Optimization of Lead and Diclofenac Removal from Aqueous Media Using a Composite Sorbent of Silica Core and Polyelectrolyte Coacervate Shell
Abstract
1. Introduction
2. Materials and Methodology
2.1. Materials
2.2. Batch Sorption and Desorption Studies
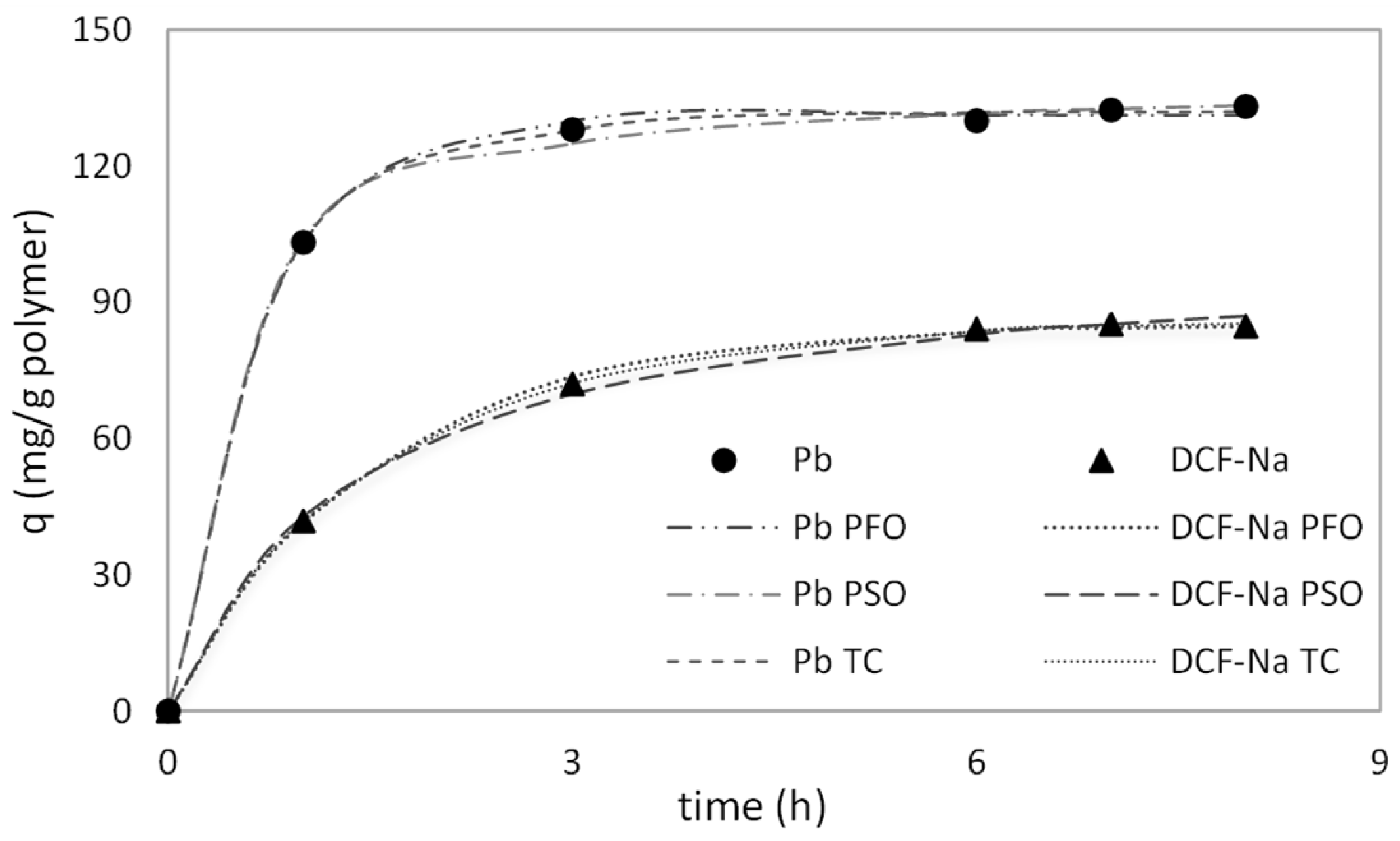
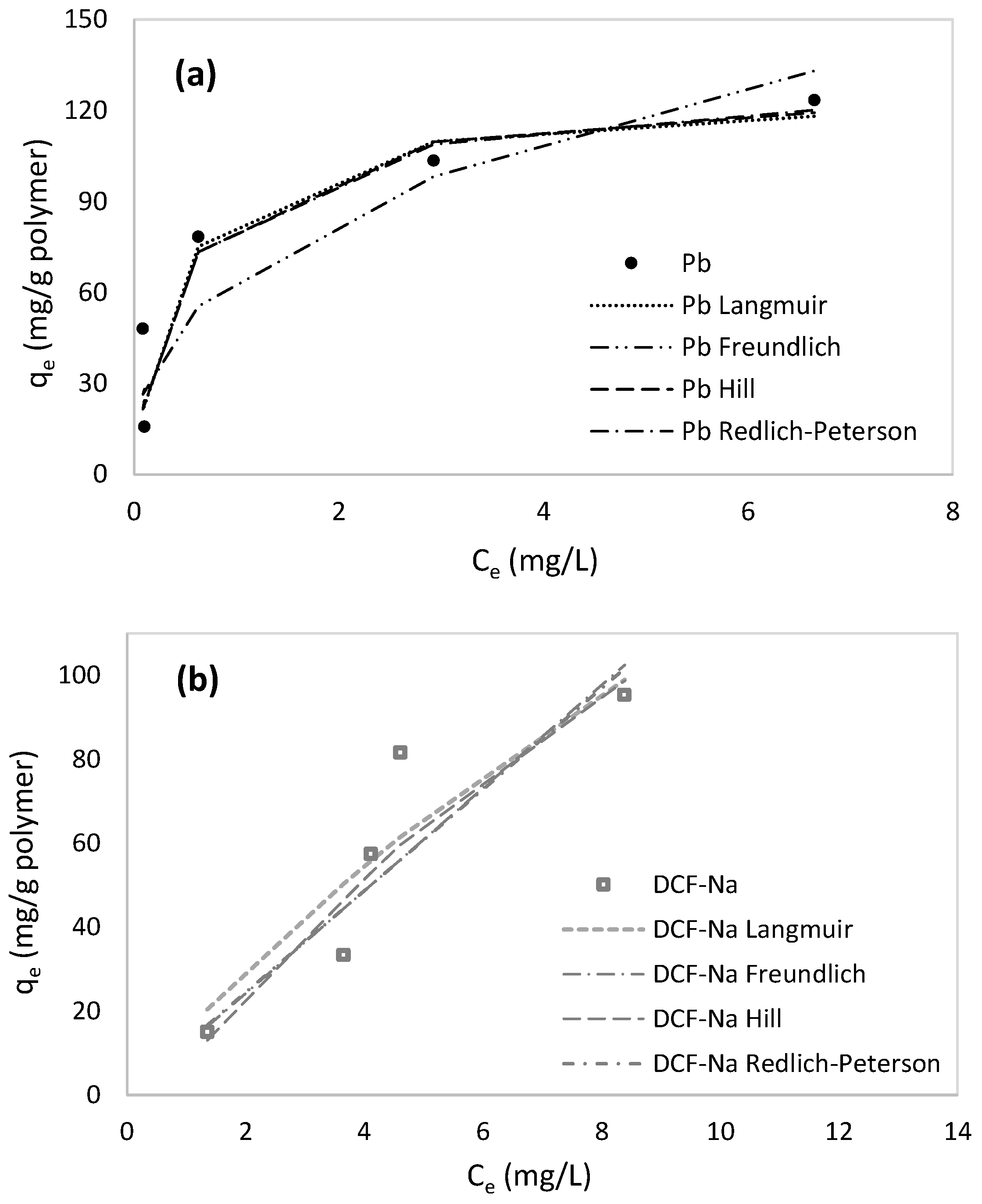
2.3. Adsorption Kinetics and Isotherm Models
2.4. Design of Experiments
3. Results and Discussion
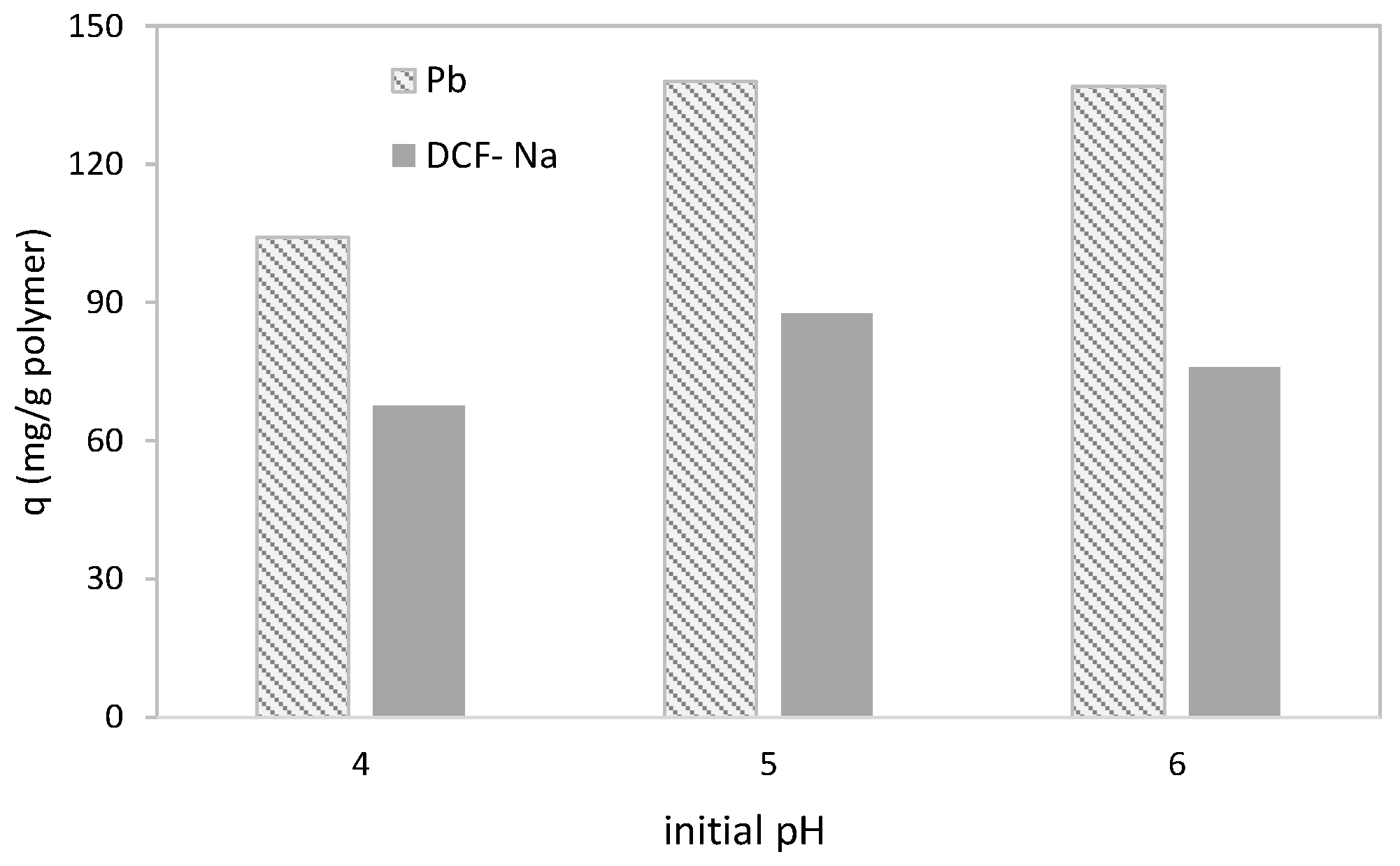
3.1. Univariate Simultaneous Experiments
3.2. Multivariate/Multi-Objective Simultaneous Process Modeling and Optimization
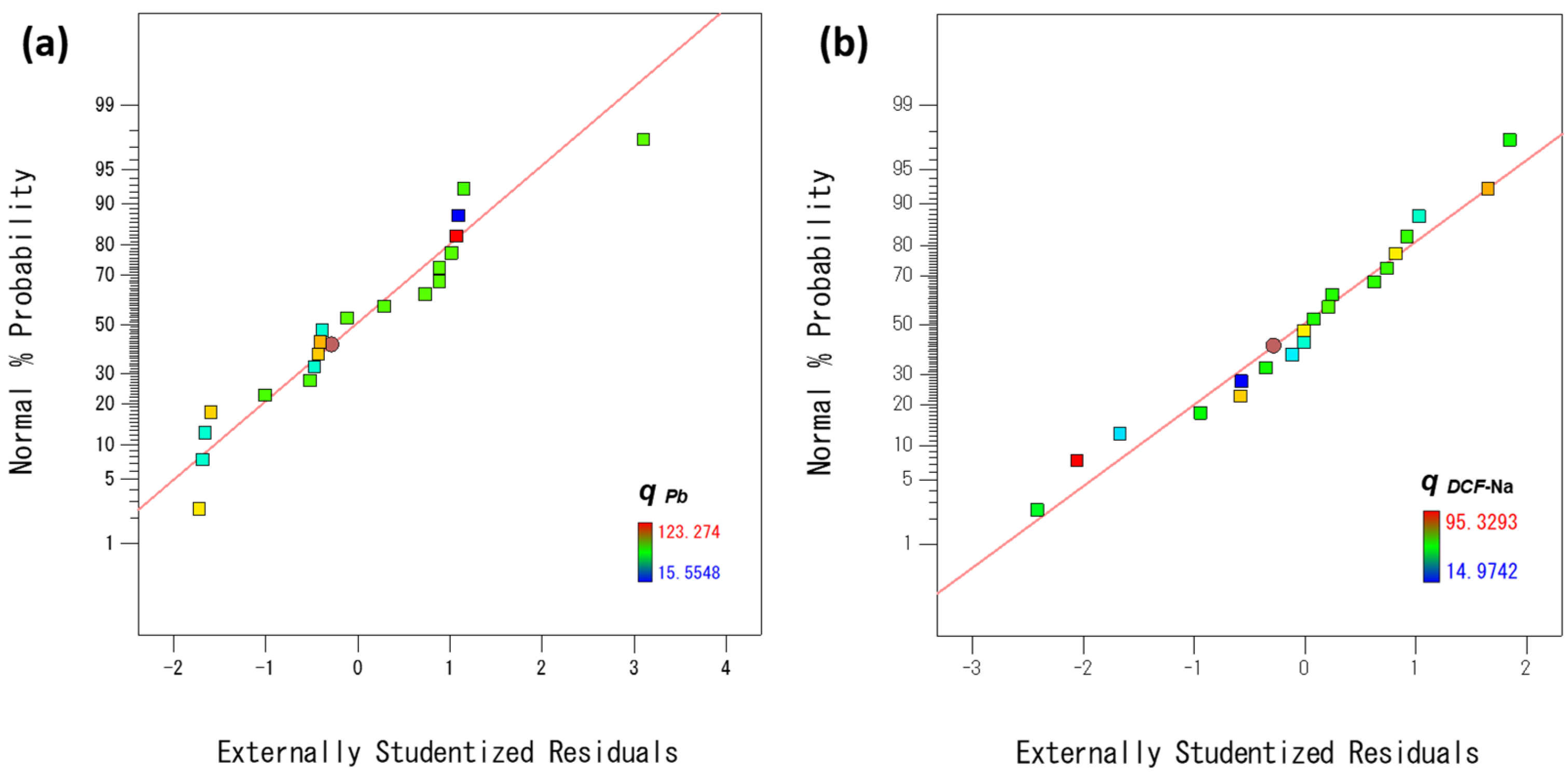
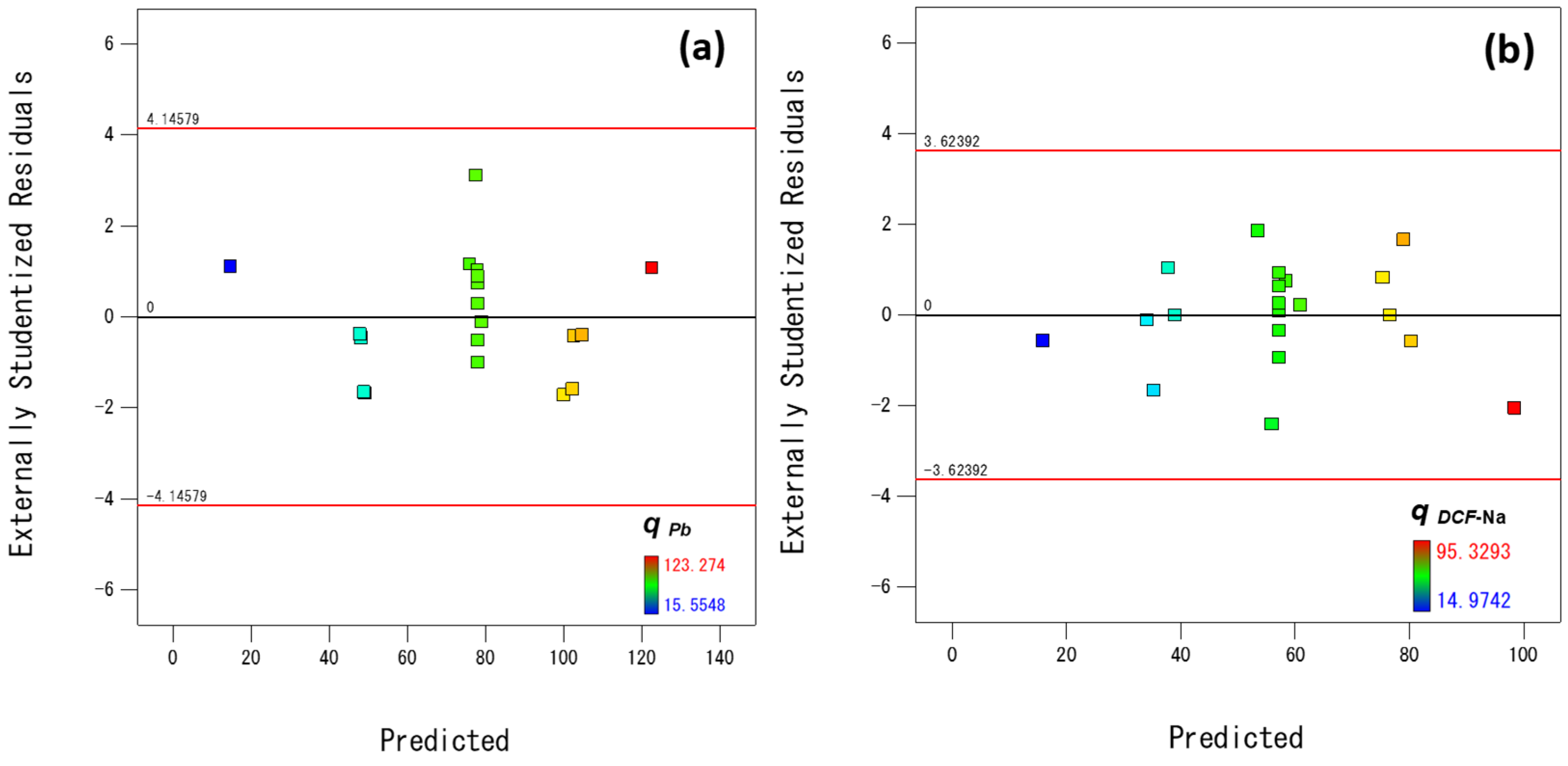
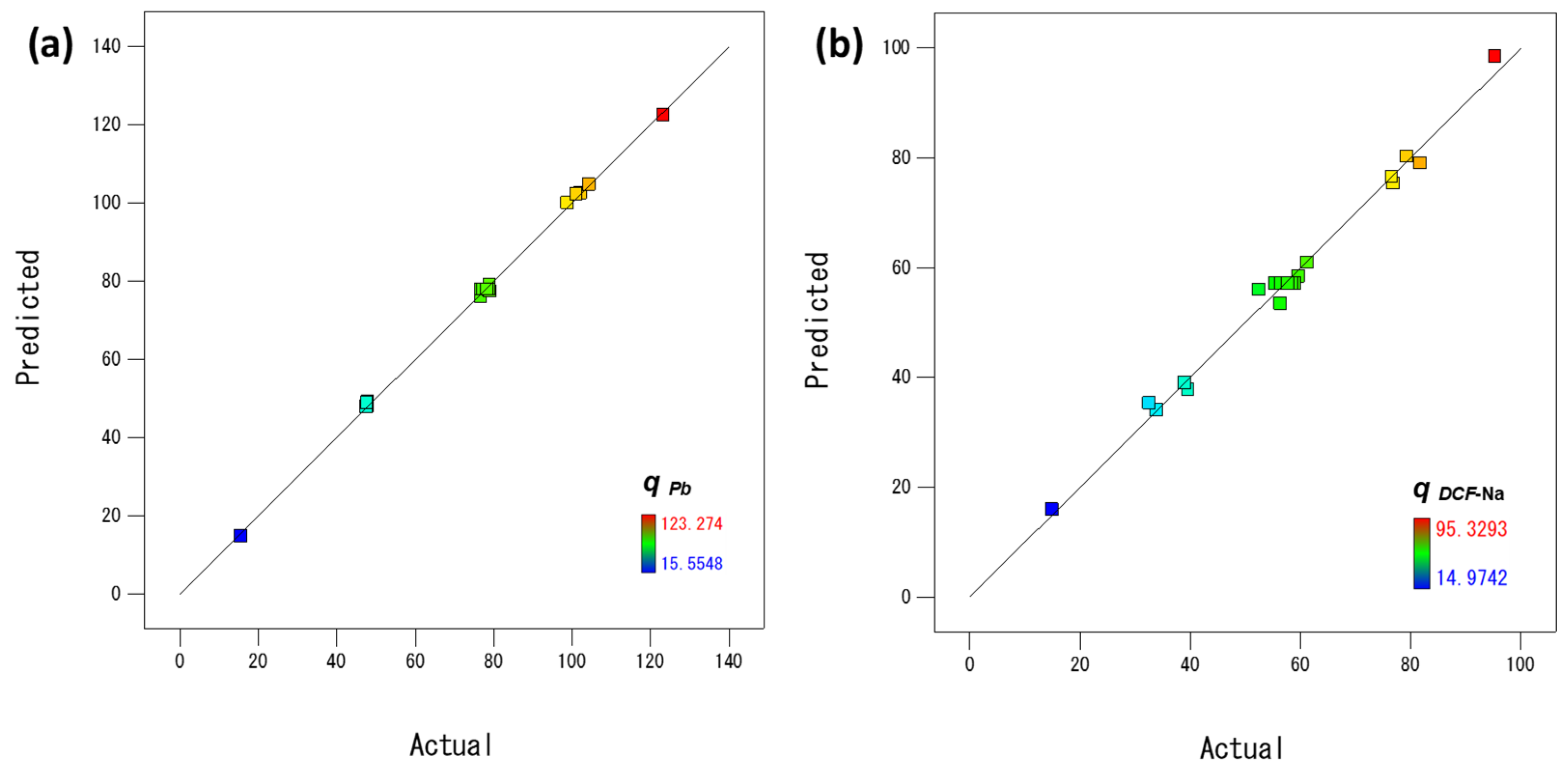
3.2.1. Statistical Analysis of the Process Model
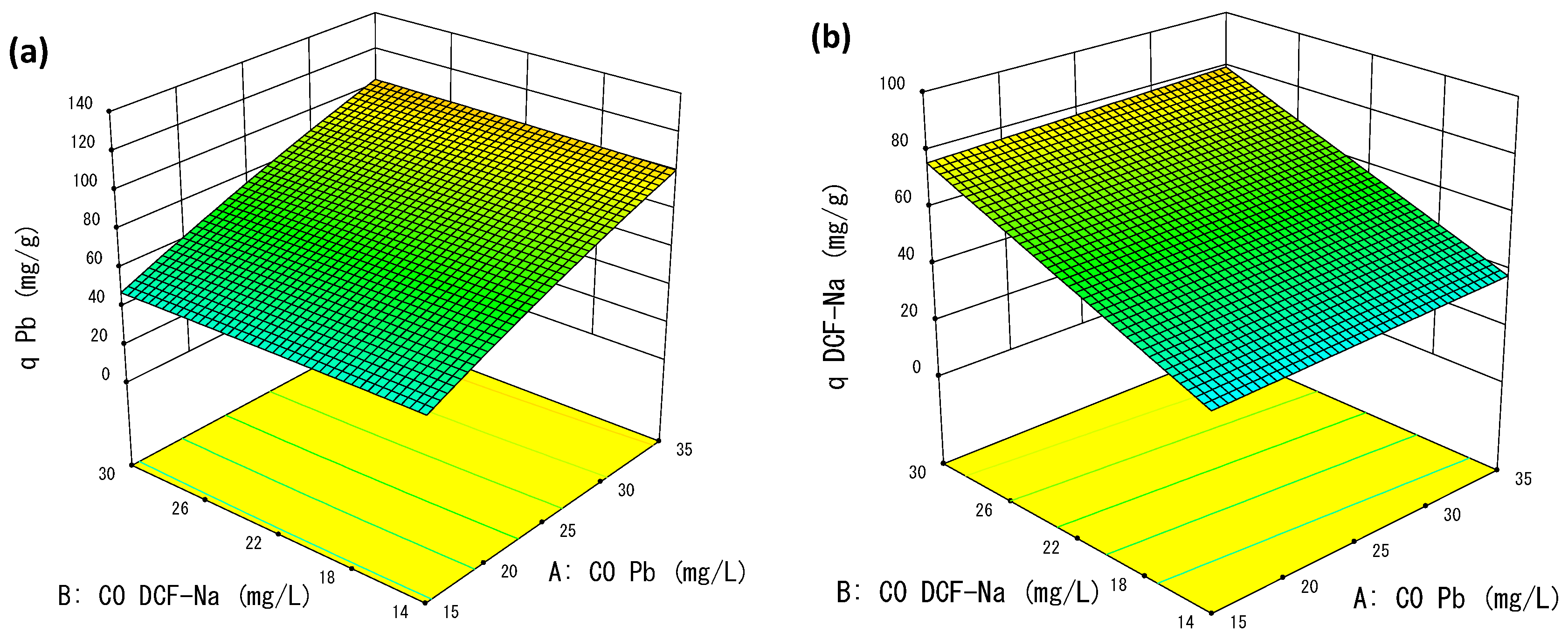
3.2.2. Effects of Factors
3.2.3. Optimization of the Adsorption Process
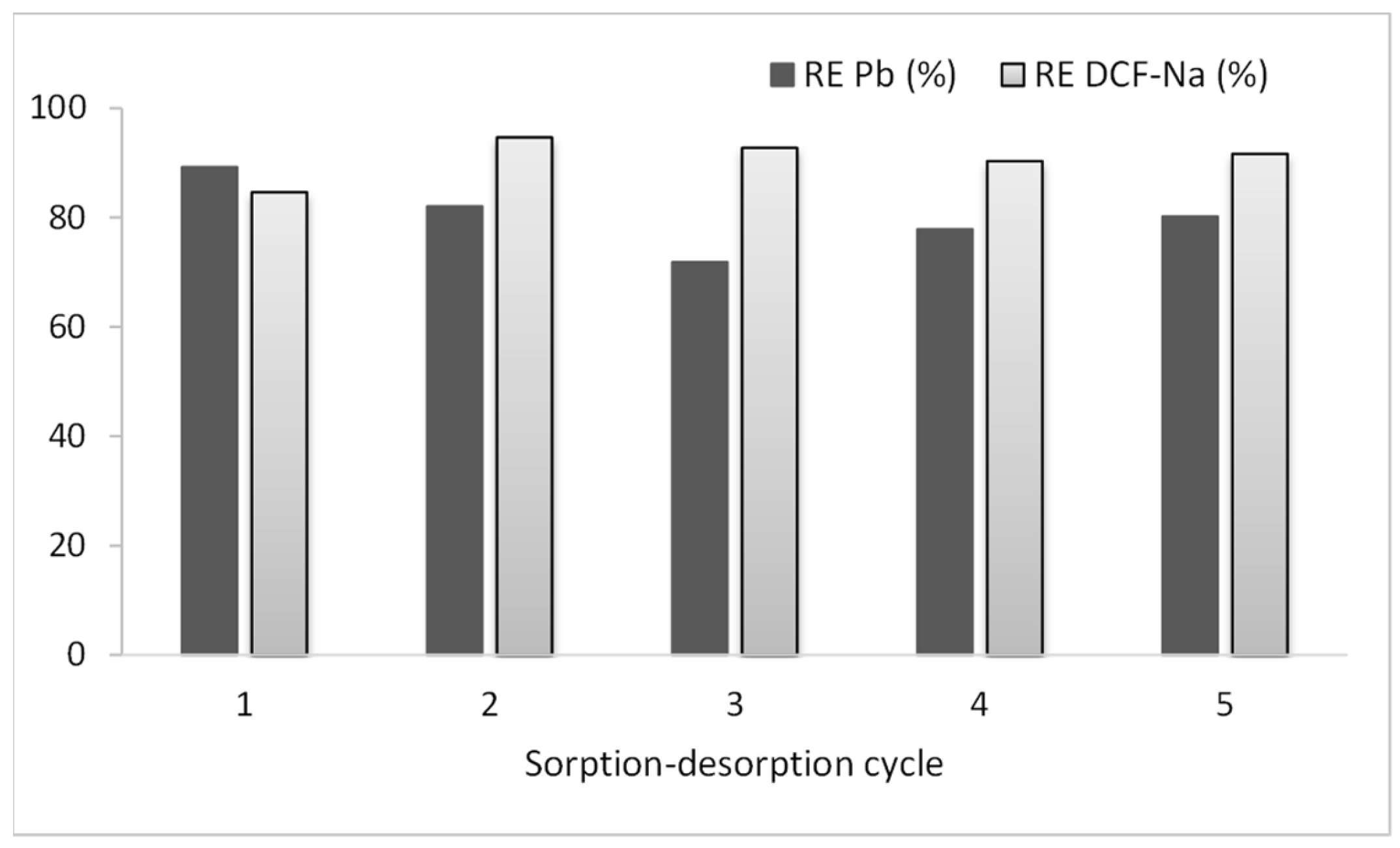
3.3. Desorption and Sorbent Regeneration
3.4. Sequential Sorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nair, A.T.; Makwana, A.R.; Ahammed, M.M. The Use of Response Surface Methodology for Modelling and Analysis of Water and Wastewater Treatment Processes: A Review. Water Sci. Technol. 2014, 69, 464–478. [Google Scholar] [CrossRef]
- Anfar, Z.; Ait Ahsaine, H.; Zbair, M.; Amedlous, A.; Ait El Fakir, A.; Jada, A.; El Alem, N. Recent Trends on Numerical Investigations of Response Surface Methodology for Pollutants Adsorption onto Activated Carbon Materials: A Review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 1043–1084. [Google Scholar] [CrossRef]
- Hiew, B.Y.Z.; Lee, L.Y.; Lee, X.J.; Gan, S.; Thangalazhy-Gopakumar, S.; Lim, S.S.; Pan, G.-T.; Yang, T.C.-K. Adsorptive Removal of Diclofenac by Graphene Oxide: Optimization, Equilibrium, Kinetic and Thermodynamic Studies. J. Taiwan Inst. Chem. Eng. 2019, 98, 150–162. [Google Scholar] [CrossRef]
- Masoumi, H.; Ghaemi, A.; Gilani Ghanadzadeh, H. Elimination of Lead from Multi-Component Lead-Nickel-Cadmium Solution Using Hyper-Cross-Linked Polystyrene: Experimental and RSM Modeling. J. Environ. Chem. Eng. 2021, 9, 106579. [Google Scholar] [CrossRef]
- Treméa, R.; Quesada, H.B.; Bergamasco, R.; de Jesus Bassetti, F. Influence of Important Parameters on the Adsorption of Diclofenac Sodium by an Environmentally Friendly Eucalyptus Wood Biochar and Optimization Using Response Surface Methodology. Desalin. WATER Treat. 2021, 230, 384–399. [Google Scholar] [CrossRef]
- Arabkhani, P.; Javadian, H.; Asfaram, A.; Ateia, M. Decorating Graphene Oxide with Zeolitic Imidazolate Framework (ZIF-8) and Pseudo-Boehmite Offers Ultra-High Adsorption Capacity of Diclofenac in Hospital Effluents. Chemosphere 2021, 271, 129610. [Google Scholar] [CrossRef]
- Kang, J.-K.; Kim, Y.-G.; Lee, S.-C.; Jang, H.-Y.; Yoo, S.-H.; Kim, S.-B. Artificial Neural Network and Response Surface Methodology Modeling for Diclofenac Removal by Quaternized Mesoporous Silica SBA-15 in Aqueous Solutions. Microporous Mesoporous Mater. 2021, 328, 111497. [Google Scholar] [CrossRef]
- Morosanu, I.; Paduraru, C.; Bucatariu, F.; Fighir, D.; Mihai, M.; Teodosiu, C. Shaping Polyelectrolyte Composites for Heavy Metals Adsorption from Wastewater: Experimental Assessment and Equilibrium Studies. J. Environ. Manag. 2022, 321, 115999. [Google Scholar] [CrossRef] [PubMed]
- Morosanu, I.; Teodosiu, C.; Coroaba, A.; Paduraru, C. Sequencing Batch Biosorption of Micropollutants from Aqueous Effluents by Rapeseed Waste: Experimental Assessment and Statistical Modelling. J. Environ. Manag. 2019, 230, 110–118. [Google Scholar] [CrossRef]
- Hannan, E.J.; Quinn, B.G. The Determination of the Order of an Autoregression. J. R. Stat. Soc. Ser. B (Methodol.) 1979, 41, 190–195. [Google Scholar] [CrossRef]
- Refat, M.S.; Mohamed, G.G.; Ibrahim, M.Y.S.; Killa, H.M.A.; Fetooh, H. Synthesis and Characterization of Coordination Behavior of Diclofenac Sodium Drug Toward Hg(II), Pb(II), and Sn(II) Metal Ions: Chelation Effect on Their Thermal Stability and Biological Activity. Synth. React. Inorg. Met. Nano-Metal Chem. 2014, 44, 161–170. [Google Scholar] [CrossRef]
- Lagergren, S.Y. Zur Theorie Der Sogenannten Adsorption Gelöster Stoffe. K. Sven. Vetenskapsakad. Handl. 1989, 24, 1–39. [Google Scholar]
- Zhou, Y.; Liu, X.; Xiang, Y.; Wang, P.; Zhang, J.; Zhang, F.; Wei, J.; Luo, L.; Lei, M.; Tang, L. Modification of Biochar Derived from Sawdust and Its Application in Removal of Tetracycline and Copper from Aqueous Solution: Adsorption Mechanism and Modelling. Bioresour. Technol. 2017, 245, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Salvestrini, S. Analysis of the Langmuir Rate Equation in Its Differential and Integrated Form for Adsorption Processes and a Comparison with the Pseudo First and Pseudo Second Order Models. React. Kinet. Mech. Catal. 2018, 123, 455–472. [Google Scholar] [CrossRef]
- Azizian, S. Kinetic Models of Sorption: A Theoretical Analysis. J. Colloid Interface Sci. 2004, 276, 47–52. [Google Scholar] [CrossRef]
- Sahoo, T.R.; Prelot, B. Adsorption Processes for the Removal of Contaminants from Wastewater. In Nanomaterials for the Detection and Removal of Wastewater Pollutants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 161–222. [Google Scholar]
- Boyd, G.E.; Adamson, A.W.; Myers, L.S. The Exchange Adsorption of Ions from Aqueous Solutions by Organic Zeolites. II. Kinetics 1. J. Am. Chem. Soc. 1947, 69, 2836–2848. [Google Scholar] [CrossRef]
- Langmuir, I. The Constitution and Fundamental Properties of Solids and Liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Freundlich, H. Über Die Adsorption in Lösungen. Zeitschrift für Phys. Chemie 1907, 57U, 385–470. [Google Scholar] [CrossRef]
- Hill, A. The Possible Effects of the Aggregation of the Molecules of Haemoglobin on Its Dissociation Curves. J. Physiol. 1910, 40, iv–vii. [Google Scholar] [CrossRef]
- Redlich, O.; Peterson, D.L. A Useful Adsorption Isotherm. J. Phys. Chem. 1959, 63, 1024. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the Use and Interpretation of Adsorption Isotherm Models: A Review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Liang, X.X.; Omer, A.M.; Hu, Z.; Wang, Y.; Yu, D.; Ouyang, X. Efficient Adsorption of Diclofenac Sodium from Aqueous Solutions Using Magnetic Amine-Functionalized Chitosan. Chemosphere 2019, 217, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Chaduka, M.; Guyo, U.; Zinyama, N.P.; Tshuma, P.; Matsinha, L.C. Modeling and Optimization of Lead (II) Adsorption by a Novel Peanut Hull-g-Methyl Methacrylate Biopolymer Using Response Surface Methodology (RSM). Anal. Lett. 2020, 53, 1294–1311. [Google Scholar] [CrossRef]
- de Luna, M.D.G.; Murniati; Budianta, W.; Rivera, K.K.P.; Arazo, R.O. Removal of Sodium Diclofenac from Aqueous Solution by Adsorbents Derived from Cocoa Pod Husks. J. Environ. Chem. Eng. 2017, 5, 1465–1474. [Google Scholar] [CrossRef]
- Salvestrini, S.; Fenti, A.; Chianese, S.; Iovino, P.; Musmarra, D. Diclofenac Sorption from Synthetic Water: Kinetic and Thermodynamic Analysis. J. Environ. Chem. Eng. 2020, 8, 104105. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, Z.; Ouyang, X.; Ji, C.; Liu, Y.; Huang, F.; Yang, L.-Y. Fabrication of Cross-Linked Chitosan Beads Grafted by Polyethylenimine for Efficient Adsorption of Diclofenac Sodium from Water. Int. J. Biol. Macromol. 2020, 145, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, Y.-F.; Zeng, J.; Zhang, Y.; Zhang, Z.-B.; Zhang, Z.-J.; Ma, S.; Tang, C.-M.; Xu, J.-Q. Chitosan/Polyethyleneimine Magnetic Hydrogels for Adsorption of Heavy Metal Ions. Iran. Polym. J. 2022, 31, 1273–1282. [Google Scholar] [CrossRef]
- Nicola, R.; Costişor, O.; Ciopec, M.; Negrea, A.; Lazău, R.; Ianăşi, C.; Picioruş, E.-M.; Len, A.; Almásy, L.; Szerb, E.I.; et al. Silica-Coated Magnetic Nanocomposites for Pb2+ Removal from Aqueous Solution. Appl. Sci. 2020, 10, 2726. [Google Scholar] [CrossRef]
- Azizkhani, S.; Mahmoudi, E.; Abdullah, N.; Ismail, M.H.S.; Mohammad, A.W.; Hussain, S.A. Synthesis and Characterisation of Graphene Oxide-Silica-Chitosan for Eliminating the Pb(II) from Aqueous Solution. Polymers 2020, 12, 1922. [Google Scholar] [CrossRef]
- Putz, A.-M.; Ivankov, O.I.; Kuklin, A.I.; Ryukhtin, V.; Ianăşi, C.; Ciopec, M.; Negrea, A.; Trif, L.; Horváth, Z.E.; Almásy, L. Ordered Mesoporous Silica Prepared in Different Solvent Conditions: Application for Cu(II) and Pb(II) Adsorption. Gels 2022, 8, 443. [Google Scholar] [CrossRef]
- Ali, A.; Alharthi, S.; Ahmad, B.; Naz, A.; Khan, I.; Mabood, F. Efficient Removal of Pb(II) from Aqueous Medium Using Chemically Modified Silica Monolith. Molecules 2021, 26, 6885. [Google Scholar] [CrossRef] [PubMed]
- Ekpenyong, M.; Antai, S.; Asitok, A.; Ekpo, B. Response Surface Modeling and Optimization of Major Medium Variables for Glycolipopeptide Production. Biocatal. Agric. Biotechnol. 2017, 10, 113–121. [Google Scholar] [CrossRef]



| Factor | Units | Level | ||||
|---|---|---|---|---|---|---|
| −2 | −1 | 0 | +1 | +2 | ||
| A: C0 Pb | mg/L | 5 | 15 | 25 | 35 | 45 |
| B: C0 DCF-Na | mg/L | 6 | 14 | 22 | 30 | 38 |
| C: T | °C | 4 | 12 | 20 | 28 | 36 |
| Run | A | B | C | Y1: qexp, Pb (mg/g Polymer) | qpred, Pb (mg/g Polymer) | Residual (qexp, Pb − qpred, Pb) | Y2: qexp, DCF-Na (mg/g Polymer) | qpred, DCF-Na (mg/g Polymer) | Residual (qexp, DCF-Na − qpred, DCF-Na) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 35 | 30 | 12 | 104.39 | 104.71 | −0.32 | 81.80 | 79.07 | 2.73 |
| 2 | 25 | 22 | 20 | 78.98 | 77.95 | 1.02 | 58.44 | 57.20 | 1.24 |
| 3 | 45 | 22 | 20 | 123.27 | 122.53 | 0.74 | 61.27 | 60.90 | 0.37 |
| 4 | 35 | 14 | 12 | 102.23 | 102.57 | −0.34 | 39.60 | 37.81 | 1.79 |
| 5 | 25 | 22 | 20 | 77.35 | 77.95 | −0.60 | 57.36 | 57.20 | 0.16 |
| 6 | 25 | 22 | 4 | 78.98 | 79.07 | −0.084 | 52.46 | 55.98 | −3.52 |
| 7 | 5 | 22 | 20 | 15.55 | 14.80 | 0.76 | 56.37 | 53.50 | 2.87 |
| 8 | 25 | 22 | 20 | 76.82 | 77.95 | −1.13 | 55.37 | 57.20 | −1.83 |
| 9 | 35 | 14 | 28 | 98.81 | 100.01 | −1.20 | 39.02 | 39.03 | −0.011 |
| 10 | 25 | 22 | 36 | 79.08 | 77.49 | 1.59 | 59.67 | 58.42 | 1.26 |
| 11 | 15 | 30 | 12 | 47.58 | 47.88 | −0.30 | 76.81 | 75.37 | 1.44 |
| 12 | 15 | 14 | 28 | 47.92 | 49.10 | −1.18 | 32.58 | 35.33 | −2.75 |
| 13 | 35 | 30 | 28 | 101.12 | 102.25 | −1.13 | 79.25 | 80.29 | −1.03 |
| 14 | 25 | 22 | 20 | 78.81 | 77.95 | 0.85 | 56.50 | 57.20 | −0.70 |
| 15 | 25 | 38 | 20 | 78.57 | 77.86 | 0.71 | 95.33 | 98.45 | −3.13 |
| 16 | 25 | 22 | 20 | 78.30 | 77.95 | 0.34 | 57.70 | 57.20 | 0.50 |
| 17 | 15 | 14 | 12 | 47.84 | 48.21 | −0.37 | 33.91 | 34.11 | −0.21 |
| 18 | 25 | 22 | 20 | 78.98 | 77.95 | 1.02 | 59.00 | 57.20 | 1.80 |
| 19 | 25 | 6 | 20 | 76.74 | 75.95 | 0.79 | 14.97 | 15.95 | −0.97 |
| 20 | 15 | 30 | 28 | 47.71 | 48.87 | −1.17 | 76.58 | 76.59 | −9.537 × 10−3 |
| Pb2+ Ions | DCF-Na | ||
|---|---|---|---|
| Pseudo-first order | qe (mg/g) | 131.27 | 85.18 |
| k1 (min−1) | 0.02562 | 0.01112 | |
| R2 | 0.984 | 0.997 | |
| SSE | 9.82 | 3.72 | |
| χ2 | 0.08 | 0.05 | |
| ARE | 0.97 | 0.96 | |
| HQC | 5.28 | 0.43 | |
| Pseudo-second order | qe (mg/g) | 139.06 | 102.06 |
| k1 (min−1) | 0.000356 | 0.000118 | |
| R2 | 0.980 | 0.991 | |
| SSE | 12.71 | 12.52 | |
| χ2 | 0.10 | 0.17 | |
| ARE | 0.96 | 1.89 | |
| HQC | 6.57 | 6.49 | |
| Two-compartment , Ffast + Fslow = 1 | qe (mg/g) | 132.02 | 86.16 |
| Ffast | 0.425 | 0.084 | |
| kfast (min−1) | 0.247 | 0.212 | |
| Fslow | 0.575 | 0.916 | |
| kslow (min−1) | 0.016 | 0.010 | |
| R2 | 0.993 | 0.999 | |
| SSE | 4.64 | 0.86 | |
| χ2 | 0.04 | 0.01 | |
| ARE | 0.53 | 0.41 | |
| HQC | 4.39 | −4.06 | |
| Film diffusion | kfd (min−1) | 0.0083 | 0.0126 |
| R2 | 0.899 | 0.996 |
| Pb2+ Ions | DCF-Na | ||
|---|---|---|---|
| Langmuir | qm,L (mg/g) | 125.68 | 388.35 |
| KL (L/mg) | 2.36 | 0.04 | |
| R2 | 0.907 | 0.837 | |
| SSE | 868.38 | 739.89 | |
| χ2 | 20.31 | 15.79 | |
| ARE | 24.90 | 23.69 | |
| HQC | 27.69 | 26.89 | |
| Freundlich | nF | 2.69 | 0.99 |
| KL (mg/g)(mg/L)1/nF | 65.84 | 12.05 | |
| R2 | 0.871 | 0.810 | |
| SSE | 1262.84 | 878.98 | |
| χ2 | 27.29 | 13.25 | |
| ARE | 33.39 | 18.84 | |
| HQC | 29.56 | 27.75 | |
| Hill | qm,H (mg/g) | 129.11 | 177.18 |
| KH | 0.49 | 20.05 | |
| nH | 0.94 | 1.52 | |
| R2 | 0.909 | 0.849 | |
| SSE | 849.74 | 692.16 | |
| χ2 | 20.25 | 11.86 | |
| ARE | 25.39 | 18.16 | |
| HQC | 28.53 | 27.51 | |
| Redlich–Peterson | KRP (L/g) | 314.28 | 13.89 |
| α (L/mg)β | 2.652 | 0.135 | |
| β | 0.96 | 0.04 | |
| R2 | 0.911 | 0.811 | |
| SSE | 839.61 | 873.70 | |
| χ2 | 20.22 | 13.26 | |
| ARE | 25.20 | 18.99 | |
| HQC | 28.47 | 28.67 |
| Sorbent | Sorption Solution | Maximum Sorption Capacity (mg/g) for Pb | Isotherm | Maximum Sorption Capacity (mg/g) for DCF-Na | Isotherm | Reference |
|---|---|---|---|---|---|---|
| Eucalyptus wood biochar | monocomponent | - | - | 33.13 | Sips | [5] |
| Hyper-cross-linked polymer (HCP) | multicomponent: Pb, Ni, and Cd | 173.10 | RSM optimization | - | - | [4] |
| Magnetic GO/ ZIF-8/γ-AlOOH | monocomponent | - | - | 2594.3 | Langmuir | [6] |
| Magnetic amine-functionalized chitosan | monocomponent | - | - | 469.48 | Langmuir | [23] |
| Peanut hull-g-methyl methacrylate biopolymer | monocomponent | 370.40 | Langmuir | - | - | [24] |
| Activated carbon from cocoa pod husk | monocomponent | - | - | 5.67 | Experimental | [25] |
| Activated carbon | monocomponent | - | - | 180 | Langmuir | [26] |
| Cross-linked chitosan beads grafted with polyethylenimine | monocomponent | - | - | 253.32 | Langmuir | [27] |
| Polyethyleneimine (PEI)-modified chitosan magnetic hydrogel | multicomponent: Pb, Ni, and Cu | 100.32 | Langmuir | - | - | [28] |
| Magnetic iron oxide-silica shell nanocomposite | monocomponent | 17.1 | Langmuir | - | - | [29] |
| Graphene oxide-silica-chitosan adsorbent | monocomponent | 256.41 | Langmuir | - | - | [30] |
| Ordered mesoporous silica nanoparticles of MCM-41 type | monocomponent | 22.2 | Sips | - | - | [31] |
| Chemically modified silica monolith | monocomponent | 574.71 | Langmuir | [32] | ||
| IS/(PEI/PMAA)c (r = 1.0) | multicomponent: Pb and DCF-Na | 125.68 | Langmuir | 388.35 | Langmuir | This study |
| qPb | qDCF-Na | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Sum of Squares | df | Mean Square | F-Value | p-Value |
| Model | 11,766.72 | 9 | 1307.41 | 856.07 | <0.0001 | 6868.48 | 3 | 2289.49 | 577.59 | <0.0001 |
| A-C0 Pb | 11,606.63 | 1 | 11,606.63 | 7599.82 | <0.0001 | 54.77 | 1 | 54.77 | 13.82 | 0.0019 |
| B-C0 DCF-Na | 3.67 | 1 | 3.67 | 2.40 | 0.1521 | 6807.79 | 1 | 6807.79 | 1717.47 | <0.0001 |
| C-T | 2.47 | 1 | 2.47 | 1.62 | 0.2319 | 5.92 | 1 | 5.92 | 1.49 | 0.2393 |
| AB | 3.05 | 1 | 3.05 | 2.00 | 0.1877 | |||||
| AC | 5.93 | 1 | 5.93 | 3.89 | 0.0770 | |||||
| BC | 5.288 × 10−3 | 1 | 5.288 × 10−3 | 3.462 × 10−3 | 0.9542 | |||||
| A2 | 135.62 | 1 | 135.62 | 88.80 | <0.0001 | |||||
| B2 | 1.72 | 1 | 1.72 | 1.13 | 0.3129 | |||||
| C2 | 0.17 | 1 | 0.17 | 0.11 | 0.7473 | |||||
| Residual | 15.27 | 10 | 1.53 | 63.42 | 16 | 3.96 | ||||
| Lack of Fit | 11.06 | 5 | 2.21 | 2.63 | 0.1561 | 54.75 | 11 | 4.98 | 2.87 | 0.1273 |
| Pure Error | 4.21 | 5 | 0.84 | 8.68 | 5 | 1.74 | ||||
| R2 | 0.9987 | 0.9909 | ||||||||
| Adjusted R2 | 0.9975 | 0.9891 | ||||||||
| Predicted R2 | 0.9920 | 0.9835 | ||||||||
| Adequate precision | 123.287 | 92.668 | ||||||||
| qPb | qDCF-Na | |||
|---|---|---|---|---|
| Factor | Coefficient Estimate | Standard Error | Coefficient Estimate | Standard Error |
| Intercept | 77.95 | 0.49 | 57.20 | 0.45 |
| A - C0, Pb | 26.93 | 0.31 | 1.85 | 0.50 |
| B - C0, DCF-Na | 0.48 | 0.31 | 20.63 | 0.50 |
| C - T | −0.39 | 0.31 | 0.61 | 0.50 |
| AB | 0.62 | 0.44 | ||
| AC | −0.86 | 0.44 | ||
| BC | 0.026 | 0.44 | ||
| A2 | −2.32 | 0.25 | ||
| B2 | −0.26 | 0.25 | ||
| C2 | 0.082 | 0.25 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morosanu, I.; Bucatariu, F.; Fighir, D.; Paduraru, C.; Mihai, M.; Teodosiu, C. Optimization of Lead and Diclofenac Removal from Aqueous Media Using a Composite Sorbent of Silica Core and Polyelectrolyte Coacervate Shell. Polymers 2023, 15, 1948. https://doi.org/10.3390/polym15081948
Morosanu I, Bucatariu F, Fighir D, Paduraru C, Mihai M, Teodosiu C. Optimization of Lead and Diclofenac Removal from Aqueous Media Using a Composite Sorbent of Silica Core and Polyelectrolyte Coacervate Shell. Polymers. 2023; 15(8):1948. https://doi.org/10.3390/polym15081948
Chicago/Turabian StyleMorosanu, Irina, Florin Bucatariu, Daniela Fighir, Carmen Paduraru, Marcela Mihai, and Carmen Teodosiu. 2023. "Optimization of Lead and Diclofenac Removal from Aqueous Media Using a Composite Sorbent of Silica Core and Polyelectrolyte Coacervate Shell" Polymers 15, no. 8: 1948. https://doi.org/10.3390/polym15081948
APA StyleMorosanu, I., Bucatariu, F., Fighir, D., Paduraru, C., Mihai, M., & Teodosiu, C. (2023). Optimization of Lead and Diclofenac Removal from Aqueous Media Using a Composite Sorbent of Silica Core and Polyelectrolyte Coacervate Shell. Polymers, 15(8), 1948. https://doi.org/10.3390/polym15081948









