakkarainen, M. Photocurable, Thermally Reprocessable, and Chemically Recyclable Vanillin-Based Imine Thermosets. ACS Sustain. Chem. Eng. 2020, 8, 17272–17279. [Google Scholar] [CrossRef]
- Ngadiwiyana Laksana, D.V.; Bima, D.N.; Ismiyarto Sarjono, P.R.; Prasetya, N.B. Synthesis of copolymer eugenol-trithiol-divinylbenzene via photoinitiated cross-linking reaction as antibacterial compound. AIP Conf. Proc. 2022, 2553, 020013. [Google Scholar]
- Kuznetsov, D.M.; Mukhina, O.A.; Kutateladze, A.G. Photoassisted Synthesis of Complex Molecular Architectures: Dearomatization of Benzenoid Arenes with Aza-o-xylylenes via an Unprecedented [2 + 4] Reaction Topology. Angew. Chem. Int. Ed. 2016, 55, 6988–6991. [Google Scholar] [CrossRef] [PubMed]
- Geng, H.; Wang, Y.; Yu, Q.; Gu, S.; Zhou, Y.; Xu, W.; Zhang, X.; Ye, D. Vanillin-Based Polyschiff Vitrimers: Reprocessability and Chemical Recyclability. ACS Sustain. Chem. Eng. 2018, 6, 15463–15470. [Google Scholar] [CrossRef]
- Fox, T.G., Jr.; Flory, P.J. Second-Order Transition Temperatures and Related Properties of Polystyrene. I. Influence of Molecular Weight. J. Appl. Phys. 1950, 21, 581–591. [Google Scholar] [CrossRef]
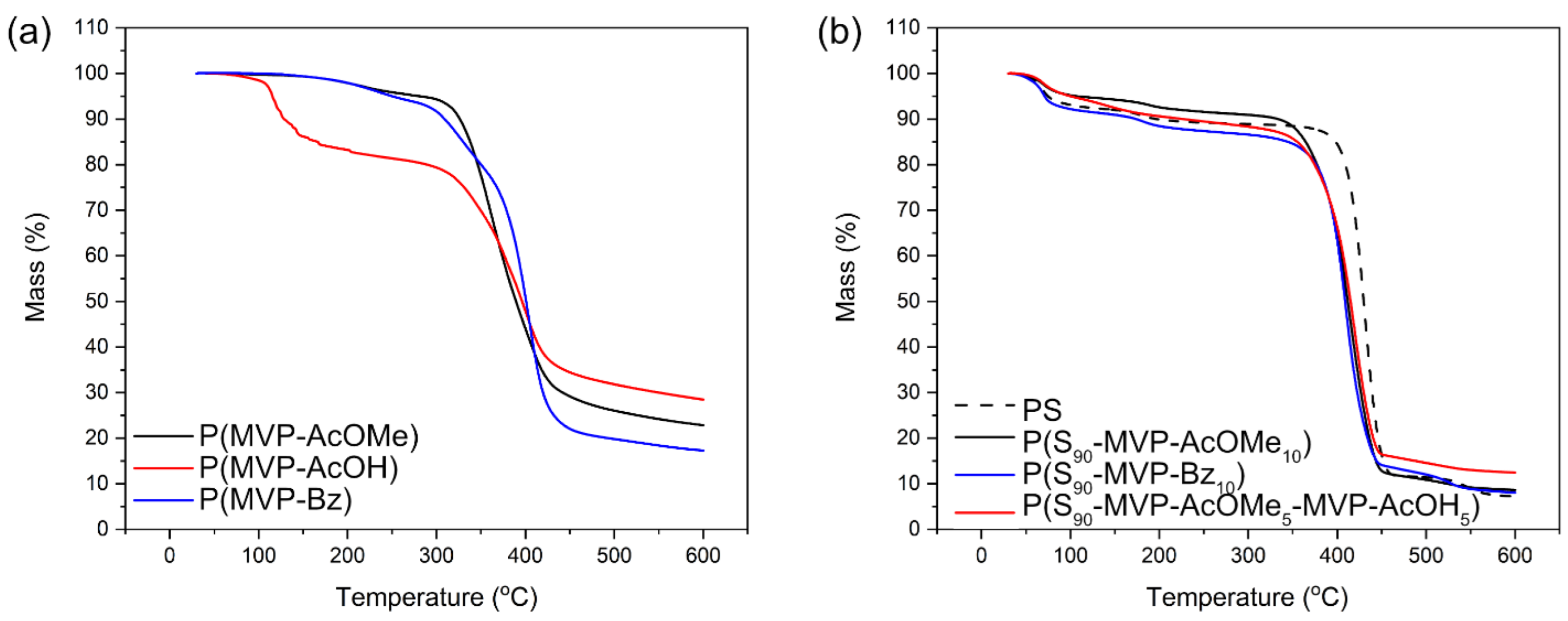
| Sample Name | Type 1 | p (%) 2 | Composition (wt.%) 3 | (g/mol) 4 | (°C) 5 | (°C) 6 |
|---|---|---|---|---|---|---|
| P(MVP-AcOMe) | S | 91 | N.A. | 25,000 (1.5) | 45.9 ± 0.2 | 289 ± 12 |
| P(MVP-AcOH) | S | 89 | N.A. | N.A. ** | 56.1 ± 0.3 | 115 ± 1 |
| P(MVP-Bz) | S | 75 | N.A. | 14,600 (1.7) | 47.1 ± 0.2 | 250 ± 1 |
| PS | E | >99 | N.A. | 170,000 (1.6) | 105.8 ± 0.3 | 381.0 ± 0.3 * |
| P(S90-MVP-AcOMe10) | E | 94 | 88:12 | 140,000 (1.4) | 99.5 ± 0.5 | 349 ± 1 * |
| P(S90-MVP-Bz10) | E | 92 | 66:34 | 75,000 (1.4) | 101.2 ± 0.8 | 344 ± 2 * |
| P(S90-MVP-AcOMe5-MVP-AcOH5) | E | 81 | 99:0.9:0.1 | 1,000,000 (1.5) | 93.7 ± 2.9 | 354 ± 8 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexakis, A.E.; Ayyachi, T.; Mousa, M.; Olsén, P.; Malmström, E. 2-Methoxy-4-Vinylphenol as a Biobased Monomer Precursor for Thermoplastics and Thermoset Polymers. Polymers 2023, 15, 2168. https://doi.org/10.3390/polym15092168
Alexakis AE, Ayyachi T, Mousa M, Olsén P, Malmström E. 2-Methoxy-4-Vinylphenol as a Biobased Monomer Precursor for Thermoplastics and Thermoset Polymers. Polymers. 2023; 15(9):2168. https://doi.org/10.3390/polym15092168
Chicago/Turabian StyleAlexakis, Alexandros E., Thayanithi Ayyachi, Maryam Mousa, Peter Olsén, and Eva Malmström. 2023. "2-Methoxy-4-Vinylphenol as a Biobased Monomer Precursor for Thermoplastics and Thermoset Polymers" Polymers 15, no. 9: 2168. https://doi.org/10.3390/polym15092168
APA StyleAlexakis, A. E., Ayyachi, T., Mousa, M., Olsén, P., & Malmström, E. (2023). 2-Methoxy-4-Vinylphenol as a Biobased Monomer Precursor for Thermoplastics and Thermoset Polymers. Polymers, 15(9), 2168. https://doi.org/10.3390/polym15092168







