Impact of ZnS/Mn on the Structure, Optical, and Electric Properties of PVC Polymer
Abstract
1. Introduction
2. Methods and Materials
2.1. Chemicals Used
2.2. Synthesis Procedure
3. Results and Discussion
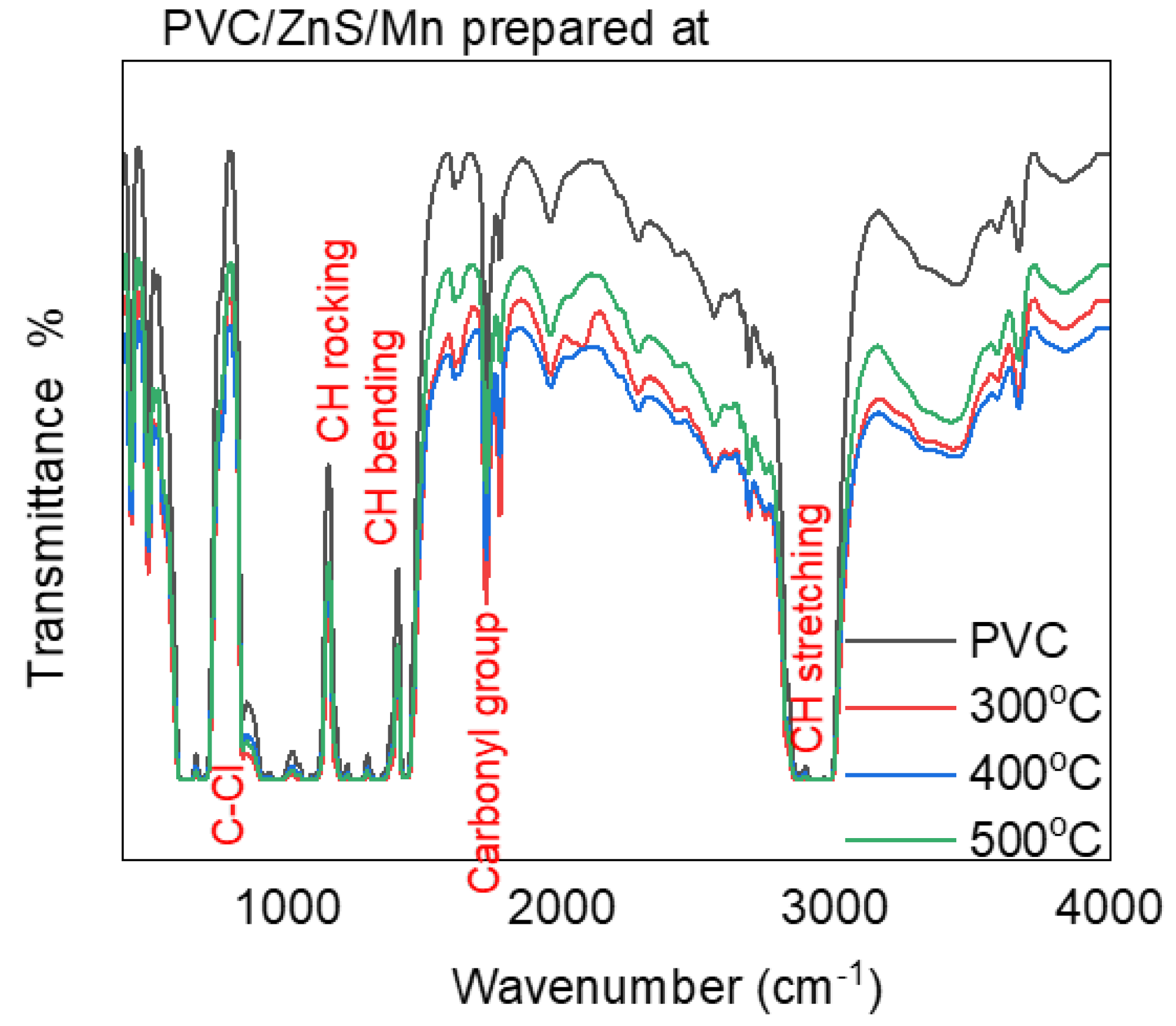
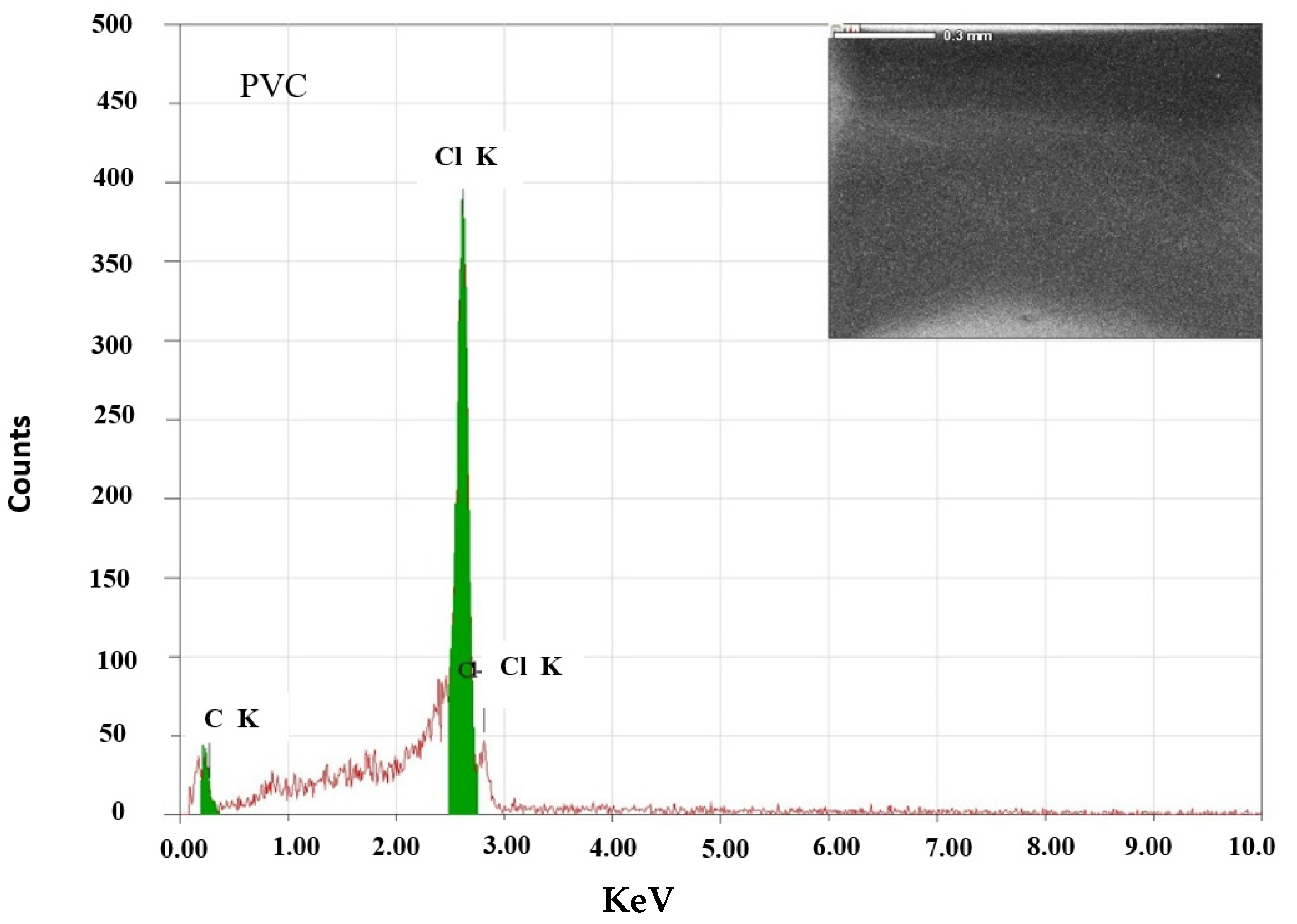
3.1. Structural Investigation
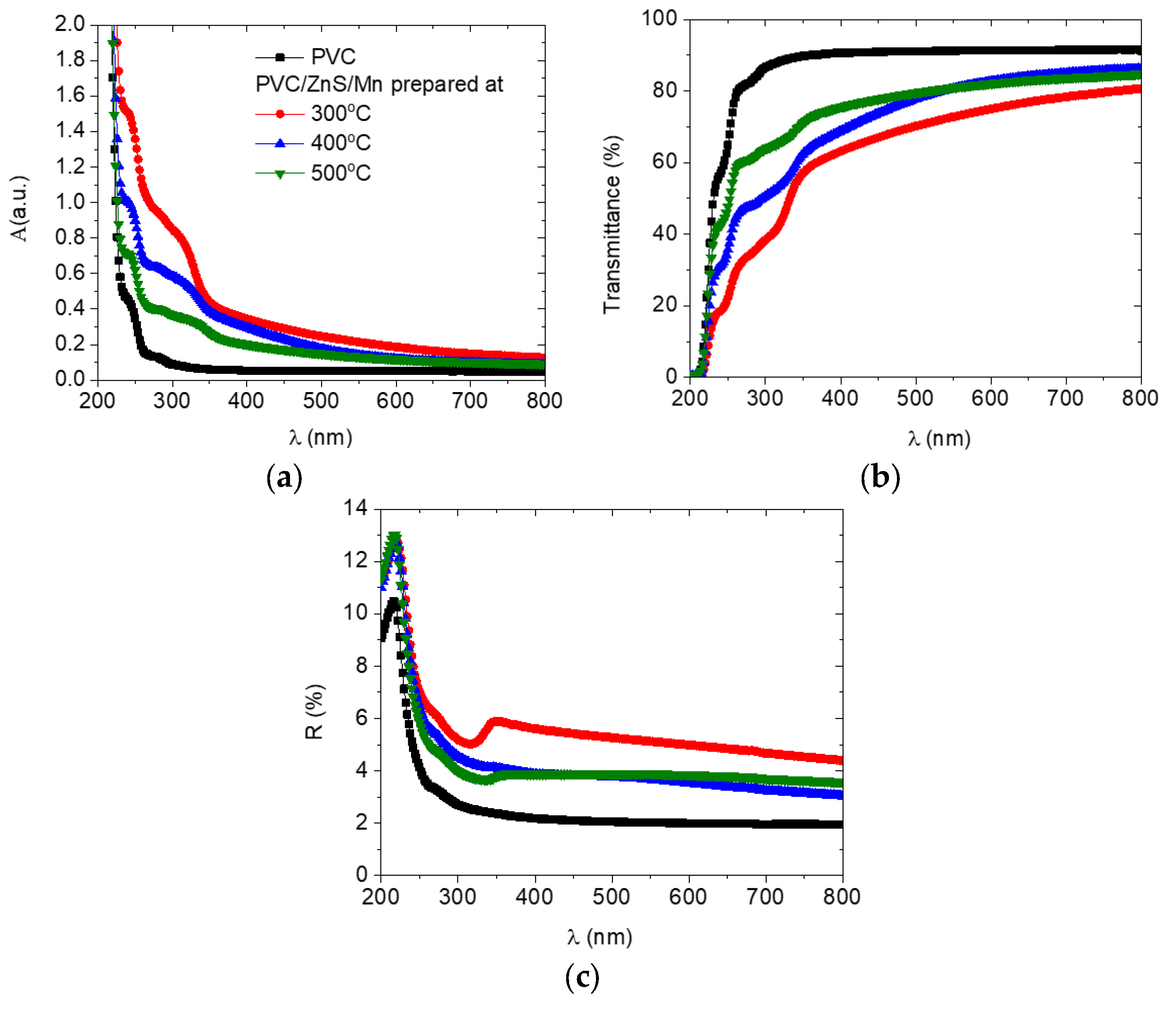
3.2. Optical Features
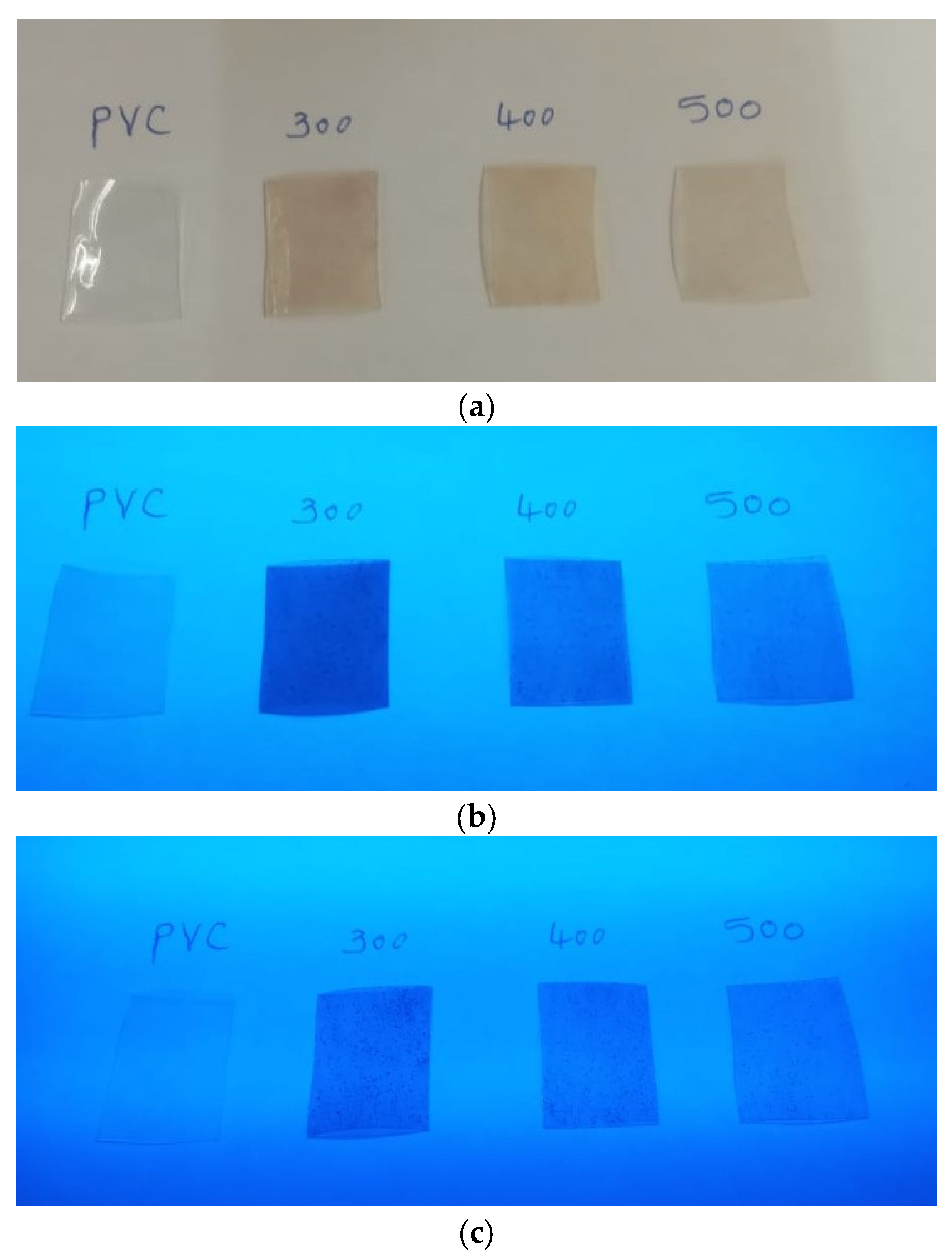
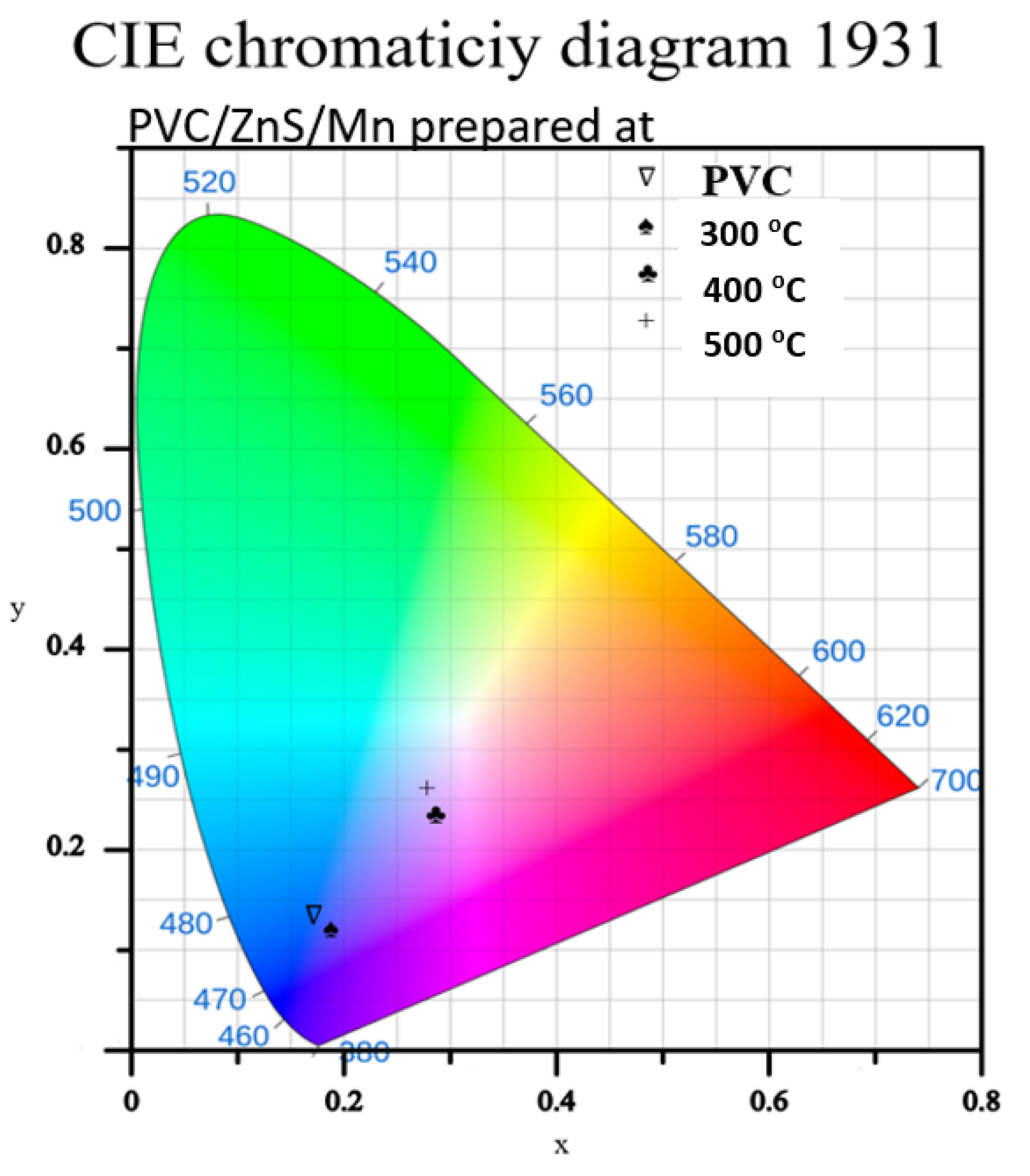
3.3. Fluorescence Analysis
3.4. Dielectric Characteristics
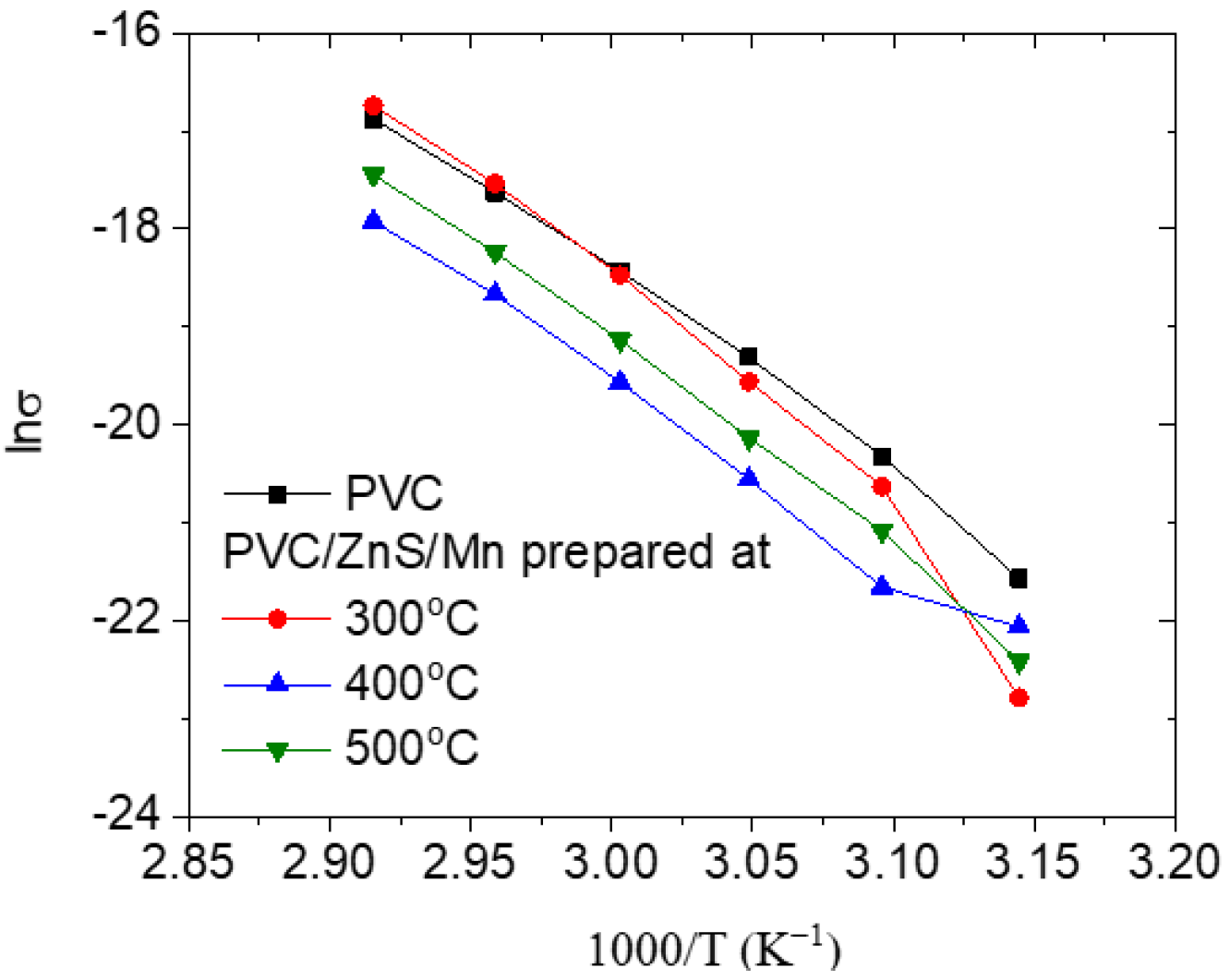
3.5. DC Electrical Conductivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, A.A.; Alsaad, A.M.; Al-Bataineh, Q.M.; Al-Akhras, M.-A.H.; Albataineh, Z.; Alizzy, K.A.; Daoud, N.S. Synthesis and characterization of ZnO NPs-doped PMMA-BDK-MR polymer-coated thin films with UV curing for optical data storage applications. Polym. Bull. 2021, 78, 1189–1211. [Google Scholar] [CrossRef]
- Alsaad, A.M.; Al-Bataineh, Q.M.; Ahmad, A.A.; Jum’h, I.; Alaqtash, N.; Bani-Salameh, A.A. Optical properties of transparent PMMA-PS/ZnO NPs polymeric nanocomposite films: UV-Shielding applications. Mater. Res. Exp. 2020, 6, 126446. [Google Scholar] [CrossRef]
- Jum’h, I.; Mousa, M.S.; Mhawish, M.; Sbeih, S.; Telfah, A. Optical and structural properties of (PANI-CSA-PMMA)/NiNPs nanocomposites thin films for organic optical filters. J. Appl. Polym. Sci. 2019, 137, 48643. [Google Scholar] [CrossRef]
- Chamroukhi, H.; Hamed, Z.B.; Telfah, A.; Bassou, M.; Zeinert, A.; Hergenröder, R.; Bouchriha, H. Optical and structural properties enhancement of hybrid nanocomposites thin films based on polyaniline doped with Zinc Oxide embedded in bimodal mesoporous silica (ZnO@SiOX) nanoparticles. Opt. Mater. 2018, 84, 703–713. [Google Scholar] [CrossRef]
- Aouachria, K.; Belhaneche-Bensemra, N. Miscibility of PVC/PMMA blends by vicat softening temperature, viscometry, DSC and FTIR analysis. Polym. Test. 2006, 25, 1101–1108. [Google Scholar] [CrossRef]
- Demirselcuk, B.; Kus, E.; Kucukarslan, A.; Sarica, E.; Akyuz, I.; Bilgin, V. Optimization of chemically sprayed ZnS films by Mn doping. Phys. B 2021, 622, 413353. [Google Scholar] [CrossRef]
- Trung, D.Q.; Tran, M.-T.; Hung, N.D.; Van, Q.N.; Huyen, N.T.; Tu, N.; Thanh, H.P. Emission-tunable Mn-doped ZnS/ZnO heterostructure nanobelts for UV-pump WLEDs. Opt. Mater. 2021, 121, 111587. [Google Scholar] [CrossRef]
- Barman, B.; Sarma, K.C. Low temperature chemical synthesis of ZnS, Mn doped ZnS nanosized particles: Their structural, morphological and photophysical properties. Solid State Sci. 2020, 109, 106404. [Google Scholar] [CrossRef]
- Gholamzadeh, L.; Sharghi, H.; Aminian, M.K. Synthesis of barium-doped PVC/Bi2WO6 composites for X-ray radiation shielding. Nucl. Eng. Technol. 2022, 54, 318–325. [Google Scholar] [CrossRef]
- Abdel Maksoud, M.I.A.; Bekhit, M.; Waly, A.L.; Awed, A.S. Optical and dielectric properties of polymer nanocomposite based on PVC matrix and Cu/Cu2O nanorods synthesized by gamma irradiation for energy storage applications. Phys. E 2023, 148, 115661. [Google Scholar] [CrossRef]
- Badawi, A.; Alharthi, S.S. Tailoring the photoluminescent and electrical properties of tin-doped ZnS@PVP polymeric composite films for LEDs applications. Superlattices Microstruct. 2021, 151, 106838. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Abd El-Fattah, Z.M.; Anad, N.S.; Attallah, M.; El-Bahnasawy, H.H. Thermo-mechanical and opto-electrical study of Cr-doped-ZnO-based polyvinyl chloride nanocomposites. Mater. Sci. Mater. Electron. 2023, 34, 113. [Google Scholar] [CrossRef]
- Telfah, M.; Ahmad, A.A.; Alsaad, A.M.; Al-Bataineh, Q.M.; Telfah, A. Doping mechanism and optical properties of as-prepared polyvinyl chloride (PVC) doped by iodine thin films. Polym. Bull. 2022, 79, 10803–10822. [Google Scholar] [CrossRef]
- Patil, H.; Marathe, K. Studies in synthesis of nano-zinc oxide mixed PVC matrix membrane and its application for ibuprofen drug removal. J. Hazard. Mater. Adv. 2023, 9, 100247. [Google Scholar] [CrossRef]
- Iribarren, A.; Rivero, P.J.; Berlanga, C.; Larumbe, S.; Miguel, A.; Palacio, J.F.; Rodriguez, R. Multifunctional Protective PVC-ZnO Nanocomposite Coatings Deposited on Aluminum Alloys by Electrospinning. Coatings 2019, 9, 921. [Google Scholar] [CrossRef]
- Rouabah, N.; Boudine, B.; Nazir, R.; Zaabat, M.; Sebais, M.; Halimi, O.; Soltani, M.T.; Chala, A. Structural, optical and photocatalytic properties of PVC/CdS nanocomposites prepared by soft chemistry method. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1102–1110. [Google Scholar] [CrossRef]
- Rasmagin, S.I.; Novikov, I.K. Optical Properties of CdSe/ZnS Nanoparticles in Heat-Treated Polyvinylchloride Films. Semiconductors 2019, 53, 499–502. [Google Scholar] [CrossRef]
- Thai, D.V.; Ben, P.V.; Thi, T.M.; Truong, N.V.; Thu, H.H. The photoluminescence enhancement of Mn2+ ions and the crystal field in ZnS:Mn nanoparticles covered by polyvinyl alcohol. Opt. Quant. Electron. 2016, 48, 362. [Google Scholar] [CrossRef]
- Anandalli, M.H.; Bhajantri, R.F.; Maidur, S.R.; Shastri, L.A.; Sasirekha, V. Dominant role of organic dye on Mn: ZnS/PMMA polymer nanocomposites: Structural, thermal, linear, and nonlinear optical properties for optoelectronic and photonic applications. Mater. Today Commun. 2023, 35, 105918. [Google Scholar] [CrossRef]
- El-naggar, A.M.; Heiba, Z.K.; Mohamed, M.B.; Kamal, A.M.; Lakshminarayana, G.; Abd-Elkader, O.H. Effect of MnS/ZnS nanocomposite on the structural, linear and nonlinear optical properties of PVA/CMC blended polymer. Opt. Mater. 2022, 128, 112379. [Google Scholar] [CrossRef]
- Huong, T.T.T.; Loan, N.T.; Loc, D.X.; Thuy, U.T.D.; Stoilova, O.; Liem, N.Q. Enhanced luminescence in electrospun polymer hybrids containing Mn-doped ZnSe/ZnS nanocrystals. Opt. Mater. 2021, 113, 110858. [Google Scholar] [CrossRef]
- Mohamed, M.B. Effect of doping and changing of the annealing temperature on the structural and optical properties of ZnS. Int. J. Appl. Ceram. Technol. 2020, 17, 823–831. [Google Scholar] [CrossRef]
- El-naggar, A.M.; Heiba, Z.K.; Kamal, A.M. Mohamed Bakr Mohamed, Assessment of performance based on structure, dielectric and linear/nonlinear optical properties of PVA/CMC/PVP blends loaded with ZnS/Co. Opt. Quant. Electron. 2023, 55, 513. [Google Scholar] [CrossRef]
- El-naggar, A.M.; Heiba, Z.K.; Kamal, A.M.; Abd-Elkader, O.H.; Mohamed, M.B. Exploring the structural, dielectric, and optical characterizations of PVA/CMC blend upon loading with nano-Zn0.9Fe0.1S. Mater. Sci. Mater. Electron. 2023, 34, 965. [Google Scholar] [CrossRef]
- El-naggar, A.M.; Heiba, Z.K.; Kamal, A.M.; Abd-Elkader, O.H.; Lakshminarayana, G.; Mohamed, M.B. Linear and nonlinear optical characteristics of PVA/CMC/PEG blended polymer loaded with ZnS formed at different temperature. Mater. Sci. Mater. Electron. 2023, 34, 114. [Google Scholar] [CrossRef]
- El-naggar, A.M.; Heiba, Z.K.; Mohamed, M.B.; Kamal, A.M.; Lakshminarayana, G. Thermal, linear and nonlinear optical properties of PVA/PVP/PEG blends loaded with nanovanadium-doped nano tin disulfide. Mater. Sci. Mater. Electron. 2022, 33, 25743. [Google Scholar] [CrossRef]
- El-naggar, A.M.; Heiba, Z.K.; Kamal, A.M.; Altowairqi, Y.; Mohamed, M.B. Impact of loading PVA/CMC/PVP blend with CdS0. 9M0. 1 non-stoichiometrically doped by transition metals (M). Opt. Mater. 2022, 133, 113085. [Google Scholar] [CrossRef]
- Sharma, S.; Basu, T.; Shahee, A.; Singh, K.; Lalla, N.P.; Sampathkumaran, E.V. Complex dielectric and impedance behavior of magnetoelectric Fe2TiO5. J. Alloys Compd. 2016, 663, 289–294. [Google Scholar] [CrossRef]
- Brunner, A.J. X-ray diffraction pattern of poly(vinyl chloride). Polym. Lett. 1972, 10, 379–383. [Google Scholar] [CrossRef]
- Mallakpour, S.; Shamsaddinimotlagh, S. Ultrasonic-promoted rapid preparation of PVC/TiO2-BSA nanocomposites: Characterization and photocatalytic degradation of methylene blue. Ultrason. Sonochem. 2018, 41, 361–374. [Google Scholar] [CrossRef]
- Jia, P.; Zhang, M.; Hu, L.; Wang, R.; Sun, C.; Zhou, Y. Cardanol Groups Grafted on Poly(vinyl chloride)—Synthesis, Performance and Plasticization Mechanism. Polymers 2017, 9, 621. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Nie, X.A.; Jiang, J.C.; Zhou, Y.H. Thermal degradation and plasticizing mechanism of poly(vinyl chloride) plasticized with a novel cardanol derived plasticizer. IOP Conf. Ser. Mater. Sci. Eng. 2017, 292, 01200. [Google Scholar] [CrossRef]
- Soman, V.V.; Kelkar, D.S. FTIR Studies of Doped PMMA–PVC Blend System. Macromol. Symp. 2009, 277, 152–161. [Google Scholar] [CrossRef]
- Rajendran, S.; Sivakumar, P.; Babu, R.S. Studies on the salt concentration of a PVdF–PVC based polymer blend electrolyte. J. Power Source 2007, 164, 815–821. [Google Scholar] [CrossRef]
- El-Zahed, H.; Dongol, M.; Radwan, M. Annealing and thickness effect on the optical absorption of Ge20Te80 and Cu6Ge14Te80 films. Eur. Phys. J. AP 2002, 17, 179–186. [Google Scholar] [CrossRef]
- Tanaka, K. Optical properties and photoinduced changes in amorphous As-S films. Thin Solid Films 1980, 66, 271–279. [Google Scholar] [CrossRef]
- El-Shamy, A.G. The optical anatomy of new polyvinyl alcohol/zinc peroxide (PVA/ZnO2) nanocomposite films for promising optical limiting applications. Prog. Org. Coat. 2021, 150, 105981. [Google Scholar] [CrossRef]
- El-naggar, A.M.; Heiba, Z.K.; Kamal, A.M.; Alzahrani, K.E.; Abd-Elkader, O.H.; Mohamed, M.B. Impact of natural melanin doping on the structural, optical and dielectric characteristics of the PVP/CMC blend. J. Taibah Univ. Sci. 2023, 17, 2190731. [Google Scholar] [CrossRef]
- Aziz, S.B.; Ahmed, H.M.; Hussein, A.M.; Fathulla, A.B.; Wsw, R.M.; Hussein, R.T. Tuning the absorption of ultraviolet spectra and optical parameters of aluminum doped PVA based solid polymer composites. Mater. Sci. Mater. Electron. 2015, 26, 8022–8028. [Google Scholar] [CrossRef]
- Wu, C.; Wicks, D.A. Use of fluorescence for the high-throughput evaluation of synergistic thermal and photo stabilizer interactions in poly (vinyl chloride). Rev. Sci. Instrum. 2005, 76, 062212. [Google Scholar] [CrossRef]
- Somersall, A.C.; Guillet, J.E. Photoluminescence of Synthetic Polymers. J. Macromol. Sci. Part C 1975, 13, 135–187. [Google Scholar] [CrossRef]
- El-Hachemi, B.; Miloud, S.; Sabah, M.; Souad, T.; Zineddine, O.; Boubekeur, B.; Toufik, S.M.; Ouahiba, H. Structural, Electrical and Optical Properties of PVC/ZnTe Nanocomposite Thin Films. J. Inorg. Organomet. Polym. Mater. 2021, 31, 3637–3648. [Google Scholar] [CrossRef]
- Silva, R.C.; Silva, L.A.; Araújo, P.L.B.; Araújo, E.S.; Santos, R.F.S.; Aquino, K.A.S. ZnS Nanocrystals as an Additive for Gamma-Irradiated Poly (Vinyl Chloride). Mater. Res. 2017, 20 (Suppl. 2), 851–857. [Google Scholar] [CrossRef]
- Khan, M.M.R.; Pal, S.; Hoque, M.M.; Alam, M.R.; Younus, M.; Kobayashi, H. Simple fabrication of PVA–ZnS composite films with superior photocatalytic performance: Enhanced luminescence property, morphology, and thermal stability. ACS Omega 2019, 4, 6144–6153. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, K.; Vadera, S.R.; Kumar, N.; Kutty, T.R.N. Energy transfer from organic surface adsorbate-polyvinyl pyrrolidone molecules to luminescent centers in ZnS nanocrystals. Solid State Commun. 2004, 129, 469–473. [Google Scholar] [CrossRef]
- Rajesh, K.; Crasta, V.; Kumar, N.B.R.; Shetty, G.; Rekha, P.D. Structural, optical, mechanical and dielectric properties of titanium dioxide doped PVA/PVP nanocomposite. J. Polym. Res. 2019, 26, 99. [Google Scholar] [CrossRef]
- Ramya, C.S.; Selvasekarapandian, S.; Hirankumar, G.; Savitha, T.; Angelo, P.C. Investigation on dielectric relaxations of PVP–NH4SCN polymer electrolyte. J. Non-Cryst. Solids 2008, 354, 1494–1502. [Google Scholar] [CrossRef]
- Choudhary, S. Dielectric dispersion and relaxations in (PVA-PEO)-ZnO polymer nanocomposites. Phys. B 2017, 522, 48–56. [Google Scholar] [CrossRef]
- Yang, W.H.; Yu, S.H.; Sun, R. Nano- and microsize effect of CCTO fillers on the dielectric behavior of CCTO/PVDF composites. Acta Mater. 2011, 59, 5593–5602. [Google Scholar] [CrossRef]
- Dang, Z.M.; Yuan, J.K.; Yao, S.H. Flexible Nanodielectric Materials with High Permittivity for Power Energy Storage. Adv. Mater. 2013, 25, 6334–6365. [Google Scholar] [CrossRef]
- Aslam, M.; Kalyar, M.A.; Raza, Z.A. Effect of Separate Zinc, Copper and Graphene Oxides Nanofillers on Electrical Properties of PVA Based Composite Strips. J. Electron. Mater. 2019, 48, 1116–1121. [Google Scholar] [CrossRef]
- Hema, M.; Selvasekerapandian, S.; Sakunthala, A.; Arunkumar, D.; Nithya, H. Structural, vibrational and electrical characterization of PVA–NH4Br polymer electrolyte system. Phys. B Condens. Matter. 2008, 403, 2740–2747. [Google Scholar] [CrossRef]
- Rayssi, C.; El Kossi, S.; Dhahri, J.; Khirouni, K. Frequency and temperature-dependence of dielectric permittivity and electric modulus studies of the solid solution Ca0.85Er0.1Ti1−xCo4x/3O3 (0 ≤ x ≤ 0.1). RSC Adv. 2018, 8, 17139–17150. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.B. Study of electrical percolation phenomenon from the dielectric and electric modulus analysis. Bull. Mater. Sci. 2015, 38, 1597–1602. [Google Scholar] [CrossRef]
- Singh, P.; Gupta, P.N.; Saroj, A.L. Ion dynamics and dielectric relaxation behavior of PVA-PVP-NaI-SiO2 based nano-composites polymer blend electrolytes. Phys. B Condens. Matter 2020, 578, 411850. [Google Scholar] [CrossRef]
- Makled, M.H.; Sheha, E.; Shanap, T.S.; El-Mansy, M.K. Electrical conduction and dielectric relaxation in p-type PVA/CuI polymer composite. J. Adv. Res. 2013, 4, 531–538. [Google Scholar] [CrossRef]
- Wadatkar, N.S.; Waghuley, S.A. Characterizing the electro-optical properties of polyaniline/poly(vinyl acetate) composite films as-synthesized through chemical route. Results Surf. Interfaces 2021, 4, 100016. [Google Scholar] [CrossRef]





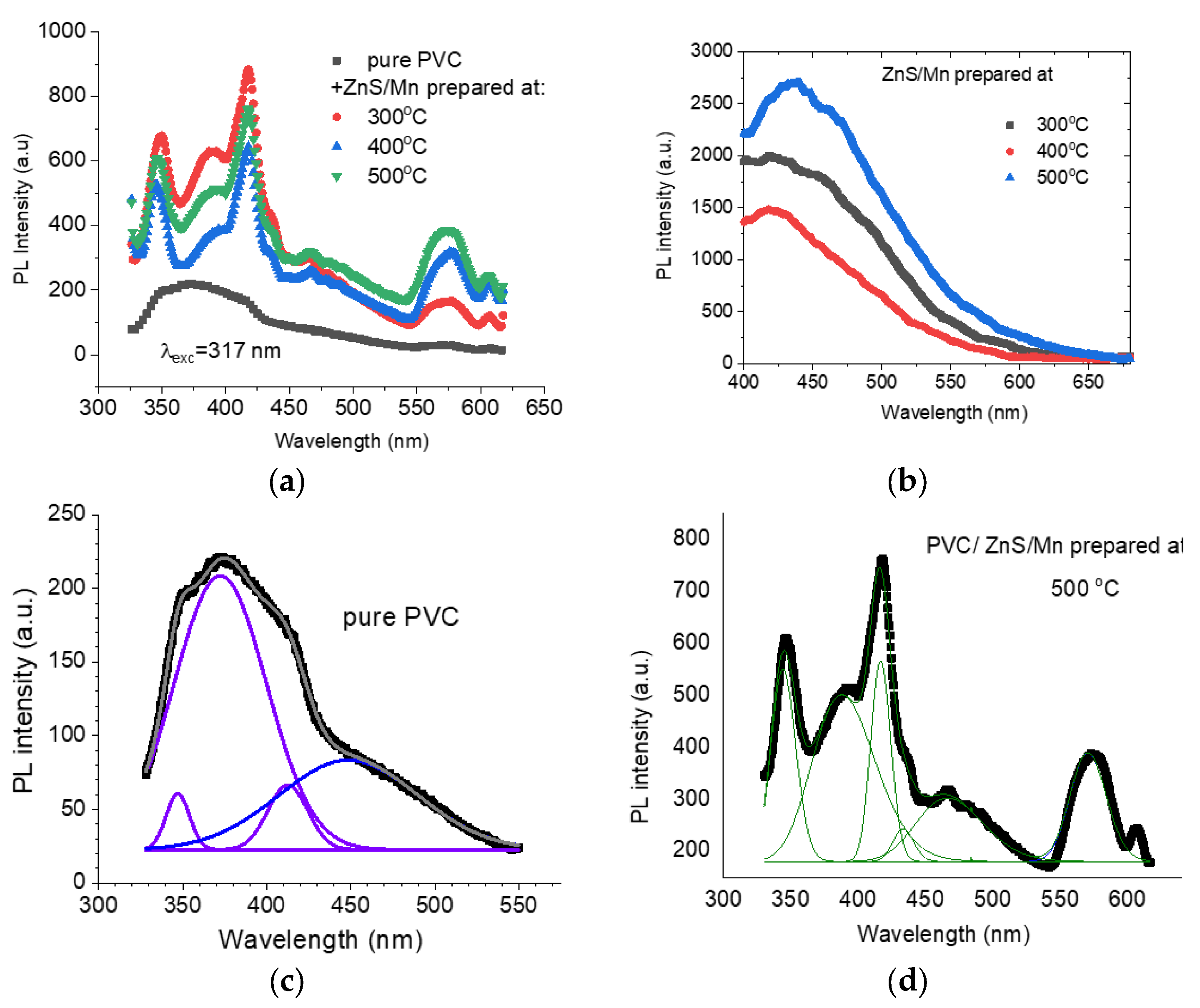
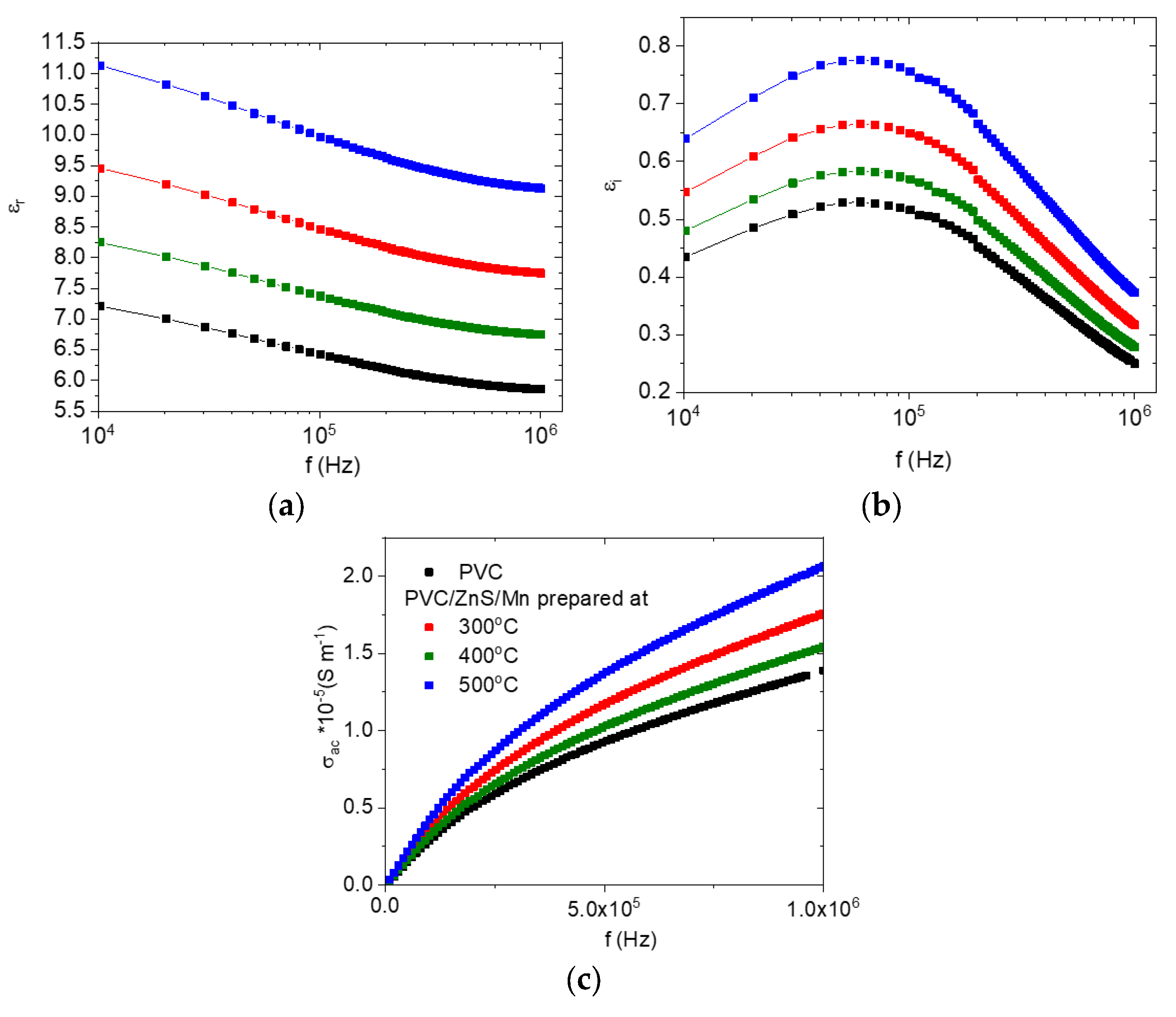
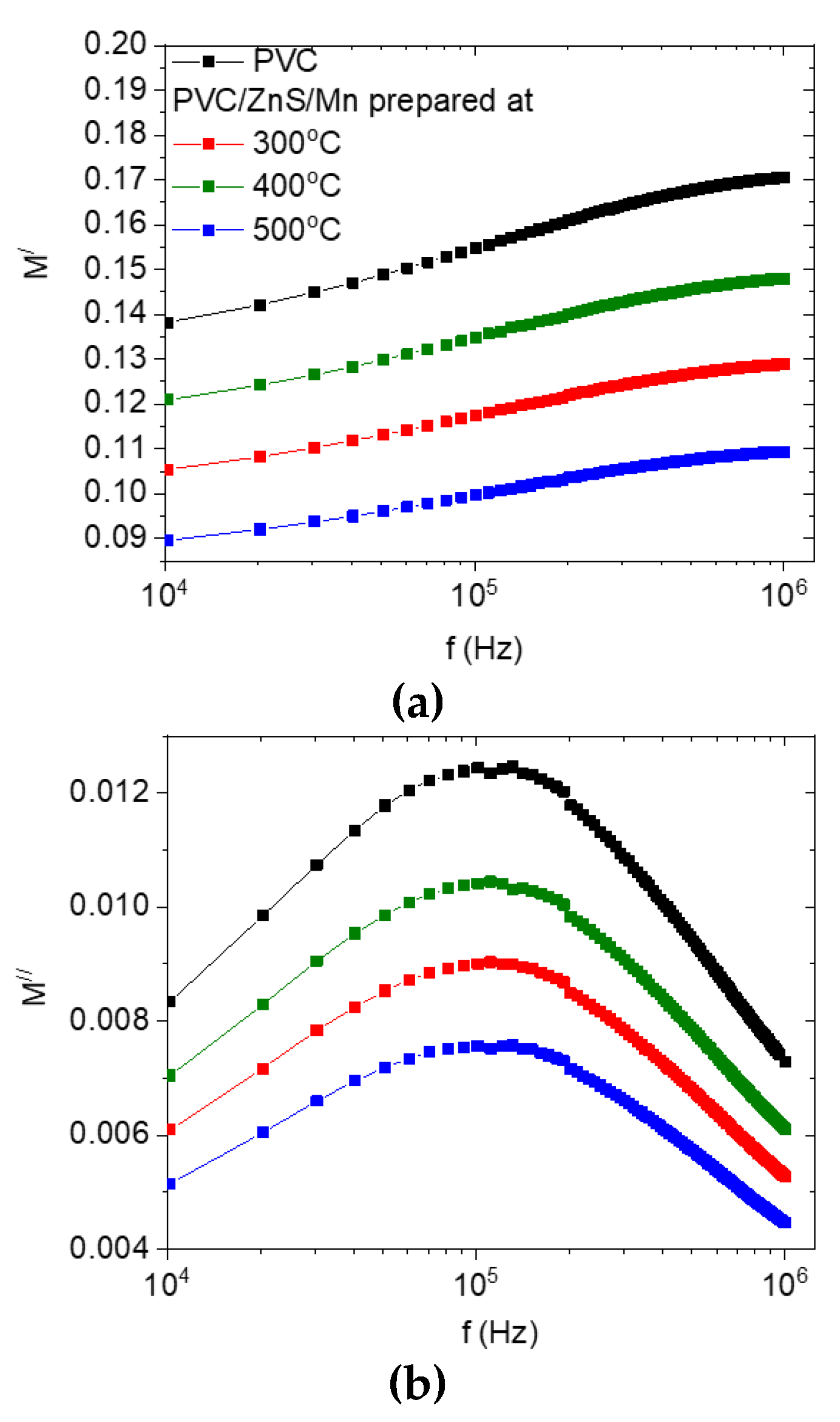
| T °C | a (A) | Size (nm) | Strain |
|---|---|---|---|
| 300 | 5.3750 (7) | 20 | 0.0115 |
| 400 | 5.3756 | 22 | 0.0102 |
| 500 | 5.3787 | 25 | 0.0075 |
| Sample | Direct Eg (eV) | Indirect Eg (eV) | EA (eV) |
|---|---|---|---|
| Undoped PVC | 5.41 | 4.52 | 0.174 |
| Doped PVC with ZnS/Mn prepared at | |||
| 300 °C | 4.56 | 3.63 | 0.187 |
| 400 °C | 4.61 | 3.8 | 0.165 |
| 500 °C | 4.67 | 4.08 | 0.185 |
| Sample | Corresponding CIE Coordinates (x, y) for Emission |
|---|---|
| Undoped PVC | (0.1707, 0.1336) |
| Doped PVC with ZnS/Mn prepared at | |
| 300 °C | (0.1880, 0.1205) |
| 400 °C | (0.2845, 0.2346) |
| 500 °C | (0.2776, 0.2608) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Naggar, A.M.; Heiba, Z.K.; Kamal, A.M.; Abd-Elkader, O.H.; Mohamed, M.B. Impact of ZnS/Mn on the Structure, Optical, and Electric Properties of PVC Polymer. Polymers 2023, 15, 2091. https://doi.org/10.3390/polym15092091
El-Naggar AM, Heiba ZK, Kamal AM, Abd-Elkader OH, Mohamed MB. Impact of ZnS/Mn on the Structure, Optical, and Electric Properties of PVC Polymer. Polymers. 2023; 15(9):2091. https://doi.org/10.3390/polym15092091
Chicago/Turabian StyleEl-Naggar, A. M., Zein K. Heiba, A. M. Kamal, Omar H. Abd-Elkader, and Mohamed Bakr Mohamed. 2023. "Impact of ZnS/Mn on the Structure, Optical, and Electric Properties of PVC Polymer" Polymers 15, no. 9: 2091. https://doi.org/10.3390/polym15092091
APA StyleEl-Naggar, A. M., Heiba, Z. K., Kamal, A. M., Abd-Elkader, O. H., & Mohamed, M. B. (2023). Impact of ZnS/Mn on the Structure, Optical, and Electric Properties of PVC Polymer. Polymers, 15(9), 2091. https://doi.org/10.3390/polym15092091






