Green Synthesis and Characteristics of Cellulose Nanocrystal/Poly Acrylic Acid Nanocomposite Thin Film for Organic Dye Adsorption during Water Treatment
Abstract
1. Introduction
2. Experimental Procedures
2.1. Materials
2.2. Extraction of Cellulose Nanocrystals (CNCs) from Sugarcane Bagasse
2.3. Fabrication and Characterization of Thin Film
2.4. Adsorption Studies
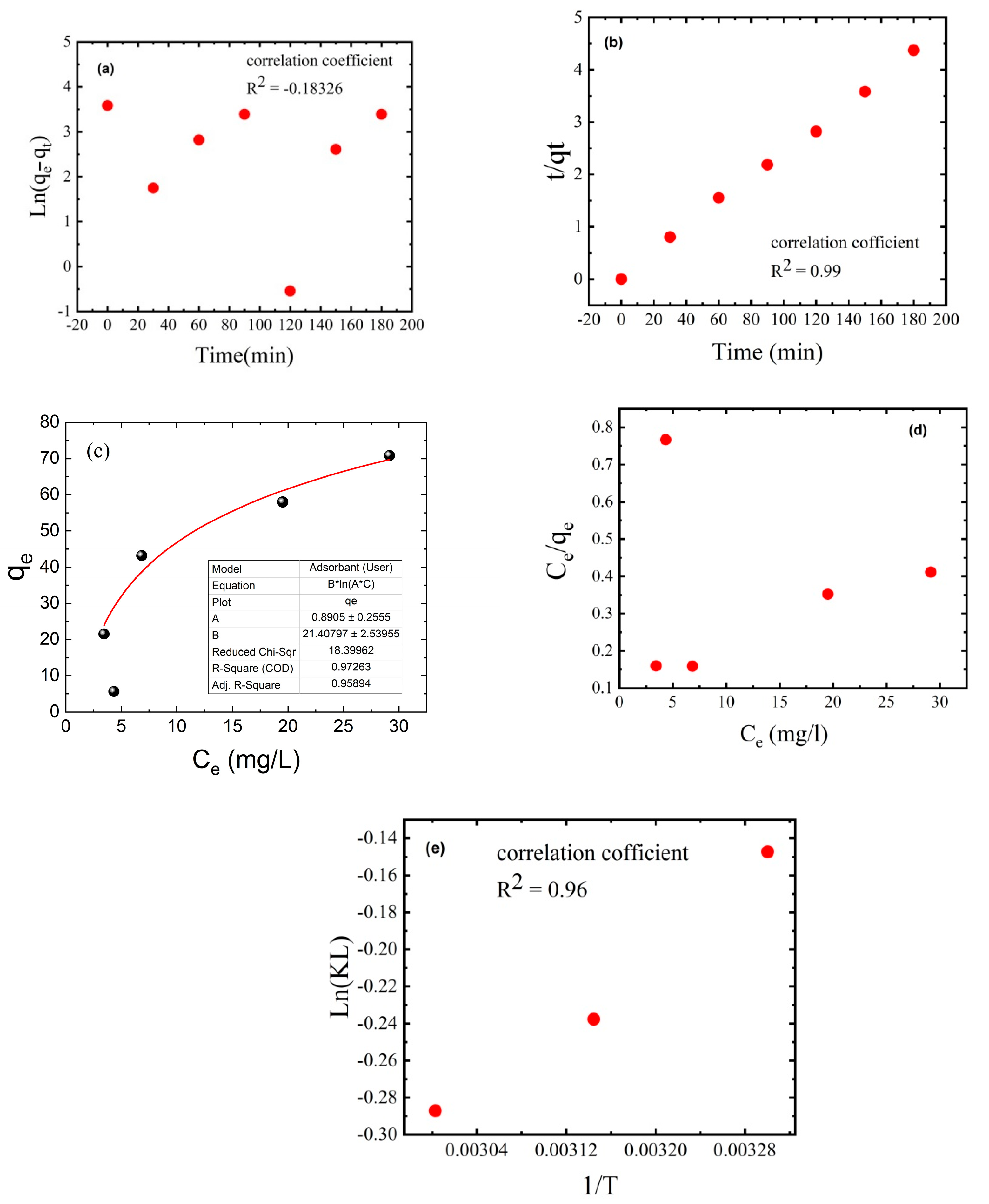
2.5. Adsorption Kinetics and Isotherm
2.6. Thermodynamic Isotherm
3. Results and Discussion
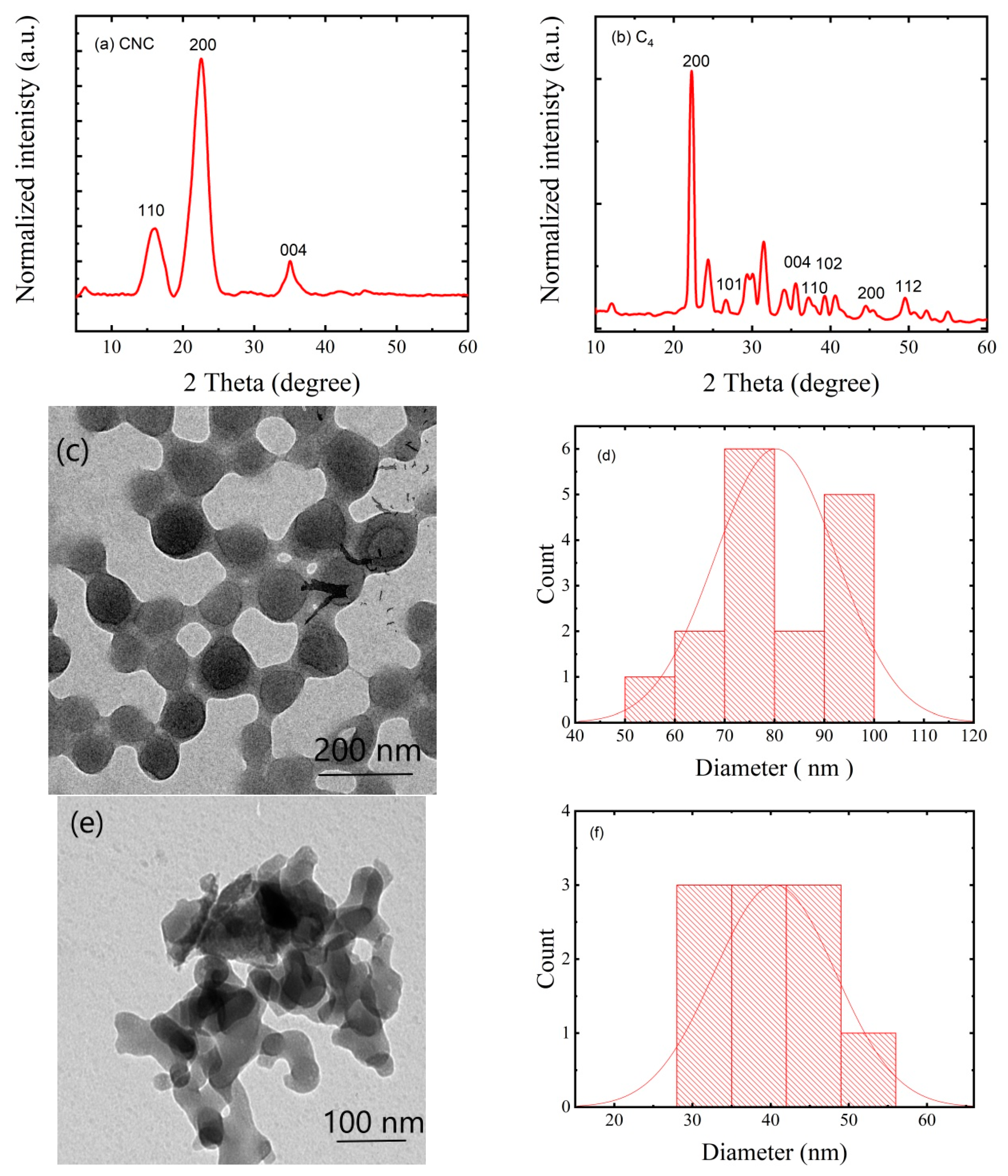
3.1. Characteristics of CNCs and AC Particles
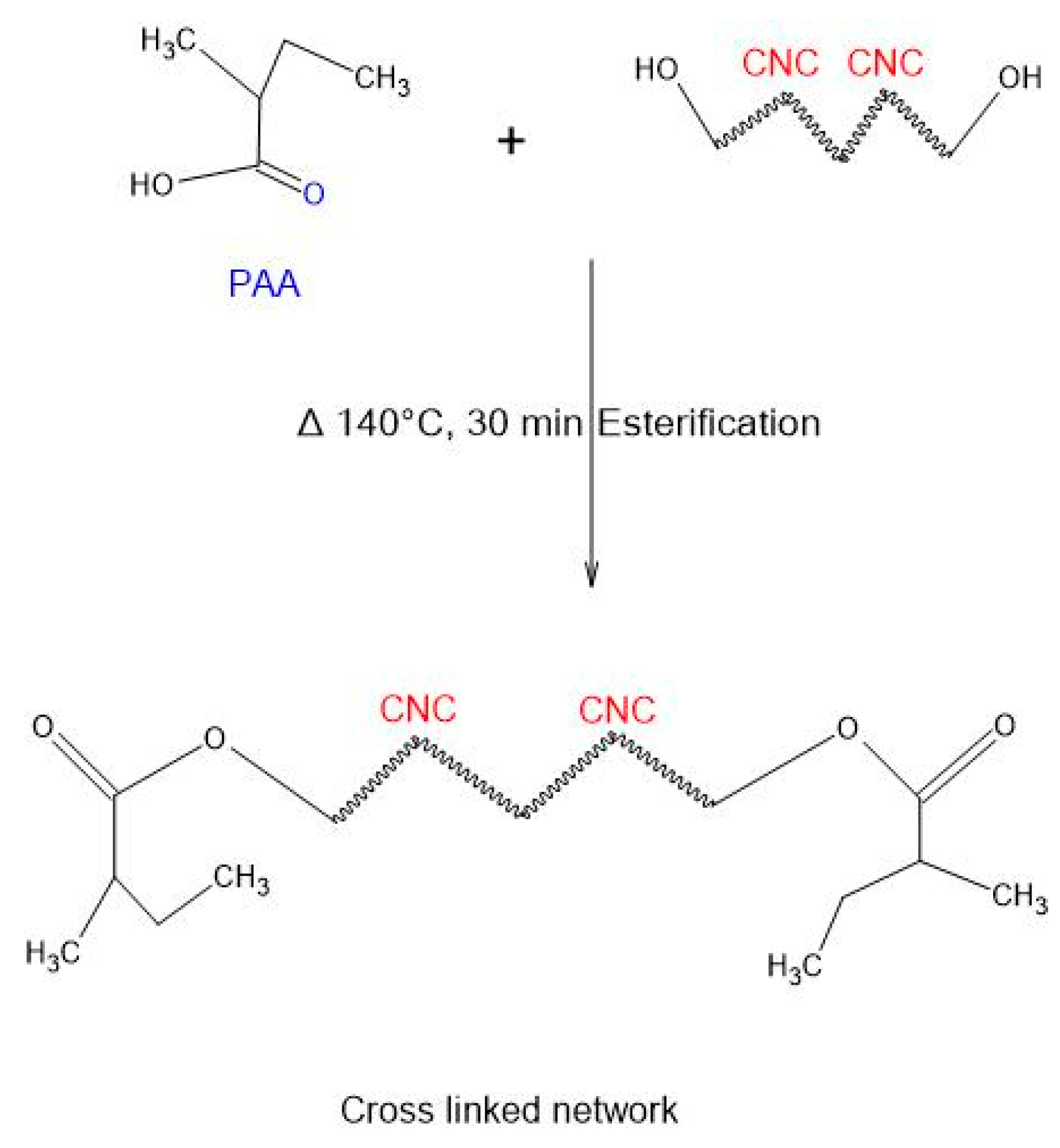
3.2. Crosslinking and Thin Film Formation
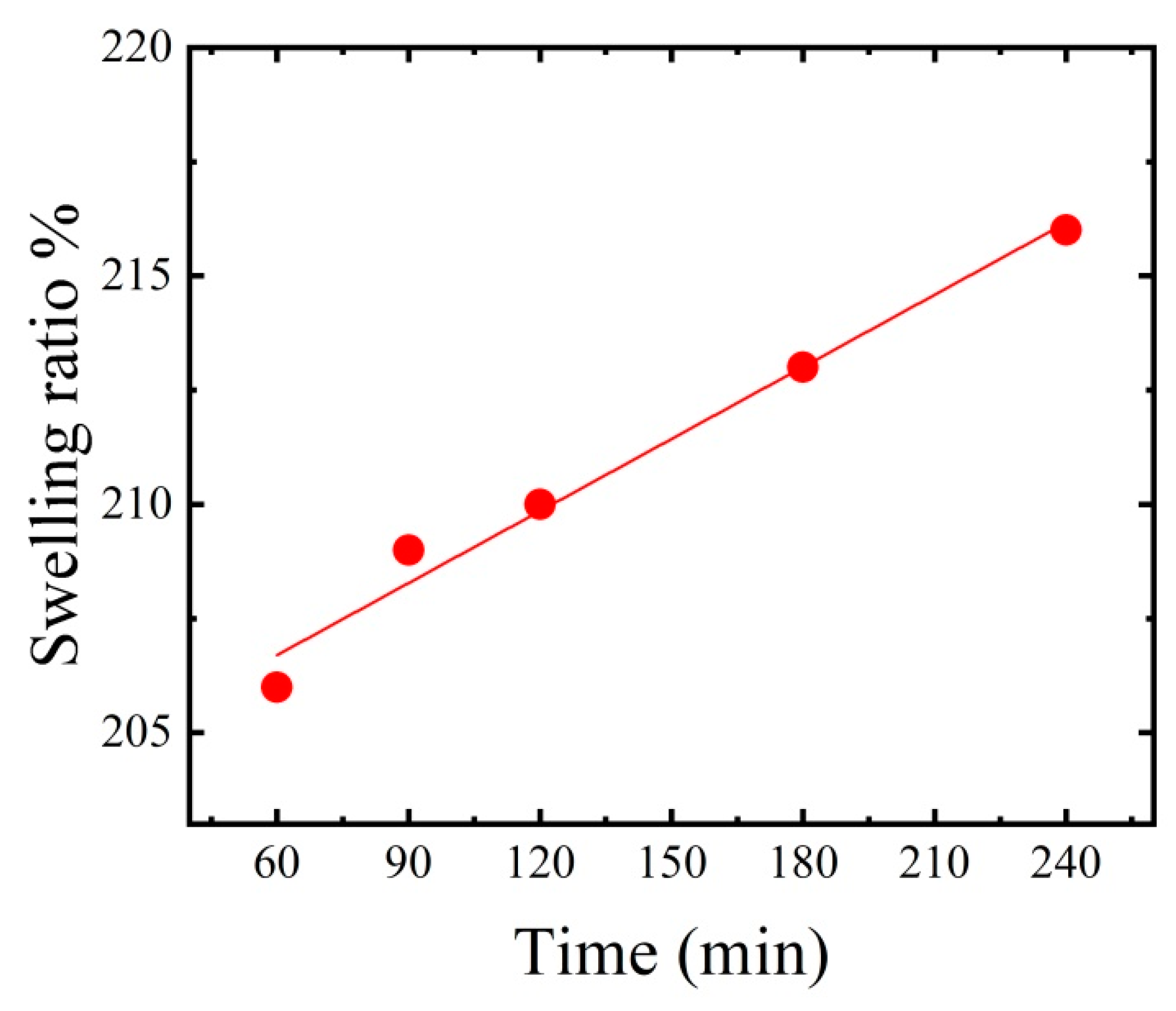
3.3. Crosslinked Thin Film Swelling Effect
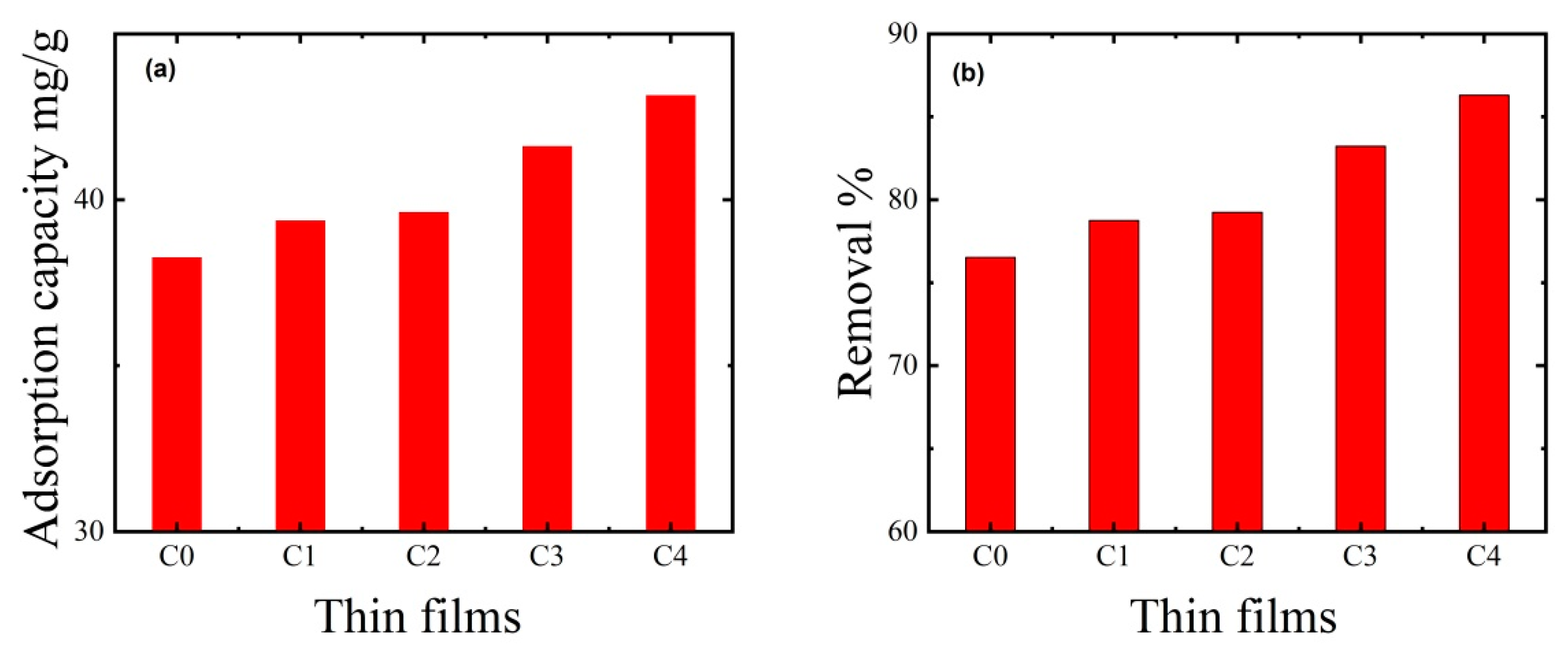
3.4. Effect of Thin Film Composition on MB Dye Removal
3.5. Effect of Initial MB Dye Concentration on Its Removal
3.6. Effect of Contact Time on MB Dye Removal
3.7. Effect of Temperature on MB Dye Removal
3.8. Effect of pH on MB Dye Removal
3.9. Adsorption Kinetics and Thermodynamic Isotherm
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faur-Brasquet, C.; Métivier-Pignon, H.; Le Cloirec, P. Adsorption of Dyes onto Activated Carbon Cloths: Approach of Adsorption Mechanisms and Coupling of ACC with Ultrafiltration to Treat Coloured Wastewaters. Sep. Purif. Technol. 2003, 31, 3–11. [Google Scholar]
- Ravikumar, K.; Deebika, B.; Balu, K. Decolourization of Aqueous Dye Solutions by a Novel Adsorbent: Application of Statistical Designs and Surface Plots for the Optimization and Regression Analysis. J. Hazard. Mater. 2005, 122, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Pearce, C.I.; Lloyd, J.R.; Guthrie, J.T. The Removal of Colour from Textile Wastewater Using Whole Bacterial Cells: A Review. Dye. Pigment. 2003, 58, 179–196. [Google Scholar] [CrossRef]
- Lee, J.-W.; Choi, S.-P.; Thiruvenkatachari, R.; Shim, W.-G.; Moon, H. Evaluation of the Performance of Adsorption and Coagulation Processes for the Maximum Removal of Reactive Dyes. Dye. Pigment. 2006, 69, 196–203. [Google Scholar] [CrossRef]
- McMullan, G.; Meehan, C.; Conneely, A.; Kirby, N.; Robinson, T.; Nigam, P.; Banat, I.; Marchant, R.; Smyth, W.F. Microbial Decolourisation and Degradation of Textile Dyes. Appl. Microbiol. Biotechnol. 2001, 56, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of Dyes in Textile Effluent: A Critical Review on Current Treatment Technologies with a Proposed Alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Banat, I.M.; Nigam, P.; Singh, D.; Marchant, R. Microbial Decolorization of Textile-Dyecontaining Effluents: A Review. Bioresour. Technol. 1996, 58, 217–227. [Google Scholar] [CrossRef]
- Ghosh, D.; Bhattacharyya, K.G. Adsorption of Methylene Blue on Kaolinite. Appl. Clay Sci. 2002, 20, 295–300. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption of Basic Dye on High-Surface-Area Activated Carbon Prepared from Coconut Husk: Equilibrium, Kinetic and Thermodynamic Studies. J. Hazard. Mater. 2008, 154, 337–346. [Google Scholar] [CrossRef]
- Tan, I.A.W.; Ahmad, A.L.; Hameed, B.H. Adsorption of Basic Dye Using Activated Carbon Prepared from Oil Palm Shell: Batch and Fixed Bed Studies. Desalination 2008, 225, 13–28. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Haghighi, R. Chemical Catalytic Reaction and Biological Oxidation for Treatment of Non-Biodegradable Textile Effluent. Chem. Eng. J. 2003, 95, 163–169. [Google Scholar] [CrossRef]
- Jain, A.K.; Gupta, V.K.; Bhatnagar, A. Utilization of Industrial Waste Products as Adsorbents for the Removal of Dyes. J. Hazard. Mater. 2003, 101, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-S.; McKay, G. Sorption of Dyes and Copper Ions onto Biosorbents. Process Biochem. 2003, 38, 1047–1061. [Google Scholar] [CrossRef]
- Derbyshire, F.; Jagtoyen, M.; Andrews, R.; Rao, A.; Martin-Gullon, I.; Grulke, E.A. Carbon Materials in Environmental Applications. Chem. Phys. Carbon 2001, 27, 1–66. [Google Scholar]
- Sleiman, M.; Vildozo, D.; Ferronato, C.; Chovelon, J.-M. Photocatalytic Degradation of Azo Dye Metanil Yellow: Optimization and Kinetic Modeling Using a Chemometric Approach. Appl. Catal. B Environ. 2007, 77, 1–11. [Google Scholar] [CrossRef]
- Abbasi, M.; Asl, N.R. Sonochemical Degradation of Basic Blue 41 Dye Assisted by NanoTiO2 and H2O2. J. Hazard. Mater. 2008, 153, 942–947. [Google Scholar] [CrossRef]
- Zaghbani, N.; Hafiane, A.; Dhahbi, M. Removal of Safranin T from Wastewater Using Micellar Enhanced Ultrafiltration. Desalination 2008, 222, 348–356. [Google Scholar] [CrossRef]
- Wu, J.-S.; Liu, C.-H.; Chu, K.H.; Suen, S.-Y. Removal of Cationic Dye Methyl Violet 2B from Water by Cation Exchange Membranes. J. Memb. Sci. 2008, 309, 239–245. [Google Scholar] [CrossRef]
- Fan, L.; Zhou, Y.; Yang, W.; Chen, G.; Yang, F. Electrochemical Degradation of Aqueous Solution of Amaranth Azo Dye on ACF under Potentiostatic Model. Dye. Pigment. 2008, 76, 440–446. [Google Scholar] [CrossRef]
- Zhu, M.-X.; Lee, L.; Wang, H.-H.; Wang, Z. Removal of an Anionic Dye by Adsorption/Precipitation Processes Using Alkaline White Mud. J. Hazard. Mater. 2007, 149, 735–741. [Google Scholar] [CrossRef]
- Sudarjanto, G.; Keller-Lehmann, B.; Keller, J. Optimization of Integrated Chemical–Biological Degradation of a Reactive Azo Dye Using Response Surface Methodology. J. Hazard. Mater. 2006, 138, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Sarria, V.; Deront, M.; Péringer, P.; Pulgarin, C. Degradation of a Biorecalcitrant Dye Precursor Present in Industrial Wastewaters by a New Integrated Iron (III) Photoassisted–Biological Treatment. Appl. Catal. B Environ. 2003, 40, 231–246. [Google Scholar] [CrossRef]
- García-Montaño, J.; Pérez-Estrada, L.; Oller, I.; Maldonado, M.I.; Torrades, F.; Peral, J. Pilot Plant Scale Reactive Dyes Degradation by Solar Photo-Fenton and Biological Processes. J. Photochem. Photobiol. A Chem. 2008, 195, 205–214. [Google Scholar] [CrossRef]
- Lodha, B.; Chaudhari, S. Optimization of Fenton-Biological Treatment Scheme for the Treatment of Aqueous Dye Solutions. J. Hazard. Mater. 2007, 148, 459–466. [Google Scholar] [CrossRef]
- Hameed, B.H.; Daud, F.B.M. Adsorption Studies of Basic Dye on Activated Carbon Derived from Agricultural Waste: Hevea Brasiliensis Seed Coat. Chem. Eng. J. 2008, 139, 48–55. [Google Scholar] [CrossRef]
- Forgacs, E.; Cserháti, T.; Oros, G. Removal of Synthetic Dyes from Wastewaters: A Review. Environ. Int. 2004, 30, 953–971. [Google Scholar] [CrossRef]
- Ahmad, A.; Rafatullah, M.; Danish, M. Removal of Zn (II) and Cd (II) Ions from Aqueous Solutions Using Treated Sawdust of Sissoo Wood as an Adsorbent. Holz Als Roh Werkst. 2007, 65, 429–436. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of Copper (II), Chromium (III), Nickel (II) and Lead (II) Ions from Aqueous Solutions by Meranti Sawdust. J. Hazard. Mater. 2009, 170, 969–977. [Google Scholar] [CrossRef]
- Dhruv Patel, D.; Bhatt, S. Environmental Pollution, Toxicity Profile, and Physico-Chemical and Biotechnological Approaches for Treatment of Textile Wastewater. Biotechnol. Genet. Eng. Rev. 2022, 38, 33–86. [Google Scholar] [CrossRef]
- Obey, G.; Adelaide, M.; Ramaraj, R. Biochar Derived from Non-Customized Matamba Fruit Shell as an Adsorbent for Wastewater Treatment. J. Bioresour. Bioprod. 2022, 7, 109–115. [Google Scholar] [CrossRef]
- Bhat, S.; Uthappa, U.T.; Sadhasivam, T.; Altalhi, T.; Han, S.S.; Kurkuri, M.D. Abundant Cilantro Derived High Surface Area Activated Carbon (AC) for Superior Adsorption Performances of Cationic/Anionic Dyes and Supercapacitor Application. Chem. Eng. J. 2023, 459, 141577. [Google Scholar] [CrossRef]
- Abd-Elhamid, A.I.; Nayl, A.A. Fabrication and Characterization of a Novel (GO/PAA/PAM) Nanocomposite as Effective Adsorbent for Cationic Dyes. J. Mater. Res. Technol. 2021, 15, 3807–3824. [Google Scholar] [CrossRef]
- Subhedar, A.; Bhadauria, S.; Ahankari, S.; Kargarzadeh, H. Nanocellulose in Biomedical and Biosensing Applications: A Review. Int. J. Biol. Macromol. 2021, 166, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Akter, M.; Bhattacharjee, M.; Dhar, A.K.; Rahman, F.B.A.; Haque, S.; Rashid, T.U.; Kabir, S.M.F. Cellulose-Based Hydrogels for Wastewater Treatment: A Concise Review. Gels 2021, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhan, C.; Wu, J.; Cui, Z.; Si, J.; Wang, Q.; Peng, X.; Turng, L.-S. Highly Efficient Removal of Methylene Blue Dye from an Aqueous Solution Using Cellulose Acetate Nanofibrous Membranes Modified by Polydopamine. ACS Omega 2020, 5, 5389–5400. [Google Scholar] [CrossRef]
- Zinge, C.; Kandasubramanian, B. Nanocellulose Based Biodegradable Polymers. Eur. Polym. J. 2020, 133, 109758. [Google Scholar] [CrossRef]
- Gupta, N.; Mahur, B.K.; Izrayeel, A.M.D.; Ahuja, A.; Rastogi, V.K. Biomass Conversion of Agricultural Waste Residues for Different Applications: A Comprehensive Review. Environ. Sci. Pollut. Res. 2022, 29, 73622–73647. [Google Scholar] [CrossRef]
- Yu, S.; Sun, J.; Shi, Y.; Wang, Q.; Wu, J.; Liu, J. Nanocellulose from Various Biomass Wastes: Its Preparation and Potential Usages towards the High Value-Added Products. Environ. Sci. Ecotechnol. 2021, 5, 100077. [Google Scholar] [CrossRef]
- Nagarajan, K.J.; Ramanujam, N.R.; Sanjay, M.R.; Siengchin, S.; Surya Rajan, B.; Sathick Basha, K.; Madhu, P.; Raghav, G.R. A Comprehensive Review on Cellulose Nanocrystals and Cellulose Nanofibers: Pretreatment, Preparation, and Characterization. Polym. Compos. 2021, 42, 1588–1630. [Google Scholar] [CrossRef]
- Mohammed, N.; Lian, H.; Islam, M.S.; Strong, M.; Shi, Z.; Berry, R.M.; Yu, H.-Y.; Tam, K.C. Selective Adsorption and Separation of Organic Dyes Using Functionalized Cellulose Nanocrystals. Chem. Eng. J. 2021, 417, 129237. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Starch, Cellulose, Pectin, Gum, Alginate, Chitin and Chitosan Derived (Nano) Materials for Sustainable Water Treatment: A Review. Carbohydr. Polym. 2021, 251, 116986. [Google Scholar] [CrossRef] [PubMed]
- Jjagwe, J.; Olupot, P.W.; Menya, E.; Kalibbala, H.M. Synthesis and Application of Granular Activated Carbon from Biomass Waste Materials for Water Treatment: A Review. J. Bioresour. Bioprod. 2021, 6, 292–322. [Google Scholar] [CrossRef]
- Raichur, A.; Sinha, N. Synthesis of Multi-Layered Nanoswabs for Simultaneous and Expeditious Removal of Antibiotic-Resistant Bacteria, Dyes, and Antibiotics from Wastewater. Sep. Purif. Technol. 2023, 308, 122830. [Google Scholar] [CrossRef]
- Stanly, S.; Jelmy, E.J.; John, H. Studies on Modified Montmorillonite Clay and Its PVA Nanohybrid for Water Purification. J. Polym. Environ. 2020, 28, 2433–2443. [Google Scholar] [CrossRef]
- Hosseini, H.; Zirakjou, A.; McClements, D.J.; Goodarzi, V.; Chen, W.-H. Removal of Methylene Blue from Wastewater Using Ternary Nanocomposite Aerogel Systems: Carboxymethyl Cellulose Grafted by Polyacrylic Acid and Decorated with Graphene Oxide. J. Hazard. Mater. 2022, 421, 126752. [Google Scholar] [CrossRef]
- Spoljaric, S.; Salminen, A.; Luong, N.D.; Seppälä, J. Crosslinked Nanofibrillated Cellulose: Poly(Acrylic Acid) Nanocomposite Films; Enhanced Mechanical Performance in Aqueous Environments. Cellulose 2013, 20, 2991–3005. [Google Scholar] [CrossRef]
- Sarfat, M.S.; Setyaningsih, D.; Fahma, F.; Indrasti, N.S. Characterization of Mono-Diacylglycerols, Cellulose Nanocrystals, Polypropylene, and Supporting Materials as Raw Materials for Synthesis of Antistatic Bionanocomposites. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022; Volume 1034, p. 12009. [Google Scholar]
- Zhang, W.; Song, H.; Zhu, L.; Wang, G.; Zeng, Z.; Li, X. High Flux and High Selectivity Thin-Film Composite Membranes Based on Ultrathin Polyethylene Porous Substrates for Continuous Removal of Anionic Dyes. J. Environ. Chem. Eng. 2022, 10, 107202. [Google Scholar] [CrossRef]
- Mostafa, M.H.; Elsawy, M.A.; Darwish, M.S.A.; Hussein, L.I.; Abdaleem, A.H. Microwave-Assisted Preparation of Chitosan/ZnO Nanocomposite and Its Application in Dye Removal. Mater. Chem. Phys. 2020, 248, 122914. [Google Scholar] [CrossRef]
- Somsesta, N.; Sricharoenchaikul, V.; Aht-Ong, D. Adsorption Removal of Methylene Blue onto Activated Carbon/Cellulose Biocomposite Films: Equilibrium and Kinetic Studies. Mater. Chem. Phys. 2020, 240, 122221. [Google Scholar] [CrossRef]
- Senthil Kumar, P.; Fernando, P.S.A.; Ahmed, R.T.; Srinath, R.; Priyadharshini, M.; Vignesh, A.M.; Thanjiappan, A. Effect of Temperature on the Adsorption of Methylene Blue Dye onto Sulfuric Acid–Treated Orange Peel. Chem. Eng. Commun. 2014, 201, 1526–1547. [Google Scholar] [CrossRef]
- Mohammed, N.; Grishkewich, N.; Berry, R.M.; Tam, K.C. Cellulose Nanocrystal–Alginate Hydrogel Beads as Novel Adsorbents for Organic Dyes in Aqueous Solutions. Cellulose 2015, 22, 3725–3738. [Google Scholar] [CrossRef]
- Sahu, S.; Pahi, S.; Tripathy, S.; Singh, S.K.; Behera, A.; Sahu, U.K.; Patel, R.K. Adsorption of Methylene Blue on Chemically Modified Lychee Seed Biochar: Dynamic, Equilibrium, and Thermodynamic Study. J. Mol. Liq. 2020, 315, 113743. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, X.; Chen, B. Stable Graphene Oxide/Poly (Ethyleneimine) 3D Aerogel with Tunable Surface Charge for High Performance Selective Removal of Ionic Dyes from Water. Chem. Eng. J. 2018, 334, 1119–1127. [Google Scholar] [CrossRef]
- Abatal, M.; Córdova Quiroz, A.V.; Olguín, M.T.; Vázquez-Olmos, A.R.; Vargas, J.; Anguebes-Franseschi, F.; Giácoman-Vallejos, G. Sorption of Pb (II) from Aqueous Solutions by Acid-Modified Clinoptilolite-Rich Tuffs with Different Si/Al Ratios. Appl. Sci. 2019, 9, 2415. [Google Scholar] [CrossRef]
- Oyewo, O.A.; Adeniyi, A.; Sithole, B.B.; Onyango, M.S. Sawdust-Based Cellulose Nanocrystals Incorporated with ZnO Nanoparticles as Efficient Adsorption Media in the Removal of Methylene Blue Dye. ACS Omega 2020, 5, 18798–18807. [Google Scholar] [CrossRef]
- Hameed, B.H.; Ahmad, A.L.; Latiff, K.N.A. Adsorption of Basic Dye (Methylene Blue) onto Activated Carbon Prepared from Rattan Sawdust. Dye. Pigment. 2007, 75, 143–149. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Hameed, B.H.; Aziz, N. Adsorption of Direct Dye on Palm Ash: Kinetic and Equilibrium Modeling. J. Hazard. Mater. 2007, 141, 70–76. [Google Scholar] [CrossRef]
- El Qada, E.N.; Allen, S.J.; Walker, G.M. Adsorption of Basic Dyes from Aqueous Solution onto Activated Carbons. Chem. Eng. J. 2008, 135, 174–184. [Google Scholar] [CrossRef]
- Yang, C. Statistical Mechanical Study on the Freundlich Isotherm Equation. J. Colloid Interface Sci. 1998, 208, 379–387. [Google Scholar] [CrossRef]
- Sunderland, N.; Catalano, T.; Kendall, E.; McAuliffe, D.; Chenoweth, L. Exploring the Concept of Moral Distress with Community-Based Researchers: An Australian Study. J. Soc. Serv. Res. 2011, 37, 73–85. [Google Scholar] [CrossRef]
- Alkan, M.; Demirbaş, Ö.; Doğan, M. Adsorption Kinetics and Thermodynamics of an Anionic Dye onto Sepiolite. Microporous Mesoporous Mater. 2007, 101, 388–396. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Gupta, V.K. Rice Husk and Its Ash as Low-Cost Adsorbents in Water and Wastewater Treatment. Ind. Eng. Chem. Res. 2011, 50, 13589–13613. [Google Scholar] [CrossRef]
- Batzias, F.; Sidiras, D.; Schroeder, E.; Weber, C. Simulation of Dye Adsorption on Hydrolyzed Wheat Straw in Batch and Fixed-Bed Systems. Chem. Eng. J. 2009, 148, 459–472. [Google Scholar] [CrossRef]
- Zhang, Z.; O’Hara, I.M.; Kent, G.A.; Doherty, W.O.S. Comparative Study on Adsorption of Two Cationic Dyes by Milled Sugarcane Bagasse. Ind. Crops Prod. 2013, 42, 41–49. [Google Scholar] [CrossRef]
- Rao, V.V.B.; Rao, S.R.M. Adsorption Studies on Treatment of Textile Dyeing Industrial Effluent by Flyash. Chem. Eng. J. 2006, 116, 77–84. [Google Scholar]
- Aygün, A.; Yenisoy-Karakaş, S.; Duman, I. Production of Granular Activated Carbon from Fruit Stones and Nutshells and Evaluation of Their Physical, Chemical and Adsorption Properties. Microporous Mesoporous Mater. 2003, 66, 189–195. [Google Scholar] [CrossRef]
- Tan, C.H.C.; Sabar, S.; Hussin, M.H. Development of Immobilized Microcrystalline Cellulose as an Effective Adsorbent for Methylene Blue Dye Removal. South African J. Chem. Eng. 2018, 26, 11–24. [Google Scholar] [CrossRef]
- Kangwansupamonkon, W.; Jitbunpot, W.; Kiatkamjornwong, S. Photocatalytic Efficiency of TiO2/Poly [Acrylamide-Co-(Acrylic Acid)] Composite for Textile Dye Degradation. Polym. Degrad. Stab. 2010, 95, 1894–1902. [Google Scholar] [CrossRef]






| Thin Film Formulation | Thin Film Composition | Dye Removal Efficiency | |||
|---|---|---|---|---|---|
| CNC | PAA | AC | Removal Capacity | Removal Percentage | |
| C0 | 0.0% | 80.0% | 20% | 38.26 mg/g | 76.53% |
| C1 | 40.0% | 40.0% | 20% | 39.37 mg/g | 78.75% |
| C2 | 53.30% | 26.65% | 20% | 39.62 mg/g | 79.24% |
| C3 | 60% | 20% | 20% | 41.61 mg/g | 83.22% |
| C4 | 64% | 16.0% | 20% | 43.15 mg/g | 86.30% |
| Adsorbent | Maximum Adsorption Capacity (mg/g) | Reference |
|---|---|---|
| Rice husk | 19.77 | [63] |
| Wheat straw | 3.82 | [64] |
| Milled sugarcane | 9.90 | [65] |
| Activated carbon | 9.81 | [66] |
| Almond shell-AC | 1.33 | [67] |
| Microcrystalline cellulose | 12.85 | [68] |
| TiO2/Poly(acrylamide-co-acrylic acid) | 21.3 | [69] |
| CNC, PAA, and AC | 43.15 | Our work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almuslem, A.S.; Alnaim, N.; Ibrahim, S.S.; Ibrahim, M.A. Green Synthesis and Characteristics of Cellulose Nanocrystal/Poly Acrylic Acid Nanocomposite Thin Film for Organic Dye Adsorption during Water Treatment. Polymers 2023, 15, 2154. https://doi.org/10.3390/polym15092154
Almuslem AS, Alnaim N, Ibrahim SS, Ibrahim MA. Green Synthesis and Characteristics of Cellulose Nanocrystal/Poly Acrylic Acid Nanocomposite Thin Film for Organic Dye Adsorption during Water Treatment. Polymers. 2023; 15(9):2154. https://doi.org/10.3390/polym15092154
Chicago/Turabian StyleAlmuslem, Amani Saleh, Nisrin Alnaim, Sobhy S. Ibrahim, and Mostafa A. Ibrahim. 2023. "Green Synthesis and Characteristics of Cellulose Nanocrystal/Poly Acrylic Acid Nanocomposite Thin Film for Organic Dye Adsorption during Water Treatment" Polymers 15, no. 9: 2154. https://doi.org/10.3390/polym15092154
APA StyleAlmuslem, A. S., Alnaim, N., Ibrahim, S. S., & Ibrahim, M. A. (2023). Green Synthesis and Characteristics of Cellulose Nanocrystal/Poly Acrylic Acid Nanocomposite Thin Film for Organic Dye Adsorption during Water Treatment. Polymers, 15(9), 2154. https://doi.org/10.3390/polym15092154






