Study of the Mechanical Properties and Microstructure of Alkali-Activated Fly Ash–Slag Composite Cementitious Materials
Abstract
1. Introduction
2. Experimental Design
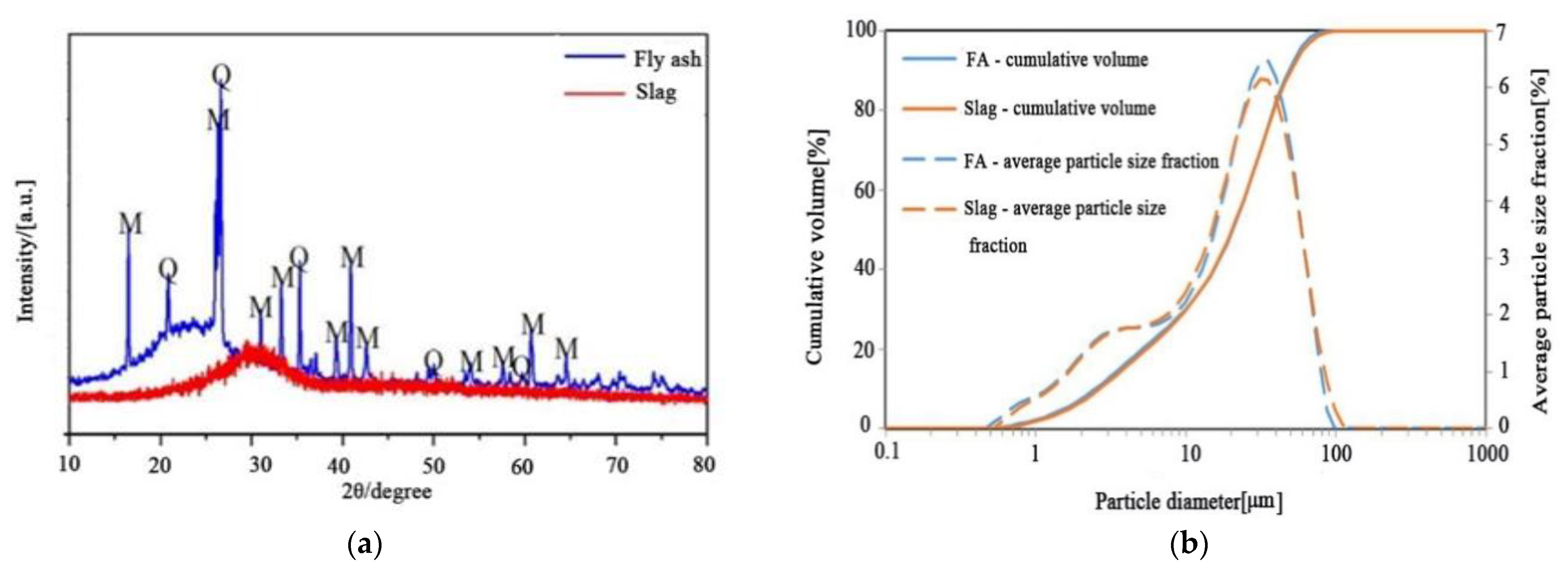
2.1. Materials
2.2. Specimen Preparation and Mixed-Ratio Design
2.3. Test Methods
2.3.1. Compressive Strength Test
2.3.2. Reaction Heat Test
2.3.3. Phase Composition Test
2.3.4. Pore Structure Test
2.3.5. Micromorphology Test
3. Results and Discussion
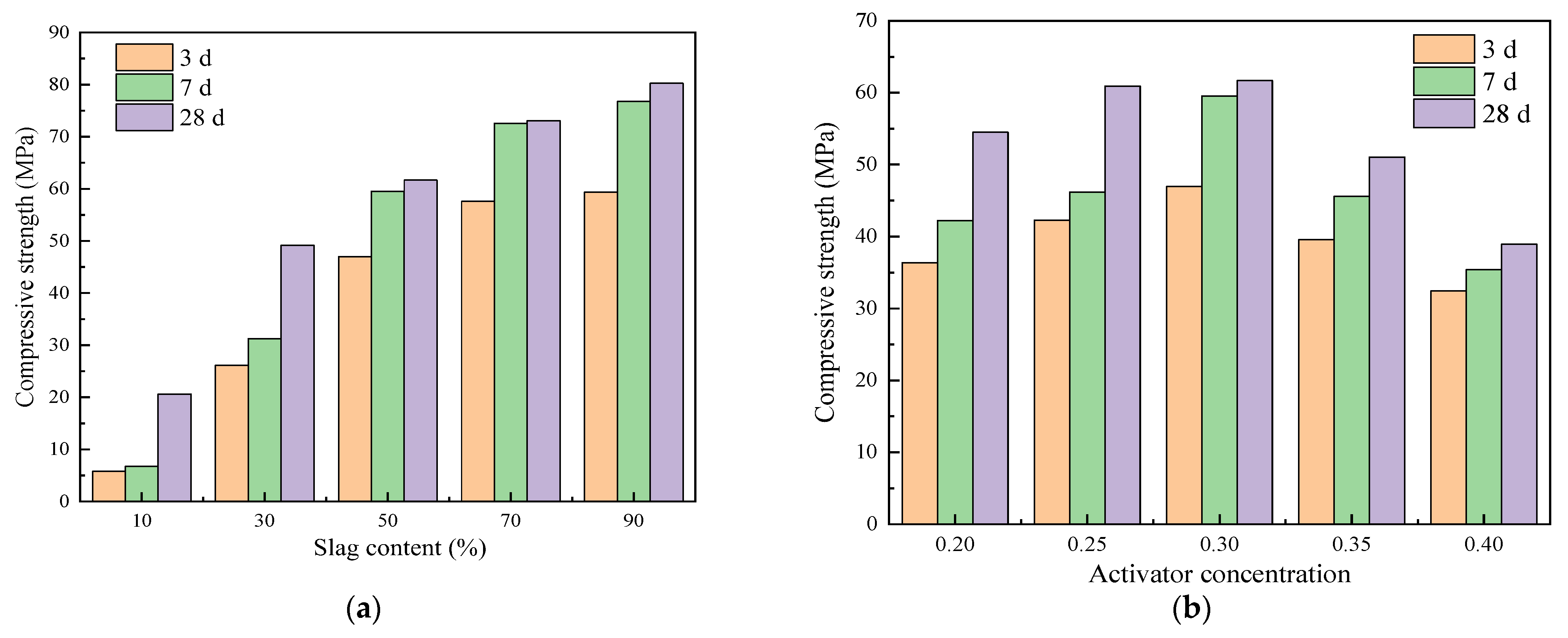
3.1. Compressive Strength Analysis
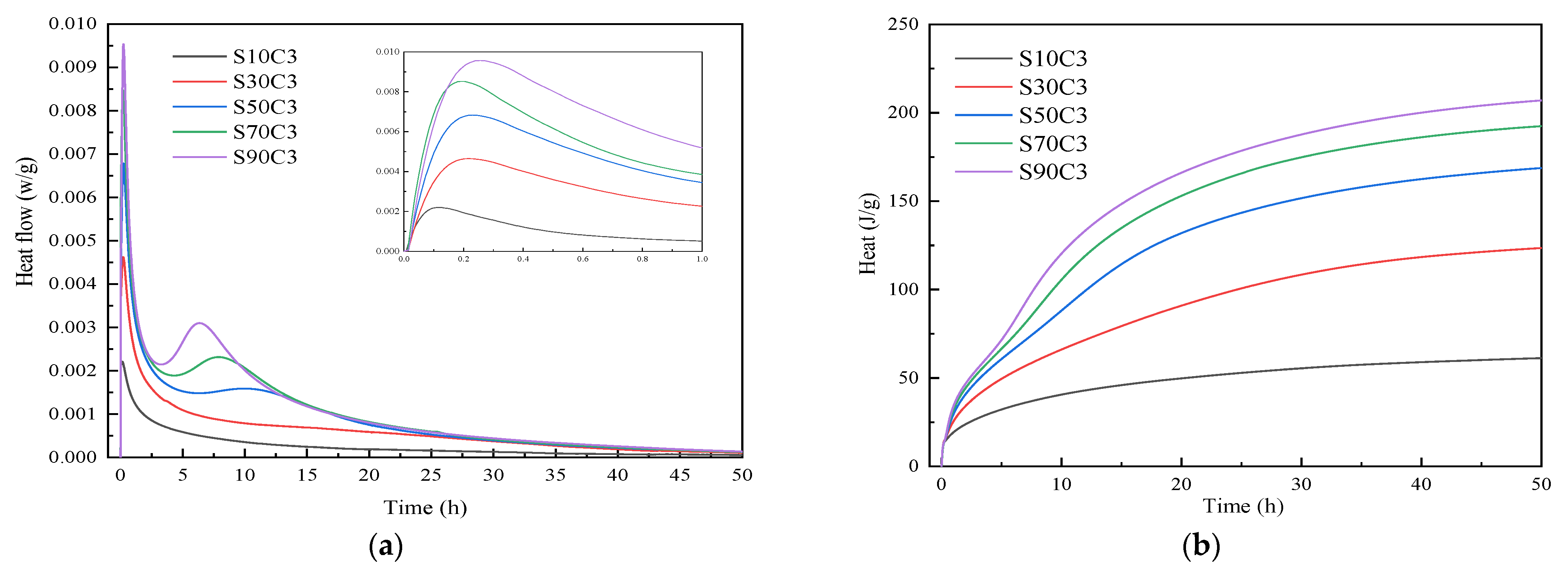
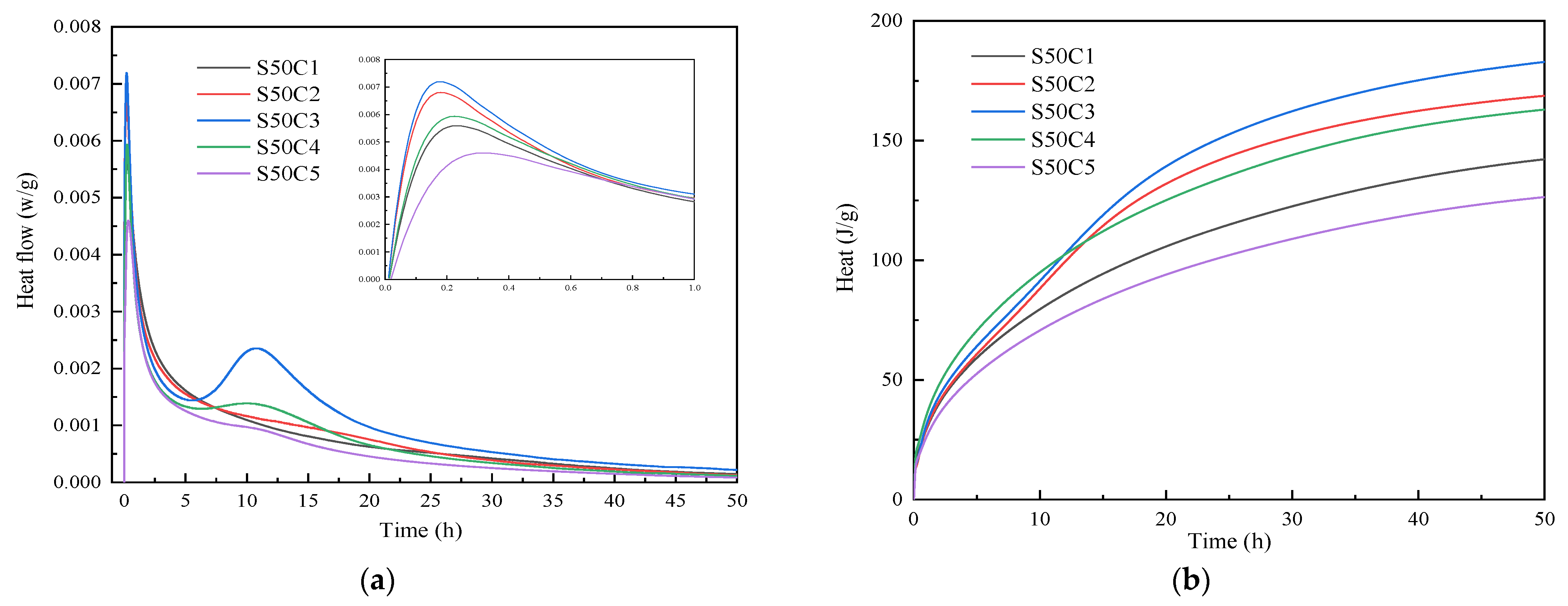
3.2. Reaction Rate Analysis
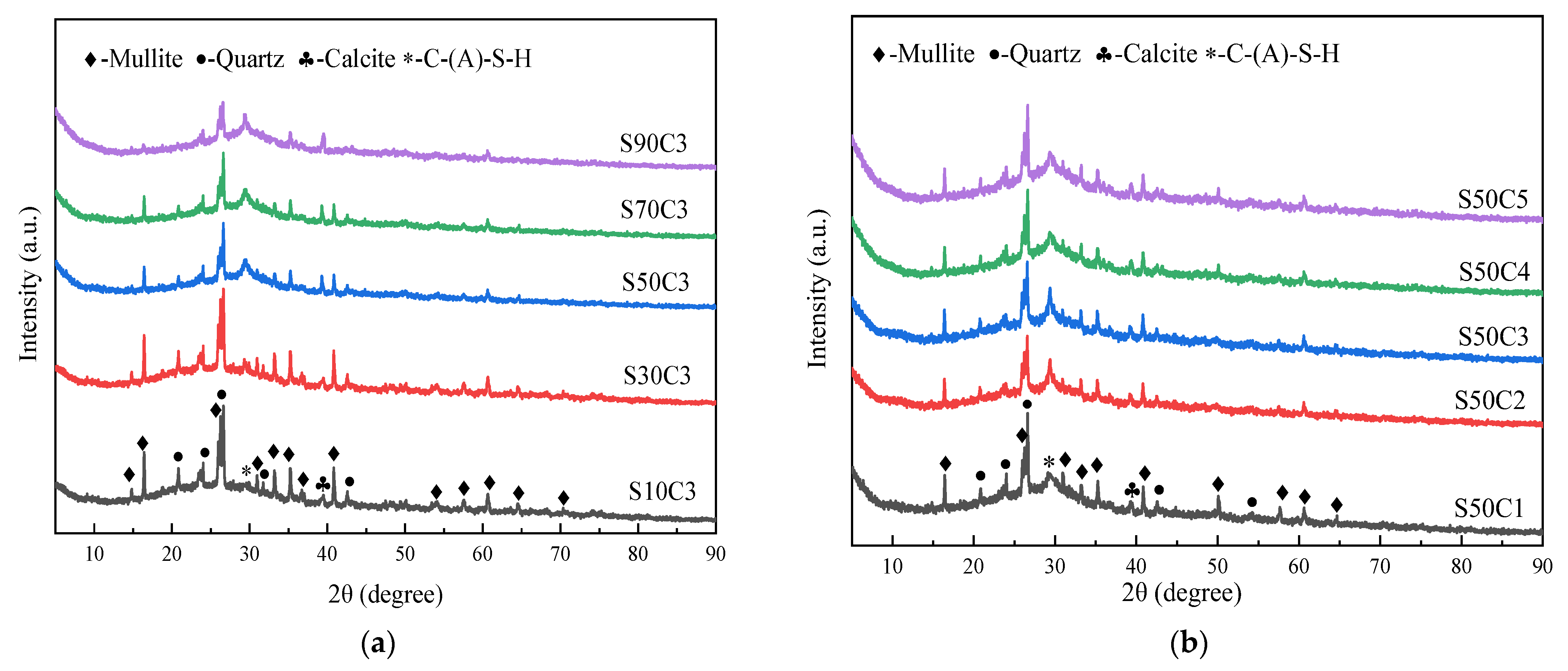
3.3. X-ray Diffraction Analysis
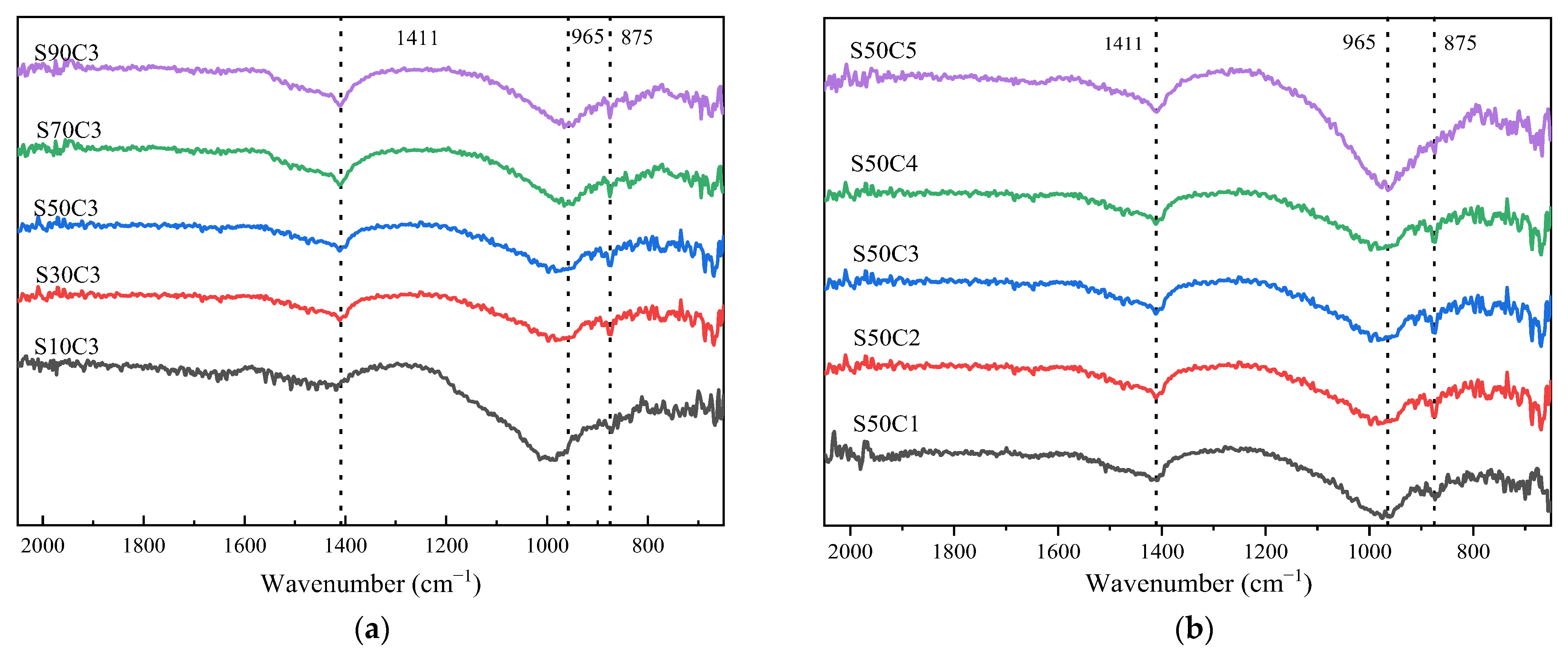
3.4. Fourier Infrared Spectroscopy Analysis
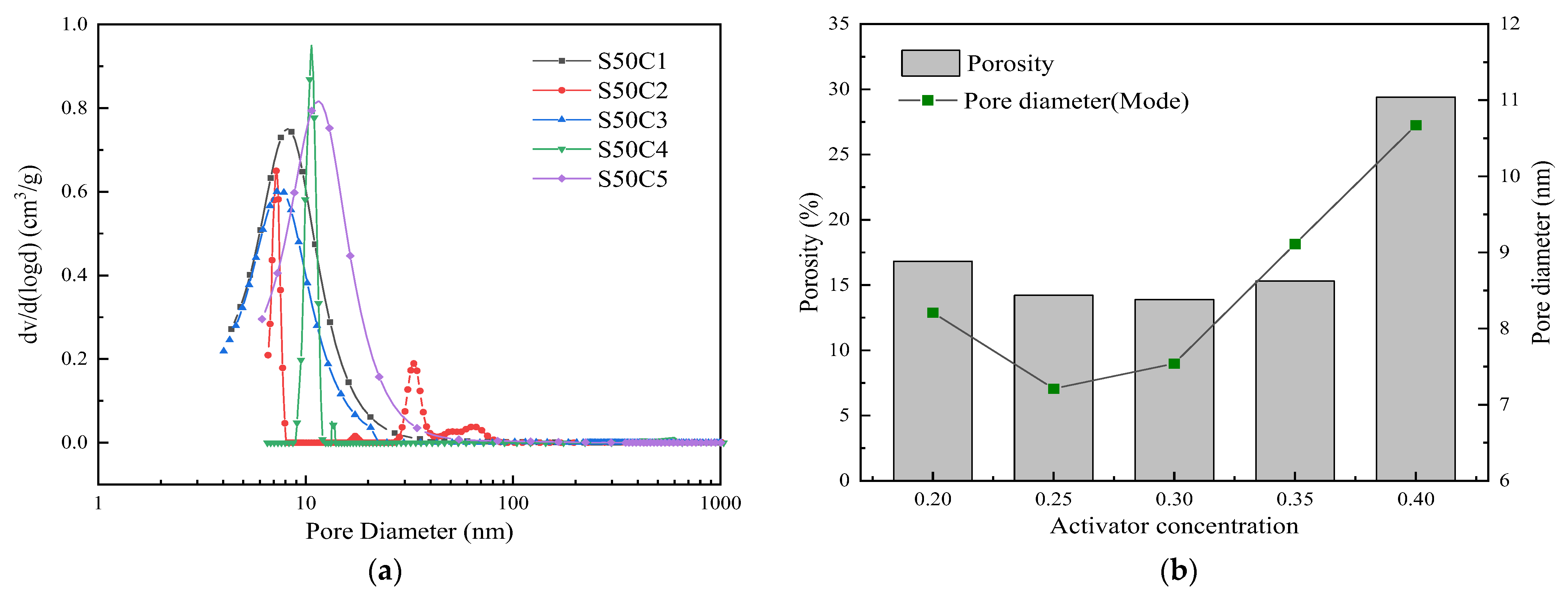
3.5. Pore Structure Analysis
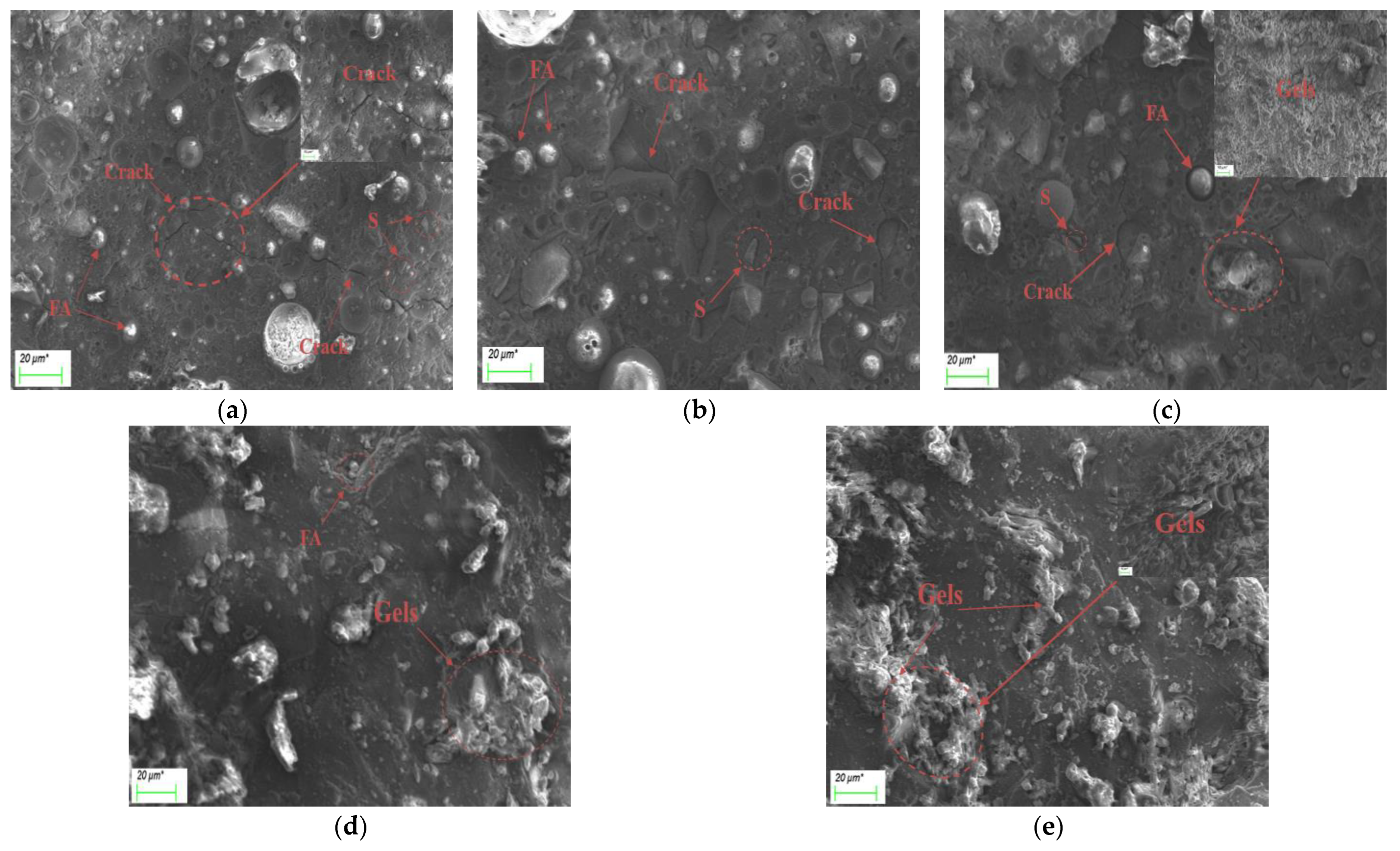
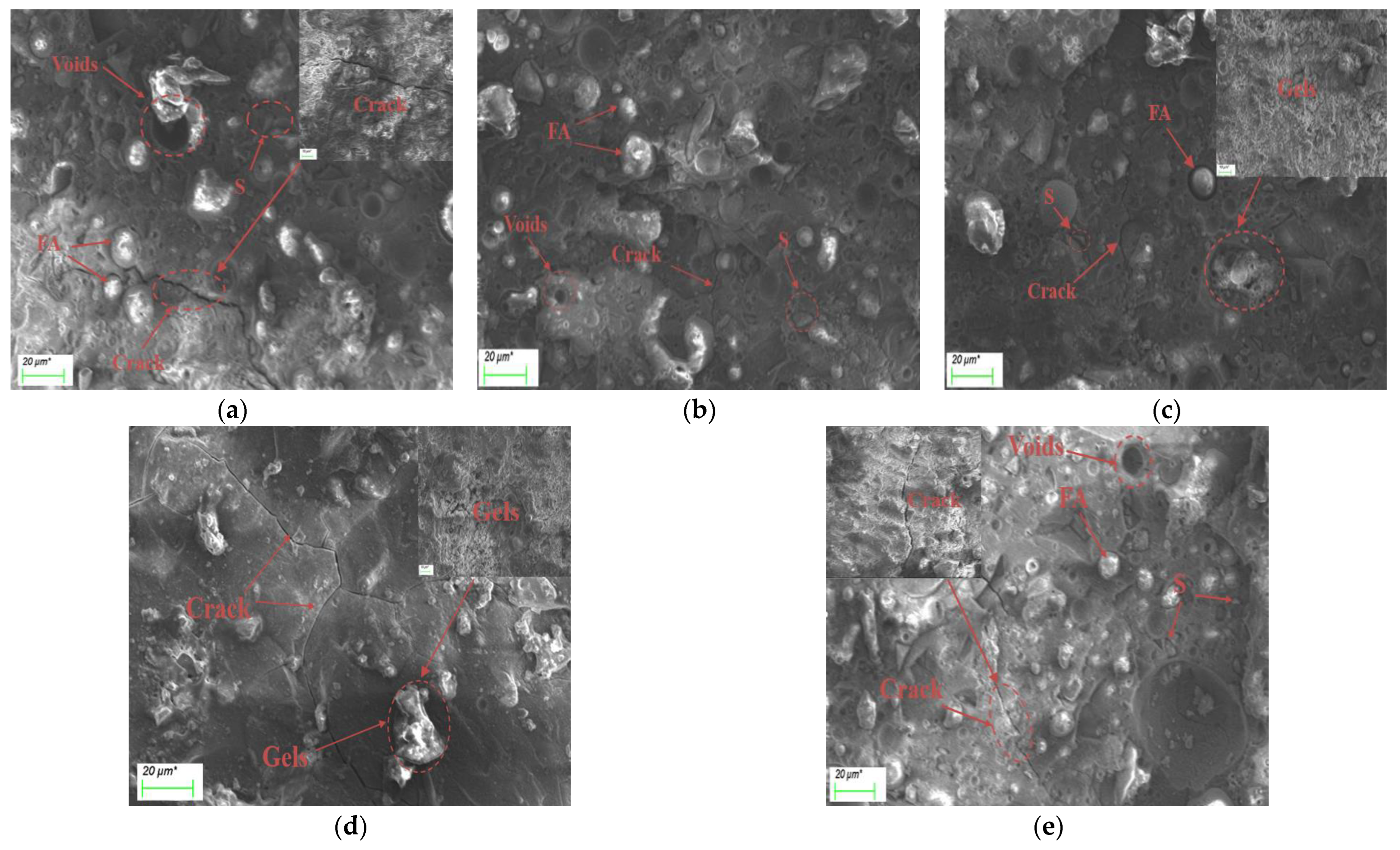
3.6. Microscopic Morphology Analysis
4. Conclusions
- The compressive strength of alkali-activated cementitious materials increases as the curing age increases. The increased curing age increases the degree of hydration reaction of alkali-activated cementitious materials, and more hydration products are generated, thereby improving the compressive strength. Moreover, the hydration reaction is relatively rapid in the early stage and is relatively slow in the later stage, showing the characteristics of early strength.
- The amount of slag is the main influencing factor in the compressive strength of alkali-activated cementitious materials, and the compressive strength of alkali-activated cementitious materials tends to increase with an increase in slag content, showing higher mechanical properties. Compared with slag, the activator concentration has less of an effect on the compressive strength of alkali-activated cementitious materials. As the activator concentration increases from 0.20 to 0.40, it first increases and then decreases, and the compressive strength reaches its maximum at 0.30.
- Based on the analysis of the results of the microstructure test, it was found that the increase in slag content accelerates the hydration reaction rate of alkali-activated cementitious materials. It promotes the formation of a large number of C-(A)-S-H and N-A-S-H gels, refines the pore size distribution, and forms a denser and more uniform microstructure, thereby improving the strength of the cementitious material. The increase in the activator concentration improves the alkalinity of the activator. This promotes the production of more gel products and increases the strength of the cementitious material. When the activator concentration increases to a certain value, the strength of the alkali-activated cementitious material begins to decrease; when the activator concentration is too large or too small, this will hinder the hydration reaction and affect the development of strength.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roy, D.M. Alkali-activated cements opportunities and challenges. Cem. Concr. Res. 1999, 29, 249–254. [Google Scholar] [CrossRef]
- Autef, A.; Joussein, E.; Gasgnier, G.; Rossignol, S. Role of the silica source on the geopolymerization rate. J. Non. Cryst. Solids. 2012, 358, 2886–2893. [Google Scholar] [CrossRef]
- Yang, N.R. Physicochemical basis of alkaline cementitious material formation (I). J. Chin. Chem. Soc. 1996, 02, 209–215. [Google Scholar]
- Wan, Q.; Rao, F.; Song, S.; García, R.E.; Estrella, R.M.; Patiño, C.L.; Zhang, Y. Geopolymerization reaction, microstructure and simulation of metakaolin-based geopolymers at extended Si/Al ratios. Cem. Concr. Compos. 2017, 79, 45–52. [Google Scholar] [CrossRef]
- Fang, G.; Ho, W.K.; Tu, W.; Zhang, M. Workability and mechanical properties of alkali-activated fly ash-slag concrete cured at ambient temperature. Constr. Build. Mater. 2018, 172, 476–487. [Google Scholar] [CrossRef]
- Kong, D.Y.; Zhang, J.Z.; Ni, T.Y.; Jiang, J.; Fang, C. Research progress of alkali-activated binders and concrete. J. Chin. Chem. Soc. 2009, 37, 151–159. [Google Scholar]
- Ishwarya, G.A.; Singh, B.; Deshwal, S.; Bhattacharyya, S.K. Effect of sodium carbonate/sodium silicate activator on the rheology, geopolymerization and strength of fly ash/slag geopolymer pastes. Cem. Concr. Compos. 2019, 97, 226–238. [Google Scholar]
- Al-Majidi, M.H.; Lampropoulos, A.; Cundy, A.; Meikle, S. Development of geopolymer mortar under ambient temperature for in situ applications. Constr. Build. Mater. 2016, 120, 198–211. [Google Scholar] [CrossRef]
- Lu, H.; Zhao, R.H.; Zhou, X.Y.; Mei, C. Research on electrical resistivity stability and compressive strength of different materials and modulus of water glass. Concrete 2019, 12, 11–17. [Google Scholar]
- Wu, Y.; Lu, B.; Bai, T.; Wang, H.; Du, F.; Zhang, Y.; Cai, L.; Jiang, C.; Wang, W. Geopolymer, green alkali activated cementitious material: Synthesis, applications and challenges. Constr. Build. Mater. 2019, 224, 930–949. [Google Scholar] [CrossRef]
- Shi, C.; Jimenez, A.F.; Palomo, A. New cements for the 21st century: The pursuit of an alternative to Portland cement. Cem. Concr. Res. 2011, 41, 750–763. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S. Geopolymer technology: The current state of the art. J. Mater. Sci. Technol. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Chi, M. Effects of modulus ratio and dosage of alkali-activated solution on the properties and micro-structural characteristics of alkali-activated fly ash mortars. Constr. Build. Mater. 2015, 99, 128–136. [Google Scholar] [CrossRef]
- Tong, G.Q.; Zhang, W.Y.; Gao, Y.T.; Tang, X.Y. Mechanical Properties and Micromechanism of Alkali-activated Fly Ash Geopolyme. Mater. Rev. 2022, 36, 129–134. [Google Scholar]
- Chen, Y.; Han, T.Y.; Deng, Y.F. Review of Fundamental Researches on Fly Ash Based Geopolymer. J. Chin. Ceram Soc. 2015, 34, 1864–1870. [Google Scholar]
- Nguyen, H.T.; Dang, P.T. Fly Ash-Based Geopolymer: Green Material in Carbon-Constrained Society. Solid State Phenomena. Solid State Phenom. 2021, 321, 65–71. [Google Scholar]
- Li, Q.H.; Ding, T.T.; Chen, S.D. Early Property and High Temperature Resistance of Alkali Activated System of Fly Ash-Slag. J. Chin. Ceram. Soc. 2017, 36, 365–368+373. [Google Scholar]
- Nath, P.; Sarker, P.K. Effect of GGBFS on setting, workability and early strength properties of fly ash geopolymer concrete cured in ambient condition. Constr. Build. Mater. 2014, 66, 163–171. [Google Scholar] [CrossRef]
- Ling, Y.; Wang, K.; Li, W.; Shi, G.; Lu, P. Effect of slag on the mechanical properties and bond strength of fly ash-based engineered geopolymer composites. Compos. B. Eng. 2019, 164, 747–757. [Google Scholar] [CrossRef]
- Yang, S.Y.; Zhao, R.D.; Jin, H.S.; Li, F.H. Analysis on Mechanical Properties and Fractal Characteristics of Micropore Structure of Geopolymer Mortar. J. South China Univ. Technol. (Nat. Sci. Ed.) 2020, 48, 126–135. [Google Scholar]
- Huang, H.; Guo, M.X.; Zhang, W.; Yang, S.L. Mechanical property and microstructure of geopolymer concrete based on fly ash and slag. J. Harbin Inst. Technol. 2022, 54, 74–84. [Google Scholar]
- Huang, K.; Ma, Y.H.; Guo, Y.Q.; Li, Z.H. Properties of Alkali-activated Fly Ash /Slag Composite System. J. Chin. Ceram Soc. 2015, 34, 2769–2774. [Google Scholar]
- Nath, S.K.; Kumar, S. Influence of granulated silico-manganese slag on compressive strength and microstructure of ambient cured alkali-activated fly ash binder. Waste Biomass Valorization 2019, 10, 2045–2055. [Google Scholar] [CrossRef]
- Deb, P.S.; Nath, P.; Sarker, P.K. The effects of ground granulated blast-furnace slag blending with fly ash and activator content on the workability and strength properties of geopolymer concrete cured at ambient temperature. Mater. Des. 2014, 62, 32–39. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Rose, V.; De Gutierrez, R.M. Evolution of binder structure in sodium silicate-activated slag-metakaolin blends. Cem. Concr. Compos. 2011, 33, 46–54. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, C.; Wang, X.; Zhang, T.; Wang, Q. Response surface methodology for multi-objective optimization of fly ash-GGBS based geopolymer mortar. Constr. Build. Mater. 2022, 315, 125644. [Google Scholar] [CrossRef]
- Hojati, M.; Radlinska, A. Shrinkage and strength development of alkali-activated fly ash-slag binary cements. Constr. Build. Mater. 2017, 150, 808–816. [Google Scholar] [CrossRef]
- Gao, X.; Yu, Q.L.; Brouwers, H.J.H. Reaction kinetics, gel character and strength of ambient temperature cured alkali activated slag-fly ash blends. Constr. Build. Mater. 2015, 80, 105–115. [Google Scholar] [CrossRef]
- Chi, M.C.; Huang, R. Binding mechanism and properties of alkali-activated fly ash/slag mortars. Constr. Build. Mater. 2013, 40, 291–298. [Google Scholar] [CrossRef]
- Lee, N.K.; Lee, H.K. Setting and mechanical properties of alkali-activated fly ash/slag concrete manufactured at room temperature. Constr. Build. Mater. 2013, 47, 1201–1209. [Google Scholar] [CrossRef]
- Elyamany, H.E.; Abd Elmoaty, M.; Elshaboury, A.M. Setting time and 7-day strength of geopolymer mortar with various binders. Constr. Build. Mater. 2018, 187, 974–983. [Google Scholar] [CrossRef]
- Tänzer, R.; Jin, Y.; Stephan, D. Alkali activated slag binder: Effect of cations from silicate activators. Mater. Struct. 2017, 50, 1–9. [Google Scholar] [CrossRef]
- Celikten, S.; Sarıdemir, M.; Deneme, I.Ö. Mechanical and microstructural properties of alkali-activated slag and slag + fly ash mortars exposed to high temperature. Constr. Build. Mater. 2019, 217, 50–61. [Google Scholar] [CrossRef]
- Yang, D.; Pang, L.X.; Song, D.; Lu, M.; Wang, J.; Guan, Z. Reaction Mechanism of Fly Ash in Alkali-Activated Slag/Fly Ash System. J. Chin. Ceram Soc. 2021, 40, 3005–3011. [Google Scholar]
- JGJ/T 70–2009; Standard for Test Methods of Basic Performance of Construction Mortar. China Architecture & Building Press: Beijing, China, 2009.
- Wang, H.; Li, H.; Yan, F. Synthesis and mechanical properties of metakaolinite-based geopolymer. Colloids Surf. A Physicochem. Eng. Asp. 2005, 268, 1–6. [Google Scholar] [CrossRef]
- Liu, J.Q.; Wang, S.Y.; Peng, H.; Zhong, Q.Y.; Lin, F.K. Study on the effect of alkali activator on the properties of fly ash-based geopolymer. J. Transp. Sci. Eng. 2020, 4, 8–13. [Google Scholar]
- Yu, P.; Kirkpatrick, R.J.; Poe, B.; McMillan, P.F.; Cong, X. Structure of calcium silicate hydrate (C-S-H): Near-, Mid-, and Far-infrared spectroscopy. J. Am. Ceram. Soc. 1999, 82, 742–748. [Google Scholar] [CrossRef]
- Yunsheng, Z.; Wei, S.; Qianli, C.; Lin, C. Synthesis and heavy metal immobilization behaviors of slag based geopolymer. J. Hazard. Mater. 2007, 143, 206–213. [Google Scholar] [CrossRef]
- Song, W.L.; Zhu, Z.D.; Pu, S.Y.; Song, S.; Peng, Y.; Gu, X.; Wei, Y. Mechanical Performance and Micro-mechanism of Alkali-activated Binary/Ternary Composite Industrial Waste Residues Cementitious Materials. Mater. Rev. 2020, 34, 22070–22077. [Google Scholar]
- Zhan, J.H.; Li, H.B.; Fu, B.; Tuo, M. Effect of Different Alkali Equivalent Fly Ash and Slag Content on the Mechanical Properties and Microstructure of Alkali-activated Fly Ash-slag Geopolymer. Sci. Technol. Eng. 2021, 21, 12218–12224. [Google Scholar]
- Zhuang, X.Y.; Chen, L.; Komarneni, S.; Zhou, C.H.; Tong, D.S.; Yang, H.M.; Yu, W.H.; Wang, H. Fly ash-based geopolymer: Clean production, properties and applications. J. Clean. Prod. 2016, 125, 253–267. [Google Scholar] [CrossRef]

| Material | Mass Fraction/% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | Fe2O3 | MgO | SO3 | K2O | TiO2 | Others | |
| Fly ash | 2.66 | 55.71 | 32.80 | 4.43 | 0.23 | 0.65 | 1.54 | 1.66 | 0.32 |
| Slag | 44.06 | 30.23 | 13.72 | 0.41 | 5.58 | 3.16 | 0.50 | 1.79 | 0.55 |
| Group | Sample No. | Alkali-Activated Materials | Activator Concentration | Water-to-Binder Ratio | Modulus | |
|---|---|---|---|---|---|---|
| Fly Ash/% | Slag/% | |||||
| Slag content group | S10C3 | 90 | 10 | 0.30 | 0.35 | 1.2 |
| S30C3 | 70 | 30 | ||||
| S50C3 | 50 | 50 | ||||
| S70C3 | 30 | 70 | ||||
| S90C3 | 10 | 90 | ||||
| Activator concentration group | S50C1 | 50 | 50 | 0.20 | 0.35 | 1.2 |
| S50C2 | 0.25 | |||||
| S50C3 | 0.30 | |||||
| S50C4 | 0.35 | |||||
| S50C5 | 0.40 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Y.; Wang, C.; Han, W.; Li, X.; Peng, H. Study of the Mechanical Properties and Microstructure of Alkali-Activated Fly Ash–Slag Composite Cementitious Materials. Polymers 2023, 15, 1903. https://doi.org/10.3390/polym15081903
Lv Y, Wang C, Han W, Li X, Peng H. Study of the Mechanical Properties and Microstructure of Alkali-Activated Fly Ash–Slag Composite Cementitious Materials. Polymers. 2023; 15(8):1903. https://doi.org/10.3390/polym15081903
Chicago/Turabian StyleLv, Yigang, Cui Wang, Weiwei Han, Xing Li, and Hui Peng. 2023. "Study of the Mechanical Properties and Microstructure of Alkali-Activated Fly Ash–Slag Composite Cementitious Materials" Polymers 15, no. 8: 1903. https://doi.org/10.3390/polym15081903
APA StyleLv, Y., Wang, C., Han, W., Li, X., & Peng, H. (2023). Study of the Mechanical Properties and Microstructure of Alkali-Activated Fly Ash–Slag Composite Cementitious Materials. Polymers, 15(8), 1903. https://doi.org/10.3390/polym15081903







