Fungal Selectivity and Biodegradation Effects by White and Brown Rot Fungi for Wood Biomass Pretreatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Biodegradation Decay Assessment
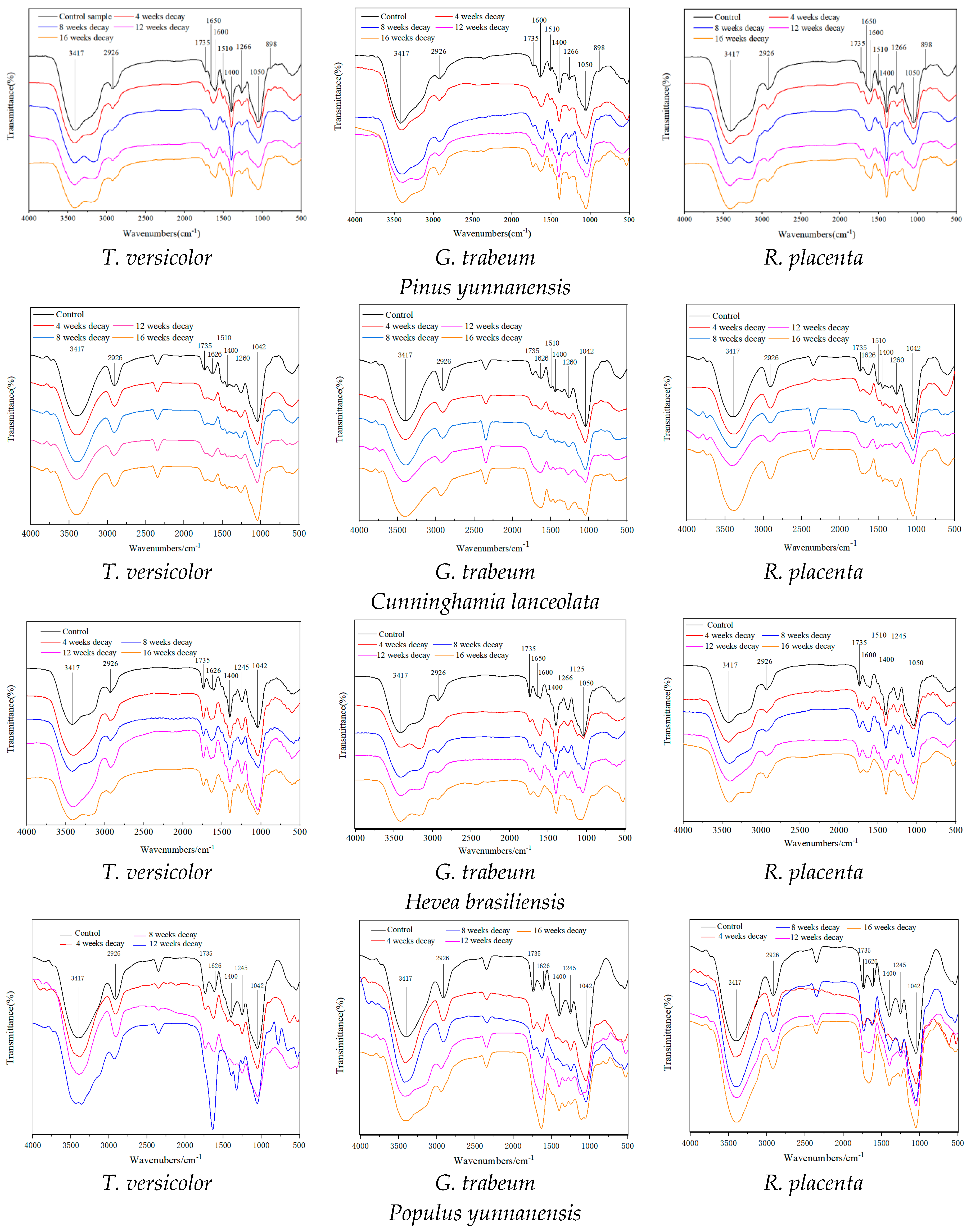
2.3. FTIR Analysis
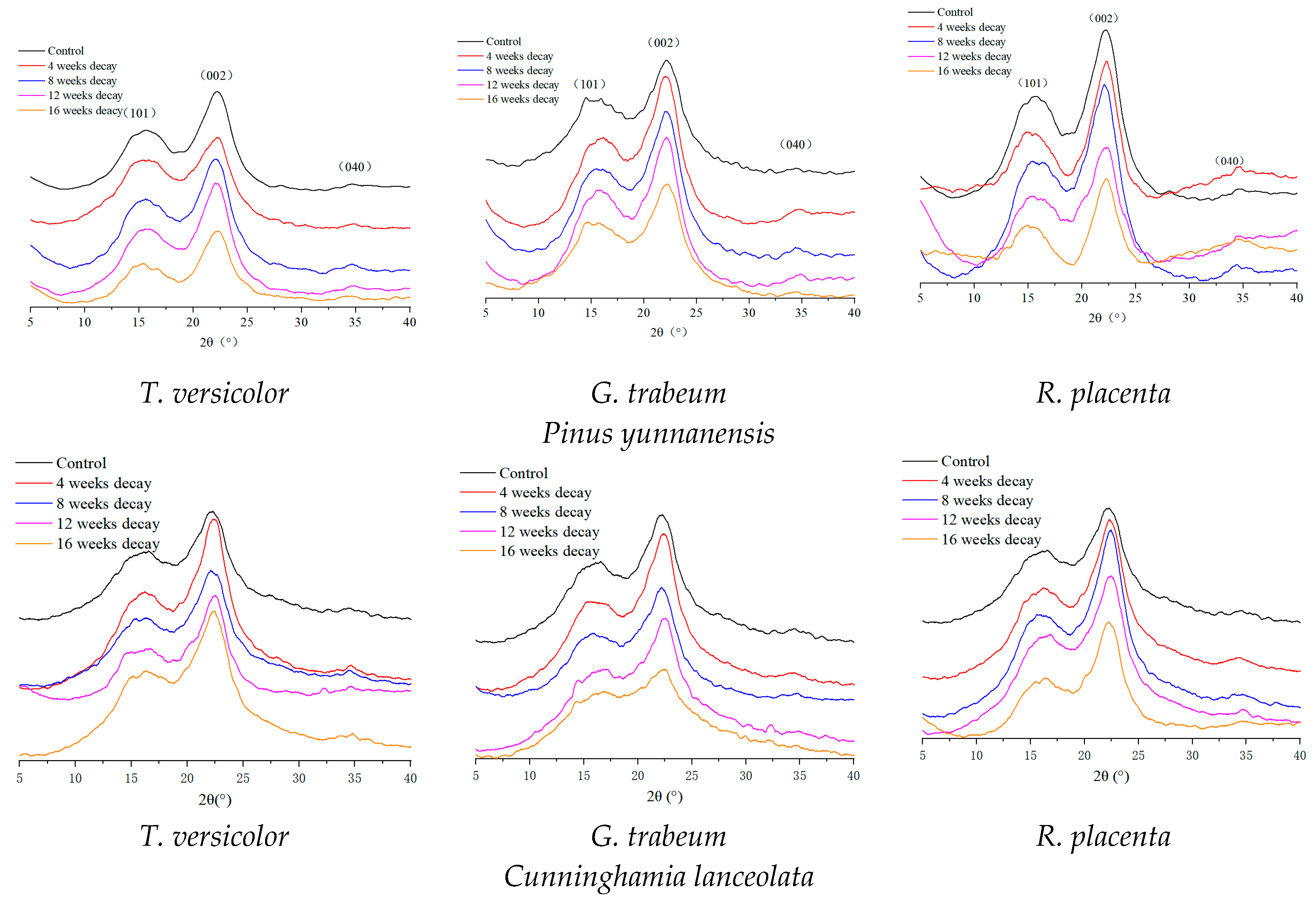
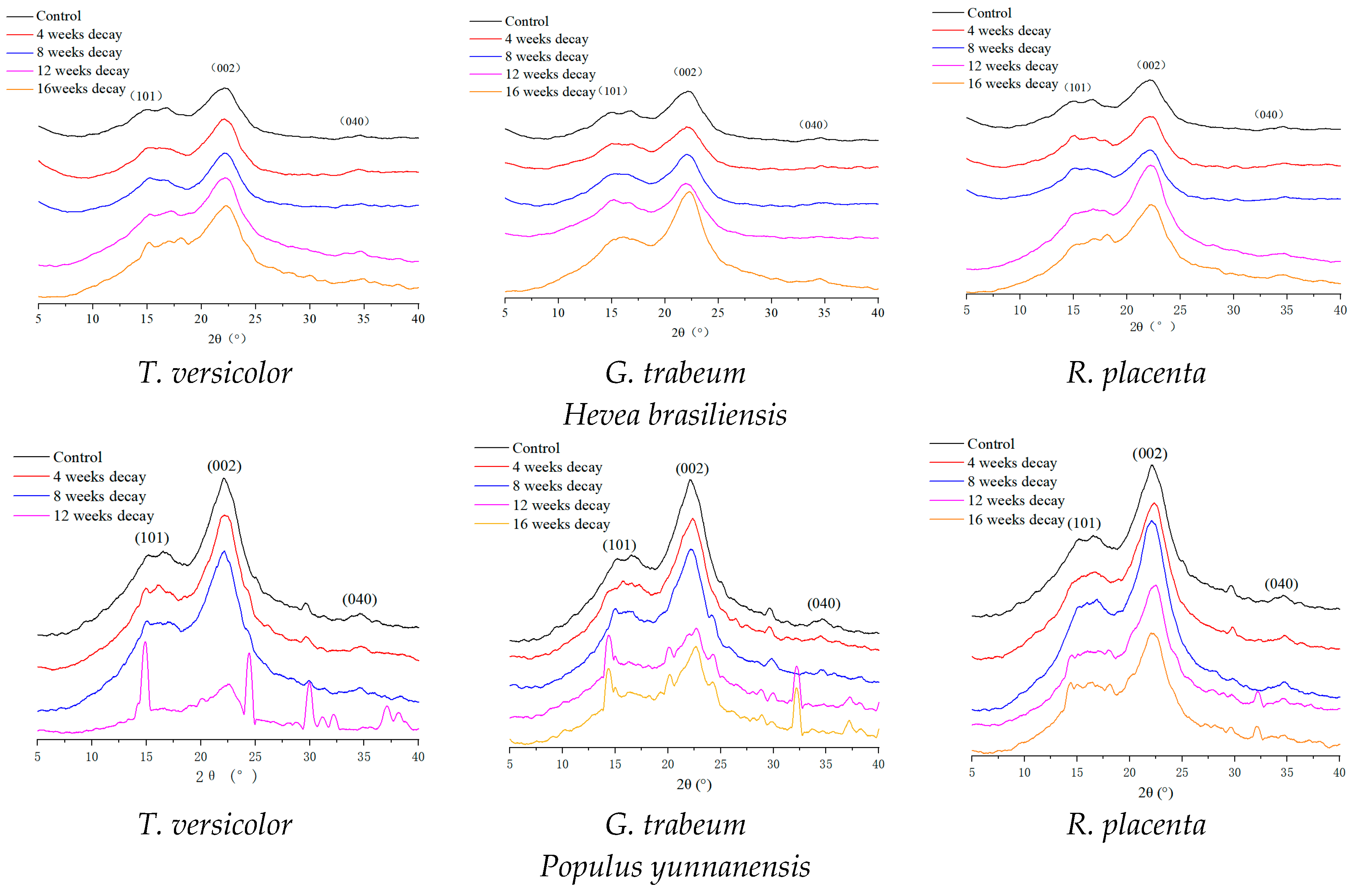
2.4. X-ray Diffraction Analysis
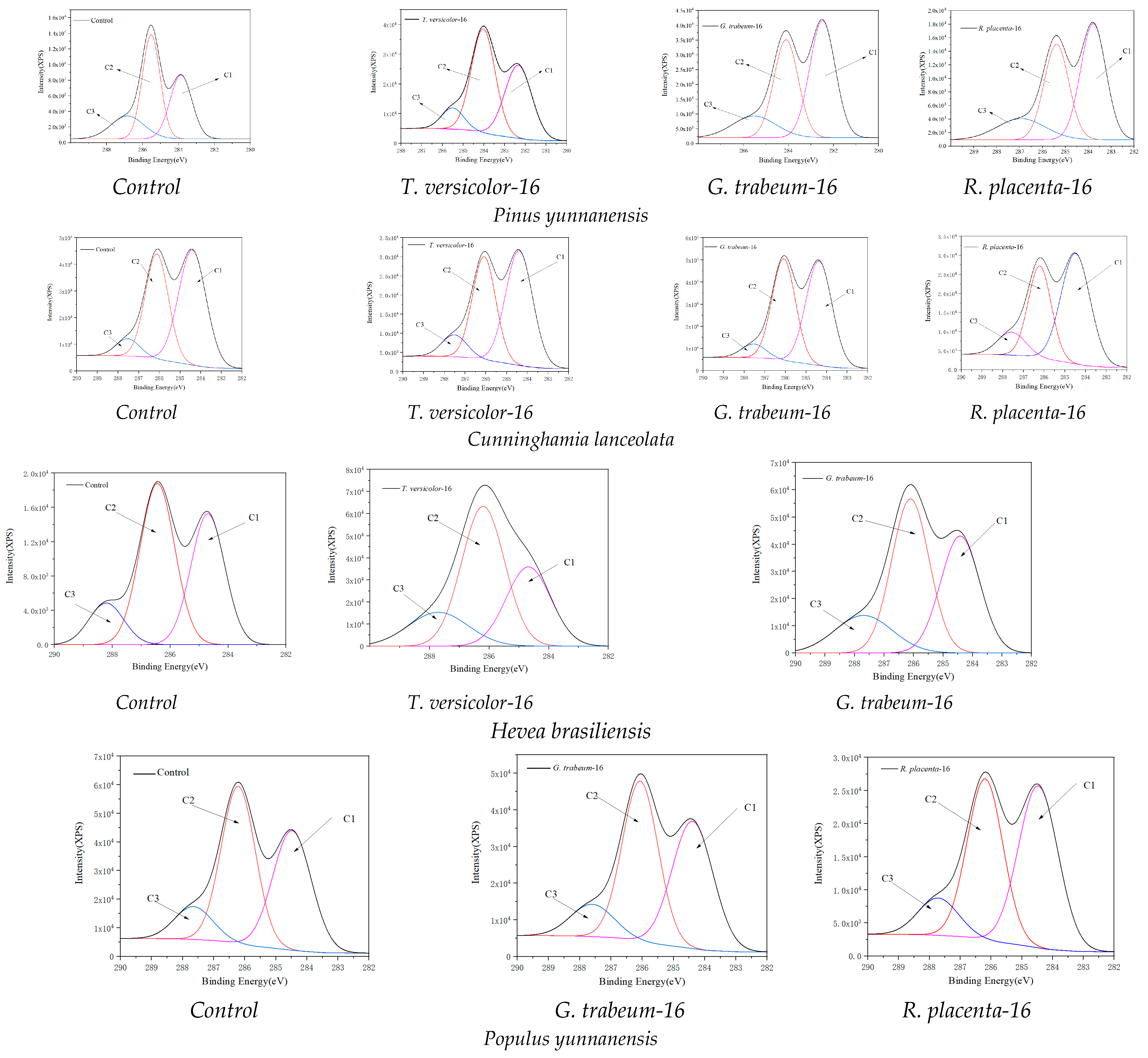
2.5. XPS Spectroscopy Analysis
2.6. Observation on Microscopic Structure
3. Results and Discussion
3.1. Wood Mass Conversion in Relation to Fungi and Wood Species
3.2. Chemical Variation of Wood Biomass after Selective Fungi Process
3.3. Change in Crystallinity
3.4. XPS Spectroscopy Analysis
3.5. Mycelial Distribution and Conversion of Constituents
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Low, Y.W.; Yee, K.F.J.B. A review on lignocellulosic biomass waste into biochar-derived catalyst: Current conversion techniques, sustainable applications and challenges. Biomass Bioenergy 2021, 154, 106245. [Google Scholar] [CrossRef]
- Heap, L.; Green, A.; Brown, D.; van Dongen, B.; Turner, N.J.C.S. Role of laccase as an enzymatic pretreatment method to improve lignocellulosic saccharification. Catal. Sci. Technol. 2014, 4, 2251–2259. [Google Scholar] [CrossRef]
- Thomsen, S.T.; Londoño, J.E.; Ambye-Jensen, M.; Heiske, S.; Kádár, Z.; Meyer, A.S. Combination of ensiling and fungal delignification as effective wheat straw pretreatment. Biotechnol. Biofuels 2016, 9, 16. [Google Scholar] [CrossRef]
- Salvachúa, D.; Martínez, A.T.; Tien, M.; López-Lucendo, M.F.; García, F.; De Los Ríos, V.; Martínez, M.J.; Prieto, A. Differential proteomic analysis of the secretome of Irpex lacteus and other white-rot fungi during wheat straw pretreatment. Biotechnol. Biofuels 2013, 6, 115. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, B.; Thakur, S.; Khasa, Y.P.; Gupte, A.; Puniya, A.K.; Kuhad, R.C. White-rot fungal conversion of wheat straw to energy rich cattle feed. Biodegradation 2011, 22, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Valadares, F.; Gonçalves, T.A.; Gonçalves, D.S.; Segato, F.; Romanel, E.; Milagres, A.M.; Squina, F.M.; Ferraz, A. Exploring glycoside hydrolases and accessory proteins from wood decay fungi to enhance sugarcane bagasse saccharification. Biotechnol. Biofuels 2016, 9, 110. [Google Scholar] [CrossRef]
- Goodell, B.; Zhu, Y.; Kim, S.; Kafle, K.; Eastwood, D.; Daniel, G.; Jellison, J.; Yoshida, M.; Groom, L.; Pingali, S.V. Modification of the nanostructure of lignocellulose cell walls via a non-enzymatic lignocellulose deconstruction system in brown rot wood-decay fungi. Biotechnol. Biofuels 2017, 10, 179. [Google Scholar] [CrossRef]
- Shirkavand, E.; Baroutian, S.; Gapes, D.J.; Young, B.R. Pretreatment of radiata pine using two white rot fungal strains Stereum hirsutum and Trametes versicolor. Energy Convers. Manag. 2017, 142, 13–19. [Google Scholar] [CrossRef]
- Kirk, T. Quantitative changes in structural components of conifer woods during decay by white-and brown-rot fungi. Phytopathology 1973, 63, 1338–1342. [Google Scholar] [CrossRef]
- Kirk, T.K.; Shimada, M. Lignin biodegradation: The microorganisms involved and the physiology and biochemistry of degradation by white-rot fungi. Biosynth. Biodegrad. Wood Compon. 1985, 579–605. [Google Scholar]
- Arantes, V.; Goodell, B. Current understanding of brown-rot fungal biodegradation mechanisms: A review. ACS Symp. 2014, 1158, 3–21. [Google Scholar] [CrossRef]
- Rytioja, J.; Hildén, K.; Yuzon, J.; Hatakka, A.; De Vries, R.P.; Mäkelä, M.R. Plant-polysaccharide-degrading enzymes from basidiomycetes. Microbiol. Mol. Biol. Rev. 2014, 78, 614–649. [Google Scholar] [CrossRef] [PubMed]
- Ayrilmis, N.; Kaymakci, A.; Güleç, T. Potential use of decayed wood in production of wood plastic composite. Ind. Crops Prod. 2015, 74, 279–284. [Google Scholar] [CrossRef]
- Xiao, P.; Mori, T.; Kamei, I.; Kondo, R. A novel metabolic pathway for biodegradation of DDT by the white rot fungi, Phlebia lindtneri and Phlebia brevispora. Biodegradation 2011, 22, 859–867. [Google Scholar] [CrossRef]
- Asgher, M.; Bhatti, H.N.; Ashraf, M.; Legge, R.L. Recent developments in biodegradation of industrial pollutants by white rot fungi and their enzyme system. Biodegradation 2008, 19, 771–783. [Google Scholar] [CrossRef]
- Kirk, T.K.; Moore, W.E. Removing lignin from wood with white-rot fungi and digestibility of resulting wood. Wood Fiber Sci. 1972, 72–79. [Google Scholar]
- Witomski, P.; Olek, W.; Bonarski, J.T. Effects of white and brown rot decay on changes of wood ultrastructure. BioResources 2014, 9, 7363–7371. [Google Scholar] [CrossRef]
- Beck, G.; Thybring, E.E.; Thygesen, L.G. Brown-rot fungal degradation and de-acetylation of acetylated wood. Int. Biodeterior. Biodegrad. 2018, 135, 62–70. [Google Scholar] [CrossRef]
- Ray, M.J.; Leak, D.J.; Spanu, P.D.; Murphy, R.J. Brown rot fungal early stage decay mechanism as a biological pretreatment for softwood biomass in biofuel production. Biomass Bioenergy 2010, 34, 1257–1262. [Google Scholar] [CrossRef]
- Antczak, A.; Szadkowski, J.; Szadkowska, D.; Zawadzki, J. Assessment of the effectiveness of liquid hot water and steam explosion pretreatments of fast-growing poplar (Populus trichocarpa) wood. Wood Sci. Technol. 2022, 56, 87–109. [Google Scholar] [CrossRef]
- Steffen, K.T.; Schubert, S.; Tuomela, M.; Hatakka, A.; Hofrichter, M. Enhancement of bioconversion of high-molecular mass polycyclic aromatic hydrocarbons in contaminated non-sterile soil by litter-decomposing fungi. Biodegradation 2007, 18, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Bari, E.; Schmidt, O.; Oladi, R. A histological investigation of Oriental beech wood decayed by Pleurotus ostreatus and Trametes versicolor. For. Pathol. 2015, 45, 349–357. [Google Scholar] [CrossRef]
- Aghajani, H.; Bari, E.; Bahmani, M.; Humar, M.; Ghanbary, M.A.T.; Nicholas, D.D.; Zahedian, E. Influence of relative humidity and temperature on cultivation of Pleurotus species. Maderas. Cienc. Y Tecnol. 2018, 20, 571–578. [Google Scholar] [CrossRef]
- Low, K.; Lee, C.; Mak, S. Sorption of copper and lead by citric acid modified wood. Wood Sci. Technol. 2004, 38, 629–640. [Google Scholar] [CrossRef]
- Brischke, C.; Hanske, M. Durability of untreated and thermally modified reed (Phragmites australis) against brown, white and soft rot causing fungi. Ind. Crops Prod. 2016, 91, 49–55. [Google Scholar] [CrossRef]
- Liu, M.; Zhong, H.; Ma, E.; Liu, R. Resistance to fungal decay of paraffin wax emulsion/copper azole compound system treated wood. Int. Biodeterior. Biodegrad. 2018, 129, 61–66. [Google Scholar] [CrossRef]
- Pointing, S.B.; Parungao, M.M.; De Hy, K.D. Production of wood-decay enzymes, mass loss and lignin solubilization in wood by tropical Xylariaceae. Mycol. Res. 2003, 107, 231–235. [Google Scholar] [CrossRef]
- Zongde, W.; Guorong, F.; Jinyun, P. Studies on the Fiber Shape and Chemical Composition of the Wood of Cunninghamia lanceolata in Jiangxi Province. Acta Agric. Univ. Jiang Xiensis 2001, 23, 112–115. [Google Scholar]
- Zhu, Y.H.; Wen, L.; Zhang, Y.L.; Wang, X.Z. Visualization of micro-structure and chemical composition of poplar tension wood. J. For. Eng. 2020, 5, 54–58. [Google Scholar] [CrossRef]
- Li, P.P.; Lei, Y.C.; Chen, C.; Meng, Q.J. Effect of Hot Water Prehydrolysis on the Major Components of the Pine and the Hydrolysate. China Pulp. Pap. 2012, 31, 1–6. [Google Scholar]
- Nannan, Z. Study on the Properties of Heat-Treated Rubber Wood Modified by Silica Impregnation; Northeast Forestry University: Harbin, China, 2019. [Google Scholar]
- Norhaslida, R.; Halis, R.; Lakarim, L.; Danial, M.; Low, J.; Naimah, M.J.B. Chemical alteration of banana pseudostems by white rot fungi. Biomass Bioenergy 2014, 61, 206–210. [Google Scholar] [CrossRef]
- Sodre, V.; Vilela, N.; Tramontina, R.; Squina, F.M.J.B. Microorganisms as bioabatement agents in biomass to bioproducts applications. Biomass Bioenergy 2021, 151, 106161. [Google Scholar] [CrossRef]
- Knapic, S.; Pirralho, M.; Louzada, J.; Pereira, H. Early assessment of density features for 19 Eucalyptus species using X-ray microdensitometry in a perspective of potential biomass production. Wood Sci. Technol. 2014, 48, 37–49. [Google Scholar] [CrossRef]
- Gu, Y.; Bian, H.; Wei, L.; Wang, R. Enhancement of hydrotropic fractionation of poplar wood using autohydrolysis and disk refining pretreatment: Morphology and overall chemical characterization. Polymers 2019, 11, 685. [Google Scholar] [CrossRef]
- Sasaki, C.; Yamanaka, S. Novel sterilization method combining food preservative use and low temperature steaming for treatment of lignocellulosic biomass with white rot fungi. Ind. Crops Prod. 2020, 155, 112765. [Google Scholar] [CrossRef]
- Zhenfu, L.; Biao, P.; Buyun, L.; Xuefeng, Z.; Shiyu, C. Study on the density and shrinkage of poplar in the beach of the Yangtze River. For. Sci. Technol. Dev. 2012, 26, 60–62. [Google Scholar] [CrossRef]
- Fan, S.; Hu, J.; Liu, J. Comparison of properties of rubber wood from two different producing areas and preliminary modification. J. Beihua Univ. 2019, 20, 256–259. [Google Scholar] [CrossRef]
- Lihong, Q.; Xiaoling, L.; Liufeng, L.; Yunlin, F.; Mei, L. Green wood properties of pinus yunnanensis var. tenuifolia. J. Northwest For. Univ. 2015, 30, 7. [Google Scholar]
- Tan, N.; Wang, X.; Huang, A.; Wang, C. Wood Density Prediction of Cunninghamia lanceolata Based on Gray Wolf Algorithm SVM and NIR. For. Sci. 2018, 54, 5. [Google Scholar] [CrossRef]
- Goodell, B. Brown-rot fungal degradation of wood: Our evolving view. ACS Publ. 2003, 845, 97–118. [Google Scholar] [CrossRef]
- Tamburini, D.; Łucejko, J.J.; Pizzo, B.; Mohammed, M.Y.; Sloggett, R.; Colombini, M.P. A critical evaluation of the degradation state of dry archaeological wood from Egypt by SEM, ATR-FTIR, wet chemical analysis and Py (HMDS)-GC-MS. Polym. Degrad. Stab. 2017, 146, 140–154. [Google Scholar] [CrossRef]
- Bouslimi, B.; Koubaa, A.; Bergeron, Y. Effects of biodegradation by brown-rot decay on selected wood properties in eastern white cedar (Thuja occidentalis L.). Int. Biodeterior. Biodegrad. 2014, 87, 87–98. [Google Scholar] [CrossRef]
- Eller, F.J.; Kirker, G.T.; Mankowski, M.E.; Hay, W.T.; Palmquist, D.E. Effect of burgundy solid extracted from Eastern Red Cedar heartwood on subterranean termites and Wood-decay fungi. Ind. Crops Prod. 2020, 144, 112023. [Google Scholar] [CrossRef]
- Li, X.; Tang, Z.; Sun, Z.; Simonsen, J.; Luo, Z.; Li, X.; Morrell, J.J. Chemical and Enzymatic Fiber Modification to Enhance the Mechanical Properties of CMC Composite Films. Polymers 2022, 14, 4127. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Xing, D.; Lia, J. FTIR studies of the changes in wood chemistry from wood forming tissue under inclined treatment. Energy Procedia 2012, 16, 758–762. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, T.-Y.; Wen, J.-L.; Zhao, Y.-L.; Qiu, J.; Sun, R.-C. Multi-analysis of chemical transformations of lignin macromolecules from waterlogged archaeological wood. Int. J. Biol. Macromol. 2017, 109, 407–416. [Google Scholar] [CrossRef]
- Bi, Z.; Zhao, Y.; Morrell, J.J.; Lei, Y.; Yan, L. The antifungal mechanism of konjac flying powder extract and its active compounds against wood decay fungi. Ind. Crops Prod. 2021, 164, 113406. [Google Scholar] [CrossRef]
- Ibarra, D.; Martín-Sampedro, R.; Wicklein, B.; Borrero-López, A.M.; Valencia, C.; Valdehíta, A.; Navas, J.M.; Eugenio, M.E. Populus alba L., an autochthonous species of Spain: A source for cellulose nanofibers by chemical pretreatment. Polymers 2021, 14, 68. [Google Scholar] [CrossRef]
- Xu, G.; Wang, L.; Liu, J.; Wu, J. FTIR and XPS analysis of the changes in bamboo chemical structure decayed by white-rot and brown-rot fungi. Appl. Surf. Sci. 2013, 280, 799–805. [Google Scholar] [CrossRef]
- Zhang, L.; You, T.; Zhou, T.; Zhang, L.; Xu, F. Synergistic effect of white-rot fungi and alkaline pretreatments for improving enzymatic hydrolysis of poplar wood. Ind. Crops Prod. 2016, 86, 155–162. [Google Scholar] [CrossRef]
- Viltres, H.; Odio, O.F.; Lartundo-Rojas, L.; Reguera, E. Degradation study of Arsenic Oxides under XPS Measurements. Appl. Surf. Sci. 2020, 511, 145606. [Google Scholar] [CrossRef]
- Okello, V.A.; Mwilu, S.; Noah, N.; Zhou, A.; Sadik, O.A. Reduction of Hexavalent Chromium Using Naturally-Derived Flavonoids. Environ. Sci. Technol. 2012, 46, 10743–10751. [Google Scholar] [CrossRef] [PubMed]
- Zhidkov, I.S.; Boukhvalov, D.W.; Akbulatov, A.F.; Frolova, L.A.; Kurmaev, E.Z. XPS spectra as a tool for studying photochemical and thermal degradation in APbX3 hybrid halide perovskites. Nano Energy 2021, 79, 105421. [Google Scholar] [CrossRef]
- Bari, E.; Daryaei, M.G.; Karim, M.; Bahmani, M.; Schmidt, O.; Woodward, S.; Tajick Ghanbary, M.A.; Sistani, A. Decay of Carpinus betulus wood by Trametes versicolor—An Anatomical and Chemical study. Int. Biodeterior. Biodegrad. 2019, 137, 68–77. [Google Scholar] [CrossRef]
- Kocaefe, D.; Kocaefe, Y.; Boluk, Y.; Krause, C. Structural analysis of heat-treated birch (Betule papyrifera) surface during artificial weathering. Appl. Surf. Sci. 2013, 264, 117–127. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Y.; Zhang, R.; Wang, W.; Cao, J. Improvement of wood against UV weathering and decay by using plant origin substances: Tannin acid and tung oil. Ind. Crops Prod. 2021, 168, 113606. [Google Scholar] [CrossRef]
- Bi, Z.; Fang, S.; Gao, Q.; Lei, Y.; Morrell, J.J.; Yan, L. Improvement of mould resistance of wood with cinnamaldehyde chitosan emulsion. Wood Sci. Technol. 2022, 56, 187–204. [Google Scholar] [CrossRef]
- Kaushal, R.; Sharma, N.; Dogra, V.J.B. Molecular characterization of glycosyl hydrolases of Trichoderma harzianum WF5—A potential strain isolated from decaying wood and their application in bioconversion of poplar wood to ethanol under separate hydrolysis and fermentation. Biomass Bioenergy 2016, 85, 243–251. [Google Scholar] [CrossRef]

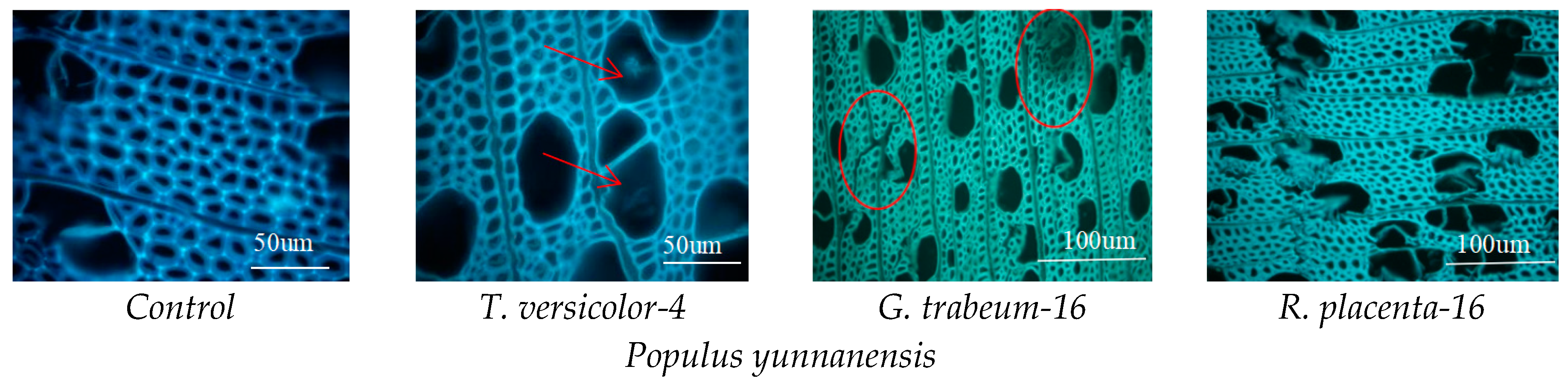

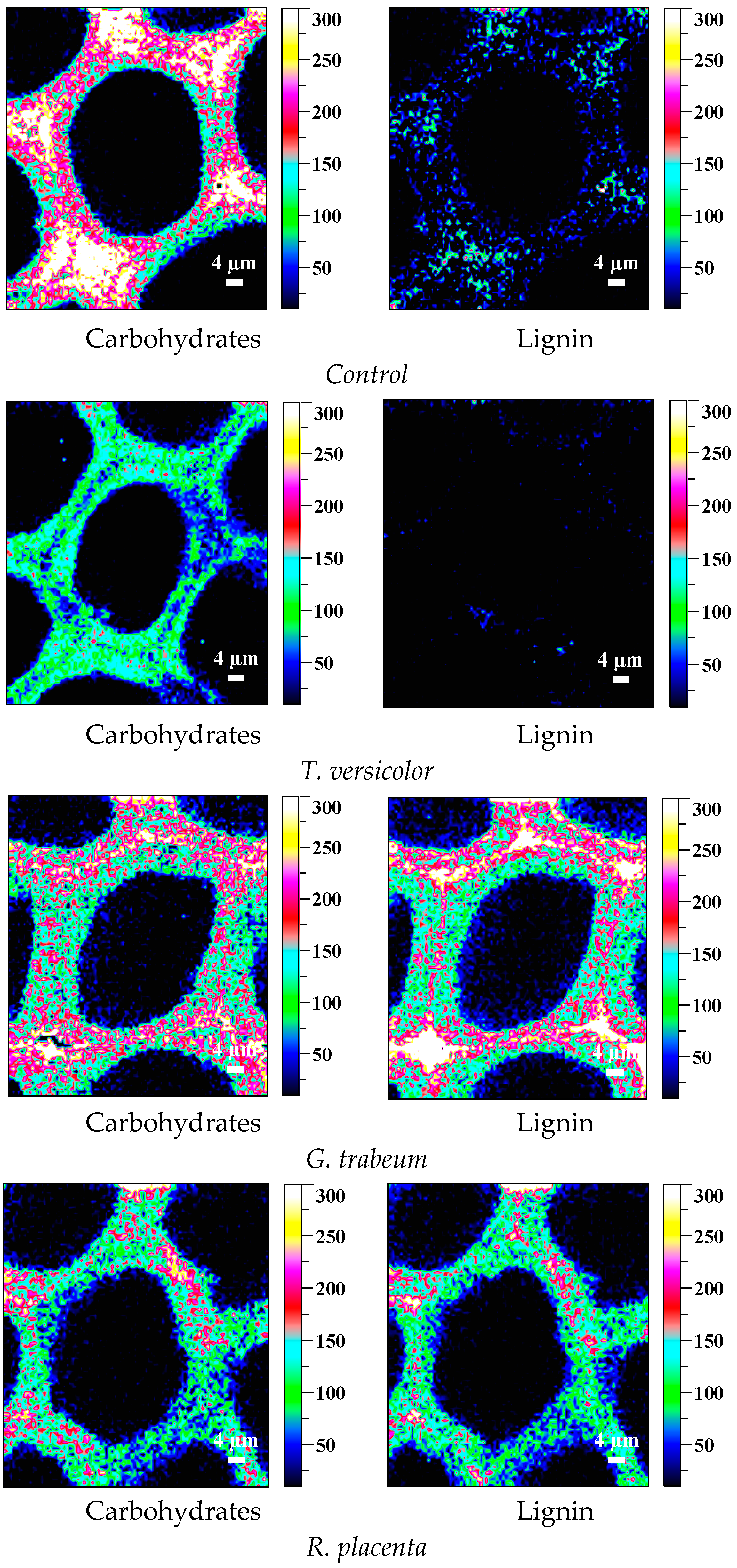
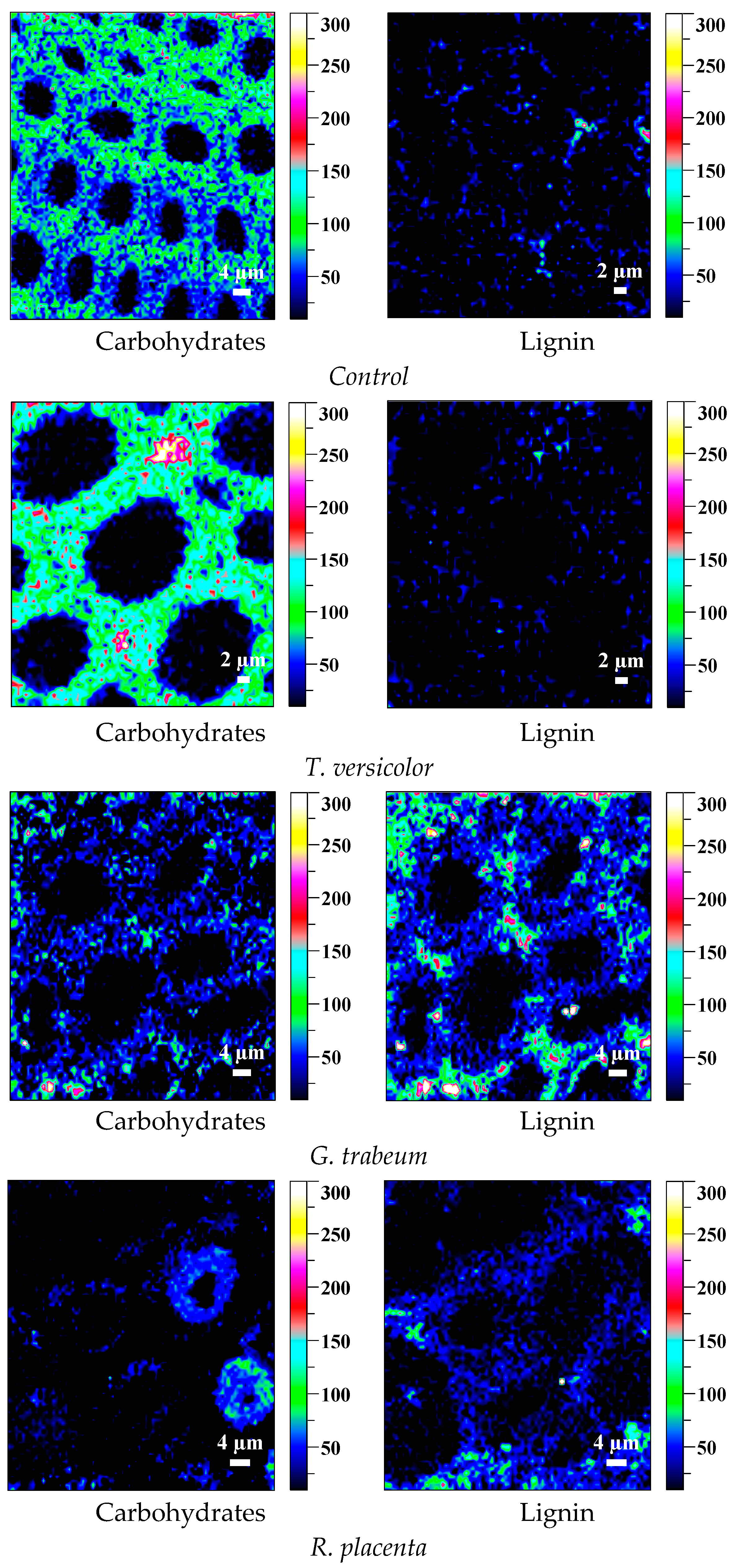
| Tree Species | Periods (Weeks) | Mass Loss/% | Standard Deviation (б) | CrI/% | Varying Ratio of CrI/% | Periods (Weeks) | Mass Loss/% | Standard Deviation (б) | CrI/% | Varying Ratio of CrI/% | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fungi | Pinus yunnanensis | Cunninghamia lanceolata | |||||||||
| T versicolor | Control | 0 | 0 | 32.82 | 0 | Control | 0 | 0 | 22.30 | 0 | |
| 4 | 1.02 | 0.2927 | 20.01 | −12.81 | 4 | 0.17 | 0.5436 | 22.42 | +0.12 | ||
| 8 | 1.41 | 0.3444 | 24.04 | −8.78 | 8 | 1.99 | 0.3649 | 22.53 | +0.23 | ||
| 12 | 10.59 | 0.8408 | 28.24 | −4.58 | 12 | 31.18 | 0.6254 | 26.45 | +4.15 | ||
| 16 | 13.09 | 0.6817 | 43.99 | +11.17 | 16 | 35.57 | 0.3583 | 22.96 | +0.66 | ||
| G. trabeum | 4 | 0.98 | 0.5464 | 27.78 | −5.04 | 4 | 0.60 | 0.4528 | 20.00 | −2.30 | |
| 8 | 5.10 | 0.4988 | 25.67 | −7.15 | 8 | 3.98 | 0.3745 | 18.46 | −3.84 | ||
| 12 | 13.03 | 0.5297 | 24.52 | −8.30 | 12 | 51.36 | 0.6283 | 17.46 | −4.84 | ||
| 16 | 28.59 | 0.3244 | 22.05 | −10.77 | 16 | 66.52 | 0.3683 | 16.42 | −5.88 | ||
| R. placenta | 4 | 7.71 | 0.5197 | 19.80 | −13.02 | 4 | 0.50 | 0.3659 | 19.78 | −2.52 | |
| 8 | 7.36 | 0.4225 | 16.66 | −16.16 | 8 | 1.71 | 0.4573 | 19.94 | −2.36 | ||
| 12 | 35.21 | 0.2407 | 15.37 | −17.45 | 12 | 38.05 | 0.6538 | 18.91 | −3.39 | ||
| 16 | 36.19 | 0.3513 | 17.11 | −15.71 | 16 | 45.87 | 0.5527 | 18.83 | −3.47 | ||
| Hevea brasiliensis | Populus yunnanensis | ||||||||||
| T versicolor | Control | 0 | 0 | 30.26 | 0 | Control | 0 | 0 | 22.97 | 0 | |
| 4 | 4.97 | 0.5593 | 36.71 | +6.45 | 4 | 4.34 | 0.6366 | 22.25 | −0.72 | ||
| 8 | 5.57 | 0.4917 | 35.69 | +5.43 | 8 | 6.56 | 0.2846 | 24.37 | +1.40 | ||
| 12 | 19.22 | 0.4489 | 30.87 | +0.61 | 12 | 80.88 | 0.7354 | 39.87 | +16.90 | ||
| 16 | 40.75 | 0.2305 | 26.15 | −4.11 | 16 | / | / | / | / | ||
| G. trabeum | 4 | 2.03 | 0.3068 | 30.15 | −0.11 | 4 | 0.25 | 0.4683 | 20.83 | −2.14 | |
| 8 | 5.61 | 0.2172 | 26.45 | −3.81 | 8 | 3.48 | 0.5739 | 20.55 | −2.42 | ||
| 12 | 27.70 | 0.6643 | 29.94 | −0.32 | 12 | 61.20 | 0.2648 | 15.24 | −7.73 | ||
| 16 | 32.65 | 0.2370 | 18.37 | −11.89 | 16 | 65.54 | 0.8464 | 18.17 | −4.80 | ||
| R. placenta | 4 | 0.08 | 0.0353 | 28.25 | −2.01 | 4 | 0.38 | 0.5628 | 18.49 | −4.48 | |
| 8 | 2.02 | 0.3779 | 26.69 | −3.57 | 8 | 0.90 | 0.6783 | 17.77 | −5.20 | ||
| 12 | 10.25 | 0.5247 | 16.64 | −13.62 | 12 | 16.46 | 0.5728 | 17.46 | −5.51 | ||
| 16 | 19.05 | 0.4543 | 14.06 | −16.20 | 16 | 36.88 | 0.3558 | 17.34 | −5.63 | ||
| Cellulose (%) | Hemicellulose (%) | Lignin (%) | |
|---|---|---|---|
| Pinus yunnanensis | 49.65 | 16.20 | 26.41 |
| Cunninghamia lanceolata | 44.39 | 11.91 | 26.76 |
| Hevea brasiliensis | 50.19 | 24.31 | 23.43 |
| Populus yunnanensis | 50.22 | 14.55 | 24.59 |
| Tree Species | Pinus yunnanensis | Cunninghamia lanceolata | Hevea brasiliensis | Populus yunnanensis |
|---|---|---|---|---|
| Densities/g.cm−3 | 0.472 | 0.401 | 0.650 | 0.364 |
| Standard deviation (б) | 0.0061 | 0.0197 | 0.0100 | 0.0355 |
| Tree Species | Fungi | Periods (Weeks) | C1/C | C2/C | C3/C |
|---|---|---|---|---|---|
| Control | 0 | 34.81 | 49.93 | 15.25 | |
| Pinus yunnanensis | T versicolor | 16 | 33.24 | 53.17 | 13.59 |
| G. trabeum | 16 | 47.56 | 40.11 | 12.33 | |
| R. placenta | 16 | 58.60 | 29.50 | 11.89 | |
| Control | 0 | 53.05 | 40.77 | 6.18 | |
| Cunninghamia lanceolata | T versicolor | 16 | 50.19 | 43.69 | 6.13 |
| G. trabeum | 16 | 53.89 | 35.43 | 10.66 | |
| R. placenta | 16 | 51.53 | 39.62 | 9.08 | |
| Control | 0 | 37.69 | 52.23 | 10.09 | |
| Hevea brasiliensis | T versicolor | 16 | 30.58 | 51.95 | 17.47 |
| G. trabeum | 16 | 36.67 | 46.15 | 17.17 | |
| Control | 0 | 35.82 | 55.70 | 8.48 | |
| Populus yunnanensis | G. trabeum | 16 | 41.99 | 46.82 | 11.19 |
| R. placenta | 16 | 46.67 | 42.10 | 11.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, J.; Li, F.; Jia, L.; Zhang, X.; Deng, S.; Luo, B.; Zhou, Y.; Fan, M.; Xia, Y. Fungal Selectivity and Biodegradation Effects by White and Brown Rot Fungi for Wood Biomass Pretreatment. Polymers 2023, 15, 1957. https://doi.org/10.3390/polym15081957
Qi J, Li F, Jia L, Zhang X, Deng S, Luo B, Zhou Y, Fan M, Xia Y. Fungal Selectivity and Biodegradation Effects by White and Brown Rot Fungi for Wood Biomass Pretreatment. Polymers. 2023; 15(8):1957. https://doi.org/10.3390/polym15081957
Chicago/Turabian StyleQi, Jiyun, Fangfang Li, Lu Jia, Xiaoyuan Zhang, Shuduan Deng, Bei Luo, Yonghui Zhou, Mizi Fan, and Yan Xia. 2023. "Fungal Selectivity and Biodegradation Effects by White and Brown Rot Fungi for Wood Biomass Pretreatment" Polymers 15, no. 8: 1957. https://doi.org/10.3390/polym15081957
APA StyleQi, J., Li, F., Jia, L., Zhang, X., Deng, S., Luo, B., Zhou, Y., Fan, M., & Xia, Y. (2023). Fungal Selectivity and Biodegradation Effects by White and Brown Rot Fungi for Wood Biomass Pretreatment. Polymers, 15(8), 1957. https://doi.org/10.3390/polym15081957








